Abstract
The recovery of wetland function after tidal flow restoration may be influenced by water pollution and sea level rise. Our objective was to examine the effects of tidal flow restoration on denitrification potential and carbon (C) mineralization across an urban coastal landscape. Soil cores were collected from 32 tidal wetlands in Connecticut, U.S.A., spanning a wide range of salinity (0.3–29 ppt) and watershed development (<1–79%). In brackish wetlands, denitrification potential increased with time since restoration, while C mineralization showed no significant relationship. Soil chemistry was also a strong predictor of process rates; best fit multiple linear regression models for denitrification included both soil chemistry variables and time since restoration. Although principal components analysis revealed soil chemistry overlapped by wetland type (freshwater, saline, or brackish), process rates in freshwater versus brackish wetlands had different relationships with soil chemistry. In freshwater wetlands, denitrification potential and C mineralization increased with soil metal content. In brackish wetlands, denitrification potential decreased with increasing salinity and C mineralization increased with increasing organic matter, soil moisture, and ammonium. Our results highlight the potential for biogeochemical processes to recover after wetland restoration, along with complex interactions between these processes and chemicals in developed coastal landscapes.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Human alteration of coastal wetland hydrology through artificial drainage networks and installation of physical barriers that restrict tidal flow, such as berms or dikes, tidal gates, and culverts, is widespread (Gedan et al. 2009). Restricted tidal flow causes marshes to become fresher and receive less inorganic sediment inputs, which can alter vegetation communities and biogeochemical cycling. Tidal flow restoration techniques are increasingly implemented to restore wetland hydrology (Gedan et al. 2009), typically to improve fish passage, restore habitat, and reduce invasive plant species (Chambers et al. 2002; Elphick et al. 2015). Even though tidally restored wetlands tend to exhibit faster biogeochemical recovery than other types of restoration, recovery can take decades, the effect of restoration on carbon (C) and nitrogen (N) storage is highly variable, and there is typically less focus on improving and monitoring biogeochemical function post-restoration (Moreno-Mateos et al. 2012). Predicting and understanding biogeochemical recovery trajectories of coastal wetlands is difficult, likely in part because tidal flow restoration often occurs in densely populated coastal areas where wetlands experience multiple stressors simultaneously, including elevated nutrient and metals loading and sea level rise, which may confound changes in C and N cycling that occur as a result of restoration.
Land development in coastal zones contributes excess nutrients (Carpenter et al. 1998) and urban contaminants such as Cu and Pb (Bergback et al. 2001; Revitt et al. 2014) to wetlands. Coastal wetlands retain nutrients and metals in their soils and vegetation, and can act as long-term sinks for nutrients (Valiela and Teal 1979) and metals (Williams et al. 1994). Nutrient enrichment can alter microbial processes, such as denitrification (the microbial reduction of nitrate to N2 gas) and soil C mineralization, directly by adding reactive N, as well as indirectly by increasing plant biomass and changing the composition of wetland plant communities (Deegan et al. 2007; Gedan et al. 2009). Carbon and N microbial processes are also linked with metals through enzymatic requirements, such as Cu for denitrification, (Glass and Orphan 2012), redox pairs for microbial metabolism (Mn and Fe reduction; Lovley and Phillips 1988; Lovley 1991; Nealson and Myers 1992; Thamdrup 2000), and toxicity effects on enzymes or microbial metabolism (Giller et al. 1998; Rajapaksha et al. 2004); however, the effects of elevated metals from developed landscapes on microbial C and N processes are not well understood in coastal wetland ecosystems.
Saltwater intrusion elevates concentrations of ions (i.e. Cl−, Na+, SO42−, Mg2+), which may have varying effects on microbial C and N cycling in wetlands. In a laboratory experiment, Magalhaes et al. (2005) found no effects of elevated salinity on denitrification, suggesting presence of salt tolerant denitrifying species. In other field and laboratory experiments, elevated salinity decreased denitrification (Rysgaard et al. 1999; Putnam Duhon et al. 2012) potentially due to osmotic stress of microbes (Panswad and Anan 1999). Denitrification may also be inhibited or enhanced by elevated sulfate (SO42−) from seawater. Hydrogen sulfide produced by SO42− reduction inhibits the last step of denitrification to N2 gas (Senga et al. 2006; Pan et al. 2013). In contrast, other forms of sulfide may also serve as an alternate electron donor for denitrification (Burgin and Hamilton 2007), potentially increasing rates of denitrification.
Carbon cycling is influenced by saltwater intrusion through effects on microbial processing (Pathak and Rao 1998), plant communities (McKee and Mendelssohn 1989), and organic matter quality and quantity (Morrissey et al. 2014). The effects of salinity on C mineralization rates are still unclear (Herbert et al. 2015). After years of in-situ saltwater treatments, C mineralization rates were lowered in experimental plots with elevated salinity compared to freshwater plots (Neubauer et al. 2013). The reduction of methane (CH4) production in soils with elevated sea salts suggests SO42− reducers outcompete methanogens (Bartlett et al. 1987; Megonigal et al. 2004). In contrast, other researchers have found increased CH4 emissions in wetland soil cores receiving saltwater treatments (Weston et al. 2011; Ardón et al. 2018; Helton et al. 2019). Effects of saltwater intrusion on C cycling are further complicated by interactions with plant nutrient cycling and community shifts (Krauss et al. 2012). Excess salinity caused plant mortality and led to a community shift dominated by salt tolerant species (Glenn et al. 1995). Factors such as the quality and quantity of autochthonous organic inputs of plants (Neubauer et al. 2013), the duration of saltwater intrusion (Weston et al. 2010; Marton et al. 2012; Neubauer et al. 2013), and the flooding regime of the wetland (Ardón et al. 2018; Helton et al. 2019) may influence the magnitude and response of C cycling. Thus, tidal flow restoration occurs within the context of many possible, and sometimes contrasting, drivers of C and N processing.
Our overall goal was to quantify patterns of denitrification potential and C mineralization in coastal wetland soils across an urban landscape with a wide range of salinity and watershed development. Specifically, the objectives of this study were to 1) measure how soil chemistry, denitrification, and C mineralization vary between tidal flow restored versus unrestored wetlands and with age of tidal flow restoration, and 2) identify potential controls on denitrification and C mineralization across this heterogeneous landscape. The patterns of ecosystem function across diverse chemical conditions offer insight into how wetlands may respond to future changes in saltwater intrusion and land development.
Methods
Study Sites
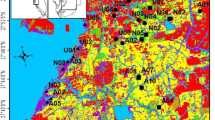
We collected soil cores from 32 tidal wetlands along the Long Island Sound in Connecticut (CT), U.S.A, including 17 restored tidal wetlands and 15 unrestored tidal wetlands (Fig. 1). Restored and unrestored sites were identified with help from CT Department of Energy and Environmental Protection (CT DEEP; P. Capotosto and R. Wolfe, personal communication, March 2, 2015). Completion of tidal flow restoration at sampling sites ranged from 1 to 23 years prior to sampling. For our purposes, tidal flow restoration included any combination of restoration practices that restored tidal hydrology to a tidally restricted wetland, for example culvert replacement, tidal gate removal, and installation of self-regulating tide gates. Unrestored wetland sites were chosen as tidal wetlands from the 1990s tidal wetlands shapefile (CT DEEP 1999) with no known tidal flow restoration. Because virtually all salt marshes were ditched or altered for mosquito control practices (Rozsa 1995), unrestored sites represent current conditions of a range of wetlands in the coastal landscape of CT, rather than reference sites. Wetland sampling sites ranged in surface water salinity from 0.03 to 29.04 ppt (556 MPS, YSI Inc., Yellow Springs, OH) at time of sampling, and included six saline, 19 brackish, and seven freshwater wetlands as defined by polyhaline (>18 ppt), oligohaline to mesohaline (0.5–18 ppt) and freshwater (< 0.5 ppt) salinity classes (Cowardin et al. 1979). Wetland sampling sites also encompassed a range of watershed land development, from less than 1% to 79% (mean ± SEM, 20 ± 2.6%) of watershed area with mean watershed size of 3867 ± 1204 km2 (NHDPlus 2011).
Wetland sampling site locations in Connecticut with developed land cover (grey) and water (black) from 2010 land cover data (CLEAR 2015)
Field Sampling and Soil Processing
At each site, three to four soil cores were collected with a slide hammer (5 cm dia., 15 cm length) within three hours of high tide during July of 2015. Samples were collected within ~500 m of the terrestrial wetland edge. We analyzed the top five centimeters of soil. Large roots were removed and soil cores were composited, sieved (2 mm), and homogenized before analysis.
Soil Chemistry
Soil organic matter was determined by loss on ignition. Samples were dried at 105 °C for 72 h to determine moisture content and combusted at 550 °C for four hours to determine percent organic matter (adapted from USDA-NRCS 1996).
We extracted soil ammonium (NH4+) and nitrate (NO3−) with 2 N KCl by adding 25 ml of KCl solution to 2.5 g of field moist soils, shaking for 30 min at 200 rpm, centrifuging for 5 min at 2400 rpm, and filtering supernatant through Whatman 589/1 filters (adapted from Keeney and Nelson 1982). Extracts were analyzed on a SmartChem®200 discrete analyzer (Westco Scientific Instruments, Brookfield, CT) by the phenate method (APHA, WEF, AWWA 1999) for NH4+ and by colorimetric determination with enzymatic reduction (Campbell et al. 1997; Patton and Kryskalla 2011) for NO3−. Ninety-seven percent of samples analyzed for soil extractable NO3− were below the detection limit (0.11 mg N L−1). Therefore, NO3− was not included in further analysis. All NH4+ concentrations were above the detection limit (0.12 mg N L−1).
Soil electrical conductivity (EC) was determined using the soil:water ratio, by volume, of 1:5 (EC1:5vol) (USDA-NRCS 2011). Soil EC measurements were made with an Oakton Con5 Acorn Series Conductivity/°C Meter (Oakton Instruments, Vernon Hills, IL). Additionally, we extracted soil chloride (Cl-) and sulfate (SO42-) by adding 25 ml of distilled, deionized water to 2.5 g of field moist soil, shaking for 30 min at 200 rpm, centrifuging for 5 min at 2400 rpm, and filtering supernatant through Whatman GF/F filters. Water extractable Cl- and SO42- concentrations were analyzed by ion chromatography (ICS-1100; Thermo Fisher Scientific, Waltham, MA). As expected, Cl− and SO42− concentrations were positively correlated with each other (r2 = 0.85, p < 0.001) and with soil EC (Cl−, r2 = 0.89; SO42−, r2 = 0.78; p < 0.001). Thus, soil EC was included in analysis as a surrogate of combined effects of both salt anions (i.e. Cl− and SO42−).
Total soil metal concentrations of redox active metals, Fe and Mn, and common urban metals, Cu, Pb, and Zn were determined by acid digestions with 70% HNO3 (trace metal grade) and 30% H2O2 according to Method 3050B (US EPA 1996). Metals were analyzed with an Inductively Coupled Plasma Mass Spectrometer (ICP-MS, Agilent 7700x with He collision cell, Agilent, Delaware, USA).
Soil Process Rates
We measured denitrification enzyme activity (DEA) on homogenized soils (Groffman et al. 1999). Denitrification enzyme activity is a measure of denitrification potential since the assays remove limiting factors; they are conducted under anoxic conditions with excess C and NO3-. Following the procedure in Groffman et al. (1999), we added 5 g of field moist soil to 10 ml of DEA media (0.72 g KNO3, 0.5 g glucose, 0.125 g Chloramphenicol per liter of distilled, deionized water) in 125 ml glass flasks with gas-tight seals and flushed the headspace with N2 gas to create anoxic conditions. We added 10 ml of acetylene gas to block the reduction of N2O to N2 gas. Headspace gas was sampled for four time-points (i.e. approximately 0, 30, 60, and 90 min after start of incubation) and analyzed for N2O on a Clarus 580 gas chromatograph (GC) equipped with an electron capture detector (PerkinElmer, Shelton, CT). Samples were delivered to the GC with a TurboMatrix 40 Trap Headspace Autosampler (PerkinElmer, Shelton, CT). Denitrification rates were calculated as the linear rate of evolved N2O-N over time per gram of dry soil.
We calculated the minimum detectable concentration difference (MDCD) for N2O following Yates et al. (2006). For fluxes above MDCD, when the r2 > 0.85 we used the slope based on the full 90 min incubation. When gas accumulation was non-linear (i.e., r2 < 0.85) over the full incubation, we calculated flux excluding time 90 and/or time 60. Nitrous oxide fluxes in four assays were below MDCD, and we set these fluxes to zero for analysis.
Soil C mineralization rates were measured as CO2 accumulation from 5 g of field moist soils in a 100-mL sealed serum bottle over a three-day incubation. Headspace CO2 was sampled immediately after bottles were sealed and after one and three days. We used the substrate-induced respiration (SIR) method (Anderson and Domsch 1978; West and Sparling 1986) as an index for soil microbial activity. SIR is positively correlated with total microbial biomass (Beare et al. 1990) and is commonly used as an indirect measurement of total microbial biomass (Bååth and Anderson 2003). SIR was measured as CO2 accumulation after adding 10 mL of yeast solution to 5 g of field moist soil in 40 mL sealed amber vials. Headspace CO2 was sampled immediately after vials were sealed and after 2 and 4 h. Headspace CO2 samples for C mineralization and SIR were immediately injected into a LI-840A CO2/H2O Gas Analyzer (LI-COR, Lincoln, NE) to measure CO2 concentration. Carbon mineralization and SIR were calculated as the linear rate of accumulated CO2-C over time per gram of dry soil.
Statistical Analyses
We determined statistically significant differences among wetland (fresh, brackish, saline) and management (restored, unrestored) categories for soil chemistry and biogeochemical processes (denitrification potential, C mineralization, and SIR) using the method of analysis of multiple means described by Herberich et al. (2010), which does not require normality, equal variances, or equal sample sizes. Because we were only able to sample one restored freshwater wetland, that category was not included in this analysis.
We used principal component analysis (Mardia et al. 1979; prcomp function in R Core Team 2015) of soil chemical variables 1) to understand patterns in soil chemistry among wetland and management categories and 2) as composite variables in regression analysis to avoid violating the multi-collinearity assumptions of regression. For the whole dataset we used simple linear regression ((R function ‘lm()’) to determine relationships between principal components and soil process rates. Because the number of restored wetlands sampled was low in freshwater (n = 1) and saline (n = 3), we used restored brackish wetlands (n = 13) to determine relationships between 1) time since restoration and soil processes using simple linear regression, and 2) time since restoration, principal components, and soil processes using multiple linear regression. We performed multiple linear regression model selection by exhaustive search (R function ‘regsubsets()’). The best fit models were selected by the lowest Akaike Information Criterion (AIC) (R function ‘extractAIC()’) given all combinations of parameters (Burnham et al. 2011). Statistical analyses were performed using R version 3.1.3 (R Core Team 2015).
Results
Soil Chemistry
Across all sites (n = 32), soil EC reflected a wide range in wetland salinity with soil EC significantly highest in saline and lowest in freshwater wetlands, as expected (Fig. 2a). Percent organic matter was also highest in saline and lowest in freshwater wetlands (Fig. 2b). Ammonium was not significantly different among wetland and management types but tended to be higher in restored versus unrestored wetlands (Fig. 2c). Metal concentrations tended to be lower in restored saline wetlands than in other wetland types; however, because of high variability in metal concentrations, the difference was only significant between restored saline wetlands and unrestored brackish wetlands for Mn and restored brackish wetlands for Cu, Pb, and Fe (Table 1).
Principal components analysis of the soil chemistry variables resulted in 10 components. Component 1 (35%), component 2 (27%), and component 3 (14%) each explained greater than 10% of the variation and the majority of cumulative variance (76%), so we focus our results on these three components (Table 2). Component 1 (PC1) is positively related to percent organic matter, soil moisture, cation exchange capacity, and soil NH4+. Component 2 (PC2) represents a continuum of metal concentration; it is negatively related to soil Fe, Pb, Zn, and Cu. Component 3 (PC3) represents a salinity continuum; it is positively related to salinity and negatively related to soil Mn.
High variation in soil chemistry among wetland types led to indistinct groupings along principal component axes. Wetland types largely overlapped along the PC1 axis (Fig. 3). Similarly, wetland types largely overlapped along the PC2 axis, except restored saline wetlands (grey triangles in Fig. 3a) tended to have higher values for PC2, representing lower metal content in these wetlands (Table 2). Component 2 was also negatively related to the percent of developed land cover in the draining watershed (r2 = 0.25, p = 0.003), suggesting metal loading increases with increasing developed watershed land cover. Freshwater and saline wetlands had distinct separation along the PC3 axis (triangles versus squares in Fig. 3b), with higher PC3 values for saline wetlands, which have lower Mn and higher salinity. Except for PC2 for saline wetlands, we did not observe distinct separation in soil chemistry between restored and unrestored wetlands for any wetland type.
Within the restored brackish wetland group (n = 13), neither PC1 (p = 0.90) nor PC2 (p = 0.51) was significantly related to time since restoration. Component 3 was negatively related to time since restoration (r2 = 0.24, p = 0.09). Manganese (which had a high loading for PC3) was the only soil chemistry variable with a significant relationship with time since restoration, increasing with time since restoration (r2 = 0.29, p = 0.05). This pattern was driven by one wetland sampled 22 years post restoration that had Mn concentration greater than four times the next highest value.
Denitrification Potential and Carbon Mineralization
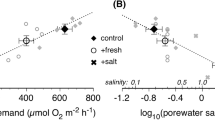
Denitrification potential tended to decrease from fresh to saline wetlands and tended to be lower for restored wetlands across wetland types; however, because of high variation for some wetland types the difference was not significant between groups (Fig. 4a). Across the whole dataset, denitrification potential was not significantly related to PC1 (p = 0.20) or PC2 (p = 0.11) but declined with PC3 (r2 = 0.23, p = 0.007) (Fig. 5a–c). Carbon mineralization was significantly higher in brackish restored wetlands than freshwater wetlands (Fig. 4b), and SIR was not significantly different among wetland types (Fig. 4c). Across the whole dataset, PC1 was a strong predictor of both C mineralization (r2 = 0.56, p < 0.001) and SIR (r2 = 0.43, p < 0.001) (Fig. 5d, g). Carbon mineralization and SIR were not significantly related to PC2 or PC3 (p > 0.10) (Fig. 5). Carbon mineralization and SIR were also strongly correlated with each other (r2 = 0.62, p < 0.001). Denitrification potential increased with SIR (r2 = 0.25, p = 0.004), but was not significantly related to C mineralization (p = 0.15).
Within wetland groups with more than three wetlands sampled (restored brackish, unrestored brackish, and unrestored freshwater), relationships between principal components suggest contrasting predictors of soil processes for fresh versus brackish wetlands. For unrestored freshwater wetlands, PC2 was a strong predictor of denitrification potential (r2 = 0.86), C mineralization (r2 = 0.94), and SIR (r2 = 0.68) (Table 3). The negative relationship between process rates and PC2 suggests that in freshwater wetlands, denitrification potential and C mineralization increase with increasing soil metal content. In contrast, PC2 was not related to soil processes for brackish wetlands. Instead, for brackish wetlands denitrification potential and SIR decreased with PC3, and SIR and C mineralization increased with PC1 (Table 3).
Within the restored brackish wetland group, denitrification potential significantly increased with time since restoration (Fig. 6a). The best fit regression model explained 65% of the variation and included a positive coefficient for time since restoration and a negative coefficient for PC3 (Tables 4, 5). Similarly, SIR increased with time since restoration (Fig. 6c). The best fit regression model explained 61% of the variation and included positive coefficients for time since restoration and PC1 and a negative coefficient for PC 3 (Tables 4, 5). Conversely, C mineralization was not related to time since restoration (Fig. 6b). The best fit regression model for C mineralization explained 88% of variation and included only a positive coefficient for PC1 (Tables 4, 5).
Discussion
Tidal flow restoration occurs within the potentially complex chemical context of developed coastal landscapes, where the intersection of saltwater intrusion and urban contaminants can affect wetland ecosystem function (Helton et al. 2014; Doroski et al. 2019). Our field study reveals two patterns that are particularly important for understanding how soil C and N cycling may change in the context of sea level rise, urban pollution, and wetland management. First, even though the restored wetlands in our study experienced a wide range of salinity, nutrient and metal concentrations, we found a strong increase in denitrification potential with time since tidal flow restoration for brackish wetlands. Denitrification potential increased more than five-fold across sites that represent one to over 20 years post restoration. Second, soil chemistry gradients were also strong predictors of wetland C and N process rates; however, fresh and brackish wetlands had different predictors. Freshwater wetlands were strongly related to metal content of soils whereas brackish wetlands were related to organic matter and salinity gradients. Thus, understanding how C and N processes change over time and after restoration may vary among these wetland types. Below we discuss potential drivers and implications of each of these patterns.
Denitrification potential increases with time since restoration
Our results show that denitrification potential increases with time since restoration, suggesting a potential recovery of denitrification over time after wetland restoration. In a 55 year chronosequence of palustrine depressional wetlands, soil properties likely linked with denitrification (organic matter and cation exchange capacity) also increased over time but did not recover to reference conditions (Ballantine and Schneider 2009). Recovery of C and N storage after restoration was highly variable in a recent meta-analysis (Zhou et al. 2017); however, denitrification was not documented well enough in the literature to be included in this meta-analysis. We also did not find clear trends in soil organic matter or chemistry with time since restoration; however, we did find a significant increase in SIR with time since restoration, suggesting an increase in microbial biomass or change in the microbial community. Research shows differences in microbial community structure in restored versus reference wetlands (e.g., Peralta et al. 2010). Less understood is how microbial biomass or community structure changes with time since restoration. Continuing to add to our measurements of denitrification and its drivers over time after restoration and across chronosequences of restoration ages is critical for understanding how N cycling in degraded wetlands responds to hydrologic restoration.
Although differences were not significant, NH4+ and C mineralization tended to be higher in restored than unrestored wetlands across wetland types (Figs. 2 and 4), although neither was related to time since restoration. Greater NH4+ and C mineralization in restored wetlands suggest enhanced nutrient deposition or transformation (Megonigal and Neubauer 2009). Our results are similar to previous studies in which restored wetland sites exhibited greater C mineralization compared to reference sites (Craft et al. 2003; Glatzel et al. 2004; Lawrence et al. 2013). Greater nutrient bioavailability after restoration may be driven by shifts in plant productivity and hydrologic regime post restoration, although we did not directly measure these drivers during our study.
Patterns of C and N process rates differ in fresh versus brackish wetlands
Although there was large overlap of soil chemistry among wetland types (i.e., Fig. 3), we found C and N process rates were most strongly associated with different aspects of soil chemistry depending on wetland type. In freshwater wetlands, denitrification potential and C mineralization increased with increasing metal content of soils (i.e., negative relationship with PC2, Table 3). Metal concentrations were comparable to those found in another Connecticut coastal wetland (Benoit et al. 1999), and were generally low. Average Cu and Zn were lower than the Effects Range-Low (ERL) and average Pb was lower than the Effects Range-Median (ERM) toxicity guidelines; maximum Cu, Zn, and Pb were all lower than the ERM guidelines. This suggests metal concentrations were less than concentrations in which biological effects in higher organisms frequently occur, but within levels at which effects would occasionally occur (Long et al. 1995).
Additions of lower concentrations of Cu or Zn to wetland sediments has been shown to increase denitrification rates, but decreases in rates were observed at higher metal concentrations that might cause toxicity effects (Sakadevan et al. 1999; Holtan-Hartwig et al. 2002). A positive association between denitrification potential and lower metal content of soils may be due to several potential mechanisms: 1) Lower metal content may be associated with more reduced conditions (Gambrell 1994), which may indicate environmental conditions conducive to a larger or more active denitrifier community. 2) Copper is a cofactor for many enzymes essential for cell growth (Samanovic et al. 2012), and metalloenzymes involved in denitrification primarily contain Fe or Cu (Glass and Orphan 2012), thus higher concentrations may reduce potential metal limitations. 3) Metals, particularly Fe, are also important redox pairs for microbial metabolism in anoxic wetland soils (Lovley and Phillips 1988; Lovley 1991; Nealson and Myers 1992; Thamdrup 2000; Zhu et al. 2013). Iron can act as an alternate electron donor for nitrate reduction, potentially increasing rates of denitrification (Burgin and Hamilton 2007). Thus, at the relatively low concentrations of metals across our study sites, elevated metal content likely led to an increase of C mineralization and denitrification potential.
For brackish wetlands, even though soil metal content spanned a similar range, we did not find a significant relationship with soil metal content. Rather, we found that denitrification potential decreased with increasing salinity and that C mineralization increased with organic matter, soil moisture, and ammonium content (see relationships with principal components, Table 3). Decreased denitrification potential with salinity may be driven by multiple mechanisms including direct microbial and enzyme effects, formation of toxic compounds (i.e., sulfide), and mobilization of nutrients. In a meta-analysis Zhou et al. 2017 found salinity as a key regulating factor decreasing denitrification in coastal ecosystems primarily as a result of sulfide toxicity and changes in microbial composition. Sulfide can have inhibitory effects on biota and certain steps of the nitrogen cycle (Joye and Hollibaugh 1995; Camargo and Alonso 2006; Pan et al. 2013). Previous research has shown inhibition of N2O reduction to N2 due to sulfide (Senga et al. 2006), which is supported by greater N2O emissions with increasing SO42− or sulfide (Brunet and Garcia-Gil 1996; Helton et al. 2014). Increased salinity enhances the flux of NH4+ to the water column which can then be exported with tides (Giblin et al. 2010; Ardón et al. 2013). Export of soil NH4+ effectively decreases the N supply for coupled nitrification-denitrification in coastal wetlands.
A recent review shows relatively consistent decreases in C mineralization from fresh to saline wetlands along natural gradients; however, the relationship with C mineralization and salinity in brackish wetlands along natural gradients is less clear (Luo et al. 2017). In our study, in situ salinity was not significantly related to C mineralization for brackish marshes. Our results suggest other factors such as C and N availability and soil moisture potentially play a stronger role in regulating C mineralization across these brackish wetlands, similar to findings in some previous studies (Blagodatskaya and Kuzyakov 2008; Setia et al. 2011).
Conclusions
Our results highlight the potential for biogeochemical recovery in restored wetlands over time, along with the complex interactions between microbial processes and urban chemicals in developed coastal landscapes. Although soil chemistry was not significantly related to time since restoration, spatial patterns of denitrification potential across brackish coastal wetlands were best explained by time since restoration and soil chemistry properties combined. The strongest predictors of denitrification potential and C mineralization also varied by wetland salinity class; process rates in freshwater wetlands were more strongly associated with soil metal content whereas process rates in brackish wetlands were associated with salinity and organic matter gradients. As tidal flow restoration techniques are increasingly used in highly developed coastal landscapes, understanding how restoration practices change biogeochemical function in the context of multiple chemical stressors will be important for predicting the future role of coastal wetlands in C and N cycling.
References
Anderson JPE, Domsch KH (1978) A physiological method for the quantitative measurement of microbial biomass in soils. Soil Biology and Biochemistry 10:215–221
APHA, WEF, AWWA (1999) Standard methods for the examination of water, Washington DC
Ardón M, Morse JL, Colman BP, Bernhardt ES (2013) Drought-induced saltwater incursion leads to increased wetland nitrogen export. Global Change Biology 19:2976–2985. https://doi.org/10.1111/gcb.12287
Ardón M, Helton AM, Bernhardt ES (2018) Salinity effects on greenhouse gas emissions from wetland soils are contingent upon hydrologic setting: a microcosm experiment. Biogeochemistry 140:217–232. https://doi.org/10.1007/s10533-018-0486-2
Bååth E, Anderson TH (2003) Comparison of soil fungal/bacterial ratios in a pH gradient using physiological and PLFA-based techniques. Soil Biology and Biochemistry 35:955–963. https://doi.org/10.1016/S0038-0717(03)00154-8
Ballantine K, Schneider R (2009) Fifty-five years of soil development in restored years of soil development freshwater wetlands depressional. Ecological Applications 19:1467–1480. https://doi.org/10.1890/07-0588.1
Bartlett KB, Bartlett DS, Harriss RC et al (1987) Methane emissions along a salt marsh salinity gradient. Biogeochemistry 4:183–202
Beare MH, Neely CL, Coleman DC, Hargrove WL (1990) A substrate-induced respiration (SIR) method for measurement of fungal and bacterial biomass on plant residues. Soil Biology and Biochemistry 22:585–594
Benoit G, Rozan TF, Patton PC, Arnold CL (1999) Trace metals and radionuclides reveal sediment sources and accumulation rates in Jordan cove, Connecticut. Estuaries 22:65–80. https://doi.org/10.2307/1352928
Bergback B, Johansson K, Mohlander U (2001) Urban Metal Flows - A Case Study of Stockholm. Water, Air, and Soil Pollution 1:3–24
Blagodatskaya E, Kuzyakov Y (2008) Mechanisms of real and apparent priming effects and their dependence on soil microbial biomass and community structure: critical review. Biology and Fertility of Soils 45:115–131. https://doi.org/10.1007/s00374-008-0334-y
Brunet RC, Garcia-Gil LJ (1996) Sulfide-induced dissimilatory nitrate reduction to ammonia in anaerobic freshwater sediments. FEMS Microbiology Ecology 21:131–138
Burgin AJ, Hamilton SK (2007) Have we overemphasized in aquatic removal of nitrate the role ecosystems? A review of nitrate removal pathways. Frontiers in Ecology and the Environment 5:89–96. https://doi.org/10.1890/1540-9295(2007)5[89:HWOTRO]2.0.CO;2
Burnham KP, Anderson DR, Huyvaert KP (2011) AIC model selection and multimodel inference in behavioral ecology : some background , observations , and comparisons. Behavioral Ecology and Sociobiology 65:23–35. https://doi.org/10.1007/s00265-010-1029-6
Camargo JA, Alonso A (2006) Ecological and toxicological effects of inorganic nitrogen pollution in aquatic ecosystems: a global assessment. Environment International 32:831–849. https://doi.org/10.1016/j.envint.2006.05.002
Campbell ER, Corrigan JS, Campbell WH (1997) Field determination of nitrate using nitrate reductase. Field Analytical Methods for Hazardous Wastes and Toxic Chemicals. Pittsburgh, PA, pp 851–860
Carpenter S, Caraco N, Correll D, Carpenter SR, Caraco NF, Correl DR (1998) Nonpoint pollution of surface waters with phosphorus and nitrogen. Ecological Applications 8:559–568. https://doi.org/10.1890/1051-0761(1998)008[0559:NPOSWW]2.0.CO;2
Chambers R, Osgood DT, Chambers RM, Osgood DT, Kalapasev N (2002) Hydrologic and chemical control of Phragmites growth in tidal marshes of SW Connecticut, USA. Marine Ecology Progress Series 239:83–91. https://doi.org/10.3354/meps239083
CLEAR (2015) https://clear.uconn.edu/projects/landscape/index.htm
Cowardin LM, Carter V, Golet FC, LaRoe ET (1979) Classification of wetlands and deepwater habitats of the United States. FWS/OBS-79/31
Craft C, Megonigal P, Broome S et al (2003) The pace of ecosystem development of constructed Spartina alterniflora marshes. Ecological Applications 13:1417–1432
CT DEEP (1999) Tidal Wetlands 1990s. https://www.ct.gov/deep/cwp/view.asp?A=2698&Q=459068
Deegan LA, Bowen JL, Drake D et al (2007) Susceptibility of salt marshes to nutrient enrichment and predation removal. Ecological Applications 17:42–63. https://doi.org/10.1890/06-0452.1
Doroski AA, Helton AM, Vadas T (2019) Greenhouse gas fluxes from wetlands at the intersection of urban pollution and saltwater intrusion: a soil core experiment. Soil Biology and Biochemistry 131:44–53. https://doi.org/10.1016/j.soilbio.2018.12.023
Elphick CS, Meiman S, Rubega MA (2015) Tidal-flow restoration provides little nesting habitat for a globally vulnerable saltmarsh bird. Restoration Ecology 23:439–446
Gambrell RP (1994) Trace and toxic metals in wetlands—a review. Journal of Environmental Quality 23:883–891. https://doi.org/10.2134/jeq1994.00472425002300050005x
Gedan KB, Silliman BR, Bertness MD (2009) Centuries of human-driven change in salt marsh ecosystems. Annual Review of Marine Science 1:117–141. https://doi.org/10.1146/annurev.marine.010908.163930
Giblin AE, Weston NB, Banta GT et al (2010) The effects of salinity on nitrogen losses from an Oligohaline estuarine sediment. Estuaries and Coasts 33:1054–1068. https://doi.org/10.1007/s12237-010-9280-7
Giller KE, Witter E, Mcgrath SP (1998) Toxicity of heavy metals to microorganisms and microbial processes in agricultural soils: a review. Soil Biology and Biochemistry 30:1389–1414. https://doi.org/10.1016/S0038-0717(97)00270-8
Glass JB, Orphan VJ (2012) Trace metal requirements for microbial enzymes involved in the production and consumption of methane and nitrous oxide. Frontiers in Microbiology 3:1–20. https://doi.org/10.3389/fmicb.2012.00061
Glatzel S, Basiliko N, Moore T, Unit LE (2004) Carbon dioxide and methane production potentials of peats from natural, harvested and restored sites , eastern Quebec, Canada. Wetlands 24:261–267
Glenn E, Thompson TL, Frye R et al (1995) Effects of Salinity on Growth and Evapotranspiration of Typha-Domingensis Pers. Aquatic Botany 52:75–91. https://doi.org/10.1016/0304-3770(95)00492-I
Groffman PM, Holland EA, Myrold DD (1999) Denitrification. In: Robertson G, Coleman D, Bledsoe C, Sollins C (eds) Standard soil methods for Long-term ecological research. Oxford University Press, Oxford, pp 272–288
Helton AM, Bernhardt ES, Fedders A (2014) Biogeochemical regime shifts in coastal landscapes: the contrasting effects of saltwater incursion and agricultural pollution on greenhouse gas emissions from a freshwater wetland. Biogeochemistry 120:133–147. https://doi.org/10.1007/s10533-014-9986-x
Helton AM, Ardon M, Bernhardt ES (2019) Hydrologic context alters greenhouse gas feedbacks of coastal wetland salinization. Ecosystems. https://doi.org/10.1007/s10021-018-0325-2
Herberich E, Sikorski J, Hothorn T (2010) A robust procedure for comparing multiple means under heteroscedasticity in unbalanced designs. PLoS One 5:e9788. https://doi.org/10.1371/journal.pone.0009788
Herbert ER, Boon P, Burgin AJ et al (2015) A global perspective on wetland salinization: ecological consequences of a growing threat to freshwater wetlands. Ecosphere 6(10):1–43. https://doi.org/10.1890/ES14-00534.1
Holtan-Hartwig L, Bechmann M, Risnes Høyås T et al (2002) Heavy metals tolerance of soil denitrifying communities: N2O dynamics. Soil Biology and Biochemistry 34:1181–1190. https://doi.org/10.1016/S0038-0717(02)00055-X
Joye SB, Hollibaugh JT (1995) Influence of sulfide inhibition of nitrification on nitrogen regeneration in sediments. Science (Washington D C) 270:623–625. https://doi.org/10.1126/science.270.5236.623
Keeney DR, Nelson DW (1982) Nitrogen-inorganic forms. In: Page AL, Miller RH, Keeney DR (eds) Methods of soil analysis. Part 2, 2nd edn. ASA, SSSA, Madison, Wisconsin, pp 643–698
Krauss KW, Whitbeck JL, Howard RJ (2012) On the relative roles of hydrology, salinity, temperature, and root productivity in controlling soil respiration from coastal swamps (freshwater). Plant and Soil 358:265–274. https://doi.org/10.1007/s11104-012-1182-y
Lawrence BA, Jackson RD, Kucharik CJ (2013) Testing the stability of carbon pools stored in tussock sedge meadows. Applied Soil Ecology 71:48–57. https://doi.org/10.1016/j.apsoil.2013.05.007
Long ER, Macdonald DD, Smith SL, Calder FD (1995) Incidence of adverse biological effects within ranges of chemical concentrations in marine and estuarine sediments. Environmental Management 19:81–97
Lovley DR (1991) Dissimilatory Fe(III) and Mn(IV) reduction. Microbiological Reviews 55:259–287
Lovley DR, Phillips EJP (1988) Novel mode of microbial energy metabolism: organic carbon oxidation coupled to dissimilatory reduction of Iron or manganese. Applied and Environmental Microbiology 54:1472–1480
Luo M, Huang JF, Zhu WF, Tong C (2017) Impacts of increasing salinity and inundation on rates and pathways of organic carbon mineralization in tidal wetlands: a review. Hydrobiologia 827:31–49. https://doi.org/10.1007/s10750-017-3416-8
Magalhaes CM, Joye SB, Moreira RM et al (2005) Effect of salinity and inorganic nitrogen concentrations on nitrification and denitrification rates in intertidal sediments and rocky biofilms of the Douro River estuary, Portugal. Water Research 39:1783–1794. https://doi.org/10.1016/j.watres.2005.03.008
Mardia KV, Kent JT, Bibby JM (1979) Multivariate Analysis. Academic Press, London
Marton JM, Herbert ER, Craft CB (2012) Effects of salinity on denitrification and greenhouse gas production from laboratory-incubated tidal Forest soils. Wetlands 32:347–357. https://doi.org/10.1007/s13157-012-0270-3
McKee KL, Mendelssohn IA (1989) Response of a freshwater marsh plant community to increased salinity and increased water level. Aquatic Botany 34:301–316
Megonigal JP, Neubauer SC (2009) Biogeochemistry of Tidal Freshwater Wetlands. In: Perillo GME, Wolanski E, Cahoon DR, Brinsom MM (eds) Coastal Wetlands: An Integrated Ecosystem Approach 535–562
Megonigal JP, Hines ME, Visscher PT (2004) Anaerobic metabolism:linkages to trace gases and aerobic processes. In: Schlesinger WH, Holand HD, Turekian KK (eds) Treatise on geochemistry, vol 8. Elsevier, pp 317–424
Moreno-Mateos D, Power ME, Comín FA, Yockteng R (2012) Structural and functional loss in restored wetland ecosystems. PLoS Biology 10:e1001247. https://doi.org/10.1371/journal.pbio.1001247
Morrissey EM, Gillespie JL, Morina JC, Franklin RB (2014) Salinity affects microbial activity and soil organic matter content in tidal wetlands. Global Change Biology 20:1351–1362. https://doi.org/10.1111/gcb.12431
Nealson KH, Myers CR (1992) Microbial reduction of manganese and iron: new approaches to carbon cycling. Applied and Environmental Microbiology 58:439–443
Neubauer SC, Franklin RB, Berrier DJ (2013) Saltwater intrusion into tidal freshwater marshes alters the biogeochemical processing of organic carbon. Biogeosciences 10:8171–8183. https://doi.org/10.5194/bg-10-8171-2013
NHDPlus (2011) NLCD 2011 Land use extension. http://www.horizon-systems.com/NHDPlus/
Pan Y, Ye L, Yuan Z (2013) Effect of H2S on N2O reduction and accumulation during denitrification by methanol utilizing Denitrifiers. Environmental Science & Technology 47:8408–8415
Panswad T, Anan C (1999) Impact of high chloride wastewater on an anaerobic/anoxic/aerobic process with and without inoculation of chloride acclimated seeds. Water Research 33:1165–1172. https://doi.org/10.1016/S0043-1354(98)00314-5
Pathak H, Rao DLN (1998) Carbon and nitrogen mineralization from added organic matter in saline and alkali soils. Soil Biology and Biochemistry 30:695–702. https://doi.org/10.1016/S0038-0717(97)00208-3
Patton CJ, Kryskalla JR (2011) Colorimetric determination of nitrate plus nitrite in water by enzymatic reduction, automated discrete analyzer methods. U.S. Geological Survey techniques and methods, 5th edn. p 34
Peralta AL, Matthews JW, Kent AD (2010) Microbial community structure and denitrification in a wetland mitigation bank. Applied and Environmental Microbiology 76:4207–4215. https://doi.org/10.1128/AEM.02977-09
Putnam Duhon LA, Gambrell LP, Rusch KA, White JR (2012) Effects of salinity on the microbial removal of nitrate under varying nitrogen inputs within the marshland upwelling system. Journal of Environmental Science and Health, Part A 47:1739–1748. https://doi.org/10.1080/10934529.2012.689221
R Core Team (2015) R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. http://www.R-project.org/
Rajapaksha RMCP, Tobor-Kaplon MA, Bååth E (2004) Metal toxicity affects fungal and bacterial activities in soil differently. Applied and Environmental Microbiology 70:2966–2973. https://doi.org/10.1128/AEM.70.5.2966
Revitt DM, Lundy L, Coulon F, Fairly M (2014) The sources , impact and management of car park runoff pollution : a review. Journal of Environmental Management 146:552–567. https://doi.org/10.1016/j.jenvman.2014.05.041
Rozsa R (1995) Human Impacts on Tidal Wetlands: History and Regulations. In: Dreyer G, Niering W (eds) Tidal Marshes of Long Island Sound: Ecology, History, and Restoration. Connecticut College, New London, CT, pp 42–50
Rysgaard S, Thastum P, Dalsgaard T et al (1999) Effects of salinity on NH4 + adsorption capacity, nitrification, and denitrification in Danish estuarine sediments. Estuaries 22:21. https://doi.org/10.2307/1352923
Sakadevan K, Zheng H, Bavor HJ (1999) Impact of heavy metals on denitrification in surface wetland sediments receiving wastewater. Water Science and Technology 40:349–355
Samanovic MI, Ding C, Thiele DJ, Darwin KH (2012) Copper in microbial pathogenesis: meddling with the metal. Cell Host & Microbe 11:106–115. https://doi.org/10.1016/j.chom.2012.01.009
Senga Y, Mochida K, Fukumori R et al (2006) N2O accumulation in estuarine and coastal sediments: the influence of H2S on dissimilatory nitrate reduction. Estuarine, Coastal and Shelf Science 67:231–238. https://doi.org/10.1016/j.ecss.2005.11.021
Setia R, Marschner P, Baldock J et al (2011) Relationships between carbon dioxide emission and soil properties in salt-affected landscapes. Soil Biology and Biochemistry 43:667–674. https://doi.org/10.1016/j.soilbio.2010.12.004
Thamdrup B (2000) Bacterial manganese and iron reduction in aquatic sediments. In: Schink B (ed) Advances in microbial ecology, vol 16, Springer. Boston, MA, pp 41–84
US EPA (1996) Method 3050B acid digestions of sediments, Sludges, and Soils
USDA-NRCS (1996) Soil survey laboratory methods manual, soil survey investigations report no. 42, Ver 3.0
USDA-NRCS (2011) Electrical conductivity and soluble salts. Washington DC
Valiela I, Teal JM (1979) The nitrogen budget of a salt marsh ecosystem. Nature 280:652–656
West AW, Sparling GP (1986) Modifications to the substrate-induced respiration method to permit measurement of microbial biomass in soils of differing water contents. Journal of Microbiological Methods 5:177–189
Weston NB, Giblin AE, Banta GT et al (2010) The effects of varying salinity on ammonium exchange in estuarine sediments of the parker river, Massachusetts. Estuaries and Coasts 33:985–1003. https://doi.org/10.1007/s12237-010-9282-5
Weston NB, Vile MA, Neubauer SC, Velinsky DJ (2011) Accelerated microbial organic matter mineralization following salt-water intrusion into tidal freshwater marsh soils. Biogeochemistry 102:135–151. https://doi.org/10.1007/s10533-010-9427-4
Williams TP, Bubb JM, Lester JN (1994) Metal accumulation within salt marsh environments: a review. Marine Pollution Bulletin 28:277–290
Yates TT, Si BC, Farrell RE, Pennock DJ (2006) Probability distribution and spatial dependence of nitrous oxide emission. Soil Science Society of America Journal 70:753–762. https://doi.org/10.2136/sssaj2005.0214
Zhou M, Butterbach-Bahl K, Vereecken H, Bruggemann N (2017) A meta-analysis of soil salinization effects on nitrogen pools, cycles and fluxes in coastal ecosystems. Global Change Biology 23:1338–1352. https://doi.org/10.1111/gcb.13430
Zhu X, Silva LCR, Doane TA, Horwath WR (2013) Iron: the forgotten driver of nitrous oxide production in agricultural soil. PLoS One 8:1–7. https://doi.org/10.1371/journal.pone.0060146
Acknowledgments
This research was funded by The Connecticut Sea Grant College Program (R/ES-26). We thank Roger Wolfe, Paul Capotosto, Harry Yamalis, Ron Rosza, and Juliana Barrett for sharing information on Connecticut wetland history and management, and Mary Zawatski, Mary Schoell, Cristina Macklem, Jason Sauer, and Hongwei Luan for field and lab assistance, and two anonymous reviewers for comments that improved the manuscript.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Doroski, A.A., Helton, A.M. & Vadas, T.M. Denitrification Potential and Carbon Mineralization in Restored and Unrestored Coastal Wetland Soils Across an Urban Landscape. Wetlands 39, 895–906 (2019). https://doi.org/10.1007/s13157-019-01128-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13157-019-01128-z