Abstract
Titanium is a metallic element known by several attractive characteristics, such as biocompatibility, excellent corrosion resistance and high mechanical resistance. It is widely used in Dentistry, with high success rates, providing a favorable biological response when in contact with live tissues. Therefore, the objective of this study was to describe the different uses of titanium in Dentistry, reviewing its historical development and discoursing about its state of art and future perspective of its utilization. A search in the MEDLINE/PubMed database was performed using the terms ‘titanium’, ‘dentistry’ and ‘implants’. The title and abstract of articles were read, and after this first screening 20 articles were selected and their full-texts were downloaded. Additional text books and manual search of reference lists within selected articles were included. Correlated literature showed that titanium is the most used metal in Implantology for manufacturing osseointegrated implants and their systems, with a totally consolidated utilization. Moreover, titanium can be also employed in prosthodontics to obtain frameworks. However, problems related to its machining, casting, welding and ceramic application for dental prosthesis are still limiting its use. In Endodontics, titanium has been used in association to nickel for manufacturing rotatory instruments, providing a higher resistance to deformation. However, although the different possibilities of using titanium in modern Dentistry, its use for prostheses frameworks still needs technological improvements in order to surpass its limitations.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Titanium is a chemical element represented by the symbol Ti, with atomic number of 22 (which represents 22 protons and 22 electrons) and atomic mass equal to 47.90 u. It is a light transition metal, strong, metallic white colored, lustrous, resistant to corrosion and solid in room temperature [1]. Titanium was initially found in 1790, in England, by Reverend William Justin Gregor, while he was working with magnetic sand. Later, this element was isolated once again by the German chemist Heinrich Klaproth, this time in the rutile mineral (TiO2). He named the mineral titanium in 1795, in honor of the Titans, sons of Uranus and Gaya, from Greek mythology [2]. However, only in 1887 the impure metallic titanium was produced and, in 1938, a process capable of producing commercial amounts of titanium was developed [3]. Metallic titanium was not used out of the laboratory until 1946, when William Justin Kroll developed a method of producing commercial titanium through the reduction of titanium tetrachloride (TiCl4) with magnesium at 800 °C in argon atmosphere. This process is universally known as “Kroll Process” and it is the most used process to obtain commercial titanium. According to this method, it is obtained a porous product known as titanium sponge which, subsequently, is purified to reach the commercial product [1, 4].
Although titanium as a metal is not found free in nature, it is the ninth most found element and the fourth metal in abundance in earth, preceded by aluminum, iron and magnesium; and it is present in most of the igneous rocks and sediments derived from these rocks [4]. It is mainly found in the minerals ilmenite, leucoxene and rutile. Titanium global reserves are estimated in 300 million tons and the most important are found in Australia, Scandinavia, United States, Canada, Finland and Malaysia [2, 4, 5].
Titanium is a metallic element known by its several attractive characteristics, such as excellent corrosion resistance (almost equivalent to platinum) and mechanical resistance. It has low thermal conductivity and high electrical conductivity. As cited previously, it is a light and strong metal, easy to manufacture and with low density (40 % of the iron density). When pure, it is ductile and easy to work. Its relatively high fusion point makes it useful as a refractory metal. In addition, titanium is strong as steel, but 45 % lighter; and 60 % heavier than aluminum, but twice stronger. Such characteristics make titanium very resistant against the usual types of fatigue. This metal forms a passive layer of oxide (Fig. 1) when exposed to air, but when it is in an oxygen-free environment it is ductile. Moreover, it burns when heated and is capable of burning when immerse in sulfuric and hydrochloric nitrogen, as well as most of organic acids [1, 4].
Titanium is a versatile material utilized in several areas such as: engineering, chemical industry (due to its corrosion resistance and chemical attack), naval industry (used in submarine equipments and to desalinate sea water), nuclear industry (used in the fabrication of heat recuperators in nuclear energy power stations), and war industry (fabrication of missiles and weapons). When used as a metal and its alloys, near 60 % of titanium is utilized in aeronautical and aerospacial industries to fabricate motor pieces and turbines, airplanes fuselage and rockets [4].
Among all of these applications, approximately 95 % of all titanium is used as titanium dioxide, an intensely white permanent pigment. Thus, it has been applied in several areas from infrared radiation reflectors used by astronomers to applications in products such as bicycles, glasses, computers and sunscreen oils [4].
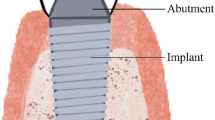
As it was described above, titanium has been historically used in several areas, mainly in engineering. However, titanium is also widely used in Medical areas and Dentistry, with high success rates [6, 7], mainly due to its characteristics of high corrosion resistance, low toxicity, very low allergenic potential and biocompatibility (Fig. 2), allowing a favorable biological response when in contact with live (Fig. 3) [6–8]. According to several authors [9–11], this favorable biological response is due to its limited ions release, stability of compounds that are formed and limited biological effects of these ions. Moreover, when titanium is in contact with oxygen (air), a layer of titanium dioxide is formed immediately, with 4 nm thickness, which is a powerful barrier against the metal dissolution [8, 12, 13]. Therefore, several authors agree that this chemically inert layer of titanium dioxide is the main responsible for the biological properties of titanium in a live organism (Fig. 4) and has only a small tendency to corrosion [13, 14]. However, Strietzel et al. [15] observed that there is a release of titanium ions in the presence of fluoride, thus recommending to avoid the use of fluorides if there are titanium pieces exposed in the mouth.
Demineralized histologic section showing the biocompatibility of implant surface of titanium commercially pure (cp-Ti). It is possible to note the bone tissue formation in contact with the implant installed in rabbit tibia. a postoperative of 30 days, b postoperative of 60 days, c postoperative of 90 days. Hematoxylin and eosin staining, increase of ×200
Mineralized histological section of cp-Ti implant installed in rabbit tibia. 30 days post-operative. a Total view of the implant showing neoformed bone tissue in contact with its surface, b Enlarged image (×200) of a implant thread highlighting neoformed bone tissue inside it. Alizarin red and Stevenl’s blue stains
Due to these characteristics, the aim of this study was to describe the different uses of titanium in Dentistry, including its historical development, and to discuss about the state of the art and future perspectives about its utilization.
State of the Art
The literature review showed that the initial utilization of titanium in Dentistry is dated from the 60’s and occurred accidentally. In 1965, the Swedish doctor Per-Ingvar Brånemark was investigating the blood microcirculation in rabbits tibiae with an observational camera made of titanium, when he noticed that metal and bone were perfectly integrated, without any rejection, and these cameras were very difficult to be removed. Based on this observation, Brånemark developed special cylinders to be implanted in rabbits’ and dogs’ tibiae; which became, later, a secure, modified and optimized base to receive long-term fixed prosthesis in maxilla and mandible (Fig. 5) for human application [16]. In this same year, a 10-year follow-up study was initiated in Gothenburg, Sweden to evaluate the clinical results from the application of this technique in humans [17].
In 1982, In Toronto, Canada, Brånemark presented the results of his research with implants [18]. Such results reached a success rate near 97 %, what promptly received the recognition of all international scientific community. Brånemark definitively introduced the technique of osseointegrated titanium implants, not only in Dentistry, but also in the medical area [18].
Titanium implants have been used with success for years in the substitution of lost dental elements. They can be manufactured both from commercially pure titanium (cp-Ti) or titanium alloys. A passive layer based on titanium oxide allows an intimate apposition of physiological fluids, proteins (Fig. 6), hard (Fig. 7) and soft tissues to the metallic surface [7, 19].
Titanium in Dentistry
Titanium can be found in different combinations for use in Dentistry. Pure titanium is composed by 99.5 % of titanium and 0.5 % of interstitial elements (carbon, oxygen, nitrogen, hydrogen and iron) and the proportion of these elements directly affects the metal properties. The ASTM (American Society of Testing and Materials) Standard F1295 [20] specifies titanium in different grades according to its purity, which is evaluated according to the amount of oxygen. The titanium melted only from titanium sponge is known as titanium grade 1, which is considered the most pure grade. When titanium sponge is mixed with titanium fragments, the amount of oxygen (O2) and iron (Fe) increases and titanium becomes harder. The more fragments are added, the harder the titanium becomes (titanium grades 2, 3 and 4). Therefore, grade 1–4 refers to cp-Ti, but with different purity grades (Table 1).
Besides grades 1–4, a variety of elements can be added to titanium, composing titanium grade 5, according to the ASTM [20]. Grade 5 refers to the three combinations of aluminum (Al) and vanadium (V). An alloy with aluminum and vanadium, for example, will increase the material hardness while an alloy with palladium (Pd) will only increase its corrosion resistance (corresponding to titanium grades 7 and 11) [20]. Formulations of alloys containing titanium have been studied and, among them, the system titanium–aluminum–vanadium (Ti–6Al–4V) has been the most utilized due to its better physical and mechanical properties in comparison to commercially pure titanium [20]. Besides presenting a higher hardness [6, 21], Ti–6Al–4V alloy also showed to be more resistant to fatigue than cp-Ti [6, 22].
Exploring the properties that indicate titanium for implants fabrication and searching for alternative cast alloys, besides its conventional use for osseointegrated implants and their systems, titanium and its alloys also became attractive to be used in dental prostheses frameworks.
Cp-Ti presents mechanical properties similar or slightly better than gold alloys type III and IV, nickel–chromium (Ni–Cr) and cobalt–chromium (Co–Cr), normally used in the fabrication of these frameworks [1]. Therefore, it has been used in the fabrication of prosthetic devices such as crowns, removable prosthesis, partial fixed prosthesis and, recently, in frameworks of implant-supported prosthesis [23]. In case of pure titanium, besides being an abundant element in nature, it is less toxic than the other materials used in dental prosthesis (cobalt and nickel, both combined with chromium). Likewise, as cited previously, titanium has a low allergenic potential and does not cause allergic reaction [6, 23, 24]. Moreover, the low density of titanium allows to fabricate extremely light and resistant prosthesis [21].
Although titanium provides some advantages to these prostheses, the high melt temperature of titanium, near 1,672 °C, requires special melt procedures, cooling cycles, investments and equipments to avoid its contamination [21]. These requirements must be followed because titanium suffers a change in its crystalline state at 883 °C, where the alpha-hexagonal phase changes to beta-cubic phase. This transformation affects the interface between titanium and ceramic and this is the reason why dental ceramics used with titanium are burned under 800 °C. Due to the gas absorption and high chemical reactivity of casting, titanium is difficult to be processed through the conventional technique of lost-wax. In high temperatures, it reacts with gaseous elements such as nitrogen, oxygen and hydrogen, and it must be manipulated in a controlled environment; otherwise the risk to form a thick layer of oxides, designated “alpha case”, may reduce the resistance and ductility of the structure obtained. Moreover, due to its low specific weight, the injection of the molten metal into the investment mould requires special care during the cast process, such as use of a vacuum chamber and controlled environment [21, 25].
There are three main types of titanium casting systems: casting under pressure/vacuum with separated chambers of melt and casting; casting under pressure/vacuum with a single chamber of melt and casting; casting under vacuum/centrifugation. Also, dental titanium casting can be accomplished through the methods of centrifugation or pressure/vacuum [26]. The metal is melt with an electric plasma arch or through inductive heating in a chamber full of inert gas or at vacuum. The metal is melt and then is transferred to the refractory mould through the centrifuge or through filling under pressure/vacuum [24, 26].
Several equipments are commercially available for casting titanium but their cost is considerably higher than the conventional casting equipments [19]. Materials with low reactivity are used to prevent superficial reactions with the melted metal while materials with high expansion are used to compensate the high shrinkage of titanium. For this purpose, it is preferable to use titanium alloys. The most common is the Ti–6Al–4V alloy, mainly because of its simple reproducibility [27].
Due to these difficulties related to titanium casting, other techniques were developed to fabricate crowns and frameworks. Such techniques comprise titanium machining from a solid metallic block. The frameworks are all fabricated in titanium grade 2 considering four generations of development. The first generation is based on pre-fabricated titanium cylinders and a bar component, which are joined through laser welding [28]. In the second generation technique, different pieces of titanium components with cylinders are used. After the leveling of the components, a titanium bar is positioned and, then, horizontally laser welded [28]. In the third generation of frameworks, small titanium components are individually cast by a technician for each titanium abutment at the main mould and, then, joined by laser weld. After that, resin teeth are joined or ceramic is cast to the titanium device [28, 29]. In the fourth generation, Procera® titanium frameworks are fabricated in a single piece through machining controlled by a computer. This technique is based on a concept where the technician makes a resin pattern simulating the final shape of the framework, and then this pattern is scanned and its image is generated by a software. Information about the implants positions are also given to the computer. When all data are collected, a framework is machined from a solid block of titanium grade 2, which is only refined and polished by a technician. Following, resin teeth are fixed or a low fusion porcelain is used to fabricate the teeth [28].
Other techniques of frameworks fabrication through titanium machining are also available. Such techniques utilize the CAD-CAM system and comprise a computed system of restoration/reconstruction that uses a scanning technique primarily in combination with machining techniques of titanium and/or porcelain in the prosthetic laboratory. The different systems can use either CAD-CAM or a wax pattern combined with CAM [30]. Some systems known are: Hint-ELs®, Procera®, DCS President System®, Cad. Esthetics, KaVo Everest® System, microDenta, Cercon®brain and Cerec® [31]. These techniques are based on a model scanning, which is digitalized for the production of a design by the software. This design would represent the final shape of the desired structure. Therefore, through the data obtained from the representation of the final structure shape, the framework is fabricated by industrial machining equipments using a single block of titanium. From this moment, also through the same system described previously, the crowns on the framework are obtained by machining a ceramic block [30, 31].
Despite of the wide availability of systems, the abrasive machining of titanium is slow and extremely expensive, what has been limiting this technique [19]. Therefore, for the fabrication of these elements in titanium, casting is still the most used technique. Thus, to obtain a satisfactory sealing between prosthesis and implants, segmented structures are fabricated and then welded in order to minimize casting distortions [29]. Laser welding aims to enhance the poorer marginal adaptation of titanium castings, because, besides the difficulties of casting and machining, titanium presents a great difficulty related to the conventional welding due to its high melting point and chemical reactivity [8, 21]. In laser welding, the intensity and duration of laser pulse is such that a sufficient energy can be provided to a junction in order to join the segmented structures and reach a weld point before a high heat is conducted to other parts of the piece. It means that there is a small generation of heat for the piece, except for the point of laser application [29]. Despite of these advantages, Sjögren et al. [32] attested that the chemical composition of the highly reactive titanium is changed in the weld point during laser welding, and this can influence the mechanical properties in this region. Moreover, these authors describe different defects in welded samples, such as pores and fissures in fractured surfaces.
According to all characteristics described previously, it is possible to verify that there is no perfect technique to fabricate titanium frameworks in dental prosthesis. Difficulties concerning its processing have limited the use of titanium in prosthesis and frameworks, even with all favorable characteristics.
However, it is important to state that, concerning osseointegrated implants, the use of titanium is consolidated. Moreover, titanium has been used in other areas of Dentistry, besides prosthodontics, such as endodontics. In this area, the use of titanium is also consolidated where, in combination with nickel, is used for the fabrication of rotatory instruments, providing them a higher resistance [33].
Conclusions
Due to the several possibilities of using titanium, it seems to be a promising material in modern Dentistry. Because of its physiological inertia, biocompatibility, corrosion resistance in oral environments, and combination of strength and lightness; titanium can be considered a versatile and utile biomaterial, which will probably increase its importance in the dental market. However, although all its advantages and being considered economic and promptly available, the technologies related to its machining, casting, welding and application of ceramic for dental prosthesis still are expensive and with important limitations. Therefore, a wide use of titanium in dental prosthesis will depend on technological advance and more laboratorial and clinical investigations in order to develop more profitable techniques proving its efficiency.
References
Van Noort R (2002) Casting alloys for metallic restorations. In: Van Noort R (ed) Introduction to dental materials. Mosby, Toranto, pp 221–230
Gegel HL, Hoch M (1973) Alloy processing. In: Jaffee RI, Burte HM (eds) Metallurgical society of AIME. Titanium science and technology. Plenum Press, New York, pp 923–931
Brown D (1997) All you wanted to know about titanium, but were afraid to ask. Br Dent J 10:393–394
Maia A (2003) Titânio. Balanço Mineral Brasileiro 1:1–23
Freese HL, Volas MG, Wood RJ (2001) Metallurgy and technological properties of titanium and titanium alloys. In: Brunette DM, Tengvall P, Textor M, Thomsen P (eds) Titanium in medicine: material science, surface science, engineering, biological responses and medical applications. Springer-Verlag, Berlin, pp 13–24
Niinomi M (2008) Mechanical biocompatibilities of titanium alloys for biomedical applications. J Mech Behav Biomed Mater 1:30–42. doi:10.1016/j.jmbbm.2007.07.001
Steinemann S (1998) Titanium—the material of choice. Periodontol 2000 17:7–21. doi:10.1111/j.1600-0757.1998.tb00119.x
Parr GR, Gardner LK, Toth RW (1985) Titanium: the mystery metal of implant dentistry. Dental materials aspects. J Prosthet Dent 54:410–414. doi:10.1016/0022-3913(85)90562-1
Williams DR (1981) Implants in dental and maxillofacial surgery. Biomaterials 2:133–146
Ducheyne P, Healy KE (1988) Surface spectroscopy of calcium phosphate ceramic and titanium implants materials. In: Ratner B (ed) Surface characterization of biomaterials. Elsevier, Amsterdam, pp 175–192
Adell R, Eriksson B, Lekholm U, Brånemark P-I, Jemt T (1990) A long-term follow-up study of osseointegrated implants in the treatment of totally edentulous jaws. Int J Oral Maxillofac Implants 5:347–359
Albrektsson T (1983) Direct bone anchorage of dental implants. J Prosthet Dent 50:255
Kasemo B (1983) Biocompatibility of titanium implants: surface science aspects. J Prosthet Dent 49:832–837
Kaus T, Pröbster L, Weber H (1996) Clinical follow-up study of ceramic veneered titanium restorations—three-year results. Int J Prosthodont 9:9–15
Strietzel R, Hösch A, Kalbfleisch H, Buch D (1998) In vitro corrosion of titanium. Biomaterials 19:1495–1499. doi:10.1016/S0142-9612(98)00065-9
Brånemark P-I, Breine U, Adell R, Hansson BO, Lindström J, Ohlsson A (1969) Intraosseous anchorage of dental prostheses. I—experimental studies. Scand J Plast Reconstr Surg 3:81–100
Brånemark P-I, Hansson BO, Adell R, Breine U, Lindström J, Hallén O, Ohman A (1977) Osseointegrated dental implants in the treatment of edentulous jaw. Experience from a 10 years period. Scand J Plast Reconstr Surg Suppl 16:1–132
Brånemark P-I, Adell R, Albrektsson T, Lekholm U, Lindström J, Rockler B (1984) An experimental and clinical study of osseointegrated implants penetrating the nasal cavity and maxillary sinus. J Oral Maxillofac Surg 42:497–505
ADA Council on Scientific Affairs (2003) Titanium applications in dentistry. J Am Dent Assoc 134:347–349
ASTM Standard, F1295 (2005) Standard specification for wrought titanium-6 aluminum-7 niobium alloy for surgical implant applications, ASTM International, West Conshohocken [ASTM International website]. Available at: http://www.astm.org. Accessed 30 March 2010
Wang RR, Fenton A (1996) Titanium for prosthodontic applications: a review of the literature. Quintessence Int 27:401–408
Zavanelli RA, Henriques GEP, Ferreira I, Rollo JM (2000) Corrosion-fatigue life of commercially pure titanium and Ti–6Al–4V alloys in different storage environments. J Prosthet Dent 84:274–279. doi:10.1067/mpr.2000.108758
Ohkubo C, Hanatani S, Hosoi T (2008) Present status of titanium removable dentures—a review of the literature. J Oral Rehabil 35:706–714. doi:10.1111/j.1365-2842.2007.01821.x
Tschernitschek H, Borchers L, Geurtsen W (2005) Nonalloyed titanium as a bioinert metal—a review. Quintessence Int 36:523–530
Bessing C, Bergman M (1992) The castability of unalloyed titanium in three different casting machines. Swed Dent J 16:109–113
Zinellis S (2000) Effect of pressure of helium, argon, krypton and xenon on the porosity, microstructure, and mechanical properties of commercially pure titanium castings. J Prosthet Dent 84:575–584. doi:10.1067/mpr.2000.109479
Lautenschlager EP, Monaghan P (1993) Titanium and titanium alloys as dental materials. Int Dent J 43:245–253
Örtop A, Jemt T (2001) Développement des aramatures en titane pour la prothése implantaire. Implant 7:169–175
Örtop A, Jemt T (1999) Clinical experiences of implant-supported prostheses with laser-welded titanium frameworks in the partially edentulous jaws. A 5-year follow-up study. Clin Implant Dent Relat Res 2:84–91
Kucey BK, Fraser DC (2000) The Procera abutment—the fifth generation abutment for dental implants. J Can Dent Assoc 66:445–449
Henriksson K, Jemt T (2003) Evaluation of custom-made Procera ceramic abutments for single implant tooth replacement: a prospective 1-year follow-up study. Int J Prosthodont 16:626–630
Sjögren G, Andersson M, Bergman M (1988) Laser welding of titanium in dentistry. Acta Odontol Scand 46:247–253
Plotino G, Grande NM, Cordaro M, Testarelli L, Gambarini G (2009) A review of cyclic fatigue testing of nickel-titanium rotatory instruments. J Endod 35:1469–1476. doi:10.1016/j.joen.2009.06.015
Conflict of interest
The authors claim to have no financial interest, directly or indirectly, in any entity that is commercially related to the products mentioned in this study.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Jorge, J.R.P., Barão, V.A., Delben, J.A. et al. Titanium in Dentistry: Historical Development, State of the Art and Future Perspectives. J Indian Prosthodont Soc 13, 71–77 (2013). https://doi.org/10.1007/s13191-012-0190-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13191-012-0190-1