Abstract
Even though, the blue carbon ecosystems are gaining keen research interest around the globe, the carbon stock of South-West coast of India was poorly reported, and this study is a pioneer attempt and will be an important document for filling the gaps in uncertainties in global carbon stock assessment and also will increase knowledge on biomass variability among mangrove species and mangrove habitats. The study also highlighted the vital role of biomass carbon pool for long-term soil carbon burial. We estimated the above and below-ground biomass carbon stock of 13 mangrove species using two common allometric equations and species-specific equations and reported a very high mean total living biomass carbon stock of Kochi mangroves at 237.19 ± 113.82 Mg C ha−1, 295.78 ± 143.14 Mg C ha−1, and 272.42 ± 132.78 Mg C ha−1 according to Chave’s, Komiyama equation and species-specific equation respectively corresponding to carbon dioxide equivalent (CO2e) of approximately 4,37,774.51 Mg CO2e. The biomass stock was significantly differed between the stations (p = 0.000) and also within species (p = 0.020). Among the mangrove species, Avicennia officinalis contributed highest and Bruguiera sexangula contributed least. Variation was observed for biomass stock of mangroves with large trunk diameter while comparing different allometric equations and therefore projected the need for the development of site- and species-specific equations for solving the uncertainty in global mangrove carbon stock. The study observed that dominant mangrove vegetation type, salinity and also the geomorphology of mangrove habitat had strong influence on variability in biomass stock within a small regional area. The study also suggests that understanding the structure and biomass carbon storage of each regional mangrove habitats can be wisely used in mangrove restoration and conservation programmes and in turn for nature-based solutions for climate change mitigation efforts.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Quantifying forest biomass is of crucial importance in the area of climate change science, forest conservation and management, as it fixes the atmospheric carbon dioxide as plant biomass. Therefore, recognising the vital role of forest biomass for carbon sequestration, the United Nations labelled forest as potential carbon storage in Article 3.3 and 3.4 of the Kyoto protocol (United Nations 1998). Mangrove forests are not an exemption and are well-known for its high biomass, high productivity and litter production (Alongi 2014), thus considered as one of the carbon-richest habitats on earth. Protection of these habitats is of greatest scientific, social and economic concern of the world as it helps in reducing atmospheric carbon dioxide through its high carbon sequestration potential and can be best utilised for mitigating climate change (Murdiyarso et al. 2015). Even though the total global area of mangroves is very small compared to tropical forest, they contributed a global average above-ground biomass (AGB) carbon stock of 7 8.0 ± 64.5 Mg C ha−1 and sequestration of 2.9 ± 2.2 Mg C ha−1 year−1 (Estrada and Soares 2017) and a net primary production rate of 92–280 Tg C year−1 which is 15% of the total carbon accumulation in marine sediments (Kusumaningtyas et al. 2019).
The never-ending human needs are rapidly depleting the forest and almost 38% loss (1998–2010 period) in the global mangroves (Thomas et al. 2017) with the highest loss in developing countries, through conversion for reclamation, shrimp cultivation that has seriously affected their valuable ecosystem services including a reduction in sediment carbon burial and carbon stock (Rani et al. 2021; Kauffman et al. 2017; Jennerjahn et al. 2017; Pérez et al. 2017). This mangrove loss will trigger the emission of precious carbon stored in these habitats such as greenhouse gases like carbon dioxide (CO2) and methane (CH4) and may degrade its status from ‘carbon sink’ to ‘carbon source’.
In India, the mangrove biomass studies are limited compared to global studies and mostly confined to the mangrove forest of Sundarban and Andaman Islands (Mitra et al. 2011; Chowdhury 2015; Hossain et al. 2016; Mall et al. 1991; Suresh et al. 2017). But an interesting global estimation highlighted that India had a potential of soil carbon restoration of 3,289,058 tonnes of carbon and a total of above-ground biomass carbon of 1,066,672 tonnes with the limited literature (Worthington and Spalding 2018). This indicates the imperative need for regional level, site-specific precise estimates and knowledge of the spatial distribution of mangrove biomass stocks to understand the actual climate mitigation potential as well as to reinforce the conservation measures for the sustainability of mangroves.
In Kerala state, south-west of India, the mangrove habitats are facing rapid deforestation (Bijoy Nandan et al. 2015) and resulted in degradation in many habitats. Above 90% of the mangrove extent is lost due to anthropogenic and developmental activities along the coast and reduced the mangrove area from 70,000 ha (Blasco 1975) to 900 ha (FSI 2019) and the Kochi mangroves are now existed as fragmented habitats. Many native mangrove species are lost and structural attributes especially density and biomass of many mangrove species are reduced tremendously along the Kerala coast (Bijoy Nandan et al. 2015; Rani et al. 2018; Sreelekshmi et al. 2018). Therefore, there is an urgent need to document the existing mangrove habitats focusing on its biodiversity, structure and also its ecosystem service for sustainable management. Another significant limitation on the negligible literature available on regional level biomass estimation was usage of wood density values from the global database (Vinod et al. 2018; Harishma et al. 2020) instead of measured values for biomass estimation. This will again contribute uncertainties in global carbon stock estimation, and therefore, the current study attempts to estimate the wood density of each mangrove species in each age class for biomass estimation for providing an accurate database for the global assessments.
Accordingly, the primary aim of this study is to estimate the ‘blue carbon’ present in the different compartments (above-ground and below-ground biomass) of the few mangrove habitats along the south-west coast of India (Kerala region), which in turn contributes to enhancing the ecosystem services of the coastal zone in a sustainable manner for its better climatic and economic management. The study also hypothetically checked whether the biomass stock varied according to species and station. It also assessed possible major reasons for this biomass variation using multivariate approach.
Materials and methods
Study area
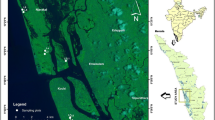
The study area, Kochi, is an important economic and vibrant coastal port city in the state of Kerala, South-West coast of India. The study area has a humid equatorial tropical monsoon-driven climate which is classified (Menon et al. 2000) as pre-monsoon (February–May), south-west monsoon (June–September) and northeast monsoon/post-monsoon (October–January). It is a very sensitive marine and estuary-influenced area with no previous studies reported so far on carbon stock along its coastal belt. The mangrove habitats bordered the Kochi estuary, which is a positive tropical tidal estuary (76°9′25″E–76°24′28″E and 9°47′31″N–10°12′N). The study area was occupied with luxurious mangrove habitats in 1970s but had declined to only a few hectares due to rapid urbanisation, Port construction, other developmental activities and aquaculture farm conversion (Blasco 1975 Bijoy Nandan et al. 2015). The aquaculture conversion seriously affected the biomass and carbon stocks of these mangrove forests. However, some significant mangrove patch still exists in small islands of Kochi estuary. Based on the scientific need on the documentation of the existing degrading mangroves in Kochi, the floristic and structural character of mangroves was carried out along eight mangrove habitats in and around Kochi estuary (Fig. 1), and description of each station is described in Table 1.
Community structure and Phytosociology
The structural analysis was completed using fixed area plot measurement based on the methodology proposed by Cintro´n and Schaffer-Novelli (1984). In each site, five quadrats were laid perpendicular to the shoreline. The size of the quadrat was fixed at 5 × 5 m (25 m2). The methodology was standardized and adopted the same from Rani et al. (2018) and Kauffman and Donato (2012). Counting and measurement of all mangrove species was done in all quadrats. Based on data obtained from quadrats, the structural parameters like diameter at breast height (DBH), density, relative density, abundance, percentage frequency, relative frequency, basal area, relative basal area, importance value index (IVI) and relative IVI were calculated by using the standard formula (Cintro´n and Schaffer-Novelli 1984). DBH was taken for each site, once during the sampling year. The girth of a tree at breast height was measured using a steel tape (in cm) and was converted into diameter, dividing by π (3.14). Breast height was determined as being approximately at 1.3 m. From DBH, the basal area was calculated. The community analysis was done using univariate analysis, such as computing various diversity indices using the software PRIMER v.6 (Clarke and Gorley 2006). The spatial difference in species diversity was compared through this analysis. The various indices used for calculations were as follows: species richness (Margalef’s index, d), species evenness (Pielou’s index, J′), species diversity (Shannon index, H′) and species dominance (Simpson’s index, λ′). Rainfall data were obtained from the India Meteorological Department (IMD) (www.imd.gov.in). The salinity of the tidal creek water was measured in the field using a Refractometer (Atago, Japan). It was then cross-checked by salinity determination through chlorinity estimation by modified Mohr-Knudsen method (Grasshoff et al. 1999). An average of 2-year data was taken as the salinity of each mangrove habitat.
Biomass and carbon pool
Biomass is an important factor in forest carbon stock assessment. As the Cochin mangroves are deforested widely, non-destructive method for mangrove trees and destructive method for ferns and herbs was selected for biomass estimation (Kauffman and Donato 2012). The ferns and herbs were harvested and separated into above-ground biomass and below-ground biomass. The weight of the separated components was measured by drying the material in 60 °C to a constant weight and expressed as dry biomass. A total of 701 samples were taken for tree biomass estimation (> 5 cm DBH). Even though many species- and site-specific equations are available for mangrove biomass estimation around the globe, such developed equations are not available to the current study area and even for the country. Also, the study site comprised many mangrove species for which species-specific allometric equation was not available; therefore, common allometric equations (described below) based on DBH and wood density were employed for the biomass calculation of mangrove trees. However, an attempt was made to calculate the biomass using species/generic equations which were developed for similar latitudinal areas with approximately same climatic regime and with approximately similar DBH matching to the study area. The list of equations used for AGB estimation of different mangrove species is described in Table 2. The variation in above-ground biomass of different mangrove species was checked by using two different common allometric equation and species/generic equation in order to check the anomalies in estimation (Chave et al. 2005, Komiyama et al. 2005):
where AGB = above ground biomass, BGB = below ground biomass, ρ = wood density, D = DBH.
The wood density estimation was done following the procedure of Chave (2006). The wood core was sampled using standard Haglöf 3-Thread Increment Borer (8″L × 0.200″ {5.15mm Dia.}). Duplicate samples were taken for each species and each DBH class present in the study area. The measured samples were kept in hot air oven at 60 °C up to constant weight, and dry weight was measured. Wood density was calculated as:
The biomass of mangroves was converted into carbon stock or mangrove carbon pool as living biomass. The carbon content in the wood core sample along with branch and leaf samples from the present study was pooled to get the conversion of biomass into carbon which revealed an average carbon content in AGB as 45%. A factor of 39% of BGB as carbon (Kauffman and Donato 2012) was taken for estimation of below-ground biomass carbon stock. The carbon content in the wood core, branches and leaves were analysed using Analytik Jena TOC analyzer HT 1300 solid module. The corresponding carbon dioxide (CO2) equivalents or CO2e of the biomass carbon stock of the study area was calculated by using IPCC conversion factor of 3.6 (IPCC 2007).
Statistical analysis
Statistical analysis was conducted with (SPSS) version 16.0. All tests were considered statistically significant at P level < 0.05. The Shapiro–Wilk W test was used to test data (Residual errors) for normality. Homogeneity of variance was examined with the Levene’s test. Homogeneous and approximately normally distributed data were tested for significant differences with the ANOVA (Analysis of Variance). Variation pattern in biomass stock of mangroves was evaluated by multivariate methods as a grouping analysis (Cluster) based on Bray-Curtis’ similarity index by using PRIMER v6 program. The square root transformation was done prior to PRIMER analyses.
Results
Environmental characters
The mean monthly rainfall in the catchment area of Kochi estuary and surrounding area was 277.37 ± 32.87 mm during the entire study period. From the average of 3-year data (2013, 2014, 2015) during the study period, the mean total rainfall at Kochi and surrounding area was 3328.46 mm/year. A major portion of the rain was received from the south-west monsoon and the rest from north-east monsoon. The salinity also varied significantly with St.8, Puthuvypin showing the highest salinity (14.34 ± 11.25), and St.5, Valanthakad Island displayed the lowest salinity (6.26 ± 7.17). The mean salinity in each mangrove habitat is shown in Fig. 2.
Stand structure
Floristic diversity study of mangroves revealed 13 species of true mangroves belonging to six families. Rhizophoraceae family constituted the greatest number of species which included Rhizophora apiculata Bl., Rhizophora mucronata Lam., Kandelia candel (L.) Druce., Bruguiera cylindrica (L.) Bl., Bruguiera gymnorrhiza (L.) Lam and Bruguiera sexangula (Lour.) Poir. Acanthaceae was represented by three species, Avicennia officinalis L., Avicennia marina (Forssk.) Vierh. and Acanthus ilicifolius L. while Euphorbaceae family was represented by only one species Excoecaria agallocha L. Sonneratia caseolaris (L). Engler. and Sonneratia alba Sm. belong to Lithraceae family and Acrostichum aureum L. is the only member belonging to Pteridaceae. Among these, Avicennia marina (Forssk.) Vierh., B. sexangula (Lour.) Poir and Sonneratia alba Sm. were rare in the study area. Shannon index of the eight major mangrove habitats in Kochi ranged between (H′ = 2.9 to H′ = 1.3). Shannon index, Simpsons index and richness (d) was high in St.1 (Aroor) where 11 true mangrove species were identified. Evenness was high at St.4 (Chellanam site) (Fig. 3).
The Kochi mangroves depict structurally developed (Detailed structural characters shown in Table 3) mangrove plants, with the highest density for Acanthus ilicifolius (9256.7 trees ha−1). However, the most dense mangrove tree was E. agallocha (2440 trees ha−1) followed by R. mucronata and A. officinalis. Overall, mangrove tree density varied from 11,440 trees ha−1 in Valanthakad Island to 3840 trees ha−1 in Mangalavanam Bird Sanctuary. ANOSIM analysis did not reflect variation in mangrove density in different stations of Kochi mangroves where global R was a low (R = 0.448, p < 0.001). The value of other structural parameters like frequency and abundance is shown in Supplementary Table 1. The diameter frequency class distribution vs mangrove density in the eight mangrove habitats of Kochi was performed, and it revealed the maturity of the forest (Fig. 4a-h). St.1, St.3 and St.8 showed structurally well-developed mangrove trees with DBH class intervals up to 31–40 cm, and other stations were in maturing and young stage. Two species, A. ilicifolius and A. aureum, were omitted from DBH frequency class analysis as the former is an herb and the latter a fern, with less than 1 cm in diameter. The basal area of each species varied significantly with the station (p < 0.05) and with species (p < 0.01). The total stand basal area of mangroves was exceptionally high at Mangalavanam (103.58 m2 ha−1) and Puthuvypin (102.23 m2 ha−1). The average stand basal area of the mangrove trees in Kochi was 67.06 ± 23.20 m2 ha−1. The basal area ranges from 0.1 to 94.32 m2 ha−1. A. officinalis species was having the highest basal area followed by R. mucronata and S. caseolaris. The Importance value index from the structural data for each mangrove species from each mangrove habitat is also shown in Table 2. It could be seen that for St.1, Avicennia officinalis was having higher values in the structural parameters and was the dominant species with an IVI of 95.5 followed by S. caseolaris (91.2), even though the latter species was less in number, it possessed higher relative frequency and high relative basal area. In St.2, E. agallocha was the dominant species with an IVI of 121.7 compared to the other seven species present in the area followed by B. gymnorrhiza. While in St.3, the important species was A. officinalis (IVI = 154.89) as in St.1. In St.4, Chellanam, the important mangrove species was E. agallocha followed by B. cylindrica. The important species at St.5 was E. agallocha followed by A. aureum. In St.6 and St.7, R. mucronata was having high IVI value (121.89 and 151.37 respectively) as well as high density and relative basal area. St.8 was having high IVI for B. cylindrica followed by A. officinalis. The mangrove species showing a high importance value index in each habitat indicates their habitat preferences. The area that has the most structural development for each species could be selected for its restoration programmes.
Mangrove living biomass
The total living biomass of mangroves at Kochi was 553.73 ± 264.81 Mg ha−1 according to Eq. 1 and 683.92 ± 329.95 Mg ha−1 according to Eq. 2 and 632.01 3 ± 306.91 according to species-specific equation. It can be perceived that, there is a slight variation in biomass estimation among two common allometric equations and even in species-specific equation, and this variation was prominent in well-structured (High basal area) mangrove trees. Biomass estimation showed similarity for young and maturing stage mangrove trees. The total biomass was highest at St.3, Mangalavanam and St.8, Puthuvypin while it was lowest at St.4, Chellanam. The total living biomass of mangrove in different habitats of Kochi is shown in Table 2.
Wood density
The wood density of mangrove trees is the first database from Kerala mangroves, and only limited data are available even from the Indian context. R. apiculata and R. mucronata were the densest mangrove species (0.83, 0.81 g cm−3). S. caseolaris (0.41 g cm−3) and E. agallocha (0.42 g cm−3) were the least dense ones (Fig. 5). Wood density displayed a parallel increase with the DBH. However, matured trees showed a constant wood density. In the case of A. officinalis, 1–10-cm DBH class was having a wood density of 0.53 ± 0.02 g cm−3, and maximum density was shown by 31–40 DBH class (0.603 ± 0.003 g cm−3). S. caseolaris was represented by DBH class of up to 41–50 cm, having a constant wood density from 31 to 40 cm DBH class (0.42 g cm−3) and a low wood density of 0.39 ± 0.02 g cm−3 for 1–10-cm DBH class. For R. mucronata, the wood density of 1–10-cm DBH class was 0.7 ± 0.06 g cm−3, and it gradually increased up to 0.812 ± 0.002 g cm−3 in 11–20-cm DBH class. Even though R. apiculata tree was represented by only 1–10-cm DBH class in the study area, even the young trees were having a high wood density of 0.83 g cm−3. The wood density of B. cylindrica in 1–10 cm DBH class was 0.68 ± 0.06 g cm−3, and such a high value was observed in 11–20-cm DBH class also (0.73 ± 0.02 g cm−3). B. gymnorrhiza was having a wood density of 0.68 ± 0.006 g cm−3 in the first DBH class and 0.763 ± 0.019 g cm−3 in 21–30-cm DBH class. B. sexangula and K. candel were represented by only 1–10-cm DBH class, and the corresponding wood density was 0.65 ± 0.005 g cm−3 and 0.557 ± 0.002 g cm−3 respectively. E. agallocha was having a wood density of 0.41 ± 0.03 g cm−3 in 1–10-cm DBH class and 0.43 ± 0.022 g cm−3 in 11–20 cm DBH class.
Above-ground biomass and below -ground biomass
The above-ground biomass and below-ground biomass of mangrove plants varied significantly with species (ANOVA F12,416 = 17.90, p = 0.000) and stations (ANOVA F7,416 = 2.408, p = 0.020). A. officinalis was having the highest biomass compared to other mangrove species in the study area and contributed 40.47 % (Eq. 2) of total living biomass (Fig. 6). Average total above-ground biomass of mangroves from Kochi was 484.18 ± 241.05 Mg ha−1 (Eq. 2), 353.99 ± 175.80 Mg ha−1 (Eq. 1) and 432.27 ± 218.33 according to species-specific equation, and the total below-ground biomass was 199.74 ± 89.32 Mg ha−1(Eq. 3).
It was observed that A. officinalis was having the highest above-ground biomass (41.13% of total AGB of mangrove trees), followed by R. mucronata (27.57%), S. caseolaris (7.73%) and E. agallocha (7.37%), and all the other mangroves contributed negligibly to the total AGB. The AGB of St. 1 was majorly contributed by S. caseolaris (255.21 Mg ha−1) followed by A. officinalis (135.19 Mg ha−1) respectively according to Eq. 2. In St.2, E. agallocha contributed more to biomass followed by A. officinalis and B. gymnorrhiza. In St.3, Mangalavanam and St.8, Puthuvypin, the total AGB of mangrove trees was exceptionally higher (879.86 Mg ha−1 and 795.72 Mg ha−1 respectively according to Eq. 2), and the lowest above-ground biomass was reported at St.4, Chellanam (185.39 Mg ha−1, Eq. 2). The above-ground biomass results obtained by three different equations at each station are described in Table 4.
Below-ground biomass of mangrove trees was obtained by using only one common equation (Eq. 2) and the average value for the study area was 199.74 ± 89.32 Mg ha−1. It was high for A. officinalis (77.62 ± 101.07 Mg ha−1, range = 9.11–311.63 Mg ha−1) followed by R. mucronata (53.60 ± 60.6 Mg ha−1) (Fig. 7). Below-ground biomass also varied significantly with stations (ANOVA F7,416 = 2.165, p = 0.036) and species (ANOVA F12,416 = 18.951, p = 0.000). The total below-ground biomass of mangroves (Table 4, including ferns and herbaceous mangrove) was high in St.3, Mangalavanam (332.06 t ha−1) followed by St.8, Puthuvypin (325.50 Mg ha−1) and lowest recorded at St.4, Chellanam (75.31Mg ha−1).
The cluster analysis (Bray-Curtis similarity) using biomass data showed 4 vegetation type groups having 60% similarity in the study area (Fig. 8). Four major types of vegetation: A. officinalis-R. mucronata dominated vegetation type (AVO-RMA veg), B. cylindrical-A. officinalis (BCA-AVO veg.) dominated vegetation type, and two E. agallocha dominated vegetation type (EXO-BRU and EXO-RMA.). St.3 and St.8 grouped together as they are the stations with highest biomass, and St.2 and St.4 grouped together having least biomass. The other stations clubbed together as a single group with average biomass stock.
Dendrogram of similarity among the different mangrove habitats of Kochi, considering the values of biomass stock (Eq. 2)
Carbon stock assessment
Average carbon stock as above-ground biomass of mangroves in Kochi was 217.88 ± 108.47 Mg C ha−1, 159.29 ± 79.11 Mg C ha−1 and 194.52 ± 98.25 Mg C ha−1 according to Eqs. 2, 1 and species-specific equation respectively. The average carbon stock from below-ground biomass of mangroves in Kochi was 77.90 ± 34.83 Mg C ha−1. St.3, Mangalavanam Bird sanctuary had the highest carbon stock as biomass followed by St.8, Puthuvypin (Table 4). The average total living biomass carbon stock of mangroves at Kochi was 295.78 ± 143.14 Mg C ha−1 (Eq.2), 237.19 ± 113.82 Mg C ha−1 (Eq. 1) and 272.42 ± 132.78 Mg C ha−1 according to species-specific equation. The biomass carbon stock was highest for A. officinalis followed by R. mucronata (Fig. 9). Among the mangrove trees, B. sexangula contributed a minimum to the total carbon stock of Kochi.
Discussion
Variation of biomass, based on two common allometric equations and species-specific equation
There was a variation in biomass of mangroves obtained through two different common allometric equations and species-specific equation in the present study. The AGB obtained through the common allometric equation of Komiyama et al. (2005) (Eq. 2) was slightly higher than those of Chave et al. (2005) (Eq. 1) and slightly closer to species-specific one. However, species-specific equations used in this study are not site-specific and also for few species, equation of another species in the same genera was used. Therefore, uncertainty will be there for the results. The variation was most prominent for mangrove species with large trunk diameter. The biomass results based on Eq. 2 were giving high estimates compared to Eq. 1. Below-ground biomass was higher in the study area compared to other mangrove ecosystems of the world (Table 4). Mangalavanam mangrove forest displayed a significant difference in both above- and below-ground mangrove biomass values due to the presence of large A. officinalis trees. Similar to the above-ground biomass estimation, the high below-ground biomass may be overestimated due to the existence of uncertainty in the calculation of biomass for large trunk size of A. officinalis species by using Komiyama et al.’s (2005) equation. This problem could be observed for other major species like S. caseolaris, R. mucronata and E. agallocha which contributed major share of biomass stock of the current study area. This problem was also discussed in Komiyama et al. (2008) while reviewing the allometric equations around the globe. Even though the present study is regional stock assessment, the results of the current study project a global uncertainty in using the common allometric equation for large mangrove trees. Therefore, it should be globally addressed with more samples and should be checked with statistical approach in order to clarify the uncertainties in global biomass carbon stock assessment. It could be noted that most of the studies from India used common allometric equation of Komiyama et al. (2005) for the biomass estimation (Ragavan et al. 2021; Harishma et al. 2020; Vinod et al. 2018; Sahu et al. 2016; Bindu et al. 2020). Common allometric equation was used for even Bangladesh Sundarbans (Ahmed and Kamruzzaman 2021; Rahman et al. 2015). Thus, the study seriously highlighted the research gap for the development of new site-specific and species-specific allometric equation for the country.
Global comparison of biomass stock and biomass carbon pool
High biomass stock is seen in mangroves of Kochi compared to other mangroves of the world. As the study area is located in a tropical environment with a high rainfall rate, with monsoonal driven climate and high discharge of upland terrestrial sediment load to the estuary through different riverine channels (Revichandran et al. 2012) made it an ideal place for this coastal vegetation to flourish. Usually, rainfall and precipitation had a strong positive relationship with productivity (Osland et al. 2018). Other recent studies were also compared with the present study (Table 5). It could be observed that Kochi mangroves showed a very high biomass carbon stock compared to Indian and Bangladesh Sundarbans (up to 214.94 Mg C ha−1 by Rahman et al. 2015, 118 ± 29.5 Mg C ha−1 by Kamruzzaman et al. 2018 respectively). Even the Amazon mangroves (145.17 Mg ha−1 of carbon, Kauffman et al., 2018) and African mangroves (Kauffman and Bhomia 2017) also documented low biomass carbon stock compared to the high carbon stock of the fragmented mangrove habitats of the present study. When comparing to the Indian scenario, Bhitarkanika mangroves showed the highest biomass (Bal and Banerjee 2019; 866.67 ± 166.10 Mg ha−1) against the present study. However, Pichavaram mangroves (Kathiresan et al. 2013) and other studies which used common allometric equation of Komiyama (Sahu et al. 2016; Suresh et al. 2017; Vinod et al. 2018) reported lesser AGB than the present study. The carbon stock assessment of Kadalundi mangroves of Kerala (Vinod et al. 2018) also had less above-ground biomass (mean= 166.64 Mg ha−1) similar to the assessment of biomass stock of the entire Kerala state also documented by Harishma et al. (2020). The previously reported low biomass stock at the regional level may be due to the insufficient sampling and usage of wood density global database for biomass calculation. Thus, it could be revealed that even though Kochi mangroves are fragmented patches of the South-West coast of India, they stock a large amount of carbon in its biomass and therefore its conservation and restoration could be considered as a good tool in climate change mitigation programmes in the coastal areas. The high biomass of mangroves at Kochi was achieved by high basal area of mangroves compared to other mangrove habitats of the world. The average basal area of Kochi mangroves (67.06 ± 23.20 m2 ha−1), was higher than Amazon mangroves (26.33 ± 1.1 m2 ha−1). The nutrient-rich Kochi estuary along with monsoonal climate subsequently leading in to inflow of freshwater discharges to the estuary from various adjacent rivers makes it an ideal place to flourish mangrove plants.
Spatial and species variation in biomass stock, influencing factors and risk of land use land cover change
The results showed that biomass significantly differed spatially and species wise. Usually, mangroves showed high biomass at riverine mangrove habitats (Kathiresan and Bingham 2001; Rahman et al. 2015; Adame et al. 2015); however, in contrast, the present study marked increased biomass stock towards the lower estuary with regular tidal flushing. Nevertheless, for those sites having high biomass stock (Mangalavanam (St.3), Puthuvypin (St.8), Vallarpadam (St.6) and Panmbukadu (St.7)), the salinity may not be a stressed character like various other permanently inundated low biomass stocked saline mangrove sites. The high biomass stocked stations are semi-enclosed mangrove habitats inundated only during high tide and therefore with minimum inundation period, shallow depth (may reach up to 1 m only during high tide) may also facilitate the growth of mangrove plants due to less stress. These sites were also closer to the open gut of the nutrient-rich Kochi estuary to the ocean, receiving a lot of nutrient input and resulted in high biomass stock. The low biomass stocked stations (St.2 and St.4) were located in front of the Arabian Sea. As the mangroves nearer to marine areas are exposed to varying environmental stresses such as permanent inundation of tidal water, salinity stress, sandy nature of sediment and extreme weather conditions, all these result in changes in hydrology and sediment biogeochemistry, inhibiting the growth of mangrove habitats in these regions (Woodroffe 1992) and probably one of the reasons for low biomass stock. Previously Sreelekshmi et al. (2018) and Rani et al. (2021) reported the sandy nature of the sediment in these stations.
These environmental characters and geomorphology also reflected in the formation of association of vegetation type which also influenced the high biomass stock and clustering of stations (Fig. 8). Among the vegetation types, AVO-RMA and BCA-AVO vegetation types showed maximum biomass stock (St.3, St.8, St.6 and St.7) and while EXO-BRU dominated vegetation type showed lowest biomass stock (St.2, St.4), St.5 is also an Excoecarea dominated site, its combination vegetation type is RMA, and also received more fresh water as it is an open mangrove with lowest salinity compared to other mangrove habitats of Kochi and therefore showed higher biomass stock compared to EXO-BRU type and grouped to AVO-RMA type group (St.1, St.6 and St.7, Fig. 8). It was reported that the above-ground biomass stock will be determined by the dominant species in each vegetation types depending on its structure especially their basal area (Ruiz-Jaen and Potvin 2010) which is true to this study. Even though density and abundance are high for E. agallocha at two stations (St.2 and St.4), its DBH and wood density are low compared to other mangrove tree species. Thus, habitats with such species even with high density will exhibit low biomass stock. Whereas presence of the high DBH class of A. officinalis and R. mucronata resulted in high biomass stock in other stations. Usually, the diameter of the tree is a determinant of above-ground biomass (Chave et al. 2004). In general, tree density did not positively correlate to biomass stock as young forests would be denser but structurally poorly developed than low-density matured forest.
The low biomass stock of St.2 and St.4 will be also alarming against the conversion of mangrove habitats to aquaculture farm. Manual thinning and cutting of mangroves will be associated with the aquaculture farming practices, and the aquaculture practices will also change the biogeochemistry and nutrient availability of mangrove habitats, and therefore, it may cause selective settling of vegetation types and forming a microhabitat which may be different from the adjacent mangrove habitats. This was evident from the present study as St.8 and St.2 are adjacent places. However, St.2 was an aquaculture converted site and reported low soil carbon burial (Rani et al. 2021). These results can be matched with the findings of Bhomia et al. (2016) who studied the impact of land use land type change on ecosystem carbon stock. Thus, land use and type had a strong influence on biomass stock and ultimately the ecosystem carbon stock of mangrove habitats and its potential for carbon sequestration. Thus, the topography, geomorphology, geochemistry, vegetation type and land use change altogether affect the growth of mangrove plants and ultimately to the carbon stock.
Carbon stock and sequestration potential
The areal extent of mangroves around the Kochi estuary was 411.13 ha. Consequently, the CO2e of mangrove carbon stock of Kochi will contribute approximately 437,774.51 Mg CO2e (Eq. 2) and 351,057.33 Mg CO2e (Eq. 1) according to IPCC (Intergovernmental Panel on Climate Change) conversion. This highlights the importance of this coastal vegetation in the climate change mitigation efforts of the city. The biomass carbon stock has a major influence on soil carbon sequestration potential of that ecosystem. It was found that even though Mangalavanam bird sanctuary was having high biomass carbon stock, its soil carbon sequestration rate was low compared to riverine semi-enclosed-type mangrove habitat (St.1 of the present study) (Rani et al. 2021) due to the anthropogenic activities (pollution) in that region and highlighted the impact of disturbance on the fate of primary productivity. That study also highlighted that sediment biogeochemistry and the faunal activities in mangrove ecosystem played a major role in controlling the soil carbon sequestration together with biomass stock. It was also interesting that Aroor (St.1) mangrove habitat with globally comparable high biomass stock, high primary productivity through litterfall (Rani et al. 2016) along with AVO-RMA vegetation type and sediment biogeochemistry was having globally noticeable high carbon sequestration rate (10.41 ± 2.50 Mg ha−1 year−1of carbon). At the same time, the low biomass recorded aquaculture site (St.2) revealed a vastly depleted carbon sequestration rate (Rani et al. 2021). The disturbance in the form of pollution or land use change especially aquaculture conversion had impact on biomass stock, and pollution may affect the fate of productivity even in high biomass stocked areas and may be resulted in less soil carbon sequestration rate. Therefore, biomass stock is an important driver for soil carbon burial or sequestration rate and its conservation without disturbance is significant for making the mangrove ecosystem a natural carbon sink.
Mangrove restoration based on the structure
Mangrove forests are facing drastic degradation at the global level, and Kochi mangroves are not an exception for this trend. Even though the diversity of mangrove is high in the study area, the total area of mangrove coverage is less. The current community analysis of the existing mangroves will be helpful in the scientific management of these degrading environments. The mangrove species showing high important value index in each habitat indicates its habitat preferences. For example, in the present study, St.2 and St.8 (Vypin Island) showed the highest IVI for B. gymnorrhiza species, and it could be understood that particular species can grow well in this region. Therefore, if some mangrove planting is planned for restoration, we could select this area for B. gymnorrhiza species for getting high positive results in mangrove restoration. The areas having the most structural development for each species can be selected for its restoration programmes, and this could be used as a potential mangrove management strategy elsewhere around the globe. These areas can be protected well for future management purposes. Thus, structural data and community analysis could be used as a good tool for understanding the status of mangroves and also the management of such degraded ecosystems.
Conclusion
The present study was able to survey the forest structure and community analysis in depth by including all the mangrove species and measured all the mangrove trees along with wood density of each mangrove species in each DBH class and provides more accurate and reliable biomass estimation than the previously reported regional studies, even though the destructive method has not opted. Also, this study outlined the primary data on the living biomass of mangroves of Kochi by using measured wood density data. The wood density obtained through this study would be useful in estimating the biomass of mangroves elsewhere in different age classes. The structural analysis of each mangrove habitat based on its IVI could be utilized for scientific management of mangrove conservation programmes. The influence of mangrove species specificity and spatial variation on biomass stock could be clearly understood from the study. The present study identified some uncertainties in biomass estimation by using common allometric equation for large trunked mangrove species which can be globally addressed in future research. The study found out that vegetation type, salinity together with geomorphology (open and semiclosed) and the land use type are the major reasons for spatial variability of biomass C stocks. The mangroves of Kochi recorded very high carbon stock through living biomass of mangroves comparing to several other mangrove habitats around the world, and the monsoonal climate and nutrient-rich estuaries flourished by the inflow of several freshwater sources may be the reason for this high biomass stock, and this indicated Kochi mangrove’s potentiality for high carbon sequestration. However, it could be alarming that all these mangrove habitats are in fragmented stage and under developmental pressure. Therefore, immediate management strategies should be taken for conservation and preservation of these valuable natural carbon sinks and therefore would be used in the carbon economy of the region for policy-making and thereby mitigating regional climate change problems.
References
Abino AC, Castillo JAA, Lee YJ (2014) Species diversity, biomass, and carbon stock assessments of a natural mangrove forest in Palawan, Philippines. Pak J Bot 46(6):1955–1962
Adame MF, Santini NS, Tovilla C, Vázquez-Lule A, Castro L, Guevara M (2015) Carbon stocks and soil sequestration rates of tropical riverine wetlands. Biogeosciences 12:3805–3818. https://doi.org/10.5194/bg-12-3805-2015
Ahmed S, Kamruzzaman M (2021) Species-specific biomass and carbon flux in Sundarbans mangrove forest Bangladesh: Response to stand and weather variables. Biomass Bioenergy 153:106215. https://doi.org/10.1016/j.biombioe.2021.106215
Alemayehu F, Richard O, James KM, Wasonga O (2014) Assessment of mangrove covers change and biomass in Mida Creek, Kenya. Open J For 4:398–413. https://doi.org/10.4236/ojf.2014.44045
Alongi DM (2014) Carbon cycling and storage in mangrove forests. Ann Rev Mar Sci 6:195–219. https://doi.org/10.1146/annurev-marine-010213-135020
Bal G, Banerjee K (2019) Carbon storage potential of tropical wetland forests of South Asia: a case study from Bhitarkanika Wildlife Sanctuary, India. Environ Monit and Assess 191:795. https://doi.org/10.1007/s10661-019-7690-y
Bhomia RK, Mackenzie RA, Murdiyarso D, Sasmito SD, Purbopuspito J (2016) Impacts of land use on Indian mangrove forest carbon stocks: implications for conservation and management. Ecol Appl 26(5):1396–1408. https://doi.org/10.1890/15-2143
Bijoy Nandan S, Sreelekshmi S, Preethy CM, Varghese R, Joseph P (2015) Manual on mangroves. Directorate of Public Relations and Publications, Cochin University of Science and Technology, Cochin
Bindu G, Rajan P, Jishnu ES, Joseph KA (2020) Carbon stock assessment of mangroves using remote sensing and geographic information system. Egypt J Remote Sens Space Sci 23(1):1–9
Blasco F (1975) The mangroves in India. Institute Francais de Pondicherry, Inde, Sri Aurobinda Ashram, India
Chave J, Condit R, Aguilar S, Hernandez A, Lao S, Perez R (2004) Error propagation and scaling for tropical forest biomass estimates. Philos Trans R Soc B 359:409–420. https://doi.org/10.1098/rstb.2003.1425
Chave J, Andalo C, Brown S, Cairns MA, Chambers JQ, Eamus D, Folster H, Fromard F, Higuchi N, Kira T, Lescure JP, Nelson BW, Ogawa H, Puig H, Riera B, Yamakura T (2005) Tree allometry and improved estimation of carbon stocks and balance in tropical forests. Oecologia 145:87–99. https://doi.org/10.1007/s00442-005-0100-x
Chave J, Muller-Landau HC, Baker TR, Easdale TA, Steege H, Webb CO (2006) Regional and phylogenetic variation of wood density across 2456 neotropical tree species. Ecol Appl 16:2356–2367. https://doi.org/10.1890/1051-0761(2006)016[2356:rapvow]2.0.co;2
Chowdhury MR (2015) Effect of salinity on the above-ground biomass and growth of dominant mangrove plants in Indian sundarbans. Ann Biol Res 6(5):20–25
Cintron G, Schaffer Novelli YS (1984) Methods for studying mangrove structure. In: Samuel CS, Jane GS (eds) The Mangrove ecosystem:Research Methods. UNESCO Publication Paris, pp 91–113
Clarke KR, Gorley RN (2006) PRIMER v6: user manual/tutorial. PRIMER-E Ltd: Plymouth 931:932
Clough BF, Scott K (1989) Allometric relationship for estimating above ground biomass in six mangrove species. For Ecol Manag 27:117–127
Dharmawan I, Siregar C (2008) Soil carbon and carbon estimation of Avicennia marina (Forsk.). Vierh. Stand at Ciasem, Purwakarta. J. Penelit. Hutan dan Konservasi Alam 5:317–328. https://doi.org/10.20886/jphka.2008.5.4.317-328
Estrada GCD, Soares MLG (2017) Global patterns of aboveground carbon stock and sequestration in mangroves. An Acad Bras Ciênc 89(2):973–989
FSI (2019) Forest Survey of India. State of Forest Report, Dehra Dun, India
Fromard F, Puig H, Mougin E, Marty G, Betoulle JL, Cadamuro L (1998) Structure, above-ground biomass and dynamics of mangroveecosystems: New data from French Guiana. Oecologia 115:39–53
Grasshoff K, Kremling K, Ehrhardt M (1999) Methods of seawater analysis, 3rd edn. Wiley-VCH, Weinheim, p 632
Hanh NTH, Tinh PH, Tuan MS (2016) Allometry and biomass accounting for mangroves Kandelia obovata Sheue, Liu & Yong and Sonneratia caseolaris (L.) Engler planted in coastal zone of Red River Delta, Vietnam. Int J Dev Res 6(5):7804–7808
Harishma KM, Sandeep S, Sreekumar VB (2020) Biomass and carbon stocks in mangrove ecosystems of Kerala, southwest coast of India. Ecol Process 9:31. https://doi.org/10.1186/s13717-020-00227-8
Hossain M, Saha C, SMR A, Saha S, MRH S (2016) Allometric biomass, nutrient and carbon stock models for Kandelia candel of the Sundarbans, Bangladesh. Trees, 30:709–717
Hossain M, Siddique MRH, Saha S, Abdullah SMR (2015) Allometric models for biomass, nutrients and carbon stock in Excoecaria agallocha of the Sundarbans, Bangladesh. Wetl Ecol Manag 23:765–777
IPCC (2007) In: Solomon S, Manning DQM, Chen Z, Marquis M, Averyt KB, Tignor M, Miller HL (eds) Contribution of Working Group I to the Fourth Assessment Report of the Intergovernmental Panel on Climate Change. Cambridge University Press, Cambridge, United Kingdom and New York, NY, USA, p 996
Jennerjahn TC, Gilman E, Krauss KW, Lacerda LD, Nordhaus I, Wolanski E (2017) Chapter 7: mangrove ecosystems under climate change. In: Rivera-Monroy V, Lee SY, Kristensen E, Twilley RR (eds) Mangrove ecosystems: a global biogeographic perspective – structure, function and services. Springer Publishing Company, New York, pp 211–244
Kamruzzaman D, Ahmed S, Paul S, Rahman M, Osawa A (2018) Stand structure and carbon storage in the oligohaline zone of the Sundarbans mangrove forest, , Bangladesh. Forest Sci Technol 14(1):21–28. https://doi.org/10.1080/21580103.2017.1417920
Kathiresan K, Bingham BL (2001) Biology of mangroves and mangrove ecosystems. Adv Mar Biol 40:81–251. https://doi.org/10.1016/S0065-2881(01)40003-4
Kathiresan K, Anburaj R, Gomathi V, Saravanakumar K (2013) Carbon sequestration potential of Rhizophora mucronata and Avicennia marina as influenced by age, season, growth and sediment characteristics in southeast coast of India. J Coast Conserv 17(3):397–408. https://doi.org/10.1007/s11852-013-0236-5
Kauffman JB, Donato D (2012) Protocols for the measurement, monitoring and reporting of structure, biomass and carbon stocks in mangrove forests. Center for International Forestry Research Center (CIFOR) Working paper, p 86
Kauffman JB, Bernardino AF, Ferreira TO, Giovannoni LR, de Gomes LE, Romero DJ, LCZ J, Ruiz F (2018) Carbon stocks of mangroves and salt marshes of the Amazon region, Brazil. Biol Lett 14:20180208. https://doi.org/10.1098/rsbl.2018.0208
Kauffman JB, Bhomia RK (2017) Ecosystem carbon stocks of mangroves across broad environmental gradients in West-Central Africa: global and regional comparisons. PLoS ONE 12(11):e0187749. https://doi.org/10.1371/journal.pone.0187749
Komiyama A, Ong JE, Poungparn S (2008) Allometry, biomass, and productivity of mangrove forests: a review. Aquat Bot 89:128–137. https://doi.org/10.1016/j.aquabot.2007.12.006
Komiyama A, Poungparn S, Kato S (2005) Common allometric equations for estimating the tree weight of mangroves. J Trop Ecol 21:471–477. https://doi.org/10.1017/S0266467405002476
Kusumaningtyas MA, Hutahaean AA, Fischer HW, Manuel Pérez-Mayo MP, Ransby D, Jennerjahn TC (2019) Variability in the organic carbon stocks, sources, and accumulation rates of Indonesian mangrove ecosystems. Estuar Coast Shelf Sci 218(5):310–323. https://doi.org/10.1016/j.ecss.2018.12.007
Mall LP, SinghVP GA (1991) Study of biomass, litter fall, litter decomposition and soil respiration in monogeneric man- grove and mixed mangrove forests of Andaman Islands. Trop Ecol 32:144–152
Menon NN, Balachand AN, Menon NR (2000) Hydrobiology of the Cochin back water system- a review. Hydrobiologia 430:149–183. https://doi.org/10.1023/A:1004033400255
Mitra A, Sengupta K, Banerjee K (2011) Standing biomass and carbon storage of above ground structures in dominant mangrove trees in the Sundarbans. For Ecol Manag 261:1325–1335. https://doi.org/10.1016/j.foreco.2011.01.012
Murdiyarso D, Purbopuspito J et al (2015) The potential of Indonesian mangrove forests for global climate change mitigation. Nat Clim Change 5:1089–1092. https://doi.org/10.1038/nclimate2734
Ong JE, Gong WK, Wong CH (2004) Allometry and partitioning of mangrove, Rhizophora apiculata. For Ecol Manag 188:395–408
Osland MJ, Gabler CA, Grace JB, Day RH, McCoy ML, McLeod JL, From AS, Enwright NM, Feher LC, Stagg CL, Hartley SB (2018) Climate and plant controls on soil organic matter in coastal wetlands. Glob Change Biol 24(11):5361–5379. https://doi.org/10.1111/gcb.14376
Pérez A, Machado W, Gutierrez D, Stokes D, Sanders L, Smoak JM, Santos I, Sanders CJ (2017) Changes in organic carbon accumulation driven by mangrove expansion and deforestation in a New Zealand estuary. Estuar Coast Shelf Sci 192:108–116. https://doi.org/10.5061/dryad.9519kj3
Ragavan P, Kumar S, Kathiresan K, Mohan PM, RSC J, Ravichandran K, Rana TS (2021) Biomass and vegetation carbon stock in mangrove forests of the Andaman Islands, India. Hydrobiologia 848:4673–4693. https://doi.org/10.1007/s10750-021-04651-5
Rahman MM, Khan MNI, Hoque AF, Ahmed I (2015) Carbon stock in the Sundarban mangrove forest: spatial variations in vegetation types and salinity zones. Wet Ecol Manag 23:269–283. https://doi.org/10.1007/s11273-014-9379-x
Rani V, Sreelekshmi S, Preethy CM, Bijoy Nandan S (2016) Phenology and litterfall dynamics structuring ecosystem productivity in a tropical mangrove stand on South West coast of India. Reg Stud Mar Sci 8:400–407. https://doi.org/10.1016/j.rsma.2016.02.008
Rani V, Bijoy Nandan S, Schwing PT (2021) Carbon source characterisation and historical carbon burial in three mangrove ecosystems on the South West coast of India. Catena 197:104980. https://doi.org/10.1016/j.catena.2020.104980
Rani V, Sreelekshmi S, Asha CV, Bijoy Nandan S (2018) Forest structure and community composition of Cochin mangroves, south-west coast of India. Pro Nat Acad Sci, India B 88(1):111–119. https://doi.org/10.1007/s40011-016-0738-7
Revichandran C, Srinivas K, Muraleedharan KR, Rafeeq M, Amaravayal S, Vijayakumar K, Jayalakshmi KV (2012) Environmental set-up and tidal propagation in a tropical estuary with dual connection to the sea (SW coast of India). Environ Earth Sci 66:1031–1042. https://doi.org/10.1007/s12665-011-1309-0
Ruiz-Jaen MC, Potvin C (2010) Tree diversity explains variation in ecosystem function in a Neotropical forest in Panama. Biotropica. https://doi.org/10.1111/j.1744.7429.2010.00631.x
Sahu SC, Kumar M, Ravindranath NH (2016) Carbon stocks in natural and planted mangrove forests of Mahanadi mangrove wetland, east coast of India. Current Science 110(12):2253–2260. https://doi.org/10.18520/cs/v110/i12/2253-2260
Santos DMC, Estrada GCD, Fernandez V, Estevam MRM, Souza BT, Soares MLG (2017) First assessment of carbon stock in the belowground biomass of Brazilian mangroves. An Acad Braz Cience 89:1579–1589. https://doi.org/10.1590/0001-3765201720160496
Sreelekshmi S, Preethy CM, Varghese R, Philomina Joseph CV, Asha SBN, Radhakrishnan CK (2018) Diversity, stand structure and zonation pattern of mangroves in southwest coast of India. J Asia Pac Biodivers 11(4):573–582. https://doi.org/10.1016/j.japb.2018.08.001
Suresh HS, Bhat DM, Ravindranath NH, Sukumar R (2017) Carbon stocks and sequestration potential of Indian Mangroves. Trop Ecol 58(3):547–553
Thomas N, Lucas R, Bunting P, Hardy A, Rosenqvist A, Simard M (2017) Distribution and drivers of global mangrove forest change 1996-2010. PLoS ONE 12:e0179302. https://doi.org/10.1371/journal.pone.0179302
United Nations (1998) Kyoto protocol to the United Nations Framework convention on climate change, Kyoto, Japan. United Nations
Vinod K, Anasu Koya A, Kunhi Koya VA, Silpa PG, Asokan PK, Zacharia PU, Joshi KK (2018) Biomass and carbon stocks in mangrove stands of Kadalundi estuarine wetland, south-west coast of India. Indian J Fish 65(2):89–99
Woodroffe C (1992) Mangrove sediments and geomorphology. In: Robertson AI, Alongi DM (eds) Tropical mangrove ecosystems. American Geophysical Union, Washington D.C, pp 7–41
Worthington T, Spalding M (2018) Mangrove restoration potential: a global map highlighting a critical opportunity. https://doi.org/10.17863/CAM.39153
Acknowledgements
The first author is thankful to DST-INSPIRE, Govt. of India, with grant number (IF110502) for the financial support to the present research work and also to Chief Minister's Navakerala PDF scheme, Govt. of Kerala for the research support. The authors are thankful to Mr. Aravind for his support during the field survey. We express our gratitude to Mr. Sathyakumar for mapping the study locations for the present study.
Author information
Authors and Affiliations
Contributions
Varghese Rani contributed to the conception and design of the work, acquisition, analysis, interpretation of data and manuscript drafting. Bijoy Nandan contributed in conception and edited the manuscript. Paravanparambil Rajkumar Jayachandran used new software to represent the data, edited the manuscript and also helped in field sampling. Chakkalakal Mani Preethy helped in field survey and also edited the manuscript. Suseela Sreelekshmi, Philomina Joseph and Chalil Veedu Asha helped in field sampling.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Responsible Editor: Philippe Garrigues
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Rani, V., Nandan, S.B., Jayachandran, P.R. et al. Carbon stock in biomass pool of fragmented mangrove habitats of Kochi, Southern India. Environ Sci Pollut Res 30, 96746–96762 (2023). https://doi.org/10.1007/s11356-023-29069-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-023-29069-5