Abstract
Esophageal cancer is one of the most fatal cancers principally because of its late presentation. CECT plays an important role in the staging of esophageal cancer but has some limitations. PET/CT which provides physiological information along with anatomical information and is a whole body imaging technique may therefore be a better alternative and thereby can facilitate selection or exclusion of patients for resection. The aim was to evaluate the performance of F18 FDG PET/CT in the staging and restaging of esophageal carcinoma compared to CECT using histopathologic findings and clinical follow-up as gold standard. Twenty eight patients with proven esophageal carcinoma, both preoperative and postoperative, were studied with CECT and F18 FDG PET/CT scan within an interval of 2 weeks. The PET/CT scan was acquired after injection of 370 MBq (10 mCi) F18-FDG and was evaluated for areas of increased focal uptake. CECT scan of chest and abdomen was done after injection of iodinated non-ionic contrast media. CECT findings suggested stage-IV disease in 16/28 (57.14%) patients and non stage-IV disease in 12/28 (42.86%) patients, whereas PET/CT suggested stage-IV disease in 23/28 (82.14%) patients and non stage-IV disease in 5/28 (17.86%) patients. Total nine patients were upstaged by PET/CT compared to CECT, out of which 7 (25%) were correctly upstaged and 2 (7.14%) were falsely upstaged. PET/CT improved our ability to detect distant metastases in 25% of patients that was missed by CECT. So, the use of F18 FDG PET/CT in esophageal cancer can alter management in significant number of patients.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Esophageal cancer is an uncommon disease but one of the leading cause of cancer mortality especially in men [1, 2]. It has the widest variation of incidence by geographic location of any cancer [3–5]. The incidence rates for most parts of the world range from 2.5 to 5.0 for men and 1.5 to 2.5 for women per 100,000 population [5]. It has a relatively poor prognosis, with a 5-year survival rate of 6%–11% [6]. In patients with early-stage malignancy at presentation, surgery is the treatment of choice. However, most patients come with locally advanced disease out of which 20%–30% have distant metastases [7]. In case of locally advanced disease without distant metastases, esophagectomy after neoadjuvant chemotherapy and radiotherapy is the treatment option if they do not develop distant metastases during therapy [8–10]. Hence in all patients with potentially resectable disease accurate staging is important as it has both prognostic and therapeutic importance.
Although esophageal cancer is associated with unfavorable prognosis, accurate determination of the extent of local invasion, tumor size, lymph node involvement, and distant metastasis provides valuable information for prognosis assessment and treatment selection. Conventional imaging modalities being used for evaluation of esophageal carcinoma are CT and Endoscopic Ultrasound (EUS). In the process of malignant transformation, cells develop significant changes in metabolism like DNA synthesis, amino acid use, and glycolysis [11]. Increase in glucose uptake by malignant cells is the basis for the use of FDG-PET imaging in oncology.
The goals of our current study were to compare F18-FDG-PET/CT results with CECT findings correlating with the pathologic findings and evaluating the accuracy of F18-FDG-PET/CT relative to CECT, which currently is the most commonly used conventional imaging modality. We set out to determine whether F18-FDG-PET/CT had an incremental value over CECT in patients with esophageal carcinoma and whether any such additional information would lead to a change in patient management. To the best of our knowledge till date, there is no study in the Indian population on this subject.
Methods
Patient Population
Twenty eight consecutive patients (22 males and 6 females; age range 38–74 years; mean age 57 years) with biopsy proven esophageal carcinoma were recruited in this study after obtaining written informed consent from each patient. Nine patients had an adenocarcinoma and 19 patients had a Squamous Cell Carcinoma. 19 patients had undergone some therapy (2 surgical resection; 11radiotherapy/ chemotherapy and 6 both radiotherapy and chemotherapy) before the F18-FDG PET/CT scan and 9 patients were treatment-naive. 16/28(57.14%) cases were having mid-esophagus involvement and the rest 12/28 (42.86%) lower esophagus and GE junction involvement. There was no case with upper esophagus involvement. All patients underwent routine evaluation, including history and physical examination, chest radiography, barium swallow, esophagoduodenoscopy and spiral CECT of the chest and abdomen. Patients with non-stage IV disease on CECT also underwent bone scan as part of routine imaging workup while in patients with stage IV disease on CECT bone scan was done only if specifically indicated.
Acquisition
CECT: All patients underwent contrast enhanced CT of the chest and abdomen. 12 patients had scans performed outside our institution; the hardcopy images were available and of acceptable quality. The remaining 16 patients had CT at this institute with a Somatom Plus 4 spiral CT scanner of Siemens Medical System. After administration of both oral (400 ml) and intravenous iodinated contrast agents, contiguous images of 10 mm slices were obtained from the neck to below the level of liver. Extent of the primary tumor, thickness of the esophageal wall, tumor invasion of adjacent structures and presence of lesions suggestive of metastases to distant sites were recorded.
Tumor was identified when the esophageal wall was more than 5 mm thick. Mediastinal nodes were considered positive if the short axis diameter was greater than 1 cm and left gastric nodes if greater than 8 mm. Mediastinal invasion was diagnosed when soft tissue extended into the mediastinal fat. The images were interpreted blinded to the results of the PET/CT.
TNM staging (AJCC; 6th edition) was used to define primary tumor, nodal and metastatic stage.
F18-FDG PET/CT: All PET/CT scans were performed within 2 weeks after completion of the conventional staging. For patients who had undergone chemotherapy and/or radiotherapy PET/CT was delayed by a minimum 6 weeks after the last therapy.
F18-FDG PET/CT scans were obtained on a PET/CT scanner (Siemens CTI, Biograph). For data acquisition CT component was operated with an X-ray tube voltage peak of 120 keV, 90 mA, a slice thickness of 5 mm and a rotational speed of 0.8 sec/rotation. The PET/CT system was used for 2- slice helical CT acquisition followed by a full ring dedicated PET scan of the same axial range. After overnight fasting, 10–15 mCi F18-FDG (radiochemical purity of >98%) was administered (6 MBq/kg). Data acquisition started 45–60 min after injection in whole body mode (i.e. from base of skull to mid thigh) at 2 min per bed position. Patients were received no oral muscle relaxants. They were asked to void just before the scan. No iodinated CT contrast agents were administered. PET scanner having 10.125 cm axial FOV reconstructed all images such that the spatial resolution was 6.3 mm in Transaxial and 6 mm in axial directions.
Both CT and PET scans were obtained during normal tidal breathing. PET images were reconstructed with CT derived attenuation correction factors and by using iterative (OSEM) method. The attenuation corrected PET images, CT images and fused PET/CT images were available for review in axial, coronal and sagittal planes, as was a cine display of Maximum Intensity Projections (MIP) of the raw data.
Image Analysis
F18-FDG PET/CT images were reviewed by two experienced nuclear medicine physicians, who were blinded to the patient’s clinical history and the results of previously performed conventional imaging tests. A site of increased F18-FDG was defined as negative when it was related to known nonmalignant process or to the physiologic biodistribution of F18-FDG. The physicians recorded the presence, number, size, SUV, character and precise location of presumed lymph nodes and other distant metastases. CECT scans done outside our institute were reviewed by an experienced oncologic radiologist who was unaware of PET/CT findings.
Gold Standard
Comparison between conventional staging methods and F18-FDG PET findings was validated by FNAC or pathologic examination of resection specimens as the gold standard for each TNM category. Surgical findings strongly suggesting tumor fixation to adjacent structures were regarded as the gold standard for T4 stage. The gold standard for nodal metastases was exclusively obtained by pathologic verification of resection specimens after 2- field lymphadenectomy or surgical node biopsies. The gold standard for M1b disease was based on pathology whenever possible or and clinical follow-up of suspected PET lesions.
Statistical Analysis
The sensitivity, specificity, accuracy, positive predictive value (PPV), negative predictive value (NPV) of CECT and PET/CT for the detection of locoregional lymph node metastases were calculated and compared using Mc Nemar test and p-value of <0.05 was considered statistically significant.
Results
Primary Tumor
Primary tumor was identified by both CECT and PET/CT in 16/28 (57.14%) patients. In 12/28 (42.86%) patients, tumor was not found because in these patients either the tumor was removed surgically or the tumor was completely/ partly resolved by chemotherapy and radiotherapy which restricted their visualization on CECT or PET/CT scans. Of 16 patients 11 had a T3 tumor and 5 had a T4 tumor on CECT, while on PET/CT 10 had T3 and 6 had a T4 tumor (Table 1).
Regional Lymph Node Metastases
CECT identified regional lymph nodes in 6/28 patients, of which 5 were confirmed by pathology and one proved to be false positive (case-13; pretracheal and paratracheal nodes). PET/CT identified regional lymph nodes in 9/28 patients, of which 6 were confirmed by pathology and 3 patients were found to be false positive. In 4 patients regional lymph nodes were correctly diagnosed which was missed by CECT. In 3 patients CECT had an incremental value over FDG PET/CT. (case no.-2, 9 and 26). The overall sensitivity, specificity, PPV, NPV and accuracy of CECT for detection of regional node metastases was calculated as 55.55%, 94.73%, 83.33%, 81.81% and 82.14% respectively while that of PET/CT was 66.67%, 84.21%, 66.67%, 84.21% and 78.57% respectively (Table 2).
Distant Nodal and Organ Metastases
22/28 patients had stage-IV (M1) disease. CECT identified 16/22 patients with M1 disease. No false positive results with M1 disease were found. PET/CT identified M1 disease in 23/28 patients out of which 22/28 were confirmed by the gold standard. PET/CT identified 12/23 patients with distant nodal metastases of which one was false positive (case-12), 6/23 with organ metastases and the rest 5/23 patients with both distant nodal and organ metastases. The organs involved were liver, skeleton and spleen. M1 disease was missed by CECT in 6 patients which was detected by PET/CT. For distant lymph node and organ metastases (M1 disease) the overall sensitivity, specificity, PPV, NPV and accuracy for CECT was calculated as 72.72%, 100%, 100%, 50% and 78.57%, respectively whereas for PET/CT it was calculated as 100%, 83.33%, 95.65%, 100% and 96.43%, respectively (Table 2). Thus the specificity and PPV of CECT was found better than PET/CT whereas sensitivity, NPV and accuracy of PET/CT were better than CECT.
Staging and Restaging Groups
Of the 9 patients in the staging group there were no true negative or false positive findings. Thus, we could calculate the sensitivity, PPV and accuracy of both the modalities in this group was 77.77%, 100% and 77.77% for CECT and 100%, 100% and 100% for PET/CT respectively.
Of the 19 patients in the restaging group the sensitivity, specificity, PPV, NPV and accuracy for CECT was respectively 70.59%, 100%, 100%, 28.57% and 73.68% whereas for PET/CT the values were respectively 100%, 33.33%, 88.88%, 100% and 89.47% (Table 3).
The high sensitivity demonstrated by PET in M staging has been noted because even very small lesions can be visualized by FDG-PET if they show high metabolic activity while. And the cause of comparatively low sensitivity was due to false positive results which occur due to benign tumors with a high metabolic rate, inflammation and physiologically increased uptake in normal tissue like muscle, bowel and brown adipose tissue. However these are relatively rare but require that disease status be confirmed by biopsy or others imaging tools before the treatment plan is altered.
Discussion
Many studies have been done to document the role of EUS and CT in staging esophageal cancer. The advent of FDG PET has been thought to be a highly useful development as far as detection of metastases is concerned. The role of PET in locoregional staging of esophageal cancer is however limited as shown by initial studies.
Primary Tumor
Endoscopy has been the most effective method for early detection of esophageal tumor especially in pre-malignant conditions like Barrett’s esophagus [12]. Endoscopic ultrasound (EUS) combines the additional utility of high-frequency US with endoscopic visualization of the tumor which has the ability to define the separate layers of esophageal wall. The resolution of CECT and PET/CT is not as good as EUS which can detect even T1/T2 tumors with the help of high resolution transducers. EUS provides accurate and cost-effective T-staging that is superior to both CT and PET and has been shown to affect preoperative management [13–15]. PET is limited in its ability to demonstrate the depth of tumor invasion into the esophageal wall due to reduced spatial resolution.
EUS however is not suited to determine resectability of esophageal cancer alone, and thus is most effective when used in conjunction with other imaging tests such as CT and PET [13].
Sun et al. (2009) performed restaging in 20 histopathologically diagnosed esophageal cancer (tumor recurrence) patients after surgical resection and radiotherapy using FDG PET/CT and concluded that it is effective in detecting relapse [16]. He found that the overall accuracy of FDG PET/CT was 85%, with negative predictive value (NPV) of 100%, and positive predictive value (PPV) of 78.6%.
Regional Lymph Node Metastasis
Lymph node stage is an important and independent prognostic indicator in esophageal cancer [17]. The number and location of the lymph nodes also affect the prognosis [18]. The 5-year survival rate with nodal disease is less than 10% [19–21]. Accurate assessment of lymph node status is therefore extremely important, not only for its prognostic implications, but also to guide treatment options.
CT shows poor sensitivity for detecting lymph node involvement because smaller nodes containing tumor cells can be missed [22, 23].
The detection of malignant lymph nodes in CT has been historically based on size criteria. Nodes >1 cm in size are usually considered as malignant whereas sub centimeter nodes are considered benign [23]. EUS show higher accuracy for regional lymph node assessment but complete tumor staging is impossible in approximately one third of esophageal cancer patients due to failure of passage through the stenotic lesion [24, 25]. Other methods like thoracoscopy and laparoscopy can detect regional nodal metastases with higher accuracy, but they are invasive methods [23].
F18-FDG PET may be more sensitive than CT in LN detection because the alterations in tissue metabolism measured by PET generally precede anatomic changes associated with tumor [26]. However, PET lacks precise localization landmarks, making it difficult to definitively characterize foci of increased F18-FDG uptake [27]. It has limited ability in detecting nodal disease in the direct vicinity of the primary tumor with high uptake which may obscure peritumoral nodes. For local nodal staging, F18-FDG PET had a sensitivity of only 33% and was significantly outperformed by EUS (sensitivity 81%). However, for regional and distant nodal involvement, PET had a similar accuracy to combined EUS-CT (sensitivity and specificity of 46 and 98% versus 43 and 90%) [28].
In our study 9 patients had regional lymph node metastases of which CECT identified 6 while PET/CT also correctly identified 6. But the false positive (FP) rate of PET/CT was higher as another 3 were found to be FP. It appears that both CECT and PET/CT cannot be wholly relied upon individually. For regional lymph nodes sensitivity and NPV was higher for PET/CT but specificity, PPV and accuracy was higher for CECT. Differences in the above parameters for CECT and PET/CT were not statistically significant. The reason for this could be the small study population. So, if the findings of CECT and PET/CT are combined together then none of the metastatic lymph node would be missed. Hence it can be postulated, that if intravenous iodinated contrast agents are used in PET/CT (i.e. Diagnostic CT) then the false positive results of PET/CT may decrease and correspondingly the sensitivity and NPV shall increase.
A comparison of CECT vs. FDG PET/CT by Kato et al. in their study of 55 patients found that FDG-PET showed 96% sensitivity, 68% specificity and 82% accuracy in demonstrating recurrent disease. The sensitivity of FDG-PET was higher than that of CT in detecting locoregional recurrence, but its specificity was lower because of FDG uptake in the gastric tube and thoracic lymph nodes [29]. Masahiro Okada et al. in 2009 examined 180 consecutive patients by integrated PET/CT and compared findings with CECT and evaluated metastatic regional lymph nodes in patients with resectable early stage esophageal cancer. The sensitivity, specificity, accuracy, positive, and NPV of PET/CT were respectively 60.0%, 99.5%, 94.8%, 93.8%, and 94.8%, whereas those of CECT were 60.0%, 95.1%, 91.0%, 62.5%. The author concluded that integrated PET/CT improves the PPV of regional lymph nodes when compared with CECT [30].
Distant Nodal and Organ Metastasis
The main incremental value of FDG PET/CT in the evaluation of oesophageal cancer lies in its ability to identify unsuspected metastatic disease, which is present in up to 30% of patients at initial diagnosis [31].
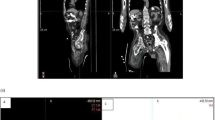
In our study out of 7 correctly upstaged patients with PET/CT, 3 had already undergone surgery. Out of these 3 patients, 2 had also received chemotherapy and radiotherapy, and underwent CECT and PET/CT scan after 6 weeks of therapy. In one patient CECT showed no evidence of disease (NED), but PET/CT clearly indicated multiple nodes in superior and anterior mediastinum, bilateral paraaortic nodes with right lung nodule (SUV max = 4.9). In the second patient CECT showed regional node (celiac) but PET/CT found non-regional node (paraaortic) which upstaged the disease from stage III to stage IV. In the third patient who had undergone surgery, PET/CT found multiple nodes in mediastinum, right paratracheal and subcarinal nodes in contrast to CECT which showed none. The clinical management changed in these patients due to PET/CT. Remaining 4 patients were scanned before therapy. All the 4 patients were upstaged from stage IIA to stage IV. In 2 patients distant node (supraclavicular) was detected on PET/CT. In 1 patient liver metastasis (Fig. 1) was seen, and in the remaining 1 patient multiple distant nodes were detected (celiac, paraaortic, left supraclavicular) which were missed on CECT scan.
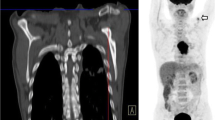
Two patients who had undergone chemotherapy were falsely upstaged by PET/CT. In one patient a regional lymph node (cervical) was falsely identified which upstaged the disease from NED to stage IIB. In another patient a focus of uptake was seen in the (right supraclavicular region) which again falsely upstaged the disease from NED to stage IV (M1 disease) (Fig. 2). The reason for this could be the inflammatory changes which occur in post-chemotherapy patients with FDG uptake not discernible from that due to malignant cause.
There were 12 patients with non-stage IV disease on CECT all of whom underwent bone scan as part of routine imaging workup at various time points but was negative for bone metastases. Also none of these patients had bone metastases on PET/CT. Rest of the 16 patients already had stage IV disease on CECT so a bone scan was not advised by the clinician except in four patients who complained of backache of which two were found to have bone metastases.
Differences in the statistical parameters apart from sensitivity for CECT and PET/CT were not statistically significant. The reason for this could be the smaller study population.
This study attempts again to define the role of FDG PET/CT in the staging of esophageal cancer. FDG PET/CT does not add much in the detection of regional nodes, but there is a significant advantage in the detection of M1 disease, avoiding unnecessary surgery. In identification of unsuspected M1 disease, FDG PET/CT performed better than CECT. FDG PET/CT correctly upstaged from N0 to N1 in 1/28 (3.57%) and from M0 to M1 in 6/28 (21.43%). Hence FDG PET/CT correctly upstaged the disease (missed by CECT) overall in 25% (7/28) of the patients. There was no downstaging seen on FDG PET/CT in comparison to CECT. The accuracy of 78.57% (22/28) for detecting M1 disease with CECT, increased to 96.43% (27/28) with FDG PET/CT.
Out of seven correctly upstaged patients six were upstaged to stage IV disease while one was upstaged to stage IIB. Those patients with stage IV disease were planned for palliative treatment whereas patient with stage IIB was converted to definitive treatment.
A prospective study by Flamen et al. demonstrated that the use of FDG PET resulted in upstaging of 15% of patients from M0 to M1 disease and in downstaging 7% of the patients from M1 to M0 [28]. Another prospective study by Heeren et al. showed that the use of PET/CT in detecting metastatic disease correctly upstaged 20% of patients and correctly downstaged 5% of patients, sparing unnecessary surgery in patients with disseminated disease [30]. Kato et al. showed that for distant organs, the sensitivity of PET in detecting lung metastasis was lower than that of CT, but its sensitivity for bone metastasis was higher. They concluded that combined PET–CT would appear to be an appropriate modality for the detection of recurrent oesophageal cancer [32].
We found an overall management change in 7/28 (25%) patients. Previous studies by Salahudeen et al. (2008) [33], Sun et al. (2009) [16] and Gillies et al. (2011) [34] found management change in 40%, 60% and 17% patients respectively.
Limitations of this study include the following. Firstly, the sample size was small i.e. 28 patients. Secondly, 12 patients were evaluated with CECT outside our institution and for those DICOM images were not available and hardcopy images were reviewed. This could potentially lead to underestimation of the extent of disease on CECT. Lastly, as there were only nine patients in the staging and 19 patients in the restaging group, individual analysis of these groups was not done. A larger study with longer follow up is currently being conducted by us at our institution.
Conclusion
To conclude, PET/CT improved our ability to detect distant metastases in patients of esophageal cancer missed by CECT. This resulted in change in clinical management in significant number of patients. However, whether added utility of PET/CT over CECT is important in regional nodal staging needs further clarification. FDG PET/CT may be therefore routinely used in esophageal cancer for staging as well as restaging of the disease.
References
Boring CC, Squires TS, Tong T (1993) Cancer statistics, 1993. CA Cancer J Clin 43:7–26
Blot WJ, Devesea SS, Kneller RW, Fraumeni JF Jr (1991) Rising incidence of adenocarcinoma of the esophagus and gastric cardia. JAMA 265:1287–1289
Schottenfeld D (1984) Epidemiology of cancer of the esophagus. Semin Oncol 11:92–100
Schoenberg BS, Bailer JC, Fraumeni JF Jr (1971) Certain mortality patterns of esophageal cancer in the United States, 1930–67. J Natl Cancer Inst 46:63–73
Aylomamitis A (1988) Epidemiology of cancer of the esophagus in Canada: 1931-1984. Gastroenterology 94:374–380
Kobori O, Kirihara Y, Kosaka N, Hara T (1999) Positron emission tomography of esophageal carcinoma using 11 C-choline and 18 F-fluorodeoxyglucose: a novel method of preoperative lymph node staging. Cancer 86:1638–1648
Flanagan FL, Dehdashti F, Siegel BA, Trash DD, Sundaresan SR, Pattersan GA et al (1997) Staging of esophageal cancer with F18-fluorodeoxyglucose positron emission tomography. Am J Roentgenol 168:417–424
Roth JA, Pass HI, Flanagan MM, Graeber GM, Rosenberg JC, Steinberg S (1988) Randomized clinical trial of preoperative and postoperative adjuvant chemotherapy with cisplatin, vindesine, and bleomycin for carcinoma of the esophagus. J Thorac Cardiovasc Surg 96:242–248
Urba SG, Orringer MB, Turrisi A, Iannettoni M, Forastiere A, Strawderman M (2001) Randomized trial of preoperative chemoradiation versus surgery alone in patients with locoregional esophageal carcinoma. J Clin Oncol 19:305–313
Kelsen DP, Ginsberg R, Pajak TF, Sheahan DG, Gunderson L, Mortimer J et al (1998) Chemotherapy followed by surgery compared with surgery alone for localized esophageal cancer. N Engl J Med 339:1979–1984
Bar-Shalom R, Valdivia AY, Blaufox MD (2000) PET imaging in oncology. Semin Nucl Med 30:150–185
May A, Ell C (2006) Diagnosis and treatment of early esophageal cancer. Curr Opin Gastroenterol 22:433–436
Pfau PR, Perlman SB, Stanko P, Frick TJ, Gopal DV, Said A et al (2007) The role and clinical value of EUS in a multimodality esophageal carcinoma staging program with CT and positron emission tomography. Gastrointest Endosc 65:377–384
Gan SI, Rajan E, Adler DG, Baron TH, Anderson MA, Cash BD et al (2007) Role of EUS. Gastrointest Endosc 66:425–434
Zhang X, Watson DI, Lally C, Bessell JR (2005) Endoscopic ultrasound for preoperative staging of esophageal carcinoma. Surg Endosc 19:1618–1621
Sun L, Su XH, Guan YS, Pan WM, Luo ZM, Wei JH et al (2009) Clinical usefulness of F18-FDG PET/CT in the restaging of esophageal cancer after surgical resection and radiotherapy. World J Gastroenterol 15(15):1836–1842
Roder JD, Busch R, Stein HJ, Fink U, Siewert JR (1994) Ratio of invaded to removed lymph nodes as a predictor of survival in squamous cell carcinoma of the oesophagus. Br J Surg 81:410–413
Choi JY, Lee KH, Shim YM, Lee KS, Kim JJ, Kim SE et al (2000) Improved detection of individual nodal involvement in squamous cell carcinoma of the esophagus by FDG PET. J Nucl Med 41:808–815
Lerut T, Coosemans W, Decker G, De Leyn P, Nafteux P, Van Raemdonck D (2001) Cancer of the esophagus and gastro-esophageal junction: potentially curative therapies. Surg Oncol 10:113–122
Greenstein AJ, Litle VR, Swanson SJ, Divino CM, Packer S, Wisnivesky JP (2008) Prognostic significance of the number of lymph node metastases in esophageal cancer. J Am Coll Surg 206:239–246
Wilson M, Rosato EL, Chojnacki KA, Chervoneva I, Kairys JC, Cohn HE (2008) Prognostic significance of lymph node metastases and ratio in esophageal cancer. J Surg Res 146:11–15
Vilgrain V, Mompoint D, Palazzo L, Menu Y, Gayet B, Ollier P et al (1990) Staging of esophageal carcinoma: comparison of results with endoscopic sonography and CT. AJR l55:277–281
Gore RM (2005) Upper gastrointestinal tract tumours: diagnosis and staging strategies. Cancer Imaging 5:95–98
Tio TL, Coene PP, Schouwink MH, Tytgat GN (1989) Esophagogastric carcinoma: preoperative TNM classification with endosonography. Radiology 173:411–417
Massari M, Cioffi U, De Simone M, Lattuada E, Montorsi M, Segalin A et al (1997) Endoscopic ultrasonography for preoperative staging of esophageal carcinoma. Surg Laparosc Endosc 7:l62–l165
Block MI, Patterson GA, Sundaresan RS, Bailey MS, Flanagan FL, Dehdashti F et al (1997) Improvement in staging of esophageal cancer with the addition of positron emission tomography. Ann Thorac Surg 64:770–776
Van Westreenen HL, Heeren PA, Jager PL, van Dullemen HM, Groen H, Plukker JT (2003) Pitfalls of positive findings in staging esophageal cancer with F18- fluorodeoxyglucose positron emission tomography. Ann Surg Oncol 10:1100–1105
Flamen P, Lerut A, Van Cutsem E, De Wever W, Peeters M, Stroobants S et al (2000) Utility of positron emission tomography for the staging of patients with potentially operable esophageal carcinoma. J Clin Oncol 18:3202–3210
Heeren PA, Jager PL, Bongaerts F, van Dullemen H, Sluiter V, Plukker JT (2004) Detection of distant metastases in esophageal cancer with F18-FDG PET. J Nucl Med 45(6):980–987
Okada M, Kumano Takamichi Murakami Seishi, Kuwabara M, Hosono Taro Shimono Makoto, Shiozaki H (2009) Integrated FDG-PET/CT compared with intravenous contrast-enhanced CT for evaluation of metastatic regional lymph nodes in patients with resectable early stage esophageal cancer. Ann Nucl Med 23:73–80
Kato H, Miyazaki T, Nakajima M, Takita J, Kimura H (2005) The incremental effect of positron emission tomography on diagnostic accuracy in the initial staging of esophageal carcinoma. Cancer 103:148–156
Kato H, Miyazaki T, Nakajima M, Fukuchi M, Manda R, Kuwano H (2001) Value of positron emission tomography in the diagnosis of recurrent oesophageal carcinoma. Br J Surg 91:1004–1009
Salahudeen HM, Balan A, Naik K, Mirsadraee S, Scarsbrook AF (2008) Impact of the introduction of integrated PET-CT into the preoperative staging pathway of patients with potentially operable oesophageal carcinoma. Clin Radiol 63:765–773
Gillies RS, Middleton MR, Maynard ND, Bradley KM, Gleeson FV (2011) Additional benefit of F18-fluorodeoxyglucose integrated positron emission tomography/computed tomography in the staging of oesophageal cancer. Eur Radiol 21(2):274–280
Conflict of interest
No conflict of interest exists with reference to this manuscript. No competing financial interests exist.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kumar, P., Damle, N.A. & Bal, C. Role of F18-FDG PET/CT in the Staging and Restaging of Esophageal Cancer: A Comparison with CECT. Indian J Surg Oncol 2, 343–350 (2011). https://doi.org/10.1007/s13193-012-0128-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13193-012-0128-4