Abstract
Moisture sorption isotherms for oat flour and rice flour were determined at 5, 23, and 45 °C using a gravimetric technique in an a w range of 0.08–0.98. The results obtained showed that, for a w values lower than around 0.75, in both flours, the sorption capacity decreased with increasing temperature, while the opposite behavior was observed at a w greater than this value. It was found that the experimental data in the water activity range considered were better represented by the four-parameter Peleg equation. The monolayer water contents for both materials were determined by the BET and GAB models. The net isosteric heats of sorption were estimated using the Clausius–Clapeyron equation. For both materials, the monolayer water content and the isosteric heat of sorption were found to decrease with increasing temperature and increasing moisture content, respectively.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Starch, quantitatively the main component of cereal flours such as oat flour and rice flour, contains two polymers that contribute to its molecular structure: amylose, mainly a linear molecule, and amylopectin, a nonlinear and highly branched molecule.1 Both molecules form semicrystalline superstructures (with crystalline and amorphous layers arranged in an onion-like structure) where most of the crystalline regions are formed by amylopectin although part of the amylose molecules are also present in them.2 Within such a system, amorphous zones are more water-accessible than crystalline regions in which the intermolecular interactions between the chains are too strong to allow solvent penetration. Consequently, water influences the structure by acting as plasticizer of the amorphous regions.3 This effect causes a depression of the glass transition temperature (T g). The plasticizing effect is very small at low a w values; therefore, the T g associated with the amorphous regions in the sample will be typically high and the material will be glassy at room temperature. Water access will be higher in the rubbery state (at T > T g) than in the glassy state, since the free volume increases above T g. Increasing water activity causes an increase in the availability of the polar sites to the water molecules.3
An important objective in food engineering is the understanding of the moisture sorption behavior of food systems. It is considered an important processing and storage parameter and it has been long addressed in the literature. Perhaps, the best way to represent food sorption characteristics is through the sorption isotherms from which food stability can be predicted. A great number of water sorption isotherm equations have been proposed in the literature for foodstuffs. They included those derived theoretically based on thermodynamic concepts, such as BET4 and GAB5 models, semiempirical and empirical models (e.g., Peleg,6 Viollaz and Rovedo7). Although, at present, there is no unique model to accurately represent moisture sorption data in the whole range of water activity, which is to be expected due to the complex sorption mechanism of food systems,8 the GAB equation has been recognized as the most versatile sorption model. For this reason, it has been recommended by the European COST90 Project.9 More recently, Peleg6 proposed a four-parameter model for a w < ∼0.9. This model fit satisfactorily published sigmoidal food isotherms. The monolayer water content is a valuable additional information that can be obtained from isothermal data determined from BET and GAB models, while the net isosteric heat can be determined from the Clausius–Clapeyron relationship. The monolayer water content gives information about the minimal water content conferring food stability, while the isosteric heat of sorption is a measure of the energy or intermolecular bonding between water molecules and adsorbing surfaces.10
Although sorption data on starchy products are extensive (e.g., Al-Muhtaseb et al.,3 McMinn and Magee11), limited water sorption data for rice flour was found in the literature consulted. Durakova and Menkov12 determined the adsorption and desorption behaviors of rice flour in a temperature range between 10 and 30 °C. These authors found that isotherms were sigmoidal type II. For rice flour, the modified Chung–Pfost and Oswin models better described the relationship between the equilibrium water content, the a w, and temperature.12 Sorption characteristics of Jasmine rice crackers were determined by Siripatrawan and Jantawat,13 finding that the GAB model gave the best fit to their experimental values. In both cases, it was found that the equilibrium moisture content (MC) decreased with an increase in storage temperature at any water activity.12, 13 No information was found for the sorption behavior of oat flour. Due to the widely spread existence of starchy flour, such as rice flour and oat flour, more sorption information is needed on these products. Consequently, the aim of this work was to determine the sorption properties of oat flour and rice flour, including the isotherms, monolayer values, and net sorption isosteric heat of these starchy flours in a wide temperature range.
Materials and Methods
Raw Material
Native rice flour and oat flour with initial MC of 10.0 and 12.7% (d.b.), respectively, were supplied by Alfonzo Rivas & Cia (Caracas, Venezuela). Standard procedure no. 925.10 of the AOAC14 was used for the determination of MC, which consisted on heating the sample in an atmospheric oven at 130 ± 3 °C for 3 h.
Sorption Isotherms
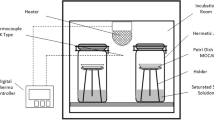
A static gravimetric method was used to determine the sorption characteristics of the flours. An approximate amount of 2 g of native sample (with its original MC) was placed in open, shallow plastic containers (3.8 cm diameter and 1 cm height) filled to a height of about 0.6 cm. The containers were placed in triplicate inside 11 desiccators, each one containing the following oversaturated salt solutions of known equilibrium relative humidity at the temperature studied:15 KOH, LiCl, CH3COOK, MgCl2, K2CO3, Mg(NO3)2, CoCl2, NaCl, (NH4)2SO4, KNO3, and K2SO4. These environments provided an a w range of 0.08 to 0.98. In this manner, part of the sorption data was obtained by adsorption and part by desorption. At water activity greater than 0.8, crystalline thymol was placed inside the desiccators to prevent the microbial spoilage of the flours. The desiccators were kept in temperature-controlled incubators (±0.5 °C) at 5, 23, and 45 °C. Samples were weighed periodically until weight variation was less than 0.001 g. It was found that equilibrium was attained within a maximum of 3 weeks. After equilibrium was reached, the MC of the equilibrated samples was determined in triplicate according to the standard method mentioned above.
Data Analysis
The experimental data obtained corresponding to the water activity and MC at the temperatures studied was adjusted to various regression models shown in Table 1. Mathematical models were chosen taking into account their applicability to starchy products and/or their versatility (BET, Peleg, GAB, and modified GAB models). Linear and nonlinear regression statistical analyses were performed using Matlab™ version 6.5. The quality of the fitting was evaluated through the R 2 and through the root mean squared error (RMSE) defined as:
where M i and \( \hat{M}_i \) are experimentally observed and predicted by the model values of the equilibrium MC, respectively, n is the number of data points, and p is the number of fitted coefficients estimated from the model.
The monolayer water content was determined from the BET and GAB equations, and the effect of temperature on the sorption isotherms was evaluated through the Clausius–Clapeyron equation:
where q st is the net isosteric heat of sorption (in joules per mole), T is the absolute temperature (in kelvins), and R is the universal gas constant (8.314 J/mol K).
Results and Discussion
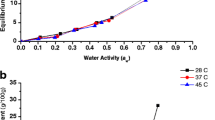
Experimental sorption isotherms obtained for oat flour and rice flour at temperatures ranging from 5 to 45 °C are presented in Figures 1 and 2, respectively. The equilibrium MC at each water activity shows the mean value of three replications. It can be seen from those figures that, for a constant temperature, the equilibrium MC increases with increasing water activity, indicating that, as with many foodstuffs, the sorption behavior of the oat flour and rice flour can be adequately represented by a sigmoidal shape, characteristic of a type II isotherm,16 according to Brunauer's classification.17 This is in agreement with several other authors who observed this behavior in starchy materials.3, 11–12
Temperature variation causes changing on water molecules mobility, apart from perturbing the dynamic equilibrium between the vapor and adsorbed phases. The effect of temperature on the sorption behavior of the flours considered in this work is also shown in Figures 1 and 2. These figures show that, albeit small, the effect of temperature below an activity water value of around 0.75 can be observed, as expected, in both oat flour and rice flour. The equilibrium MC decreases with increasing temperature, indicating that the flours become less hygroscopic. This fact was attributed to the higher energy levels and lower stability of water molecules as temperature increases, promoting their separation from the binding sites within the food matrix.18 A change in this behavior was observed for both oat flour and rice flour (Figures 1 and 2) for water activity values above 0.75 where a crossover of isotherms was noticed, i.e., for a given a w value, the equilibrium MC increased with increasing temperature. This effect is more noticeable for the case of oat flour (Figure 1).
Isotherm crossover behavior with temperature has been widely observed in carbohydrate-rich foods such as sugar beet root,19 sultana raisins,20 barley malt,21 and texturized soy protein with 20% of sugar content,22 among others. In these studies, the inverted behavior has been attributed to the endothermic dissolution of sugars at elevated water activity. In the starchy flours considered in this work, the sugar content is expected to be very small as values less than 1% have been reported for oat grains23 and milled rice.24 The trends exhibited by the oat flour (Figure 1) and rice flour (Figure 2), mainly based on starch, could then be more related to a greater exposure of active sites or hydrophilic groups at high a w or water contents as the temperature increases. Under such conditions, molecules in the glassy state could go through their glass transition region and became plasticized, leading to enhanced molecular mobility.25 Consequently, an increase of the available hydroxyl groups for sorption enhancement would be promoted. The role of glass transition in starchy products and the way it affects their sorption mechanism have been mentioned by Al-Muhtaseb et al.3 and Perdomo et al.25 The results obtained in this work seem to corroborate the suggestions made by Van den Berg26 regarding the link in the sorption capacity deviation of starches to the onset of the glass transition range where the starch amorphous regions begin to plasticize, increasing the availability of hydroxyl groups for sorption enhancement.
Sorption data obtained for the three temperatures studied were fitted to various models presented in Table 1. The results for oat and rice flours are shown in Tables 2 and 3, respectively. Evaluation of the quality of model fittings was carried out through the determination of relative (R 2) and absolute (RMSE) error measurements and the corresponding values are also shown in Tables 2 and 3. The closer to zero the RSME value is, the better the fit. Tables 2 and 3 show that all the models satisfactorily described the experimental behavior of oat and rice flours. It is worth noticing that the quality of the fit for these models was slightly lower at 45 °C, which could be attributed to changes in molecular mobility due to the onset of the glass transition of these materials, as already mentioned. As it can be observed, for both products, the best fit to the experimental data in the temperature range studied was obtained with the Peleg model as evidenced by the highest R 2 and lowest RMSE values. Dashed, dotted, and solids lines in Figures 1 and 2 represent the Peleg model fitted to the sorption data at 5, 23, and 45 °C, respectively. The Peleg model has been recognized for predicting the sorption behavior of foodstuffs and, in particular, starchy products as in this case.3
The monolayer water content, as determined by the BET and GAB models at the different temperatures, are represented by the constant A in Table 2 (oat flour) and Table 3 (rice flour) for each of these equations. In the temperature range 5–45 °C, the water content associated to the monolayer of oat flour varied from 0.068 to 0.048 g water/g dry solids when the BET model was considered and from 0.089 to 0.061 g water/g dry solids from the GAB model. On the other hand, the corresponding range found for the rice flour was 0.080–0.064 g water/g dry solids (BET model) and 0.111–0.065 g water/g dry solids (GAB model). As can be noted from these values, irrespective of the equation used in their calculation, the monolayer water contents were inversely related to temperature, as expected (Tables 2 and 3). Likewise, Tables 2 and 3 show that MCs associated to the monolayer calculated using the BET equation were lower than those calculated using the GAB equation, which is in agreement with results observed by several authors and summarized by Rahman27 and Timmermann et al.28 It is also worth noticing in Tables 2 and 3 that the smaller monolayer values obtained for the oat flour when compared with rice flour might be attributed to the higher lipid content in oat flour compared to rice flour, which would prevent the oat flour from adsorbing more water. Lipid content has been reported to be around 5.8% for oat flour and 1.2% for rice flour.29
Monolayer MC values for rice flour are in the order of 0.068–0.084 g water/g dry solids for a temperature range between 10 and 40 °C,12 which were in agreement with the values reported in this study. Values for oat flour monolayer water content were not found in the revised literature. Nevertheless, the values determined in this work for oat flour are also in agreement with values normally found for cereal products, which have been reported to be between 0.051 and 0.086 g water/g dry solids.27
Variations of the net isosteric heat of sorption with MCs for a range between 7.5 and 16.3 g water/g dry solids are shown in Figures 3 and 4 for oat and rice flours, respectively. Net isosteric heat values were calculated for data below the crossover point where trend with temperature followed the expected pattern. It can be observed in these figures that, as expected, the net isosteric heat of sorption decreases with increasing MC. It was found that this variation was better described by a linear relationship, although the normally used exponential relationship could also satisfactorily represent the changes in the heat of sorption with MC. A significant linear regression (α = 0.05) was obtained with R 2 values of 0.967 and 0.943 for oat and rice flours, respectively. The net isosteric heat of sorption of these flours can then be determined in the range of MC of 7.5–11.9% (d.b.; at) and 8.6–16.3% (d.b.; rice) through Eqs. 7 and 8, respectively (MC in fraction):
p values for the intercept and slope linear regression were 0.001 and 0.003 for the oat flour and 0.0004 and 0.0007 for the rice flour.
The net isosteric heat of sorption for oat flour varied from 8.5 to 22.6 kJ/mol and for rice flour from 1.4 to 19.4 kJ/mol. Similar values have been reported by Durakova and Menkov12 for rice flour (2.3–22.3 kJ/mol) with MC ranging from 10% to 22% (d.b.). Stripatrawan and Jantawat13 reported values of 0.1–24.9 kJ/mol for MCs between 1% and 28% (d.b.) for Jasmine rice crackers. Although no data was found for oat flour, values obtained in this work for this product fall within the values presented by Rahman27 for starchy products.
Conclusions
Moisture sorption isotherms of oat and rice flours were determined in a temperature range of 5–45 °C. In both cases, a sigmoidal shape, characteristic of type II isotherms, was found. An inversion (or crossover) point occurred at a water activity of around 0.75 for both oat and rice flours. The experimental sorption data were fitted to several models; however, the Peleg model was the more appropriate according to their RMSE and R 2 values. The monolayer water contents, whether determined by the BET or GAB models, were inversely related to temperature. The net isosteric heat of sorption was found to decrease with increasing MC, as expected. A significant linear relationship was found to better describe this variation within the MC range considered in this work.
References
S. Damodaran, K.L. Parkin, O.R. Fennema, Fennema's food chemistry, 4th edn. (CRC, Boca Raton, FL, 2007), p.1160
H.F. Zobel, Molecules to granules: a comprehensive starch review. Stärke 40, 44–50 (1988)
A.H. Al-Muhtaseb, W.A. McMinn, T.R. Magee, Water sorption of starch powders part 1: mathematical description of experimental data. J. Food Eng. 61, 297–307 (2004)
S. Brunauer, P.H. Emmett, E. Teller, Adsorption of gases in multimolecular layers. J. Am. Chem. Soc. 60, 309–320 (1938)
C. Van den Berg, S. Bruin, Water activity and its estimation in food systems, in Water activity: influences on food quality, ed. by L.B. Rockland, G.F. Stewart (Academic, New York, 1981), pp. 147–177
M. Peleg, Assessment of a semi-empirical four parameter general model for sigmoid moisture sorption isotherms. J. Food Process Eng. 16, 21–37 (1993)
P.E. Viollaz, C.O. Rovedo, Equilibrium sorption isotherms and thermodynamic properties of starch and gluten. J. Food Eng. 40, 287–292 (1999)
C.J. Lomauro, A.S. Bakshi, T.P. Labuza, Evaluation of food moisture sorption isotherms equations. Part II: milk, coffee, tea, nuts, oilseeds, spices and starchy foods. Lebensm.-Wiss. Technol. 18, 118–124 (1985)
W.E.L. Speiss, The result of the COST90 project of water activity. In: R. Jowitt, F. Escher, B. Hallstrom, H. F. Meffert W. E. L. Spiess (Eds.), Physical properties of Foods (Applied Science Publisher, London, 1983)
A. Mulet, J. García-Reverter, R. Sanjuán, J. Bon, Sorption isosteric heat determination by thermal analysis and sorption isotherms. J. Food Sci. 64, 64–68 (1999)
W.A. McMinn, T.R. Magee, Studies on the effect of temperature on the moisture sorption characteristics of potatoes. J. Food Process Eng. 22, 113–128 (1999)
A.G. Durakova, N.D. Menkov, Moisture sorption characteristics of rice flour. Nahrung 48, 137–140 (2004)
U. Stripatrawan, P. Jantawat, Determination of moisture sorption isotherms of Jasmine rice crackers using BET and GAB models. Food Sci. Technol. Int. 12, 459–465 (2006)
Association of Official Analytical Chemist, Official Methods of Analysis of the Association of Official Analytical Chemist (Association of Official Analytical Chemist, Washington, DC, 1990)
L. Greenspan, Humidity fixed points of binary saturated aqueous solutions. J. Res. Natl. Bur. Stand. 81A, 89–96 (1977)
A.H. Al-Muhtaseb, W.A. McMinn, T.R. Magee, Moisture sorption isotherm characteristics of food products: a review. Food Bioprod. Process. 80(Part C), 118–127 (2002)
S. Brunauer, L.S. Deming, W.E. Deming, E. Troller, On the theory of Van der Waals adsorption of gases. J. Am. Chem. Soc. 62, 1723–1732 (1940)
K.B. Palipane, R.H. Driscoll, Moisture sorption characteristics of inshell macadamia nuts. J. Food Eng. 18, 63–76 (1992)
H. Iglesias, J. Chirife, J. Lombardi, Water sorption isotherms in sugar beet root. J. Food Technol. 10, 299–308 (1975)
G.D. Saravacos, D.A. Tsiourvas, E. Tsami, Effect of temperature on the water adsorption isotherms of sultana raisins. J. Food Sci. 51, 381–383 (1986)
J.A. Barreiro, S. Fernández, A.J. Sandoval, Water sorption characteristics of six row barley malt (Hordeum vulgare). Lebensm.-Wiss. Technol. 36, 37–42 (2003)
A.S. Cassini, L.D. Marczak, C.P. Noreña, Water adsorption isotherms of texturized soy protein. J. Food Eng. 77, 194–199 (2006)
P. Åman, K. Hesselman, Analysis of starch and other main constituents of cereal grains. Swed. J. Agric. Res. 14, 135–139 (1984)
B.O. Juliano, The rice caryopsis and its composition, in Rice: chemistry and technology, ed. by D.F. Houston (American Association of Cereal Chemists, St. Paul, MN, 1972), pp. 16–74
J. Perdomo, A. Cova, A.J. Sandoval, L. García, E. Laredo, A.J. Müller, Glass transition temperatures and water sorption isotherms of cassava starch. Carbohydr. Polym. 76, 305–313 (2009)
C. Van den Berg, Vapour sorption equilibria and other water–starch interactions: physico-chemical approach, Ph.D. Thesis, Agricultural University Wageningen, The Netherlands, 1981
S. Rahman (Ed.), Water activity and sorption properties of foods. In: Food properties handbook (CRC Press Inc, USA, pp. 1–86, 1995)
E.O. Timmermann, J. Chirife, H.A. Iglesias, Water sorption isotherms of foods and foodstuffs: BET or GAB parameters? J. Food Eng. 48, 19–31 (2001)
A.C. Eliasson, K. Larsson, Components in other cereals. In: A. C. Eliason (Author), Cereals in breadmaking. A molecular colloidal approach (Marcel Dekker, Inc. New York, pp. 203–240, 1993)
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Brett, B., Figueroa, M., Sandoval, A.J. et al. Moisture Sorption Characteristics of Starchy Products: Oat Flour and Rice Flour. Food Biophysics 4, 151–157 (2009). https://doi.org/10.1007/s11483-009-9112-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11483-009-9112-0