Abstract
Mature green ‘Maradol’ papaya fruits were exposed to ultraviolet (UV)-C irradiation (1.48 kJ·m−2) and stored at 5 or 14 °C. Changes in total phenols, total flavonoids, enzymatic activities of superoxide dismutase (SOD), catalase (CAT) and peroxidase (POD), as well as the scavenging activity against 2,2-diphenyl-1picrylhydrazyl (DPPH) and 3-ethylbenzothiazoline-6-sulfonic acid (ABTS) radicals were investigated in peel and flesh tissues at 0, 5, 10 and 15 days of storage. UV-C irradiation increased significantly (P < 0.05) the flavonoid content (2.5 and 26 %) and ABTS radical scavenging activity (5.7 and 6 %) in flesh and peel at 14 °C respectively; and CAT activity (16.7 %) in flesh at 5 °C. Flavonoid contents, CAT and SOD activities were positively affected under low storage temperature (5 °C). DPPH and ABTS radical scavenging activities increased in both control and UV-C treated papaya peel during storage at 5 °C. UV-C irradiation effect on radical scavenging of papaya peel could be attributed to increased flavonoid content. Papaya antioxidant system was activated by UV-C and cold storage by increasing phenolic content and antioxidant enzymatic activities as a defense response against oxidative-stress.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The market for tropical fruits has increased in the last years due to an increasing consumer demand for exotic products, changes in diet habits, attractive sensorial properties and because they supply an optimal mixture of antioxidants. Tropical fruits contain polyphenols, carotenoids and vitamins C and E that could contribute to improve health by reducing incidence of chronic-degenerative diseases (Yahia 2010). Among tropical fruits, papaya is one of the most popular because of its characteristic taste and nutraceutical value (González-Aguilar et al. 2008). However, papaya fruits are very susceptible to deterioration and postharvest losses mainly by fungal decay, physiological disorders such as chilling injury, pests, mechanical injury and over-ripeness (Perez-Carrillo and Yahia 2004; da Silva et al. 2007; Chávez-Sánchez et al. 2011).
The main cause of postharvest deterioration of fruit is enhanced metabolism, whether due to natural senescence physiology or biotic or abiotic stress, which could be inhibited by controlling storage temperature and relative humidity of the atmosphere around the product (Azene et al. 2011). Controlled postharvest abiotic stresses, including temperature and Ultraviolet (UV)-B and (UV)-C irradiation, can be applied to fresh fruits to induce specific secondary metabolites synthesis with antioxidant properties (Cisneros-Zevallos 2003). Postharvest UV-C treatment induced accumulation of phytoalexins as a defense mechanism, which has been positively correlated with the resistance against different pathogens and the reduction of physiological disorders that occur during cold storage of fruits (Rodov et al. 1992; D’hallewin et al. 1999). UV-C irradiation could also delay deterioration processes, ripening and senescence in tropical fruit (Pongprasert et al. 2011; González-Aguilar et al. 2007b).
It is well known that secondary metabolism is activated together with the enzymatic antioxidant system. Exposure to UV irradiation and low temperatures induce oxidative stress in plant tissues by increasing reactive oxygen species (ROS) (Gonzalez-Aguilar et al. 2010; Matsui and Li 2003). To cope with this stress, the most effective protection mechanism stimulated under UV exposure is the accumulation of flavonoids and other UV-absorbing phenolic compounds with (Frohnmeyer and Staiger 2003). UV-C irradiation at hormetic doses (0.5–9.0 kJm2) has been proven to increase the activity of phenylalanine ammonia-lyase (PAL) (key enzyme in phenol biosynthesis) and the antioxidant capacities of fruit and vegetables (Erkan et al. 2008; Gonzalez-Aguilar et al. 2007a; Vunnam et al. 2012; Alothman et al. 2009; Goyal et al. 2011). When exposed to low temperatures, the plant antioxidant system is also activated at different levels, but the precise mechanisms remain to be elucidated (Karpinski et al. 2002).
Despite the large amount of reports on the positive effects of UV-C postharvest treatment for the activation of antioxidant defense mechanisms of fruits, there are no information on the combined effect of storage temperature and UV-C irradiation on the antioxidant system of papaya fruit. This study evaluated the effects of UV-C treatment in conjunction with low storage temperatures on bioactive compounds, antioxidant enzymes and radical scavenging activity of papaya fruit.
Materials and methods
Plant material
Mature-green ‘Maradol’ papayas were obtained from a wholesale market in Hermosillo, Sonora, Mexico. Fruits of uniform maturity stage (mature-green), size and free from defects were selected and randomized in two groups of 48 fruits. Half of the samples on each group were treated with UV-C and immediately stored for 15 days at 5 or 14 °C. Sampling was performed at 5 days intervals during storage (0, 5, 10, 15). Fruits were peeled with a sharp stainless steel manual peeler, frozen immediately and stored at −30 °C until use, to avoid sample degradation. Papaya flesh was cut into 5 × 5 cm cubes, frozen and stored at −30 °C. Every measurement was done in triplicate. The experimental unit was the peel or flesh of three fruits. The experiment was replicated twice.
Chemicals
Folin-ciocalteu reagent, aluminum chloride, sodium carbonate, sodium nitrate, sodium hydroxide, potassium persulphate, gallic acid, quercetin, β-mercaptoethanol, tris-hidrochloride, sodium acetate, potassium phosphate, L-methionine, EDTA, riboflavin, nitro blue tetrazolium (NBT), guaicol, bradford reagent, bovine serum albumin, 1-diphenyl-2-picrylhydrazyl (DPPH), 2, 2’-azinobis (3-ethylbenzothiazoline-6-sulfonic acid) (ABTS.-) were obtain from Sigma-Aldrich (St. Louis, MO, USA). Methanol (HPLC grade) and ethanol (reagent grade) were purchased from JT Baker (Xalostoc, Edo. Mex., Mex.).
UV-C treatment
Papaya fruits were washed and sanitized by immersion in hypochlorite solution (200 ppm free chlorine) and let to dry at room temperature before exposure to a UV-C dose in order to eliminate impurities and reduce fungal attack risk that could influence treatment effect. UV-C irradiation exposure for 3 min was selected since does not caused visible scalds, according to preliminary experiments carried out in our laboratory (data not shown). UV-C treatment was applied using two unfiltered General Electric 15 Watts (G15 T8) germicidal lamps placed 15 cm above and below fruits. Integral value of spectral irradiance for the wavelength range of 250–280 nm was determined as 8,220 mWm−2 using an Optronic Model 752 UV–vis spectroradiometer (Optronic Laboratories Inc., Orlando, FL, USA). Total energy administered during 3 min was 1.48 kJ·m−2. Immediately after treatment, control and treated fruits were stored for 15 days at 5 or 14 °C in the absence of light.
Total phenols and flavonoids content
Phenolic compounds of papaya were extracted from 5 or 10 g of peel or flesh tissue, respectively. Those were homogenized in 20 mL of methanol solution (80 %). The homogenate was sonicated for 30 min at 40 °C, and then centrifuged at 9,400 ×g for 15 min at 5 °C in an Allegra 64R Beckman Coulter centrifuge (Palo Alto, Calif., USA). Supernatants were collected after filtered through Whatman paper (grade1). Same procedure was repeated 3 times, these extracts were used to measure total phenolics and flavonoid contents, as well as antioxidant capacity.
Total phenols were measured as reported by (González-Aguilar et al. 2007a), where 50 μL of extract were mixed with 3 mL of H2O and 250 μL of (1 N) Folin-Ciocalteu phenol reagent. After equilibrating for 5 min, 750 μL of 20 % Na2CO3 and 950 μL of H2O were added to the reaction mixture. After vortexing, the reaction was incubated for 30 min at room temperature protected from light. Then, absorbance was then measured at 765 nm in a UV–vis spectrophotometer (Cary50 Bio model, Varian, Italy). Total phenol content was calculated based on a gallic acid standard curve; results were expressed as mg of gallic acid per 100 g of fresh weight (mg ga/100gfw).
Flavonoid content was determined as described by (Zhishen et al. 1999) with some modifications. One mL of methanolic extract was mixed with 4 mL of H2O and 300 μL of 5 % NaNO2. After agitation followed by a 5 min incubation, 300 μL of 10 % AlCl3 were added and incubated for one more minute. Two mL of 1 M NaOH and H2O were added to make up 10 mL of reaction volume. After agitation, absorbance was measured at 415 nm, in a UV–vis spectrophotometer (Cary50 Bio model, Varian, Italy). Results were expressed as mg of quercetin equivalents per 100 g of fresh weight (mg quercetin/100 gfw).
Enzymatic activity assays
Catalase (EC. 1.11.1.6; CAT) activity was determined as described by (Blackwell et al. 1990) with the following modifications. Acetone powders were obtained by continuous homogenization of frozen sample tissue with pure chilled acetone and vacuum filtered until residue gets colorless and dry. The enzyme was extracted from 0.2 or 0.5 g of acetone powder from peel or flesh tissue, respectively. It was homogenized in 10 mL of 0.1 M Tris–HCl, pH 8.5, containing 5 mM β-mercaptoethanol. The mixture was then stirred for 20 min at 4 °C and centrifuged for 20 min at 12 000 ×g and 4 °C. The reaction mixture contained 3 mL of 10 mM Tris–HCl, pH 8.5 and 0.1 mL of 0.88 % H2O2 in 100 mM Tris–HCl; it was started by adding 0.2 mL of enzyme extract. CAT activity was monitored at 240 nm for 5 min at room temperature (24–26 °C) with a UV–vis spectrophotometer. One unit of CAT specific activity was reported as the decomposition of 1 μmol of H2O2/min·mg of protein.
Peroxidase (EC. 1.11.1.7; POD) activity was determined as described by (Pérez-Tello et al. 2009) with some modifications. Enzyme was extracted from 0.1 or 0.2 g of acetone powder from peel or flesh tissue, respectively, homogenized in 5 mL of 0.1 M Tris–HCl (pH 8.0), containing 5 mM β-mercaptoethanol. The mixture was stirred for 20 min at 4 °C, centrifuged for 30 min at 12 000 xg and 4 °C, and the supernatant was decanted. POD activity was measured at 470 nm for 2 min at 30 °C in 2.15 mL reaction solution with 10 mM sodium acetate (pH 5.3) containing 0.5 % guaiacol, 0.25 mL of 0.1 % H2O2 and 0.1 mL of enzyme extract. POD specific activity was reported as the decomposition of 1 mmol of guaicol/min·mg of protein.
Superoxide dismutase (1.15.1.1; SOD) activity was determined according to (Tejacal et al. 2005) with slight modifications. The enzyme was extracted from 0.2 g of acetone powder from peel or flesh tissue, homogenized in 10 mL of potassium phosphate at pH 7.8. The mixture was stirred for 20 min at 4 °C, centrifuged for 30 min at 12 000 xg and 4 °C and the supernatant was decanted. Reaction mixture consisted on 27 mL of 0.05 M phosphate buffer (pH 7.8) containing 0.1 mM EDTA, 1.5 mL of L-methionine solution (30 mg mL−1), 1 mL of nitroblue tetrazolium (1.41 mg mL−1) and 0.75 mL of X-100 triton solution (1 %). Following 0.03 mL of riboflavin solution (4.4 mg 100 mL−1) and 0.4 mL of enzyme extract were added to 3 mL of reaction mixture and homogenized. Then the reaction mixture was exposed to fluorescent light emitted by two lamps of 20 W for 15 min, and absorbance was measured at 560 nm on a UV–vis spectrophotometer (Cary Bio50, Varian, Italy). Reaction velocity was determined as absorbance increment due to nitroblue tetrazolium formazan formation per unit of time. One unit of SOD was defined as enzyme extract concentration that inhibits 50 % of nitroblue tetrazolium formazan formation. Assays were performed at room temperature (24–26 °C). SOD specific activity was expressed as units of activity per gram of protein (U·min−1·g protein−1).
Total protein concentration was determined according to (Bradford 1976) in all enzymatic extracts for specific enzymatic activity calculation using bovine serum albumin as standard.
Radical scavenging activities, DPPH• and ABTS•+
Radical scavenging activity of papaya was measured in methanolic extracts by the DPPH• (2,2-diphenyl-1picrylhydrazyl) and ABTS•+ methods, described by (Jimenez-Escrig et al. 2000) and (Re et al. 1999) with some modifications. For DPPH scavenging activity, 0.1 mL of methanolic extracts as used for phenol and flavonoid analyses was added to 3.9 mL of DPPH• methanolic solution (0.025 g/L). Fading of DPPH• color solution upon reduction was measured in a UV–vis spectrophotometer (Cary50 Bio model, Varian, Italy) at 515 nm after a 30 min room temperature dark incubation. Samples were substituted with 80 % methanol in the blank reaction, and methanol was used for baseline correction. Results were expressed as DPPH• radical inhibition percentage.
For the ABTS radical scavenging assay, 2.45 mmol of potassium persulphate were added to 7 mM ABTS dissolved in water, stirred and incubated for 12–16 h in darkness to give a dark blue solution. Solution was diluted with ethanol until absorbance reached 0.7 at 734 nm. 0.1 mL of sample extract was added to 3.9 mL of resulting radical solution in a quartz spectrophotometer cell. Reaction was monitored for 5 min until end point, when absorbance became stable. Sample was substituted with 80 % methanol for blank reaction, and ethanol was used for baseline correction. For comparison between methods, radical scavenging activity was expressed as ABTS•+ radical inhibition percentage.
Statistical analysis
Data were analyzed for UV-C treatment, storage temperature, storage time and type of tissue effects by analysis of variance (ANOVA) of general linear models (GLM) where differences were considered significant at a P < 0.05, based on a Duncan’s multiple comparison test using the NCSS (2007) statistical software. Values are expressed as mean of six replicate determinations (n=6) ± standard error.
Results and discussion
Phenolic and flavonoid contents
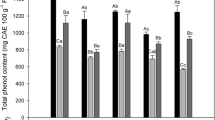
Total phenolic content (TPC) changes were evaluated in peel and flesh of papaya fruit stored at 5 and 14 °C in response to UV-C treatment. The TPC of papaya was significantly (P<0.05) affected by UV-C irradiation treatment, storage time and type of tissue analyzed. Phenolic contents were higher in control and treated papaya peels after 15 days of storage at 14 °C (5 and 33 % respectively) as compared with those stored at 5 °C. Although a decreasing tendency on TPC was observed in peel tissue through storage time (Table 1), no significant differences between days 5 and 10 were noticed. Also no significant (P>0.05) effect of storage temperature on TPC was found in papaya peel or flesh.
A similar tendency was found for TPC of peel and flesh tissues, at the beginning of storage, controls at 5 °C had the highest phenolic content. Increased TPC in UV-C treated papaya peel was observed only at day 10 of storage at 14 °C, as compared with controls.
Accumulation of phenolic compounds varied strongly in relation to fruit physiological state and results from the equilibrium between biosynthesis and further metabolism including turnover and catabolism (Oufedjikh et al. 2000). During ripening, a decreasing tendency on total phenols content as well as on antioxidant capacity has been reported on “Maradol” papaya peel and flesh (Gayosso-García Sancho et al. 2010, 2011). The effect of UV-C irradiation on phenylpropanoid metabolism has been described previously in various fruit, where phenylalanine ammonia-lyase (PAL), a key regulatory enzyme of phenylpropanoid biosynthetic route is activated by UV-C light, leading to enhanced phenolic contents (Stevens et al. 1998, 1999; Gonzalez-Aguilar et al. 2007a, b; Erkan et al. 2008; Gonzalez-Aguilar et al. 2010).
Total flavonoid content (TFC) of papaya fruit was significantly affected by UV-C treatment, type of analyzed tissue, storage temperature and time. At day 10, UV-C treated papaya peel and flesh, presented significantly higher flavonoid contents than controls at all storage temperatures. Cold storage (5 °C) had a positive effect on TFC, despite treatment or tissue analized, while a linear positive effect of storage time was observed reaching highest mean values at day 15 (Table 1). A positive effect of UV-C treatment was observed during storage at 14 °C in both tissues at days 10 and 15. Thus, cold storage had a bigger impact on TFC in both peel and flesh tissues of papaya than UV-C treatment.
It had been demonstrated that flavonoids can act as UV protective agents, participating in several plant resistance mechanisms by interfering with oxidative processes, both by chelating metal ions or by scavenging reactive oxygen species, forming less reactive “antioxidant radicals” that disappear by dismutation, recombination or reduction (Bors et al. 1992, 1998).
Our results are in accordance to previous research reporting that UV-C irradiation enhanced flavonoid content in kumquats and oranges (D’hallewin et al. 1999; Rodov et al. 1992), grapes (Cantos et al. 2001, 2003), broccoli (Costa et al. 2006), and mangoes (Gonzalez-Aguilar et al. 2007a). However, UV-C treatment effect in papaya was transitory. In contrast, no clear effect was found in total phenolic contents, which is in agreement with findings in UV irradiated shiitake mushrooms by Jiang et al. (2010).
In this study, low temperature storage affected positively some components of papaya antioxidant system, particularly flavonoid compounds. This is in accordance to (Connor et al. 2002), whom described a cultivar-dependent increase in antioxidant capacity and phenolic contents of blueberry during cold storage. Similarly, cold storage retained or increased phenolic contents and antioxidant capacity of strawberries and fresh-cut mangoes (Robles-Sanchez et al. 2009; Ayala-Zavala et al. 2004). In this context, low temperature storage could increase antioxidant capacity in fruits were phenols and flavonoid compounds contribution to antioxidant system is greater than that of ascorbic acid, (Shivashankara et al. 2004).
Antioxidant enzymes (CAT, POD, SOD)
A significant effect (P<0.05) of storage time and temperature on catalase (CAT) specific activities of papaya peel and flesh was found. Fruit stored at 5 °C reached highest levels of CAT activity after 5 days, and then decline at the end of storage period. UV-C treatment increased CAT activity in peel after 5 days at 5 °C (688 μMH2O2*min−1· mg protein−1). An inhibitory effect of 14 °C storage temperature on CAT activity was observed in control and UV-C treated papaya peel and flesh. On the other hand, UV-C treatment increased CAT activity (33 %) in flesh of papaya during the first 10 days of storage at 5 °C, as compared to controls (Table 1).
CAT protects cells against ROS because catalyzes the decomposition of hydrogen peroxide into oxygen and water, with high stability under cold storage conditions (Sala and Lafuente 1999). Our results are in agreement with those observed in maize seedlings and mandarin fruits, where, increases in CAT could be attributed to the induce-oxidative stress, promoted by low temperature storage (Prasad 1997; Sala and Lafuente 1999).
SOD activity of papaya fruit was significantly affected by UV-C treatment, temperature, time and type of tissue analyzed. UV-C irradiation reduced SOD levels of papaya fruit at the storage conditions analyzed. Diminished SOD levels were also accounted as effect of storage time with respect to initial values in papaya peel; however, no significant differences were found after day 5 of storage. Maximum SOD activity was found in control flesh samples stored at 5 °C (16.8 Units· mg protein−1) and a linear increment was observed with storage time. A positive effect of cold storage (5 °C), particularly in flesh tissue, was observed in SOD activity despite other factors (Table 1).
Superoxide dismutases (SOD), a class of metal-containing proteins, catalyze the dismutation reaction of superoxide radical anions into H2O2 and molecular oxygen (Scandalios 1993). SOD removes singlet oxygen, prevents hydroxyl radicals formation and has been implicated as an essential defense against oxygen toxicity (Fridovich 1986; McCord 1979). Since SOD is the first line of cell defense against free radicals, its high activity in fruits has been related to a higher resistance to stress and a longer commercial life (Wang and Jiao 2001; Mondal et al. 2004). Others reported a decreased SOD activity in strawberries stored at 10 °C, when compared to initial levels, but after 15 d of storage, SOD activities of UV-C treated fruit were higher than controls suggesting that treatment could activate fruit defensive responses (Erkan et al. 2008). On the other hand, UV-C treatment reduced SOD activity during ripening of tomato, but increased activity of other antioxidant enzymes, as a defense mechanism against oxidation (Barka 2001). Therefore, increase in SOD activities in papaya flesh could be a defense response against oxidative stress caused by cold storage and in a minor degree to UV-C treatment.
Activity of POD in papaya flesh was not detectable and no effect of experimental conditions was found (data not shown). However, POD activity of papaya peel was significantly (P<0.05) affected by UV-C treatment, storage time and temperature. Decreased POD activity was observed on papaya as a result of UV-C treatment and storage at 5 °C; also, average activities diminished after storage (Table 1). Contrary to what we found for the other two enzymes, POD activity increased with storage temperature, since at 14 °C, activity levels remain constant or increased in both UV-C treated and control samples reaching the highest values in control peel samples at day 10 (765 mM guaicol/min·mg protein).
Guaicol peroxidase and ascorbate peroxidase, are peroxidase enzymes found in animal, plant and microorganism tissues, catalyzing oxide-reduction between hydrogen peroxide (H2O2) and various reducing compounds (Hiraga et al. 2001). There is evidence suggesting that UV-C irradiation can induce a rapid accumulation of photo-oxidation products. Plants respond by increasing POD activity in grapes, fresh-cut melon and tomato, extending postharvest life of these fruits (Nigro et al. 1998; Barka et al. 2000; Lamikanra et al. 2005). Nevertheless, we found in papaya peel an inhibitory effect of cold storage on POD activities and no significant effect of UV-C treatment despite storage conditions that could be attributed to ripening or senescence delay, as it has been described in sapote mamey fruits (Tejacal et al. 2005).
DPPH· and ABTS radical scavenging activity
The results of antioxidant capacity of peel and flesh tissues determined as DPPH· and ABTS· radical inhibition percentage show a significant effect of tissue type, storage time and temperature in papaya fruit. DPPH· radical scavenging capacity of peel was twice as high as that in flesh (69 and 35 % total average respectively). In papaya peel, DPPH· radical scavenging was increased in samples stored at 5 °C, while at 14 °C, radical inhibition was lower than initial levels with no differences among treatments (Table 1). Nevertheless, a positive effect of UV-C treatment in peel antioxidant capacity was observed at 14 °C during all storage period. In contrast, there was no effect of UV-C treatment in DPPH• antioxidant activity of flesh samples at both storage temperatures.
A similar tendency was found for both antioxidant capacity methods; however the values of antioxidant capacity determined with the ABTS method were in average 20 % higher than the ones obtained with the DPPH assay. UV-C treatment, type of tissue, and storage time and temperature had a significant effect (P<0.05) on ABTS radical scavenging in papaya fruit. A reduction on ABTS levels by effect of UV-C treatment and storage at 14 °C was observed in peel samples. Peel average radical scavenging was 32 % higher than that of flesh, however, irradiation treatment increased radical inhibition significantly (P<0.05) in flesh tissue samples stored at 14 °C, in which radical scavenging activity oscillated in a range of 51–84 % (Table 1).
It had been described that UV-C postharvest treatment in shiitake mushrooms increase total flavonoids, ascorbic acid, antioxidant activities of CAT, SOD, ascorbate-POD, and delayed the increase in singlet oxygen and H2O2 production rate; indicating an enhance of antioxidant capacity (Jiang et al. 2010). Also, an enhanced antioxidant capacity attributed to total flavonoid content on UV-C treated fresh-cut mangoes and tomatoes was reported earlier (Gonzalez-Aguilar et al. 2007a; Vicente et al. 2005). However, in this study, UV-C effect on papaya antioxidant capacity was intermittent during storage, since, significant positive effect of UV-C on flavonoid and radical scavenging activities was found only at day 10.
Some authors attributed the delay in ripening and senescence of UV-C treated tomatoes to significant increases in the levels of non-specific antioxidants such as polyamines and phenols, suggesting that maybe the singlet oxygen scavenging mechanism is not affected by UV treatment to the same extent as the superoxide scavenging mechanism (Maharaj et al. 1999). In the same manner, in papaya, the antioxidant response against UV-C and cold storage appear to be mediated mainly by flavonoids in peel and by CAT or other antioxidants in flesh.
Since UV-C irradiation has very low penetration in solids, treatment effects were expected to appear mostly at surface level, which was confirmed in this case for papaya peel. The more marked effect of UV-C on papaya peel could also be explained by the fact that this particular tissue contain higher levels of flavonoids and UV-C treatment promotes the activation of this particular biosynthetic route. The activation of flavonoids synthesis appears to be part of the defense mechanisms induced by the UV-C treatment and the increased resistance against pathogen attack and development of other physiological disorders such as chilling injury that occur at low temperature storage of papaya.
Conclusion
UV-C treatment and cold storage promoted changes in different components of the antioxidant system of ‘Maradol’ papaya fruit. Main effects of UV-C irradiation were enhanced flavonoid contents in peel and higher CAT enzymatic activity in flesh. While in response to cold storage, only SOD enzymatic activity was enhanced as a result of a defense response to oxidative stress. Our study shows that UV-C irradiation affect antioxidant response of papaya mainly by a mechanism that involves flavonoids synthesis; however, such effect is dependent upon storage conditions and is tissue-specific. Thus, UV-C efficiency as a postharvest treatment to enhance antioxidant system of papaya fruit is compromised by storage conditions.
References
Alothman M, Bhat R, Karim AA (2009) UV radiation-induced changes of antioxidant capacity of fresh-cut tropical fruits. Innov Food Sci Emerg Technol 10(4):512–516
Ayala-Zavala JF, Wang SY, Wang CY, Gonzalez-Aguilar GA (2004) Effect of storage temperatures on antioxidant capacity and aroma compounds in strawberry fruit. Food Sci Technol 37(7):687–695
Azene M, Workneh T, Woldetsadik K (2011) Effect of packaging materials and storage environment on postharvest quality of papaya fruit. J Food Sci Technol. doi:10.1007/s13197-011-0607-6
Barka EA (2001) Protective enzymes against reactive oxygen species during ripening of tomato (Lycopersicon esculentum) fruits in response to low amounts of UV-C. Aust J Plant Physiol 28(8):785–791
Barka EA, Kalantari S, Makhlouf J, Arul J (2000) Impact of UV-C irradiation on the cell wall-degrading enzymes during ripening of tomato (Lycopersicon esculentum L.) fruit. J Agric Food Chem 48(3):667–671
Blackwell RD, Murray AJS, Lea PJ (1990) Enzymes of photorespiratory carbon pathway. In: Lea PJ (ed) Methods in plant biochemistry. Academic, New York, pp 129–144
Bors W, Heller W, Michel C, Saran M (1992) Structural principles of flavonoid antioxidants. In: Csomó G, Fehér J (eds) Free radicals and the liver. Springer, Berlin, pp 77–95
Bors W, Heller W, Michael C (1998) The chemistry of flavonoids. In: Rice-Evans CA, Packer L (eds) Flavonoids in health and disease, vol 1. Marcel Dekker, New York, pp 111–136
Bradford M (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72(1–2):248–254
Cantos E, Espin JC, Tomas-Barberan FA (2001) Postharvest induction modeling method using UV irradiation pulses for obtaining resveratrol-enriched table grapes: a new “functional” fruit? J Agric Food Chem 49(10):5052–5058
Cantos E, Espin JC, Fernandez MJ, Oliva J, Tomas-Barberan FA (2003) Postharvest UV-C-irradiated grapes as a potential source for producing stilbene-enriched red wines. J Agric Food Chem 51(5):1208–1214
Chávez-Sánchez I, Carrillo-López A, Vega-García M, Yahia E (2011) The effect of antifungal hot-water treatments on papaya postharvest quality and activity of pectinmethylesterase and polygalacturonase. J Food Sci Technol. doi:10.1007/s13197-011-0228-0
Cisneros-Zevallos L (2003) The use of controlled postharvest abiotic stresses as a tool for enhancing the nutraceutical content and adding-value of fresh fruits and vegetables. J Food Sci 68(5):1560–1565
Connor AM, Luby JJ, Hancock JF, Berkheimer S, Hanson EJ (2002) Changes in fruit antioxidant activity among blueberry cultivars during cold-temperature storage. J Agric Food Chem 50(4):893–898
Costa L, Vicente AR, Civello PM, Chaves AR, Martinez GA (2006) UV-C treatment delays postharvest senescence in broccoli florets. Postharvest Biol Technol 39(2):204–210
D’hallewin G, Schirra M, Manueddu E, Piga A, Ben-Yehoshua S (1999) Scoparone and scopoletin accumulation and ultraviolet-C induced resistance to postharvest decay in oranges as influenced by harvest date. J Am Soc Hortic Sci 124(6):702–707
da Silva J, Rashid Z, Nhut D, Sivakumar D, Gera A, Souza M Jr, Tennant P (2007) Papaya (Carica papaya L.) Biology and Biotechnology. Tree For Sci Biotech 1:47–73
Erkan M, Wang SY, Wang CY (2008) Effect of UV treatment on antioxidant capacity, antioxidant enzyme activity and decay in strawberry fruit. Postharvest Biol Technol 48(2):163–171
Fridovich I (1986) Superoxide dismutase. Adv Enzymol 58:61–64
Frohnmeyer H, Staiger D (2003) Ultraviolet-B radiation-mediated responses in plants. Balancing damage and protection. Plant Physiol 133(4):1420–1428
Gayosso-García Sancho LE, Yahia EM, González-Aguilar GA (2010) Effect of maturity stage of papaya maradol on physiological and biochemical parameters. Am J Agric Biol Sci 5(2):194–203
Gayosso-García Sancho LE, Yahia EM, González-Aguilar GA (2011) Identification and quantification of phenols, carotenoids, and vitamin C from papaya (Carica papaya L., cv. Maradol) fruit determined by HPLC-DAD-MS/MS-ESI. Food Res Int 44(5):1284–1291
Gonzalez-Aguilar GA, Villegas-Ochoa MA, Martinez-Tellez MA, Gardea AA, Ayala-Zavala JF (2007a) Improving antioxidant capacity of fresh-cut mangoes treated with UV-C. J Food Sci 72(3):S197–S202
González-Aguilar GA, Zavaleta-Gatica R, Tiznado-Hernández ME (2007b) Improving postharvest quality of mango ‘Haden’ by UV-C treatment. Postharvest Biol Technol 45(1):108–116
González-Aguilar GA, Robles-Sánchez RM, Martínez-Téllez MA, Olivas GI, Álvarez-Parrilla E, de la Rosa L (2008) Bioactive compounds in fruits: health benefits and effect of storage conditions. Stewart Postharvest Rev 4:1–10
Gonzalez-Aguilar GA, Villa-Rodriguez JA, Ayala-Zavala JF, Yahia EM (2010) Improvement of the antioxidant status of tropical fruits as a secondary response to some postharvest treatments. Trends Food Sci Technol 21(10):475–482
Goyal A, Siddiqui S, Upadhyay N, Soni J (2011) Effects of ultraviolet irradiation, pulsed electric field, hot water and ethanol vapours treatment on functional properties of mung bean sprouts. J Food Sci Technol. doi:10.1007/s13197-011-0538-2
Hiraga S, Sasaki K, Ito H, Ohashi Y, Matsui H (2001) A large family of class III plant peroxidases. Plant Cell Physiol 42(5):462–468
Jiang T, Jahangir M, Jiang Z, Lu X, Ying T (2010) Influence of UV-C treatment on antioxidant capacity, antioxidant enzyme activity and texture of postharvest shiitake (Lentinus edodes) mushrooms during storage. Postharvest Biol Technol 56:209–215
Jimenez-Escrig A, Jimenez-Jimenez I, Sanchez-Moreno C, Saura-Calixto F (2000) Evaluation of free radical scavenging of dietary carotenoids by the stable radical 2,2-diphenyl-1-picrylhydrazyl. J Sci Food Agric 80(11):1686–1690
Karpinski S, Wingsle G, Karpinska B, Hällgren J (2002) Low-temperature stress and antioxidant defense mechanisms in higher plants. In: Inzé D, Van Montagu M (eds) Oxidative stress in plants. Taylor & Francis, London and New York, pp 69–103
Lamikanra O, Kueneman D, Ukuku D, Bett-Garber KL (2005) Effect of processing under ultraviolet light on the shelf life of fresh-cut cantaloupe melon. J Food Sci 70(9):C534–C539
Maharaj R, Arul J, Nadeau P (1999) Effect of photochemical treatment in the preservation of fresh tomato (Lycopersicon esculentum cv. Capello) by delaying senescence. Postharvest Biol Technol 15(1):13–23
Matsui S, Li J (2003) Environmental stress for crops and their antioxidative mechanisms. J Regul Plant Growth Dev 38:118–124
McCord J (1979) Superoxide dismutases: occurrence, structure, function and evolution. In: Rattazi M, Scandalios J, Whitt GS (eds) Isozyme: Current topics in biological and medical research, vol 3. Liss, New York, pp 1–21
Mondal K, Sharma NS, Malhotra SP, Dhawan K, Singh R (2004) Antioxidant systems in ripening tomato fruits. Biol Plant 48(1):49–53
Nigro F, Ippolito A, Lima G (1998) Use of UV-C light to reduce Botrytis storage rot of table grapes. Postharvest Biol Technol 13(3):171–181
Oufedjikh H, Mahrouz M, Amiot MJ, Lacroix M (2000) Effect of gamma-irradiation on phenolic compounds and phenylalanine ammonia-lyase activity during storage in relation to peel injury from peel of Citrus clementina Hort. ex. Tanaka. J Agric Food Chem 48(2):559–565
Perez-Carrillo E, Yahia EM (2004) Effect of postharvest hot air and fungicide treatments on the quality of ‘maradol’ papaya (Carica papaya L.). J Food Qual 27(2):127–139
Pérez-Tello G, Martínez-Téllez M, Vargas-Arispuro I, González-Aguilar G (2009) Chilling injury in mamey sapote fruit (pouteria sapota): biochemical and physiological responses. Am J Agric Biol Sci 4(2):137–145
Pongprasert N, Sekozawa Y, Sugaya S, Gemma H (2011) The role and mode of action of UV-C hormesis in reducing cellular oxidative stress and the consequential chilling injury of banana fruit peel. Int Food Res J 18(2):741–749
Prasad T (1997) Role of catalase in inducing chilling tolerance in pre-emergent maize seedlings. Plant Physiol 114(4):1369–1376
Re R, Pellegrini N, Proteggente A, Pannala A, Yang M, Rice-Evans C (1999) Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic Bio Med 26(9–10):1231–1237
Robles-Sanchez RM, Islas-Osuna MA, Astiazaran-Garcia H, Vazquez-Ortiz FA, Martin-Belloso O, Gorinstein S, Gonzalez-Aguilar A (2009) Quality index, consumer acceptability, bioactive compounds, and antioxidant activity of fresh-cut “ataulfo” mangoes (mangifera indica L.) as affected by low-temperature storage. J Food Sci 74(3):S126–S134
Rodov V, Ben-Yehoshua S, Kim J, Shapiro B, Ittah Y (1992) Ultraviolet illumination induces scoparone production in kumquat and orange fruit and improves decay resistance. J Am Soc Hortic Sci 117:788–792
Sala JM, Lafuente MT (1999) Catalase in the heat-induced chilling tolerance of cold-stored Hybrid Fortune mandarin fruits. J Agric Food Chem 47(6):2410–2414
Scandalios J (1993) Oxygen stress and superoxide dismutases. Plant Physiol 101(1):7–12
Shivashankara KS, Isobe S, Al-Haq MM, Takenaka M, Shiina T (2004) Fruit antioxidant activity, ascorbic acid, total phenol, quercetin, and carotene of Irwin mango fruits stored at low temperature after high electric field pretreatment. J Agric Food Chem 52(5):1281–1286
Stevens C, Khan VA, Lu JY, Wilson CL, Pusey PL, Kabwe MK, Igwegbe ECK, Chalutz E, Droby S (1998) The germicidal and hermetic effects of UV-C light on reducing brown rot disease and yeast microflora of peaches. Crop Prot 17(1):75–84
Stevens C, Khan VA, Lu JY, Wilson CL, Chalutz E, Droby S, Kabwe MK, Haung Z, Adeyeye O, Pusey LP, Tang AYA (1999) Induced resistance of sweetpotato to Fusarium root rot by UV-C hormesis. Crop Prot 18(7):463–470
Tejacal I, León M, Damián M, Hernández R (2005) Chilling in sapote mamey (Pouteria sapota (Jacq.) H. E. Moore and Stearn). II. Changes in total phenols and enzymatic activity. Rev Fitotec Mex 28(1):25–32
Vicente AR, Pineda C, Lemoine L, Civello PM, Martinez GA, Chaves AR (2005) UV-C treatments reduce decay, retain quality and alleviate chilling injury in pepper. Postharvest Biol Technol 35(1):69–78
Vunnam R, Hussain A, Nair G, Bandla R, Gariepy Y, Donnelly DJ, Kubow S, Raghavan GSV (2012) Physico-chemical changes in tomato with modified atmosphere storage and UV treatment. J Food Sci Technol:1–7. doi:10.1007/s13197-012-0690-3
Wang SY, Jiao HJ (2001) Changes in oxygen-scavenging systems and membrane lipid peroxidation during maturation and ripening in blackberry. J Agric Food Chem 49(3):1612–1619
Yahia E (2010) The contribution of fruit and vegetable consumption to human health. In: de la Rosa LA, Alvarez-Parrilla E, González-Aguilar GA (eds) Fruit and vegetable phytochemicals: Chemistry, nutritional value and stability. Wiley-Blackwell, Ames, p 3
Zhishen J, Mengcheng T, Jianming W (1999) The determination of flavonoid contents in mulberry and their scavenging effects on superoxide radicals. Food Chem 64(4):555–559
Acknowledgments
The authors gratefully acknowledge the technical assistance of Monica Villegas-Ochoa and Chrystian M. Rodriguez, and the National Council on Science and Technology (CONACYT) financial support Grant 80511 “Evaluación analítica, enzimática y molecular del metabolismo de compuestos fenólicos durante la maduración del mango, papaya, piña y aguacate”.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Rivera-Pastrana, D.M., Gardea, A.A., Yahia, E.M. et al. Effect of UV-C irradiation and low temperature storage on bioactive compounds, antioxidant enzymes and radical scavenging activity of papaya fruit. J Food Sci Technol 51, 3821–3829 (2014). https://doi.org/10.1007/s13197-013-0942-x
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13197-013-0942-x