Abstract
In this work, the effect of particle size and severity factors was investigated to find the optimum condition of steam explosion pretreatment on xylan recovery of beech wood. The beech wood particles with sizes of 0.16, 1, or 2 mm were steamed at 150–210 °C for 2.5–15 min before an explosive decompression. The results showed that the maximum xylan recovery was about 10% w/w wood with low concentrations of the inhibitors, which were obtained when the particle size is 1 mm and R0 = 3.65 (190 °C, 10 min). The smallest particle size may result in overcooking of biomass, leads to easily and high degradation of hemicelluloses sugars, whereas the largest particle sizes may result in incomplete autohydrolysis in biomass and lower extractability of hemicelluloses sugars. The obtained optimum condition for xylan recovery will improve the subsequent utilization (such as in food industry and other chemical products), prior to subsequent transformation of steam explosion pretreated wood (bioethanol and pellet).
Graphical Abstract

Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Statement of Novelty
The limitations of SE process due to the lack of particle size information has been addressed. The optimum SE condition has been found and may improve the extraction of xylan of beech sawmill waste.
Introduction
The steam explosion (SE) process was developed by Mason in 1924 for the production of chipboard panels [1] and then improved for lignocellulosic materials disintegration used in the manufacture of paper, board pulp and the like [2]. The main equipments of this process are a steam generator, a high-pressure reactor and a discharge tank to receive the material after explosion [3]. SE is a pretreatment process to open up the biomass fibers. It uses high temperature and pressure steam for certain residence time proceeded by a sudden pressure drop causing a rupture of the biomass fibers rigid structure of lignocellulosic structure and lower the bulk density of biomass [4, 5]. SE is a thermo-mechanico-chemical pretreatment which induces a breakdown of lignocellulose by chemical modifications from in situ generated acids which cause the depolymerization of hemicelluloses and by mechanical action due to high shearing forces applied to the biomass during the explosive release. This process improves the recovery of sugars (or other useful compounds) and so, makes the biopolymers more accessible for subsequent processes such as enzymatic hydrolysis, fermentation or densification. It can be used as a pretreatment process to produce solid biofuel pellets, to increase the calorific value, to improve the pelletizing properties of the biomass or to make cellulose more accessible to enzymes in order to convert it to fermentable simple sugars [6, 7].
SE has been extensively described for pretreatment to enhance (1) the enzymatic hydrolysis of the resulting cellulosic pulp (poplar wood, sugar cane bagasse, pine wood, Douglas wood, corn stover, and wheat bran) [8,9,10,11,12,13] and (2) the properties of pellets from poplar wood, Douglas wood, and agricultural-based biomass [11, 12, 14]. In addition to the pretreated cellulosic pulp, high yields of hemicellulosic sugars are recovered in the water effluent of SE process. These sugars consist of monomers, oligomers, and polymers which constitute attractive building blocks for further transformations. The kinetics of hydrolysis of corn cob and eucalyptus globulus xylans have been accurately described during hydrothermal treatments (150–190 °C, 6.5–7.5 h) [15, 16]. However, the parametric factors affecting the hemicellulosic sugars recovery during the cooking step of SE treatment are not fully understood. No clear correlations between temperature, residence time, biomass particle size and hemicellulosic sugars yields have been found in the literature.
Severity factor is a model broadly used for evaluating the process of pretreatment. It is based on the assumption that the overall pretreatment process is hydrolytic, and it follows a first law concentration dependence, the constant rate obeying the Arrhenius law [17]. It combines the treatment temperature (T) and residence time (ts) in one value.
During the cooking step of SE, hydronium ions from both water and in-situ generated compounds catalyze the hemicelluloses depolymerization into oligomers and monomers (auto-hydrolysis process) [15, 16]. Under harsh conditions (high temperature, low pH), monomeric sugars can be further degraded to furan derivatives (furfural from C5 and hydroxymethylfurfural from C6 sugars).
However, one of the limitations of the severity index model is the lack of information on particle size which has a major influence on the kinetics of the hydrolytic process. Particle size strongly affects the kinetics of the hydrolytic processes, the efficiency of the vapor soaking, the particles heat transfer and physical modifications of the biomass [12, 17, 18]. Size reduction of biomass before a pretreatment is crucial for the optimization of sugars conversion but also strongly affects the milling power and the overall cost of the process [10].
Large particle sizes can hamper heat transfer and may result in incomplete auto-hydrolysis of its interior part whereas small particle may result in overcooking and in the degradation of their components. In the literature, the effect of chip size on SE process has been examined regarding the sugars recovery starting from agricultural residues [12] and on softwood [10, 19]. It was concluded that larger particles produced higher enzymatic cellulosic pulp digestibility. The effect of the chips size on the sugars recovery and the furans production have also been examined during the SE of Douglas-fir [20]. To the best of our knowledge, the effect of hardwood chips particle size on SE performance and on sugars recovery in the liquid stream of the SE has never been examined.
European Beech (Fagus sylvatica L.) is one of the most abundant species and the most harvested hardwood in France. It is used to produce sawn wood with an average yield of 43–50% [19, 21]. As a result, a considerable amount of wood residues is produced which could constitute a potential source of biopolymers and/or bioenergy. Xylans are the main hemicellulosic components of beech wood. They constitute a valuable potential source for the production of high-added value ingredients for functional foods (xylo-oligosaccharides and/or xylitol from xylose) [22, 23].
In this present study, the effect of the size of beech sawdust pretreated by varying experimental SE conditions (temperature, residence time) on the sugars conversion was examined in order to assess which SE conditions produce the maximum concentration of sugars (monomers and oligomers) in the water stream. Finding the optimum SE condition that improves the extraction of xylan of beech sawmill waste would be a novelty of this study.
Materials and Methods
Process Flowchart
Figure 1 presents the different steps used to characterize beech wood (extractives, ash, lignin, cellulose, and hemicelluloses) and to process to beech wood pre-treatment by using steam explosion (SE) process.
Sample Preparation
The wood used in this study was beech (Fagus sylvatica L.) from Lorraine forest collected in 2016. The tree was about 150 years old. The wood samples were previously sun-dried, then milled and sieved with three different sizes: 2 mm, 1 mm, and 0.160 mm. Then the samples were oven-dried and stored at room temperature. The moisture contents of those samples before steam explosion pretreatment were 6.32%, 8.39%, and 11.86%, respectively.
Chemical Composition
Extractives (Adapted from TAPPI T204-cm97)
In a Soxhlet apparatus, 2 g dry sample with particle size 0.160 mm were extracted for 16 cycles with 300 mL of toluene and ethanol (2/1; v/v). The solvent was then evaporated under vacuum and the extracts were weighed, whereas the beech sample was dried with temperature 40 °C for 24 h. Extractives content were calculated by using these two-final masses.
Holocellulose
1.5 g of beech free extractive samples with particle size 0.160 mm, 1 g of sodium chlorite (NaClO2), 1 mL acetic acid (CH3COOH) glacial (99.7%; w/w) and 125 mL ultrapure water (UPW) were mixed into a 250 mL flask. The flask was brought to reflux with continuous stirring for 2 h at 70 °C. Every 2 h, 1 g NaClO2 and 1 mL CH3COOH were added until the wood sample is completely white, meaning a total delignification. The solid fraction of holocellulose was then washed with ultrapure water then filtered, and finally dried at 40 °C for 24 h and then weighed. The holocellulose content was calculated by using the mass change.
Cellulose
1 g of the previous holocellulose was mixed with 50 mL of sodium hydroxide (NaOH-17.5%; w/v) in a 100 mL Erlenmeyer flask for 30 min at 25 °C. Then, about 5 mL of ultrapure water were added and stirred for 30 min at 25 °C. The solid part, composed of cellulose, was filtered and washed with 50 mL of a solution of acetic acid (1%; v/v). The solid was dried in an oven at 40 °C for 24 h and weighed. Cellulose content was calculated by using the mass variation.
Lignin (Adapted from TAPPI T222-cm11)
0.175 g of wood free extractive with particle size 0.160 mm was mixed with about 1.5 mL sulfuric acid (H2SO4—72%; w/v) and then incubated in a rotary water bath for at 30 °C for 1 h. These samples were taken out and 42 mL of ultrapure water were added. They were then autoclaved for 1 h 30 min. The solid, composed of insoluble lignin—also known as Klason lignin-, was filtered, washed, dried at 105 °C for 24 h and finally weighed. The liquid phase, containing monomeric sugars from cellulose and hemicelluloses, was completed with ultrapure water to 100 mL and froze for further analyses. Lignin content was calculated by using the mass variation, whereas monomeric sugars were quantified by high-performance anion-exchange chromatography coupled with pulsed amperometric detection (HPAE-PAD).
Ash (Adapted from TAPPI T211 om-02)
1 g of dry sample was weighed into a tared crucible and then put inside a muffle furnace (Carbolite WF-1100) with a temperature of 525 °C for 24 h. After 24 h, the crucible was stored in a desiccator and weighed. The ash content was be obtained by the decrease in mass.
Oligomeric Sugars Post-hydrolysis
To be quantified, oligomeric sugars contained in the liquid fraction after SE were first hydrolyzed to monomeric sugars. 10 mL of SE liquid phase was mixed with 349 µL of sulfuric acid (H2SO4—72%; w/v) and autoclaved at 121 °C for 1 h. After a subsequent dilution (200 times), the monosaccharide content of the sample was then determined by HPAE-PAD.
Chromatographic Analyses
HPAE-PAD Analysis
Monosaccharide contents of soluble fractions were analyzed by HPAE-PAD (ICS-3000 Dionex) equipped with a Dionex CarboPac™ PA-20 (3 × 150 mm) analytical column. Filtered samples (20 µL) were eluted at 35 °C and at 0.4 mL/min with the following composition: UPW 99.2%/250 mM NaOH 0.8%: 0 → 20 min ; UPW 75%/250 mM NaOH 20%/NaOAc (1M)-NaOH (20 mM) 5% 20 → 37 min ; UPW 40%/250 mM NaOH 20%/NaOAc (1M)-NaOH (20 mM) 40% 37 → 41 min. Each elution was followed by a wash and subsequent equilibration time. External sugar and uronic acids standards were used for calibration (7 points per curve): fucose, glucose, xylose, galactose, mannose, rhamnose, arabinose and galacturonic acid, glucuronic acid (all provided by Sigma-Aldrich).
High-Performance Liquid Chromatography (HPLC) Analysis
During SE, acetic acid can be released from the wood because of the partial hydrolysis of its cell wall components; whereas furfural and 5-hydroxymethylfurfural (HMF) can be produced respectively from pentose sugars and hexose sugars by a dehydration process. At last formic and levulinic acids are resulting from both furans degradation.
All these “by-products” were analyzed by HPLC (Ultimate 3000) using a Rezex™ RHM-Monosaccharide H+ (8%) column with sulfuric acid (H2SO4—5 × 10−3 M) as the mobile phase with an isocratic flow rate of 0.8 mL/min and a temperature of 65 °C. The wavelength for detecting the three acid compounds was 210 nm, whereas it was 280 nm for furfural and HMF. Each elution was followed by a wash and subsequent equilibration time. External standards provided by Sigma-Aldrich were used for calibration (5 points per curve).
Steam Explosion Pretreatment
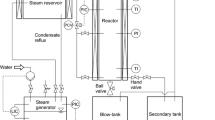
The wood samples (1 mm, 2 mm, and 0.160 mm) were impregnated in the ultrapure water for 24 h at the room temperature beforehand; with a ratio between water and wood sample of 20:1 (v/w). After the sample was soaked, it was filtered; the filtered-water was stored for the analysis of sugar content. The framework of the steam explosion process is shown in Fig. 2; the apparatus is composed of three main parts: a vapor generator, a reactor, and a discharge tank.
20 g of beech wood sample were loaded into the reactor and exposed to the several high-pressure steams (4.1 × 105 Pa–18.4 × 105 Pa) for several residence times (2.5–15 min) and temperatures (150–210 °C). The samples were then exploded by sudden-dropped in pressure and released in the discharge tank. The exploded solid biomass was separated from the liquid fraction by vacuum filtration. It was dried at 40 °C for 48 h, whereas the volume of the liquid fraction was measured; only a small part was frozen until it was analyzed for monomeric and oligomeric sugars content and other byproducts.
Severity Factor [17]
where Ro is the severity factor, Tr is temperature of reaction (°C), Tb is base temperature (100 °C), ts is residence time (min), 14.75 is empirical temperature value based on the activation energy when assuming first order kinetics.
Results and Discussion
Chemical Composition
Based on the sample with a size of 0.160 mm and moisture content of 11.86%, the untreated beech wood used in this study comprises 37.8% cellulose, 35.7% hemicelluloses, 24.5% Klason lignin, 1.8% extractives (solubility in toluene and ethanol; 2/1; v/v, respectively) and 0.2% ash. Compared to some reports from literature, the sample has a lower amount of cellulose and higher or comparable content of lignin [24,25,26,27].
Overall Mass Loss to Severity Factor
The SE experimental conditions used were as follows: temperature from 150 to 210 °C, residence time from 2.5 to 15 min, sawdust sizes of 0.16 mm, 1 mm, and 2 mm. The corresponding severity factors are gathered in Table 1. As seen in Fig. 3, the mass loss was increasing with the severity factor. It appears that below a severity factor of 3.0, the mass loss was varying very slightly whatever the size of the particle was. For R0 > 3.0, a dramatic increase in the mass loss was observed. At the highest severity (R0 = 4.41), ~ 40% of the wood was solubilized. This result is in accordance with previous works performed starting with hardwood chips using both continuous [28] or batch [25, 26] SE systems which showed that a significant degree of solubilization of wood requires treatment severity beyond R0 = 3.0 with a maximum (~ 30% of mass loss) for R0 > 3.75. From Fig. 3 it can be seen that the particle sizes impact the mass loss. The larger particles exhibited a significantly lower degree of wood solubilization.
Xylan and Glucan Yields
Figure 4a–c give respectively the total monosaccharides yields, the monomeric glucose and the monomeric xylose released in the liquid phase during the steam explosion process as a function of the severity factor. As expected, the sugars yields recovered in the liquid phase follow the same trend as the mass losses (Fig. 3). For R0 < 3.0, the sugars yields were very low and slightly increase with R0. For R0 > 3.0, an increase is observed.
As expected, the xylose concentrations (Fig. 4c) are higher than those of the other sugars, xylan being the dominant hemicelluloses of beech. For instance, with a severity factor of 4.41, xylose represents about 75% of the sugars released whatever the sawdust size. Its yield was increasing drastically when the R0 > 3.0, confirming that hydrolysis of hemicelluloses is more efficient when increasing the severity factor. Regarding glucose content, its value is very low compared to the total yield of monosaccharides. Glucose yields are more difficult to interpret because glucose is produced by the hydrolysis of both hemicelluloses and cellulose. The low glucose content in the hydrolysates showed that cellulose was not or few affected by the steam explosion process in our conditions.
Interestingly, higher monosaccharides content is recovered in solution for the intermediate particle size (1 mm); for larger (2 mm) or smaller (0.16 mm) particles lower sugars concentrations were detected. This result is in agreement with previous studies performed on softwood (Douglas-Fir), in which fine particles (0.42 mm) released fewer sugars than woodchips [20].
The yields of the oligomeric total sugars, glucans, and xylans released during the SE process in the liquid phase are shown in Fig. 5a–c respectively. Figure 5b, c confirm that xylans are the dominant polysaccharides (> 77% of total sugars). Compared to monosaccharides, the oligomeric contents are higher and the shape of the curves is different; the oligosaccharides are released at lower R0 (from R0 = 2.5) and reach a maximum at R0 = 3.65 (190 °C, 10 min). At that severity, the amount of extracted oligosaccharides was 10 g/100 g of dry wood for xylans and 0.8 g/100 g of dry wood for glucans.
A further increase in severity led to a decrease in the recovery of oligosaccharides. This can be explained by the kinetic pathways in which oligomers are released first during the steam treatment and then are further hydrolyzed into monosaccharides [15, 29]. Very similar results were previously reported for the non-catalyzed SE of poplar using a continuous 4t/h pilot plant [28]. These authors described a maximum pentosans recovery (monomers and oligomers) of 65% at R0 = 3.8. In contrast, using acid-catalyzed SE, pentosans, and hexosans were primarily recovered as monosaccharides. At R0 = 3.03, less than 20% of xylans were found as oligomers [30]. The maximum xylan yield in this study was lower than the xylan yield resulted from diluted acid pretreatment (3% H2SO4) of 55.3% [31] and from alkaline pretreatment (7% NaOH) of 57% [32].
Regarding the influence of the particle size, as it was previously observed for monomeric sugars, the oligomeric recovery in the liquid phase is higher starting from a sawdust size of 1 mm.
Furfural and 5-HMF Yields
During the cooking step of SE process, the increase of severity led to an increasing content of hemicelluloses solubilized and to an increasing dehydration rate of sugars derived from hemicelluloses, producing byproducts such as furfural and 5-hydroxymethylfurfural (HMF) [33]. In a biomass-to-biofuel process, furfural and HMF might affect enzymes in the hydrolysis process and reduce glucose conversion during fermentation process [34]. As shown in Fig. 6, the concentration of HMF and furfural in the SE liquid effluents were undetectable or very low for R0 < 3.5 and increased above this value. For the highest severity (R0 = 4.41) and the smallest particle size (0.16 mm), maximum concentration of HMF (0.2% w/w) and furfural (0.7% w/w) were detected. Stoffel et al. reported comparable trends but the generation of higher levels of degradation products during the SE of pine sawdust [30]. In this study, the higher furans content in the hydrolysates can be rationalized by the utilization of 1–3% of sulfuric acid as a catalyst which catalyzes the sugars dehydration reactions.
The impact of the wood particle size on the production of degradation products is clearly shown in Fig. 6 the smaller the particle size is, the higher the furfural and HMF concentration is. Similar results were described by Cullis et al. who observed during the SE of softwood a substantial decrease of the production of furfural and HMF as the wood chip size increased (from 1.5 to 5 cm) [20]. Overcooking of the small particles during the cooking step of SE may promote hemicelluloses depolymerization and degradation. Surprisingly, Liu et al. reported the opposite trend for the SE of corn stover: the amount of furan derivatives was higher and the sugars recoveries were lower for larger biomass particles [12].
Conclusion
The optimization of pretreatment of beech wood by uncatalyzed SE for hemicelluloses recovery in the forms of monomeric and oligomeric is as a function of the severity factor and particle sizes. The maximum of oligomers recovery was obtained when the particle size is 1 mm and R0 = 3.65, 10% w/w wood with low inhibitors concentration. The particle size is a very important factor in the hemicelluloses recovery and in this work the optimum size was found to be 1 mm. Smaller particle size (0.16 mm) resulted in overcooking of biomass, leading to higher degradation of hemicelluloses sugars, whereas larger particle size (2 mm) resulted in incomplete autohydrolysis of biomass and lower extractability of hemicelluloses sugars. The optimum condition for xylan recovery will optimize the subsequent utilization (such as in the food industry and other chemical products), prior to subsequent transformations of SE pretreated wood (bioethanol and pellet).
References
Jacquet, N., Maniet, G., Vanderghem, C., Delvigne, F., Richel, A.: Application of steam explosion as pretreatment on lignocellulosic material: a review. Ind. Eng. Chem. Res. 54, 2593–2598 (2015). https://doi.org/10.1021/ie503151g
Mason, W.H.: Process and apparatus for disintegration of wood and the like (1926)
Akinlabi, E.T., Anane-Fenin, K., Akwada, D.R.: Bamboo as fuel. In: Bamboo: The Multipurpose Plant, pp. 149–178. Cham, Springer (2017)
Arenas-Cárdenas, P., López-López, A., Moeller-Chávez, G.E., Léon-Becerril, E.: Current pretreatments of lignocellulosic residues in the production of bioethanol. Waste Biomass Valoriz. 8, 161–181 (2017). https://doi.org/10.1007/s12649-016-9559-4
Gong, L., Huang, L., Zhang, Y.: Effect of steam explosion treatment on barley bran phenolic compounds and antioxidant capacity. J. Agric. Food Chem. 60, 7177–7184 (2012). https://doi.org/10.1021/jf301599a
Lam, P.S., Lam, P.Y., Sokhansanj, S., Bi, X.T., Lim, C.J.: Mechanical and compositional characteristics of steam-treated Douglas fir (Pseudotsuga menziesii L.) during pelletization. Biomass Bioenergy 56, 116–126 (2013). https://doi.org/10.1016/j.biombioe.2013.05.001
Mosier, N., Wyman, C., Dale, B., Elander, R., Lee, Y.Y., Holtzapple, M., Ladisch, M.: Features of promising technologies for pretreatment of lignocellulosic biomass. Bioresour. Technol. 96, 673–686 (2005). https://doi.org/10.1016/j.biortech.2004.06.025
Grous, W.R., Converse, A.O., Grethlein, H.E.: Effect of steam explosion pretreatment on pore size and enzymatic hydroxlysis of poplar. Enzyme Microb. Technol. 8, 274–280 (1986)
Silva, T.A.L., Zamora, H.D.Z., Varão, L.H.R., Prado, N.S., Baffi, M.A., Pasquini, D.: Effect of steam explosion pretreatment catalysed by organic acid and alkali on chemical and structural properties and enzymatic hydrolysis of sugarcane bagasse. Waste Biomass Valoriz. 0, 1–11 (2017). https://doi.org/10.1007/s12649-017-9989-7
Ballesteros, I., Oliva, J.M., Navaro, A.A., González, A., Carrasco, J., Ballesteros, M.: Effect of chip size on steam explosion pretreatment of softwood. Appl. Biochem. Biotechnol. 84–86, 97–110 (2000)
Kumar, L., Chandra, R., Saddler, J.: Influence of steam pretreatment severity on post-treatments used to enhance the enzymatic hydrolysis of pretreated softwoods at low enzyme loadings. Biotechnol. Bioeng. 108, 2300–2311 (2011). https://doi.org/10.1002/bit.23185
Liu, Z., Qin, L., Pang, F., Jin, M., Li, B., Kang, Y., Dale, B.E., Yuan, Y.: Effects of biomass particle size on steam explosion pretreatment performance for improving the enzyme digestibility of corn stover. Ind. Crop. Prod. 44, 176–184 (2013). https://doi.org/10.1016/j.indcrop.2012.11.009
Jiang, S., Guo, N.: The steam explosion pretreatment and enzymatic hydrolysis of wheat bran. Energy Sources 38, 295–299 (2016). https://doi.org/10.1080/15567036.2012.744118
Adapa, P., Tabil, L., Schoenau, G.: Grinding performance and physical properties of non-treated and steam exploded barley, canola, oat and wheat straw. Biomass Bioenergy 35, 549–561 (2011). https://doi.org/10.1016/j.biombioe.2010.10.004
Nabarlazt, D., Farriol, X., Montane, D.: Kinetic modeling of the autohydrolysis of lignocellulosic biomass for the production of hemicellulose-derived oligosaccharide. Ind. Eng. Chem. Res. 43, 4124–4131 (2004). https://doi.org/10.1021/ie034238i
Garrote, G., Domınguez, H., Parajo, J.C.: Mild autohydrolysis: an environmentally friendly technology for xylooligosaccharide production from wood. J. Chem. Technol. Biotechnol. 74, 1101–1109 (1999)
Chornet, E., Overend, R.P.: Phenomenological kinetics and reaction engineering aspects of steam/aqueous treatments. In: Proceeding of the International Workshop on Steam Explosion Techniques: Fundamentals and Industrial Applications. pp. 21–58. Gordon and Breach Science Publisher (1988)
Xiao, L.-P., Song, G.-Y., Sun, R.-C.: Effect of hydrothermal processing on hemicellulose structure. In: Hydrothermal Processing in Biorefineries: Production of Bioethanol and High Added-Value Compounds of Second and Third Generation Biomass. pp. 45–93. Springer (2017)
Monavari, S., Galbe, M., Zacchi, G.: Impact of impregnation time and chip size on sugar yield in pretreatment of softwood for ethanol production. Bioresour. Technol. 100, 6312–6316 (2009). https://doi.org/10.1016/j.biortech.2009.06.097
Cullis, I.F., Saddler, J.N., Mansfield, S.D.: Effect of initial moisture content and chip size on the bioconversion efficiency of softwood lignocellulosics. Biotechnol. Bioeng. 85, 413–421 (2004). https://doi.org/10.1002/bit.10905
Demartini, J.D., Foston, M., Meng, X., Jung, S., Kumar, R., Ragauskas, A.J., Wyman, C.E.: How chip size impacts steam pretreatment effectiveness for biological conversion of poplar wood into fermentable sugars. Biotechnol. Biofuels 8, 1–16 (2015). https://doi.org/10.1186/s13068-015-0373-1
Agreste Lorraine: La récolte de bois récoltés en Lorraine en 2014 (2015)
Institut Technologique FCBA (Forêt Cellulose Bois-construction Ameublement): Mémento FCBA (2016)
Fengel, D., Wegener, G.: Wood: Chemistry, Ultrastructure, Reactions. De Grutyter, Berlin (1989)
Demirbas, A.: Biofuels from beech wood via thermochemicals conversion methods. Energy Sources A 32, 346–354 (2010). https://doi.org/10.1080/15567030802466201
Yildiz, U.C., Yildiz, S., Gezer, E.D.: Mechanical and chemical behavior of beech wood modified by heat. Wood Fiber Sci. 37, 456–461 (2005)
Bodirlau, R., Teaca, C.A., Spiridon, I.: Chemical modification of beech wood: effect on thermal stability. BioResources 3, 789–800 (2008). https://doi.org/10.15376/biores.3.3.789-800
Heitz, M., Capek-Ménard, E., Koeberle, P.G., Gagné, J., Chornet, E., Overend, R.P., Taylor, J.D., Yu, E.: Fractionation of Populus tremuloides at the pilot plant scale: optimization of steam pretreatment conditions using the STAKE II technology. Bioresour. Technol. 35, 23–32 (1991). https://doi.org/10.1016/0960-8524(91)90078-X
Tunc, M.S., Van Heiningen, A.R.P.: Hemicellulose extraction of mixed southern hardwood with water at 150 ??C: effect of time. Ind. Eng. Chem. Res. 47, 7031–7037 (2008). https://doi.org/10.1021/ie8007105
Stoffel, R.B., Neves, P.V., Felissia, F.E., Ramos, L.P., Gassa, L.M., Area, M.C.: Hemicellulose extraction from slash pine sawdust by steam explosion with sulfuric acid. Biomass Bioenergy 107, 93–101 (2017). https://doi.org/10.1016/j.biombioe.2017.09.019
Miazek, K., Remacle, C., Richel, A., Goffin, D.: Bioresource Technology Beech wood Fagus sylvatica dilute-acid hydrolysate as a feedstock to support Chlorella sorokiniana biomass, fatty acid and pigment production. Bioresour. Technol. 230, 122–131 (2017). https://doi.org/10.1016/j.biortech.2017.01.034
Miazek, K., Remacle, C., Richel, A., Goffin, D.: Effect of enzymatic beech fagus sylvatica wood hydrolysate on Chlorella biomass, fatty acid and pigment production. Appl. Sci. 7, 1–9 (2017). https://doi.org/10.3390/app7090871
Lam, P.S., Lam, P.Y., Sokhansanj, S., Lim, C.J., Bi, X.T., Stephen, J.D., Pribowo, A., Mabee, W.E.: Steam explosion of oil palm residues for the production of durable pellets. Appl. Energy 141, 160–166 (2015). https://doi.org/10.1016/j.apenergy.2014.12.029
Cantarella, M., Cantarella, L., Gallifuoco, A., Alfani, A.S.F.: Effect of inhibitors released during steam-explosion treatment of poplar wood on subsequent enzymatic hydrolysis and SSF. Biotechnol. Prog. 20, 200–206 (2004). https://doi.org/10.1021/bp0257978
Acknowledgements
We acknowledge the financial support of LERMAB which supported by the French National Research Agency through the Laboratory of Excellence ARBRE (ANR-12-LABXARBRE-01) and Double Degree Master Program of Indonesia Ministry of Education and Culture.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Simangunsong, E., Ziegler-Devin, I., Chrusciel, L. et al. Steam Explosion of Beech Wood: Effect of the Particle Size on the Xylans Recovery. Waste Biomass Valor 11, 625–633 (2020). https://doi.org/10.1007/s12649-018-0522-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12649-018-0522-4