Abstract
In a tropical coastal environment, mangrove ecosystems store large amount of atmospheric carbon in biomass and soil. West coast of India host 29% of the mangrove ecosystems of the country. Putting aside the few scientific studies about the Indian mangroves system, there exist gaps in our scientific knowledge of Blue Carbon domain. This study aims to quantify the ecosystem carbon stocks potential and community structure of mangrove vegetation/ ecosystem species of Vypin– Cochin, a coastal region of the Southwest coast of India. The information on species-specific carbon storage will benefit to choose best species in the afforestation and conservation initiatives. Distribution, community structure and carbon pools of above and below ground biomass of the study area were assessed. From the analysis, it is evident that the amount of carbon stored in mangroves varies according to species, functional attributes (basal area, DBH, mean height etc.) and region. Avicennia officinalis, Rhizophora mucronata, Acanthus ilicifolius, Acrostichum aureum, Bruguiera cylindrica and Sonneratia caseolaris are the mangrove species observed from the study area. As per the community structure assessment, A. officinalis showed large number, frequency, density, and evenness. While considering biomass, R.mucronata species possess high value. The mean overall above ground biomass of the region was 69.55 ± 80.38 t ha−1. Ecosystem carbon stock of the area ranges from 17.7–138.8 with an average of 54.3 ± 36 t ha−1. Therefore, the findings described will serve as a baseline on carbon stock and mangrove stand structure of the region and their role in Blue Carbon dynamics of the Southwest Coast of India.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Global warming and climate change have emerged as the most crucial environmental problem, faced by humanity. While considering these issues, wetlands have become ‘significant nonentities’ in the world of global climate change (Field 2014; Cinco-Castro and Herrera-Silveira 2020). Wetlands are the major sink of carbon-containing 40% of soil organic carbon at the global level and they represent the most significant component of the terrestrial biological carbon pool (Dixon and Krankina 1995). The vegetated coastal ecosystems such as tidal marshes, mangroves and seagrass meadows store carbon in the living organisms, decomposed organic materials and in the soil column. The carbon stored, sequester and re-emit from these ecosystems refers as ‘Blue Carbon’ (Chen et al. 2012; Herr et al. 2012; Pendleton et al. 2012; Rozainah et al. 2018; Saderne et al. 2019) which plays a vital role in the global carbon balance. Carbon cycle mainly happens through processes such as photosynthesis, respiration, decomposition, and combustion (Sruthi and Ramasamy 2018). Anthropogenic emissions and deforestation cause a significant increase in carbon dioxide in the atmosphere, results in the needs of enhancement of carbon stock and sequestration potential of different ecosystems (Pachauri et al. 2014) as well as in mangroves (Sharma et al. 2020).
Thus in the context of climate change mitigation both the reduction in CO2 emission and the carbon stocking in different systems have great significance (Liu et al. 2014). For the mitigation and management of climate change impacts, the United Nations Framework Convention on Climate Change (UNFCCC) encourages, its member countries to make a proper computation on stocked, sequestered and emitted carbon (Goodwin et al. 2020; Koh and Teh 2020; Kuhl et al. 2020). Mangrove ecosystems provides a valuable ecosystem service by mitigating the climate change through carbon sequestration and storage (Inoue 2019). From the reported studies, mangroves are the potential ecosystem to store 3–5 times more organic carbon than the terrestrial ecosystem (Donato and others 2011) and this source of carbon is considered as a crucial form of Blue Carbon (Mcleod et al. 2011). However, extensive deforestation and climate change impacts such as rise in sea level, increase in temperature, intensification of storms as well as tsunamis are the major disasters that have been faced by the mangrove community (Atwood and others 2017;Kathiresan and Faisal 2006).
Recently, carbon assimilated by mangrove ecosystems receives considerable attention and immense importance globally especially in the tropics (Castillo et al. 2018;Thorhaug et al. 2019). In most of the terrestrial systems, soil carbon saturation occurs through decades, but in the case of the coastal ecosystem, the sediments can store carbon dioxide for a long time and it might be over millennia. Disturbances to such carbon sinks, will release the stored carbon (for a long time) into the atmosphere and oceans, which results increase in greenhouse gases and global warming (Spivak et al. 2019).
At present, carbon stock by the coastal system in the form of ‘Blue Carbon’ has been reducing alarmingly due to the deterioration of such systems and the increase of carbon flux results severe effects on global climate (Hamilton and Friess 2018; Ouyang and Lee 2020). This has fuelled the research interest in estimating the carbon stock potential of Blue Carbon systems especially the mangroves in tropical ecosystems. Even though few literatures are available in the Indian mangrove systems (Banerjee et al. 2013; Joshi Gupta and Ghose 2014; Sanyal et al. 2018; Shrestha et al. 2019), there is diminutive data available from the Southwest coast of India on carbon dynamics (Vinod et al. 2019; Bindu et al. 2020). Even if the Southwest Coast of India has an extensive coastal zone, the baseline data on biomass analysis in mangrove ecosystem and their role in carbon stock are inattentive so far. Hence, the present study focuses to generate a baseline data on species-specific Blue Carbon storage by mangroves species of Vypin Island, Ernakulum District of Kerala, India.
Materials and Methods
Study Area
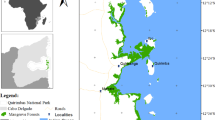
The study area is the selected mangrove forests of brackish water environments in Vypin Island of Ernakulum District on the Southwest Coast of India which extends from 76o12’ – 78o18’ E; 9o57’ – 10o03’N (Fig. 1). The area is characterized as backwater bodies, marshy lands, sandy flats and alluvial plains (Shyleshchandran and Ajaykumar 2018). Mangrove vegetation in the study area were distributed along the banks of brackish water channels, estuarine and coastal flood plains as narrow belts and as continuous patches. Besides that, Ernakulam region encompasses 24% of the mangrove areas of the state (Madhusoodhanan and Vidyasagar 2012).
Plot Selection and Sample Collection
For the analysis, simple random sampling method is adopted based on extent of the mangrove patch and accessibility to the selected plot. Nine plots from the study area (Goshree - L1,Belbopalam - L2, Rajagiri - L3, Ochanthuruthu - L4, Njarackel 1 - L5, Njarackel 2 - L6, Mundenveli 1 - L7, Mundenveli 2 - L8, Mundenveli 3 -L9) were selected for sampling. The minimum extent of each selected mangrove patch was One hectare. In each plot, subplots having50 X 50 m were deployed for the enumeration of the mangrove species. For the estimation of the total biomass, above ground and below ground biomass of each species were also assessed separately. In order to calculate the biomass of each species multiple plots having size of ‘10m X 10m’ were laid randomly in each plot. Each plant species of the subplot were analysed in terms of diameter at the base, middle and top, total height, number of branches, and total number of leaf. For the biomass estimation, five species as representatives (size class - < 6 m, 6–10 m,>10 m basal diameter) from each group of species were identified from the study area. Leaves, branches and stem of the collected plant samples were oven dried at 70 °C for 12 h, and dry weight of each species were recorded separately (Chidumayo 1990; Banerjee et al. 2013).
Community Structure Assessment
For the community structure assessment, an intensive survey was carried out on each sampling plots (n = 9) to record the species diversity, dominance, evenness, abundance, density, complexity index and Importance Value Index. Species diversity and dominance were calculated for all species of the plots (Magurran 2013). Shannon’s Index (Spellerberg and Fedor 2003) is employed for the biodiversity assessment and Simpson’s Index is used to measure the dominance. Abundance, density, relative abundance and relative density were also calculated in this study (Chen et al. 2018). Complexity index (Pool et al. 1977), and Importance value index (Joshi Gupta and Ghose 2014) were calculated by compounding relative density (RD), relative frequency (RF) and relative dominance(R Do).
Estimation of above Ground Biomass
Vegetation biomass was calculated using the parameters such as wood density, DBH, height, number of twigs, branches and number of individuals. Wood density values of each species was also computed. Dry biomass of each plant part was estimated as per the reported studies (Chidumayo 1990; Komiyama et al. 2005; Ray et al. 2011) In the case of fern/ herbs, which have scarcely branched stems, the whole plant biomass was accounted during the study.
Estimation of Stem Biomass
Stem biomass of the plant was calculated by using the allometric equation of Komiyama et al. (2000) and it is given below:
Where B is the biomass in kg, ρ is the Specific gravity (g/cm3) and D is the Diameter at breast height (cm). The biomass of the plant was then multiplied with the number of species in the plot and the value were converted into t ha−1. Diameter at Breast Height (DBH) utilized to assess the stem biomass and was measured at 1.37 m above the ground (Clough and Scott 1989; Komiyama et al. 2005).
Estimation of Leaf Biomass
Leaves and branches of each species were collected from each plot and were dried in an oven at 70 °C until it acquired a constant dry weight. The moisture content of the leaf samples and branches were calculated on the basis of its dry-weight. Fresh weight of the leaf per twig was converted to dry weight and thus the leaf biomass of each stem was estimated.
Above-Ground Branch Biomass Estimation
Based on the field observations, branch samples were categorised into the size class of diameter < 6, 6–10,>10 cm. The collected samples were oven dried for attaining constant dry weight. Multiplying the biomass of a single branch with the total number of branches of a tree accounts the total biomass of the plant. The resultant biomass again multiply with the total number of species, which accounts the total biomass of that particular species occupied in a particular area. Values were expressed in t ha−1(Ray et al. 2011).
Carbon Pool Estimation of above Ground Biomass
Carbon pools of aboveground biomass (AGB) are determined by multiplying the biomass of individual components of a plant with their specific carbon concentration (percentage). Conversion of biomass to carbon was done by multiplying with a conversion factor having value of “0.46” (Kauffman and Donato 2012).
Estimation of Plant Litter Biomass
Organic materials, excluding woody particles were collected from the surface layer of a mini plot of 50 X 50 cm. The total dry mass of this sub plot was scaled to a per-hectare approximation. The mean carbon concentration of the litter was calculated by multiplying with conversion factor of “0.45”(Kauffman et al. 1995).
Estimation of Pneumatophore Biomass
Pneumatophore density was determined by counting the number of pneumatophore in the single plot of 50 X 50 cm. The mean mass of a single pneumatophore was used to calculate the total mass of pneumatophores in the sampled stand and the mean dry weight of a single one was scaled to the total area and the value is expressed in t ha−1. The carbon conversion factor used for pneumatophore assessment is “0.39”(Mueller-Dombois and Ellenberg 1974).
Estimation of below Ground Carbon Pool
For the estimation of the soil carbon pool in mangrove systems, the samples were taken from each sampling location using a core having a diameter of 8 cm and 30 cm in length. The collected soil column was sliced in to two and its length vary from 0 to 10 cm, 10–20 cm and it is not reach beyond 30 cm and were packed in a polyethylene cover. Total 20 soil samples were collected, for the analysis of bulk density and the organic carbon. Soil organic carbon was determined by Walkley - Black chromic acid wet oxidation method (Jackson and Barak 2005).
Carbon Estimation
The total carbon stock (t ha−1) of the sample was computed by adding carbon stored in above ground carbon of tree species with the carbon stored in soil. The Carbon stock and carbon dioxide equivalents (CO2e) of the study area were calculated as per the procedure of Kauffman and Donato (2012).
Results and Discussion
Mangrove Vegetation Community Structure
A total of six species of mangrove plants, ie., Avicennia officinalis, Rhizophora mucronata, Acanthus ilicifolius, Acrostichum aureum, Bruguiera cylindrica and Sonneratia caseolaris were recorded from the study area. Their scientific name, common name and life form are given in Table 1. As per the diversity assessment, A. officinalis (100) and A. ilicifolius (77.77) showed relatively high frequency than A. aureum (55.55),R. mucronata (44.44) and S. caseolaris (22.22) while the least frequency (11.11) was observed in B. cylindrica. Species density showed the following trend, A. officinalis (63.89) > R. mucronata (37.44) > A. ilicifolius (33.22) > A. aureum (22.22) > S. caseolaris (11.22) > B. cylindrica (0.55) (Table 2).
The most abundant species observed from the study area is R. mucronata (84.25) followed by A. officinalis (63.88), S. caseolaris (50.5), A. ilicifolius (42.71) and B. cylindrica (5.0). Whereas very less evenness value was observed in R. mucronata (0.66) and A. ilicifolius (0.62). Less evenness in communities might be due to the presence of dominated species like A. officinalis. While calculating the Shannon index highest value was observed with A. officinalis (2.02) due to its high evenness and richness, followed by A. aureus (1.49), A. ilicifolius (1.48), R. mucronata (0.97) and S. caseolaris (0.68). Whereas B. cylindrica showed zero value may be due to the least evenness and its presence only in one plot. Simpson’s index showed the trend A. officinalis (0.84) > A. aureum (0.75) > A. ilicifolius (0.68) > R. mucronata (0.50) > S. caseolaris (0.49) and B. cylindrica (showed zero value). The index represents the probability that, if two individuals randomly selected from a sample will belong to different species, A. officinalis possess the high value, as it was the common mangrove species observed from the study area and B. cylindrica was observed only from a single plot.
Parameters such as basal area, density complexity index were recorded and are given in Table 3. The basal area of trees varying from 2.3 to 51.65m2 ha−1, and the highest recorded basal area was from R. mucronata and the least value (2.3 m2 ha−1) was observed from A. ilicifolius. Mean tree height ranges from 0.75 m to 7.3 m and R. mucronata showed the maximum tree height. The complexity index (Ic) ranged from 0.002 to 94.23 and the highest complexity index showed in A. officinalis, because of high density, number of species and basal area. B. cylindrica showed the least value (0.002) might because of less in number and distribution.
When considering the importance value index (IVI) A. officinalis was observed with the high value of 75.95 because of high relative density, relative frequency and relative dominance, while S. caseolaris resulted very less IVI value of 32.24 (Table 3).
The highest species diversity was observed in L8 with five species and least was found in L2, L3 and L7. Each location was occupied with only two species, which may be attributed to the magnitude of anthropogenic pressure, intense human activities or salinity profile of the area. A total of 1517 trees belong to six species were observed from the study. Among the six species, A. officinalis is the dominant one with highest number (575) of species (37.90%), followed by R. mucronata with 337 (22.21%), A. ilicifolius with 299 (19.71%), A. aureum with 200 (13.18%), S. caseolaris by 101 (6.66%) and B. cylindrica was found in least number of 5 (0.33%).
Above Ground Biomass
The stored biomass in the above-ground exhibited the trend; Stem>branch>leaf. In the above ground biomass provide a significant contribution and it varies according to the number and species. Average tree height and the number of species were counted separately from each quadrate.
In the above-ground components of the woody trees, the allocation of biomass ranged between 51% and 69% to stem, 22%–33% to branch,1%–18% to leaf in A. officinalis and 55% to 63% in stem, 26%–33% in branch and 2%–18% for R. mucronata (Table 4). For stem biomass, the values extend from 1.55 t ha−1 to 54.84 t ha-1 , for A. officinalis and 2.92 t ha−1 to 146.66 t ha−1 and for R.mucronata 0.23 t ha−1 for B.cylindrica and 33.01 t ha−1 to 45.23 t ha−1was observed from S.caseolaris. The branch biomass of A. officinalis ranged from 0.82 t ha−1 to 22.5 t ha−1 and for R. mucronata it ranged from 1.4 t ha−1 to 76.77 t ha−1. The value of B.cylindrica is 0.15 t ha−1 and for S. caseolaris it ranged from 6.55 t ha−1 to 11.61 t ha−1.
In the case of A. officinalis, the observed leaf biomass ranges from 0.64 to 4.93 t ha−1. Whereas for R.mucronata, it varies from 0.96 to 6.62 t ha−1. The value for B.cylindrica was 0.08 t ha-1 and for S.caseolaris, it ranged from 2.77 to 4.48 t ha-1. In the study area, litter and pneumatophore contribute only a small fraction of biomass.
While considering the above-ground biomass of the study area, the species wise biomass contribution is in the order R. mucronata< A. officinalis < S. caseolaris < B.cylindrica< A. ilicifolius< A. aureum. A. officinalis has a mean AGB of 23.29 t ha−1 and R. mucronata has a mean AGB of 78 t ha−1. B. cylindrica was observed from a single plot and it possess very less in number, while its AGB was 0.46 t ha−1. S. caseolaris resulted a mean AGB of 51.83 t ha−1. A. ilicifolius and A. aureum were considered as dwarf species and their total AGB have calculated with allometric equations. A. ilicifolius and A. aureum had an average AGB of 0.02 t ha−1 and 0.006 t ha−1 respectively.
The L1 region showed the highest value of AGB (257.36 t ha−1) and plot in L7 had the least value of 3.02 t ha−1. Comparatively L1, L2, L3, L8 and L9 showed relatively high AGB value. Whereas L4, L5, L6 and L7 showed the least value where species occurrence and number was very less (Fig. 2).
Soil Carbon Stock
Variation in temperature, bulk density, organic carbon and soil carbon were analysed and are given in Table 5. Soil temperature extends from 27.3 °C to 31.9 °C and the bulk density values ranges from 0.43 to 2.21 g/cm3. Values of organic carbon varies from 0.5% to 2.7%. Soil carbon stock in plots extends from 9.87 to 23.49 t ha−1. The average soil carbon stock of all plots was 18.26 ± 3.9 t ha−1. Studies postulates the increase in soil temperature due to climate change that might have a negative impact on the carbon stock potential of mangrove ecosystems and it possess negative correlation between soil temperature and carbon stock potential (Matsui et al. 2012).
Carbon Stock
Carbon Stock in Stem, Branch and Leaf
As per the results of carbon stock analysis in stem, R. mucronata showed very high carbon (23.32 t ha−1) followed by S.caseolaris (17.99 t ha−1), A. officinalis (7.05 t ha-1) and B.cylindrica (0.10 t ha−1). In case of branch carbon stock, the trend observed was similar to stem (Table 6). R. mucronata showed high carbon stock (11.35 t ha−1) followed by S.caseolaris with 4.17 t ha−1, A. officinalis with of 3.89 t ha-1.and least carbon content was found in B.cylindrica with 0.06 t ha−1. Where as in the case of leaf carbon stock S.caseolaris showed high mean value of carbon (1.66 t ha−1) followed Mucronata with 1.75 t ha−1 and A. officinalis with of 0.81 t ha−1, B.cylindrica showed least mean value of 0.03 t ha−1.
Carbon Stock in Species
Based on the species wise carbon stock analysis R.Mucronata showed very high mean above-ground carbon stock (AGCS) of 35.88 t ha−1 and A.aureum was observed with least mean value of 0.004 t ha−1. A. officinalis and Caseolaris showed mean AGCS values of 10.83 t ha−1 and 23.84 t ha−1 respectively. B. cylindrica and A.aureum possessed a mean value of 0.57 t ha−1 and 0.003 t ha−1 respectively.
The present study reveals that among all mangrove species, R. mucronata exhibit higher potential in carbon stock, it clearly can be observed in plot one. Carbon stock in litter had an average value of 0.35 t ha−1 and pneumatophore had a mean value of carbon stock 0.010 t ha−1. Therefore, litter and pneumatophores in the study area do not possess any significant carbon stock potential as comparable to the AGCS (Table 6).
Ecosystem Carbon Stock
Carbon stock in mangrove biomass and sediment carbon stock are the important carbon sinks in coastal and inland wetlands of tropical countries. The ecosystem carbon stock of the area ranges from 17.6–138.8 with an average of 54.3 ± 36 t ha−1. The greenhouse gas inventories generally represent the carbon stock in terms of CO2 equivalents. The observed CO2 equivalent of the study area was in the range of 64.6 to 509.6 with an average of 179.9 ± 132.2 t ha−1. Total above ground carbon stock, soil carbon stock, carbon stock of the study plot and CO2 Equivalents were depicted in Fig. 3.The ecosystem carbon stock of each plot was calculated and the values were comparable with the carbon stock value of mangroves in China which ranges from 28.8 t ha−1 to 355.25 t ha−1 (Liu and others 2014).
Tree Biomass Allocation
The mean AGB of the present study (69.55 ± 80.38 t ha−1) is comparable to that in Mahanadi Mangrove Wetland (Sahu 2016),Sundarbans (Mitra 2011, Choudhuri 1991) and Shigaki Island of South Japan (Suzuki and Tagawa 1983). The AGB of study possess similarity with CGM Biosphere Reserve (72.8 t ha−1) and in Mekong Delta (86.5 t ha−1)of Vietnam where Rhizophora sp. was the dominant species (Nam et al. 2016). Above ground biomass mean value of Avicennia officinalis showed close similarity with that observed from Avicennia alba (26.03 t ha−1) in the central region of Sunderbans (Mitraet al. 2011). AGB value of Sonneratia caseolaris showed a comparable result with that of Sonneratia apetala (47.68 t ha−1) in Sundarbans (Banerjee and others 2013). Acanthus ilicifolius and Acrostichum aureum were observed with a comparable AGB values (0.03 t ha−1 to 1.4 t ha−1) that reported from the herbs of Sundarbans (Joshi Gupta and Ghose 2014).
The leaf biomass of A. officinalis and R.mucronata, is not comparable with other species. Litter and pneumatophore contributed only a small fraction of biomass in the study area. The litter biomass of the present study concurs with the results of Bay of Sabol (Sitoeet al. 2014). The observed pneumatophores biomass result (0.2 t ha−1) were as close to that reported from Sundarbans (Joshi Gupta and Ghose 2014).
Bulk density of soil was similar to the value reported in Can Gio Mangrove Biospheres Reserve in Mekong delta (0.5 g/cm3) of Vietnam. Values of organic carbon percentage was comparable to that (2.29%) in the KienVang Protection Forest in Mekong delta (Nam et al. 2016).
Conclusion
The present study examined the community structure, and carbon stock potential of mangrove systems in Vypin region of Cochin coast, and the observed results vary according to spatial locations, species composition, and soil properties. It is evident that the carbon stock potential of mangroves has proven their efficiency to store more carbon and can consider it as a proficient sink of carbon. As per the community structure assessment, Avicennia officinalis dominated with large number, frequency, density and evenness. While considering the biomass, the species Rhizophora mucronata hold very high value. The assessment result of carbon stock proved that the species Rhizophora mucronata have a significant potential to capture the atmosphere carbon to soil. Mangrove ecosystems have a remarkable role to reduce the atmospheric CO2 by plant biomass and simultaneously enhance the soil carbon pool. The ecosystem carbon stock of the study area ranged between 17.7–138 with an average of 54.3 ± 36 t ha−1 and it reveal that the mangrove system of the region play a significant role in Blue Carbon dynamics of the region. The findings of the study would help in assessing carbon stock of Southwest Coast of India to a greater extent and can support the mangrove conservation and restoration programme. In addition, the information on species-specific carbon storage will help us to choose best species in the afforestation and conservation initiatives. Hence, the present study has the potential to become a blueprint for the carbon stock of the region and will be highly significant for climate change mitigation strategies.
References
Atwood TB, Connolly RM, Almahasheer H, Carnell PE, Duarte CM, Ewers Lewis CJ, Irigoien X, Kelleway JJ, Lavery PS, Macreadie PI, Serrano O, Sanders CJ, Santos I, Steven ADL, Lovelock CE (2017) Global patterns in mangrove soil carbon stocks and losses. Nature Climate Change. 7:523–528. https://doi.org/10.1038/nclimate3326
Banerjee K, Sengupta K, Raha A, Mitra A (2013) Salinity based allometric equations for biomass estimation of Sundarban mangroves. Biomass and bioenergy 56:382–391
Bindu G, Rajan P, Jishnu ES, Ajith Joseph K (2020) Carbon stock assessment of mangroves using remote sensing and geographic information system. Egyptian Journal of Remote Sensing and Space Science 23:1–9. https://doi.org/10.1016/j.ejrs.2018.04.006
Castillo JAA, Apan AA, Maraseni TN, Salmo SG (2018) Tree biomass quantity, carbon stock and canopy correlates in mangrove forest and land uses that replaced mangroves in Honda Bay, Philippines. Regional Studies in Marine Science 24:174–183. https://doi.org/10.1016/J.RSMA.2018.08.006
Chen L, Zeng X, Tam NFY, Lu W, Luo Z, du X, Wang J (2012) Comparing carbon sequestration and stand structure of monoculture and mixed mangrove plantations of Sonneratia caseolaris and S. apetala in southern China. Forest Ecology and Management 284:222–229
Chen L, Comita LS, Wright SJ, Swenson NG, Zimmerman JK, Mi X, Hao Z, Ye W, Hubbell SP, Kress WJ, Uriarte M, Thompson J, Nytch CJ, Wang X, Lian J, Ma K (2018) Forest tree neighborhoods are structured more by negative conspecific density dependence than by interactions among closely related species. Ecography. 41:1114–1123. https://doi.org/10.1111/ecog.03389
Chidumayo EN (1990) Above-ground woody biomass structure and productivity in a Zambezian woodland. Forest Ecology and Management 36:33–46
Choudhuri PKR (1991) Biomass production of mangrove plantation in Sundarbans, West Bengal (India) - a case study. Indian Forester. 117:3–12.
Cinco-Castro S, Herrera-Silveira J (2020) Vulnerability of mangrove ecosystems to climate change effects: the case of the Yucatan peninsula. Ocean and Coastal Management 192:105196. https://doi.org/10.1016/j.ocecoaman.2020.105196
Clough BF, Scott K (1989) Allometric relationships for estimating above-ground biomass in six mangrove species. Forest Ecology and Management 27:117–127. https://doi.org/10.1016/0378-1127(89)90034-0
Dixon RK, Krankina ON (1995) Can the terrestrial biosphere be managed to conserve and sequester carbon? BT - carbon sequestration in the biosphere. In: Beran MA (ed)Springer. Berlin Heidelberg, Berlin, Heidelberg, pp 153–179
Donato DC, Kauffman JB, Murdiyarso D, Kurnianto S, Stidham M, Kanninen M (2011) Mangroves among the most carbon-rich forests in the tropics. Nature Geoscience 4:293–297. https://doi.org/10.1038/ngeo1123
Field CB (2014) Climate change 2014–impacts, adaptation and vulnerability: regional aspects. Cambridge University Press
Goodwin E, Pathways MR-B briefs for 2020 O, 2019 U (2020) Mangroves and Coastal Ecosystems
Hamilton SE, Friess DA (2018) Global carbon stocks and potential emissions due to mangrove deforestation from 2000 to 2012. Nature Climate Change 8:240–244. https://doi.org/10.1038/s41558-018-0090-4
Herr D, Pidgeon E, Laffoley D (2012) Blue carbon policy framework 2.0: based on the discussion of the international blue carbon policy working group. Gland, Switzerland
Inoue T (2019) Carbon sequestration in mangroves. Blue Carbon in Shallow Coastal Ecosystems. Springer Singapore, Singapore, pp 73–99
Jackson ML, Barak P (2005) Soil chemical analysis : advanced course : a manual of methods useful for instruction and research in soil chemistry, physical chemistry of soils, soil fertility, and soil genesis. Parallel Press, University of Wisconsin-Madison Libraries
Joshi Gupta H, Ghose M (2014) Community structure, species diversity, and aboveground biomass of the Sundarbans mangrove swamps. Tropical Ecology 55:283–303
Kathiresan, K. and Faisal AM (2006) Managing Sundarbans for uncertainity and sustainability. International Conference and Exhibition on Mangroves of Indian and Western Pacific Oceans, ICEMAN. Kuala Lumpur, Malaysia, pp 1–31
Kauffman JB, Donato DC (2012) Protocols for the measurement, monitoring and reporting of structure, biomass and carbon stocks in mangrove forests. Center for International Forestry Research (CIFOR), Indonesia
Kauffman JB, Cummings DL, Ward DE, Babbitt R (1995) Fire in the Brazilian Amazon: 1. Biomass, nutrient pools, and losses in slashed primary forests. Oecologia 104:397–408. https://doi.org/10.1007/BF00341336
Koh HL, Teh SY (2020) Climate change mitigation and adaptation: role of mangroves in Southeast Asia. Springer, Cham, pp 224–236
Komiyama A, Havanond S, Srisawatt W, Mochida Y, Fujimoto K, Ohnishi T, Ishihara S, Miyagi T (2000) Top/root biomass ratio of a secondary mangrove (Ceriops tagal (Perr.) CB Rob.) forest. Forest Ecology and Management 139:127–134
Komiyama A, Poungparn S, Kato S (2005) Common allometric equations for estimating the tree weight of mangroves. Journal of Tropical Ecology 21:471–477
Kuhl L, Van Maanen K, Scyphers S (2020) An analysis of UNFCCC-financed coastal adaptation projects: assessing patterns of project design and contributions to adaptive capacity. World Development 127:104748. https://doi.org/10.1016/j.worlddev.2019.104748
Liu H, Ren H, Hui D, Wang W, Liao B, Cao Q (2014) Carbon stocks and potential carbon storage in the mangrove forests of China. Journal of Environmental Management 133:86–93
Madhusoodhanan V, Vidyasagar K (2012) Keralathille Kandalkkadukkal. Kerala Sastra Sahithya Parishad
Magurran AE (2013) Measuring biological diversity. John Wiley & Sons
Matsui N, Morimune K, Meepol W, Chukwamdee J (2012) Ten year evaluation of carbon stock in mangrove plantation reforested from an abandoned shrimp pond. Forests 3:431–444. https://doi.org/10.3390/f3020431
Mitra A, Sengupta K, Banerjee K (2011) Standing biomass and carbon storage of aboveground structures in dominant mangrove trees in the Sundarbans. Forest Ecology and Management 261:1325–1335. https://doi.org/10.1016/j.foreco.2011.01.012
Mcleod E, Chmura GL, Bouillon S, Salm R, Björk M, Duarte CM, Lovelock CE, Schlesinger WH, Silliman BR (2011) A blueprint for blue carbon: toward an improved understanding of the role of vegetated coastal habitats in sequestering CO 2. Frontiers in Ecology and the Environment 9:552–560. https://doi.org/10.1890/110004
Mueller-Dombois D, Ellenberg H (1974) Aims and methods of vegetation ecology. John Wiley & Sons
Nam VN, Sasmito SD, Murdiyarso D, Purbopuspito J, MacKenzie RA (2016) Carbon stocks in artificially and naturally regenerated mangrove ecosystems in the Mekong Delta. Wetlands Ecology and Management 24:231–244. https://doi.org/10.1007/s11273-015-9479-2
Ouyang X, Lee SY (2020) Improved estimates on global carbon stock and carbon pools in tidal wetlands. Nature Communications 11:1–7. https://doi.org/10.1038/s41467-019-14120-2
Pachauri RK, Allen MR, Barros VR, et al (2014) Climate change 2014: synthesis report. Contribution of working groups I, II and III to the fifth assessment report of the intergovernmental panel on climate change. Intergovernmental panel on climate change
Pendleton L, Donato DC, Murray BC, et al (2012) Estimating global “blue carbon” emissions from conversion and degradation of vegetated coastal ecosystems. PLoS One 7:
Pool DJ, Snedaker SC, Lugo AE (1977) Structure of mangrove forests in Florida, Puerto Rico, Mexico, and Costa Rica. Biotropica 9:195. https://doi.org/10.2307/2387881
Ray R, Ganguly D, Chowdhury C, Dey M, Das S, Dutta MK, Mandal SK, Majumder N, de TK, Mukhopadhyay SK, Jana TK (2011) Carbon sequestration and annual increase of carbon stock in a mangrove forest. Atmospheric Environment 45:5016–5024. https://doi.org/10.1016/j.atmosenv.2011.04.074
Rozainah MZ, Sofawi AB, Joharee NA, Pauzi AZ (2018) Stand structure and biomass estimation in the Klang Islands mangrove Forest, peninsular Malaysia. Environmental Earth Sciences 77:486. https://doi.org/10.1007/s12665-018-7636-7
Saderne V, Geraldi NR, Macreadie PI, Maher DT, Middelburg JJ, Serrano O, Almahasheer H, Arias-Ortiz A, Cusack M, Eyre BD, Fourqurean JW, Kennedy H, Krause-Jensen D, Kuwae T, Lavery PS, Lovelock CE, Marba N, Masqué P, Mateo MA, Mazarrasa I, McGlathery KJ, Oreska MPJ, Sanders CJ, Santos IR, Smoak JM, Tanaya T, Watanabe K, Duarte CM (2019) Role of carbonate burial in blue carbon budgets. Nature Communications 10:1106. https://doi.org/10.1038/s41467-019-08842-6
Sahu SC, Kumar M, Ravindranath NH (2016) Carbon stocks in natural and planted mangrove forests of Mahanadi Mangrove Wetland, East Coast of India. Current Science. 110:2253–2260.
Sanyal R, Inamdar AB, Agnihotri SB (2018) A comparative analysis of mangrove cover at two mangrove sites of India for climate adaptation strategy. 20th EGU general assembly, EGU2018, Proceedings from the conference held 4–13 April, 2018 in Vienna, Austria, p.1300. Vienna, Austria, p 1300
Sharma S, MacKenzie RA, Tieng T et al (2020) The impacts of degradation, deforestation and restoration on mangrove ecosystem carbon stocks across Cambodia. Science of the Total Environment 706:135416. https://doi.org/10.1016/j.scitotenv.2019.135416
Shrestha S, Miranda I, Kumar A, Pardo MLE, Dahal S, Rashid T, Remillard C, Mishra DR (2019) Identifying and forecasting potential biophysical risk areas within a tropical mangrove ecosystem using multi-sensor data. International Journal of Applied Earth Observation and Geoinformation 74:281–294. https://doi.org/10.1016/J.JAG.2018.09.017
Shyleshchandran MS, Ajaykumar B (2018) Quantitative geomorphological analysis to infer the hydrological behaviour of streams draining into the Vembanad Lake – a Ramsar site along the southwest coast of India. Journal of the Geological Society of India 92:45–53. https://doi.org/10.1007/s12594-018-0951-5
Sitoe A, Mandlate L, Guedes B (2014) Biomass and Carbon Stocks of Sofala Bay Mangrove Forests. Forests. 5:1967–1981. https://doi.org/10.3390/f5081967
Spellerberg IF, Fedor PJ (2003) A tribute to Claude Shannon (1916-2001) and a plea for more rigorous use of species richness, species diversity and the ‘Shannon-wiener’ index. Global Ecology and Biogeography 12:177–179. https://doi.org/10.1046/j.1466-822X.2003.00015.x
Spivak AC, Sanderman J, Bowen JL, Canuel EA, Hopkinson CS (2019) Global-change controls on soil-carbon accumulation and loss in coastal vegetated ecosystems. Nature Geoscience 12:685–692. https://doi.org/10.1038/s41561-019-0435-2
Sruthi SN, Ramasamy EV (2018) Enrichment of soil organic carbon by native earthworms in a patch of tropical soil, Kerala, India: first report. Scientific Reports 8:5784. https://doi.org/10.1038/s41598-018-24086-8
Suzuki E, Tagawa H (1983) Biomass of a mangrove forest and a sedge marsh on Ishigaki Island, South Japan. Japanese Journal of Ecology. 33:231–4
Thorhaug AL, Poulos HM, López-Portillo J, Barr J, Lara-Domínguez AL, Ku TC, Berlyn GP (2019) Gulf of Mexico estuarine blue carbon stock, extent and flux: mangroves, marshes, and seagrasses: a north American hotspot. Science of the Total Environment 653:1253–1261. https://doi.org/10.1016/J.SCITOTENV.2018.10.011
Vinod K, Asokan PK, Zacharia PU, Ansar CP, Vijayan G, Anasukoya A, Kunhi Koya VA, Nikhiljith M (2019) Assessment of biomass and carbon stocks in mangroves of Thalassery estuarine wetland of Kerala, south west coast of India. Journal of Coastal Research 86:209. https://doi.org/10.2112/si86-031.1
Acknowledgements
The first author gratefully acknowledges the support from the Director, Institute for Climate Change Studies (ICCS), Kerala. The authors are thankful to Dr. George Chackacherry (former Director, ICCS), and Dr. E V Ramasamy, Director, School of Environmental Sciences (SES), Mahatma Gandhi University, Kerala. The authors express their gratitude to the Director,National Centre for Earth Science Studies, Ministry of Earth Sciences, Government of India and for the encouragement. Authors would like to thank Dr. Joby Paul, Assistant Professor, Department of Botany, St. Thomas’ College, Thrissur, Kerala. The authors are grateful to the editors and anonymous reviewers for their insightful comments and suggestions.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
ShyleshChandran, M.S., Ravi, A., John, S.M. et al. Ecosystem Carbon Stock of Selected Mangrove Forests of Vypin – Cochin Region, Southwest Coast of India. Wetlands 40, 2263–2273 (2020). https://doi.org/10.1007/s13157-020-01365-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13157-020-01365-7