Abstract
Aims
A study was undertaken to investigate the value of pretreatment PET–CT in predicting survival in patients with oesophageal cancer (OC).
Methods
Between June 2010 and December 2011, 18 consecutive OC patients median (61.00 ± 12.07 years) with median survival of 7.5 month had a pretreatment PET–CT scan. Staging of the disease was made in accordance to the American Joint Committee on Cancer staging system (7th edition) and grouped as stage I–IIA and stage IIB–IV. Maximum standardized uptake value (SUVmax), size of a primary tumour and the presence of fluorodeoxyglucose (FDG)-avid lymph nodes were evaluated for all patients. Survival was analysed using the Kaplan–Meier product limit method and Cox proportional hazards regression model.
Results
PET–CT stages I–IIA and IIB–IV had a 1-year survival of 50% and 25%, respectively. Patient with size of primary tumour (<4.5 cm) had significantly (p < 0.036) better survival than those with large size (>4.5 cm). Multivariate Cox regression analysis showed that SUVmax of >5.5 in the primary tumour [hazard ratio (HR) 23.017; 95% confidence interval, p = 0.038] and the presence of FDG-avid lymph node (HR 1.248; p = 0.028) were strongly predictive of poor overall survival on multivariate analysis.
Conclusion
Pretreatment 18F-FDG PET–CT SUVmax of a primary tumour and the presence of FDG-avid lymph nodes independently predict survival in patients with oesophageal carcinoma which may potentially be used as surrogate markers for prognostic and therapeutic purposes.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Survival rates are not readily available for each stage in the American Joint Cancer Committee (AJCC) staging system for oesophageal cancer. The data on surveillance epidemiology and end results (SEER) have shown that patients presenting with regional nodal involvement and metastatic nodal involvement have worse prognosis [1]. The role of functional imaging, i.e. positron emission tomography–computed tomography (PET–CT) in evaluating oesophageal carcinoma, is emerging and promising in determining the survival and staging of the disease based on the AJCC staging scheme.
PET–CT has emerged as an essential investigation for patient with a potentially resectable oesophageal cancer given its role in both diagnostic information and as a prognostic role. PET–CT using fluorodeoxyglucose (FDG) as a tagging radionuclide tracer has been shown to concentrate in 90% of oesophageal cancer [2].
Each of the adenocarcinoma and the squamous cell carcinoma types make up 49% of cases of oesophageal cancer [3]. There were many studies that evaluated treatment outcome following the American Joint Cancer Committee Tumour-Nodes-Metastasis (AJCC TNM) on pathological scheme with less focus being discussed on the survival of the patient. Some of those remain inconclusive with survival prognostication. Currently published data assessing the prognostic value of Standardized uptake value (SUVmax) are mainly based on the tumour staging using AJCC TNM 6th edition.
The semi-quantitative parameter of PET may predict overall survival in patient with oesophageal cancer. Several studies have suggested that tumour with an elevated SUVmax tends to be more aggressive and be associated with worse survival [4].
Evaluation of oesophageal cancer staging as a survival function using on a new AJCC 7th edition is by far lacking. This study is sought to determine whether PET marker, lesion morphology and the abnormal lymph node has influence on the patient survival.
Materials and methods
Patient accrual
After approval from the Institutional Ethical Board was obtained, between June 2010 and December 2011, 18 consecutive OC patients had a pretreatment PET–CT scan. This cohort of patient was followed up until the close-out date at 2 years. Disease stage was made in accordance to the American Joint Committee on Cancer staging system (7th edition) and grouped as stage I–IIA and stage IIB–IV reflecting broad groupings that determine therapeutic choice.
[18F]-FDG PET/CT
All 18 subjects were fasted for at least 6 h prior to PET/CT. Venous blood glucose was taken to ensure the desired peripheral blood glucose was maintained at <7.0 mmol/l, patients received an IV injection of 370 MBq (10 mCi) of FDG and then rested for approximately 1 h prior to imaging. Image acquisition was performed with an integrated PET/CT device (Siemens Biograph-64) consisting of a PET scanner (Advance LiSo4) and a 64-MDCT scanner. Patients were allowed a normal shallow respiration during acquisition of CT scan. The patients were given at least 1000 ml gastrografin contrast orally in a 3-divided dose at 15 min apart. The CT was performed with IV contrast (Iopamidol 300) of spiral acquisition at 5.0 mm sectioning from the base of skull until the proximal thigh. It was followed by PET acquisition at 3 min per bed position. CT data were resized from a 512 × 512 matrix to a 128 × 128 matrix to match the PET data to allow image fusion, and CT transmission maps were generated. PET image datasets were reconstructed iteratively with the ordered-subsets expectation maximization algorithm with segmented measured attenuation correction (two iterations, 28 subsets) with the CT data. Co-registered images were displayed on the Siemens Leonardo workstation.
Interpretation
An experienced nuclear medicine physician/radiologist who read the results was blinded to the clinical data and the histological findings. For the purpose of the interpretation, results were analysed for the maximum standardized uptake value (SUVmax), size of a primary tumour and the presence of FDG-avid lymph nodes. The FDG avidity for visible lymph nodes was done on the grading system: Grade 1 = background, Grade 2 = mediastinum, Grade 3 = Liver and Grade 4 = brain [5]. The primary tumour was measured on the widest cross-sectional dimension, and the margin of the tumour bulk or thickening is based on the exact accumulation of the F-18 FDG uptake on the correlated PET image.
Statistics
General information on the patient characteristics were described using tables for categorical data, and medians and range for continuous variables. Comparison of categorical variables was done by dual analysis and continuous variables by t test. Survival analysis was done using the Kaplan–Meier method, with comparison of survival using the log-rank test. A two-sided p value of <0.05 was considered significant.
Results
Patient characteristics
Eighteen prospective patients (16 males and 2 females) with median age for cohort of 61.00 ± 12.07 years. All patients underwent pretreatment PET/CT for the primary staging of oesophageal cancer with median survival of 7.5 month. There was a predominance adenocarcinoma (83.3%) with the majority of the tumour site at the lower 1/3 of the oesophagus and/or gastrooesophageal junction accounting for (61.10%) (Table 1). All but 1 had died at the close-out date. No significant differences in the SUVmax and SUVmean with respect to patient sex, patient age or tumour localization were detected.
Comparison of CT and PET–CT stage
As outlined in Table 2, there was discordance between the CT stage groups determined by the CT and the PET/CT stage groups of IIB-IV with four of nine patients (44.44%). Overall, PET/CT upstaged four (22.22%) patients. Among these 4 patients, PET/CT stage detected distant metastatic bony lesions (Fig. 1). In group IIB–IIIC, PET/CT detected additional regional nodal deposit in one patient (Fig. 2).
Analysis on the imaging results validation
PET/CT findings had resulted in the change of management in four of eighteen patients (22.22%). Of these 18 patients, imaging findings were all validated by either pathologic or intraoperative results. Of these 18 patients, PET/CT was correctly determined the disease status in 18 (100%), CT was correct in 14 (12%) (Table 2).
Management impact
Some of the cancer is in the upper part of the oesophagus (in the neck); chemoradiation was a choice given the difficulty in the tumour accessibility but none the subject has tumour at this location. The treatment for an advance stage tumour, i.e. T4, treatment for these cancers is most often chemoradiation followed by palliative surgery.
PET/CT changed management in 4 of 18 patients (22.22%). In nine patients of grouped IIB–IIIC by CT staging, PET/CT had induced management change in four (44.44%) from surgery to multimodality treatment/palliative surgery. In five patients of group IIB–IIIC, one patient (20%) had further modification on the N staging (N0–N1) on the extent of the disease status on the PET–CT (Fig. 2). The details of the PET/CT induced management changes are outlined in Table 2.
Overall survival
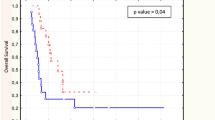
The median survival of the cohort was 7.5 months. The FDG PET/CT had enabled prognostic stratification with the separation of survival curves according to stage group to be made. The trend across the survival curves was not statistically significant for the two groups (p > 0.05). The 12-month survival rate for stage IIB–IV patients was 25% for PET/CT stage, whereas a better survival of 50% was noted for stage 1–IIA (Fig. 3).
SUVmax and survival
Patients with high SUVmax (SUV > 5.5) had a significant worse survival rate than patients with low SUVs (p = 0.015). There were 10 deaths in the patient with SUVmax > 5.5 with only three who had survived as compared to all the five were survived in the patient with SUVmax < 5.5. One survived patient in the group with SUVmax > 5.5 was planned for surgery, and two patients were planned for combined surgery and chemotherapy (Fig. 4).
FDG-avid lymph node and patient survival
As outlined in Fig. 5, the survival of the 2 patient groups was significantly different (p < 0.005). After corroborating PET/CT findings into the patients with FDG-avid lymph nodes or without, there was a significant better survival of those without FDG-avid lymph nodes as compared to patients with FDG-avid lymph nodes. Among the group with non FDG-avid lymph node, there were more survived patients (6/10) as compared to 1/8 in the group with FDG-avid lymph node. There were no significant evidence of the number of lymph nodes involved to predict patient survival in this cohort of patients (p > 0.05) (Table 3).
Size of primary tumour and survival
There was a significant difference in the findings on the size of primary oesophageal lesion between the two groups of patients (p < 0.036) (Fig. 6). Patient with size of primary tumour (<4.5 cm) had better survival than those with large size (>4.5 cm). There was one death 1/5 in the group of smaller primary tumour as compared to 9/12 deaths in the group of tumour.
Hazard curve
Given the possible interplay of the relationships among prognostic factors, multivariate analysis was performed. Multivariate Cox regression analysis showed that SUVmax of >5.5 in the primary tumour [hazard ratio (HR) 23.017; 95% confidence interval, p = 0.038] and the presence of FDG-avid lymph node (HR 1.248; p = 0.028) were strongly predictive of poor overall survival on multivariate analysis (Table 4). The type of treatment did not have an additional impact on estimating patient survival independent of PET–CT stages.
Discussion
FDG PET emphasizes on the intrinsically altered glucose metabolic requirements as compared to the structural information as provided by CT. Therefore, functional information on PET overscores the CT assessment in a wide variety of cancers, including more accurate staging. This advantage is potentially true in oesophageal cancer, with PET, compared with CT, providing superior assessment of remote nodal and systemic metastatic disease [6, 7].
The results from this study show that patients with high SUVmax have a poorer survival rate than patients with low SUVmax. This was found for the SUVmax (cutoff value 5.5) of the tumour. This is in line with several previous reports which stated that survival was significantly lower in patients who had high SUVs and reported a great variety in the cutoff values, ranging from 3 to 7 [8–10]. These findings also support the hypothesis that low 18FDG uptake within a tumour may represent more indolent tumour biology, and the high or altered glucose metabolism within the cancer cells may signify aggressive tumour behaviour [11, 12]. In the group SUVmax > 5.5, only one patient with the totally resected tumour had changed to showed comparable survival to those with three patients with multimodality treatment approach. This is much attributed to the apparently lower-staged tumour at the time of diagnosis despite an intrinsically aggressive tumour.
In the new seventh edition of AJCC TNM [13], the N stage has been subclassified on the basis of the number of positive regional lymph nodes rather than nodal location. Nevertheless, there was no significant evidence to suggest that the number of lymph nodes could prognosticate the patients’ survival in this study. On the other note, the presence of FDG-avid lymph node with grades 3 and 4 on visual assessment; we found that there was a significant evidence to suggest poor survival in patient with FDG avid lymph nodes. The PET–CT staging on N stage has modified one patient with CT stage IIA–IIIC by detection of more metastatic lymph node on PET. This result is also in accordance to other report, suggesting patients with PET-detected nodal metastatic disease have a worse prognosis than those whose nodal disease is undetectable on PET and is a potentially independent predictor of overall survival [14–17]. This had led to the change in the management intent from surgical resection to a multimodality treatment approach with radiotherapy combination. There were 22.22% of patients who had been upstaged on the PET–CT rendering a consequence impact on the management change. This is in accordance to other studies that revealed that PET upstages from 4.8% to 36.5% of patients previously deemed as surgical candidate for treatment of oesophageal cancer. [18, 19] In these patients, PET had further detected remote bony metastasis which was imperceptible on the CT images [20, 21]. In accordance to the new staging system for which the stage category of some patients may have been altered in our cohort, our results remain highly relevant to the therapeutic choices pertinent to multidisciplinary care of oesophageal cancer. There is no significant evidence that suggests pretreatment SUVmax could provide better stratification on the treatment intent as shown in this study. Among suggested reasons, a low SUV might be associated with hypoxic tumours, that when left untreated may be less aggressive, but paradoxically may make the tumour more resistant to chemoradiotherapy [20].
On the other note, the size of primary tumour is also a pertinent criteria for survival in this cohort of patient. Using the cutoff size of 4.5 cm, the survival drops at the close-out date at 2 years. The large tumour (>3.0 cm) has a tendency to be more aggressive in their growth [21–25]. However, multivariate analysis showed that both SUVmax (cutoff value of 5.5) and the FDG-avid lymph node were the only independent factor for the prediction of patient survival. The fact on the size of lymph node has no significant contribution to the revised AJCC staging (7th edition) given the range of minute to the cutoff size of 4.5 cm were unable to differentiate between T1 and T2 stages and the substages of T1a (muscularis propria) and T1b (submucosa) in this study design.
This study has several limitations. A large trial is deemed required to further reveal the use of SUVs in the prediction of survival in patients with oesophageal cancer. The analysis of the visual PET lymph node avidity was done by a radiologist who bears a potential bias on the results. Some of the patients had high glucose level of more than 7.0 mmol/l which was put on insulin regimen to normalize the value for which modification could result in inaccuracy of the images reading. It is noteworthy to highlight that the strengths of this study are that the inclusion of the prospective patients on the new AJCC TNM staging 7th edition and the available pathologic results of the resected tumour were possible in every patient in this study cohort. Both evaluations on the value of the T and N staging on the new AJCC 7th edition are by far limited in this study evaluation given that the significance of the size and the number for the T and N staging, respectively, were insignificant on the CT and PET comparison. Further studies that look at the correlation on the sonographic or histological involvement of the tumour on T and the inclusion a more comprehensive assessment of the tumour total glucose activity would ensure the thoroughness of the revised AJCC 7th edition values [26].
Conclusion
Pretreatment PET–CT SUVmax of a primary tumour and the presence of FDG-avid lymph nodes could potentially predict survival in patients with oesophageal carcinoma based on the PET–CT staging using AJCC TNM 7th edition.
References
Surveillance Epidemiology and End Results (SEER). Available at: http://seer.cancer.gov/statistics/. Accessed April 16, 2012
Skehan SJ, Brown AL, Thompson M, et al. (2000) Imaging features of primary and recurrent esophageal cancer at FDG PET. Radiographics 20(3):713
American Cancer Society (2003) Cancer facts and figures 2003. Atlanta: American Cancer Society
Westerterp M, Sloof GW, Hoekstra OS, et al. (2008) 18FDG uptake in esophageal adenocarcinoma: linking biology and outcome. J Cancer Res Clin Oncol 134:227–236
Choi JY, Lee KH, Shim YM, et al. (2000) Improved detection of individual nodal involvement in squamous cell carcinoma of the esophagus by FDG PET. J Nucl Med 41:808–815
Lina C-Y, Dingb H-J, Chenc Y-K, et al. (2008) F-18 FDG PET in detecting uterine leiomyoma. Clin Imaging 32(1):38–41
van Vliet EP, Heijenbrok-Kal MH, Hunink MG, Kuipers EJ, Siersema PD (2008) Stag-ing investigations for oesophageal cancer: a meta-analysis. Br J Cancer 98:547–557
Fathinul Fikri AS, Kroiss A, Ahmad ZF, et al. (2014) Localization and prediction of malignant potential in recurrent pheochromocytoma/paraganglioma (PCC/PGL) using 18F-FDG PET/CT. Acta Radiol 55(5):631–640. doi:10.1177/0284185113504330
Fukunaga T, Okazumi S, Koide Y, Isono K, Imazeki K (1998) Evaluation of esophageal cancers using fluorine-18-fluorodeoxyglucose PET. J Nucl Med 39:1002–1007
Rizk NP, Tang L, Adusumilli PS, et al. (2009) Predictive value of initial PET-SUVmax in patients with locally advanced esophageal and gastroesophageal junction adenocarcinoma. J Thorac Oncol. 4(7):875–879
Fathinul F, Nordin AJ, Lau WFE (2013) 18[F] FDG-PET/CT is a useful molecular marker in evaluating tumour aggressiveness; a revised understanding of an in-vivo FDGPET imaging that alludes the alteration of cancer biology. Cell Biochem Biophys 66:37–43. doi:10.1007/s12013-012-9395-5
Fathinul Fikri AS, Nordin AJ, Mokhtarudin N, Hemalata A, Lau WFE (2011) 18[F] FDG-PET/CT is a useful molecular marker in evaluating thymoma aggressiveness. Eur J Radiol Extra. 78(2):89–92
Rice TW, Blackstone EH, Rusch VW (2010) 7th Edition of the AJCC Cancer Staging Manual: esophagus and esophagogastric junction. Ann Surg Oncol 17(7):1721–1724
Kato H, Kimura H, Nakajima M, et al. (2008) The additional value of integrated PET/CT over PET in initial lymph node staging of esophageal cancer. Oncol Rep 20:857–862
Roedl JB, Prabhakar HB, Mueller PR, Colen RR, Blake MA (2009) Prediction of metastatic disease and survival in patients with gastric and gastroesophageal junction tumors: the incremental value of PET-CT over PET and the clinical role of primary tumor volume measurements. Acad Radiol 16:218–226
Yasuda T, Higuchi I, Yano M, et al. (2012) The impact of 18F-fluorodeoxyglucose positron emission tomography positive lymph nodes on postoperative recurrence and survival in resectable thoracic esophageal squamous cell carcinoma. Ann Surg Oncol 19:652–660
Gillies RS, Middleton MR, Han C, et al. (2012) Role of positron emission tomography-computed tomography in predicting survival after neoadjuvant chemotherapy and surgery for oesophageal adenocarcinoma. Br J Surg 99(2):239–245
Williams RN, Ubhi SS, Sutton CD, et al. (2009) The early use of PET-CT alters the management of patient with esophageal cancer. J Gastrointes Surg 13:868–873
Boris S, Daniel PR, Marek P, et al. (2009) Does the value of PET-CT extend beyond pretreatment staging? An analysis of survival in surgical patients with esophageal cancers. J Gastrointest Surg. doi:10.1007/s11605-009-1038-9
Facey K, Bradbury I, Laking G, et al. (2007) Overview of the clinical effectiveness of positron emission tomography imaging in selected cancers. Health Technol Assess 11:iii-iv, xi-267
Vliet EPM, Heijenbrok-Kal MH, Hunink MGM, et al. (2008) Staging investigation for esophageal cancer: a meta-analysis. Br J Cancer 98:547–557
Vaupel P, Mayer A (2007) Hypoxia in cancer: significance and impact on clinical outcome. Cancer Metastasis Rev 26:225–239
Wang BY, Goan YG, Hsu PK, Hsu WH, Wu YC (2011) Tumor length as a prognostic factor in esophageal squamous cell carcinoma. Ann Thorac Surg 91(3):887–893. doi:10.1016/j.athoracsur.2010.11.011
Wu N, Pang LW, Chen ZM, Ma QY, Chen G (2012) Tumour length is an independent prognostic factor of esophageal squamous cell carcinomas. Chin Med J. 125(24):4445–4448
Wang BY, Liu CY, Lin CH, et al. (2012) Endoscopic tumor length is an independent prognostic factor in esophageal squamous cell carcinoma. Ann Surg Oncol 19(7):2149–2158
Hyun SH, Choi JY, Shim YM, et al. (2010) Prognostic value of metabolic tumor volume measured by 18F-fluorodeoxyglucose positron emission tomography in patients with esophageal carcinoma. Ann Surg Oncol 17(1):115–122
Acknowledgments
We are grateful to Dr R. Dharmendran and his colleagues of the Hospital Tuanku Ja’afar Seremban for their joint effort in the data collection process for all patients in this study.
Conflict of interest
None.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Fathinul Fikri, A.S., Dharmendran, R., Vikneswaran, P. et al. 18F-FDG PET/CT as a potential predictor of survival in patient with oesophageal cancer: a preliminary result. Abdom Imaging 40, 1457–1464 (2015). https://doi.org/10.1007/s00261-014-0343-2
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00261-014-0343-2