Abstract
Wetlands can be significant sinks for Nr, via denitrification. There is a lack of understanding about factors controlling denitrification. Research suggests that hydrology, geomorphology, and nitrogen loading are dominant controls. We compared site-scale characteristics with denitrification enzyme activity (DEA) in wetlands along gradients of drainage basin land use to explore the relative importance of landscape and site-scale factors for determining denitrification potential. DEA rates ranged between 0.01–1.69 (μg N gdw−1 h−1), with most sites falling at the lower end. Sites with higher DEA rates had higher percentages of soil carbon and nitrogen, concentrations of soil extractable NO3 and percent loss on ignition. Sites with upstream agricultural activity had higher DEA rates than more natural sites, but there existed a wide range of DEA rates along both agricultural and urban land gradients. When multiple site and landscape-scale explanatory factors were compared to DEA rates, two site and one landscape scale characteristic (Soil NO3, Soil Percent N, and Percent Agriculture) had significant (p < 0.001, cum. r2 = 0.77) correlations with DEA rates. Our results suggest that DEA is controlled mainly by local-scale site characteristics with more work needed to determine the interdependencies and relative importance among these and potentially related landscape-scale factors.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Urban land use extent in the Tampa Bay region of Florida, USA was projected to double between 1992 and 2025 (Xian and Crane 2005) and population has grown at close to 2% a year since 1990. The region around Tampa Bay housed a population of 2.9 million in 2010 and the regional transportation authority forecasts a 2040 population of 4.2 million due to the continuation of a 1.4% annual growth rate (http://www.tbrta.com/ accessed 10/1/2016). This presents the regional authorities with a challenge to balance urban growth with the provision of valuable ecosystem services that contribute to human well-being. Rapid urban growth is the main driver of stressors on ecosystem services for the Tampa Bay region wherein the effects of increased reactive nitrogen (Nr) loading due to an increasing human population have been given prominent attention (Greening and Janicki 2006). Increasing nitrogen loads and subsequent eutrophication of estuarine systems can lead to alterations in food-web structure, degraded habitats, detrimental dissolved oxygen levels, and changes in water quality (Howarth 2008). Tampa Bay supports commercial and recreational fishing, as well as other recreational activities wholly or partially dependent on water of sufficient quality for designated uses. Over the last few decades, improvements in waste water treatment have resulted in some recovery of water quality and seagrass coverage in the Tampa Bay estuary (Greening et al. 2011; Sherwood et al. 2015). These gains, however, could be lost if nitrogen removal processes in the Tampa Bay region decline, while nitrogen loading increases. The significance of this potential decline is dependent on the magnitude of further losses to freshwater and marine wetlands as well as how effective these ecosystems are in removing nitrogen.
Non-point sources account for the majority of nitrogen loads from the Tampa Bay watershed and will present a particular challenge to managing its effects in the bay. Atmospheric deposition is considered the dominant source of nitrogen (46%) delivered to the coastal areas of the Tampa Bay watershed, according to a recent SPARROW (SPAtially-References Regression On Watershed attributes) modeling paper (Hoos and McMahon 2009) and more recent CMAQ (Community Multi-scale Air Quality) model output which estimates the direct and indirect delivery from atmospheric nitrogen at 71% of the total nitrogen loads reaching the estuary (Poor et al. 2013). Atmospheric nitrogen contributions will likely become more significant in the future because of increased emissions of combustion exhausts often associated with expanding transportation needs resulting from the spread of urbanization, although increasing motor vehicle efficiencies may offset this. Urbanization may also necessitate the formation of large tracts of impermeable surfaces, which will reduce the landscape’s ability to attenuate nitrogen, and may lead to increased delivery of nitrogen to stream channels and Tampa Bay (Xian et al. 2007; Brush 2009).
Land coverage in the Tampa Bay region includes urban areas, row crop agricultural lands, range lands, upland forests, and wetlands. It is the latter land cover type that has a prominent role in the removal of excess Nr from non-point source surfaces and groundwater flows before freshwater enters the estuary. Because of frequent anoxic conditions, high concentrations of labile carbon, and the presence of facultative microbes and available nitrogen sources, wetlands can be a significant sink for Nr, with denitrification a major pathway for transformations that result in the release of N2 (Reddy and DeLaune 2008) and N2O (an important greenhouse gas; Anderson et al. 2010). In a meta-analysis, Jordan et al. (2011) reported approximately 20% of the Nr load reaching wetlands in the contiguous U.S. was removed through plant uptake, denitrification, absorption, burial, and anaerobic ammonium oxidation. Wetland type (e.g., palustrine forested, palustrine emergent marsh) affects nitrogen removal determinants such as carbon quality and microbial composition (Boon and Sorrell 1991). Ullah and Faulkner (2006) found nitrate amended and control palustrine forested wetlands had significantly higher potential denitrification rates than palustrine herbaceous submergent/emergent systems, while conversely Dodla et al. (2008) reported significantly higher potential denitrification rates in non-amended freshwater palustrine marsh systems than palustrine forested wetlands or saline (i.e., estuarine) marshes, suggesting that finer wetland classification (e.g., Comer et al. 2005) may be necessary to better estimate denitrification rates at landscape scales. Similarly, wetland spatial location vis-à-vis sources of nitrogen-laden overland flow or groundwater and/or atmospheric deposition affects the availability of Nr for denitrification or immobilization (Rheinhardt and Brinson 2002; Ullah and Faulkner 2006; Jordan et al. 2007; Racchetti et al. 2011).
There is a lack of full quantitative understanding of factors controlling denitrification across different scales and landscape types (McClain et al. 2003; Groffman et al. 2009). Many studies of denitrification are not suitable for scaling measurements up to ecosystems because laboratory measurements, at scales of centimeters or less, are not able to capture the range of in-situ ecosystem conditions (Davidson and Seitzinger 2006). There is much still to be learned about the controlling factors of denitrification from comparisons of measurements across multiple ecosystems and scales, but a review of existing research suggests that interactions among hydrology, geomorphology, and nitrogen loading are the dominant controls of denitrification rates (Seitzinger et al. 2006). These controls act at scales of a few millimeters up to the landscape scale (McClain et al. 2003). Which control, and thus what scale, is more important for controlling denitrification in specific wetland systems is difficult to establish or predict.
The water quality improvement functions in wetlands, specifically Nr removal, were identified as important resource management knowledge gaps based on a combination of stakeholder engagement examining what the Tampa Bay regional community value and a review of existing information (Russell et al. 2011). We compared regional landcover types to local, site-scale characteristics with regard to changes in denitrification potentials in a set of wetlands. Sites were grouped by wetland type but selected along a range of land use in their local drainage basin in order to explore the relative importance of landscape and site scale factors for determining removal rates. We hypothesized that at the landscape scale, freshwater wetlands, and within that group those wetlands receiving drainage from agricultural areas, would have generally higher denitrification potentials. We also expected to find higher denitrification rates in freshwater wetlands experiencing generally wetter soil conditions and higher available nutrient concentration regimes. Our results will help inform management decisions by providing a preliminary assessment of the capacity of wetlands to remove non-point source nitrogen loads based on watershed land cover type and by determining what scale of factors are more influential on that capacity.
Methods
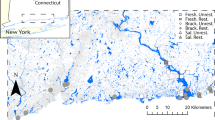
Twenty-seven wetland sites were identified in the southeastern section of the Tampa Bay drainage basin which includes a mix of urban, agricultural, and natural lands (Fig. 1 and Online Resources 1). To include the wide range of possible landscape and site-scale attributes, wetlands were selected to be representative of a wide range of conditions based on a combination of wetland habitat type and drainage basin land cover. Wetland habitat classes in the Florida Department of Transportation’s Florida Land Use/Land Cover Classification System (FLUCCS) GIS data layer for 2006 (http://www.swfwmd.state.fl.us/data/gis/layer_library/) were grouped into one of three wetland categories adapted from the Cowardin et al. (1979) classification system (Table 1 and Online Resource 2): Palustrine Emergent Marsh (primarily freshwater marsh or wet prairie, and identified as “PEM”); Palustrine Forested wetland (primarily wetland hardwood forest, cypress, or river/lake swamp, and identified as “PFO”); and Estuarine Shrub-Scrub (primarily mangroves, and identified as “ESS”). Also, the FLUCCS GIS data layer for 2006 was summarized within the National Hydrography Dataset (NHD) drainage basins (http://nhd.usgs.gov/) associated with each wetland to estimate the amount of agriculture (e.g., row crops, citrus groves, tree nurseries), urban (e.g., high, medium and low density residential; commercial and industrial; transportation, communication, and utilities), or natural (e.g., open land, rangeland, upland forests) land draining to each wetland site. For ease of reference, site names reflect sites with higher relative percentages of agricultural (A), urban (U) or natural (N) land types in their drainage basins. Land cover percentages in PEM and PFO site watersheds were refined using high-resolution LiDAR elevation data (details in Sherwood and Greening 2014) to more accurately delineate the part of the watersheds directly upstream of the freshwater wetland sites. The intertidal mangrove dominated wetland watershed have hard to identify elevation changes due to the low elevation near the coast. These mangrove wetland upstream drainage basins were identified using the NHD+ V2 drainage network (http://www.horizon-systems.com/nhdplus/NHDPlusV2_home.php) and so may contain a small amount of downstream, mostly natural land cover in their results.
Wetland sampling sites to the east of Tampa Bay, Florida (Insets) USA. ESS, PEM, and PFO sites are donated by triangles, circles, and squares respectively. Letters (A, U, and N) denote relatively higher percentages of agricultural, urban, or natural in upstream site drainage basins (See Online Resource 1 for detailed maps of each site)
Triplicate soil sample cores (4.5 cm diameter taken to 10 cm depth) were randomly extracted at each wetland site in August 2010, and again in May and October of 2011. Denitrification potentials were estimated under non-limiting conditions using a denitrification enzyme activity (DEA) assay. Denitrification enzyme activity assay incubations of the homogenized soil cores mixed with deionized water were completed using the acetylene block technique developed by Smith and Tiedje (1979), as modified by Groffman et al. (1999) with an excess of nitrate and an inhibitor of enzyme synthesis added to produce denitrification potential measurements. The DEA solution, in the reaction vessel, contained, 1.4 mg KNO3, 1.0 mg glucose and 0.05 mg chloramphenicol per gram wet weight of soil. This short-term anaerobic assay is based on the ability of acetylene to block the reduction of nitrous oxide (N2O) to nitrogen gas (N2) and gives an estimate of denitrification potential under amended, non-limiting conditions. Denitrification potential rates were averaged across the three sampling dates which span the warmer months of the growing season. Denitrification enzyme activity rates were deemed acceptable if a steadily increasing N2O concentration was observed through time during incubations. The DEA rates were estimated using the linear portion of the curve. Soil samples were analyzed for physical and chemical parameters using standard methods (following Lane et al. 2015) including: soil moisture (dry weight (g)), nitrate (NO3) (mg/kg), ammonia (NH4) (mg/kg), %N, %C, C:N, total phosphorus (soil TP, mg/kg), pH, and conductivity (μS/cm).
Porewater was collected from each wetland site to better resolve the microbial environment. A porewater sample was collected at each site from a depth of 30 cm and within 1 meter of core samples using a sipper tube apparatus (McKee et al. 1988). Within each site, one unfiltered porewater aliquot was collected for in-situ measurements of pH, salinity, and temperature. The second unfiltered aliquot was collected for measurements of total nitrogen (TN) and total phosphorus (TP). The third aliquot was filtered (0.45 μm membrane) and analyzed for dissolved nutrients (ortho-phosphate, NO3, NH4). The water samples were shipped overnight, along with the soils, on ice to the laboratory for analyses that included: total (TOC, mg L−1) and dissolved organic carbon (DOC in mg L−1), color (PTU), pH, alkalinity (mg L−1CaCO3), TP (μg L−1), TN (μg L−1), orthophosphate (μg L−1), NH4 (μg L−1), NOx (μg L−1), and dissolved inorganic nitrogen (DIN, μg L−1). TOC and DOC were preserved with H2PO4 and measured by UV-sodium persulfate oxidation and nondispersive infrared (NDIR) detection with the Dohrmann Phenix 8000 TOC analyzer (5310-C; APHA 1998). Color was measured with a Perkin Elmer UV–vis Model 20S spectrometer (Perkin Elmer, Waltham, MA, USA) and alkalinity following EPA Method 130.2 (USEPA 1979). Unfiltered and filtered (0.45 μm pore membrane) samples for N and P species were preserved frozen. Nutrient samples were analyzed using a Lachat flow-injection analyzer 8000 with QuickChem methods (Lachat Instruments, Loveland, CO, USA). The unfiltered subsamples were digested using the persulfate method for TN and TP (APHA 1998). Concentration of TP was determined by the molybdate-ascorbic acid method (10–115–01-1 B). TN and dissolved NOx were analyzed using the cadmium reduction method (10–107-04-1O), and NH4 was analyzed with the phenolate method (10–107–06-1-F).
Overall differences in site characteristics among types of wetland were explored using ANOVA. Then, the relationship between individual soil and pore water site characteristics and DEA rates across wetland types and land cover gradients were explored using simple linear regressions in R (Core Team 2013) to determine if site scale factors and DEA rates varied consistently among wetland types and along gradients of upstream land use.
The range in DEA rates for each type of wetland (e.g., PEM, PFO, and ESS) were summarized for future use by resource managers. Soil DEA rates were then regressed against drainage basin land cover characteristics, soil characteristics, and pore water quality using step-wise multiple linear regression analysis in R to determine if landscape-scale or site-scale factors explained more of the variability in DEA across individual sites regardless of wetland type.
Results
Soil characteristics were not significantly different (ANOVA p > 0.1) among palustrine emergent marsh (PEM), palustrine forested (PFO) and estuarine shrub-scrub wetlands (ESS), although ESS wetlands generally had more soil water content (p = 0.09). Likewise, there was no significant difference among mean DEA rates in PEM (mean ± SE, 0.24 ± 0.07 μg N gdw−1 h−1), PFO (mean ± SE 0.26 ± 0.10 μg N gdw−1 h−1), and ESS (mean ± SE 0.10 ± 0.01 μg N gdw−1 h−1) sites. Most PEM and PFO sites had DEA rates less than 0.5 μg N gdw−1 h−1 with only four sites, two with the highest percentages of agricultural (PEMA02 = 80.6% and PFOA04 = 77.8%) and two with relatively large percentages of urban (PEMU03 = 37% and PFOU08 = 62%) land in their upstream drainage basin, having higher rates (Table 2). ESS sites had DEA rates similar to the majority lower DEA rates at PEM and PFO sites. Site results, thus, tended to cluster into two groups based on observed DEA rates above or below 0.5 μg N gdw−1 h−1.
Higher mean DEAs (>0.5 μg N gdw−1 h−1) tend to occur in wetland soils with higher soil extractable NO3 (>4 mg kg dw−1) (linear regression p < 0.001 r2 = 0.58), higher percent organics (LOI > 40%) (linear regression p < 0.001 r2 = 0.38), and in wetland soils with lower percent natural lands in their drainage basin (%Nat < 40%) but this last factor was statistically uncorrelated at the 95% confidence level (linear regression p < 0.1 r2 = 0.11) (Fig. 2). Percent soil carbon and nitrogen covaried with LOI (r2 = 0.82 and 0.66 respectively). Porewater concentrations did not correlate with DEA rates.
Denitrification enzyme activity compared to extractable soil nitrate (NO3), percent loss on ignition (LOI) and percent natural land in the upstream drainage basin. Higher DEA rates indicated by larger circles were expected and found in the upper right-hand quarter of this plot where high percentages of agricultural and urban development, likely high NO3 loading rates, and high percentages of soil OM are present
After running stepwise multiple linear regression two soil and one drainage basin characteristics (Soil extractable NO3, Soil Percent N, and Percent Agriculture) were identified as having significant (p < 0.001, r2 = 0.77) correlations with mean DEA rates (μg N gdw−1 h−1) (Table 3).
To facilitate comparisons with other studies’ DEA measurements at wetland sites in similar landscapes, we converted our volumetric rates (μg N gdw−1 h−1) into areal rates (g N m−2 d−1) by using soil bulk densities and assuming the top 10 cm of soil is the active zone for denitrification (Lane et al. 2015), although Lane notes that others have used 30 cm or deeper. After running stepwise multiple regression with these areal rates, three soil characteristics (Soil extractable NO3, Soil Percent N, and Loss on Ignition) were identified as having significant (p < 0.001, r2 = 0.74) correlations with average site DEA areal rates (g N m−2 d−1) (Table 4).
Discussion
Potential denitrification rates varied among wetland sites, but fell within expected ranges. Our measurements range between 0.01 g N m−2 d−1 at a palustrine forested site (PFONO2) surrounded by and receiving drainage from a mostly natural (70.1%) upstream area and 1.69 g N m−2 d−1 at a marsh site (PEMU03) which receives drainage directly from an urban area and golf course, remains continuously wet due to a weir, and had one of the highest soil bulk densities. Similar patterns were seen by Gale et al. (1993), who reported a denitrification rate for natural wetlands as 0.070 g N m−2 d−1 while for constructed wetlands receiving wastewater runoff, it was 0.917 g N m−2 d−1. The average of our palustrine wetlands (0.26 g N m−2 d−1) compares well to the average DEA rate of 0.365 g N m−2 d−1 (Lane et al. 2015) measured in geographically isolated wetlands located in the similar geographic area as our study. Our measurements also compare well to DEA rates of 0.526 g N m−2 d−1 measured in riparian forest wetlands (Pinay et al. 1993), but our measurements fall on the low side of other palustrine wetlands (Lindau et al. 1991; Hanson et al. 1994; Seitzinger 1994).
Our DEA measurements in mangroves (ESS sites) averaged 0.09 μg N gdw−1 h−1. This converts to a relatively high average DEA rate of 0.13 g N m−2 d−1 in mangrove wetlands, but the measurements are bi-modal with four of the nine ESS sites having DEA > 0.1 g N m−2 d−1 and the rest having DEA < 0.06 g N m−2 d−1. These groupings within ESS sites are consistent with and strongly influenced by differences in soil bulk density with the calculations of higher areal rates corresponding to areas with high bulk densities. Denitrification in mangrove sediment is also constrained to the near surface layers so our use of 10 cm depth in the conversion from volumetric to areal rates may have resulted in an overestimation of areal DEA rates (Chiu et al. 2004). An adjustment to a conversion using a narrower active denitrification zone depth (~5 cm or less) at our mangrove sites would bring our converted to areal DEA rate results into range with other similar systems (0.0004–0.05 g N m−2 d−1) (Nedwell et al. 1994; Kristensen et al. 1998; Alongi et al. 2002; Lee and Joye 2006), but here we are more interested in the relative magnitudes among sites than in absolute magnitudes so we kept our 10 cm depth assumption to be consistent with our palustrine sites.
Confirming our hypotheses, we observed DEA rates that followed predicted patterns of higher DEA in wetlands receiving drainage from upstream agricultural or urban areas (Fig. 2). There is past evidence that wetland DEA rates tend to track with anthropogenic nutrient additions to the watershed. Seitzinger (1994) calculated that denitrification rates in cedar swamp sediments increased from less than or equal to 2.46 to 7.37 to 22.71 g N m−2 d−1 for undisturbed wetlands, wetlands with intermediate anthropogenic inputs, and wetlands with significant anthropogenic inputs, respectively. The denitrification rates in a freshwater forested wetland downstream from an undeveloped forested area ranged from 0.48–1.63 g N m−2 d−1, while those downstream from a highly developed, residential development with septic systems ranged from 0.71–3.85 g N m−2 d−1 (Hanson et al. 1994). Lindau et al. (1991) measured a 67% increase in denitrification rate from 0.27 to 0.45 g N m−2 d−1 when experimentally increasing the nitrate concentration from 100 to 300 kg N ha−1 to simulate nutrient loading from anthropogenic sources. In a freshwater forested and a marsh wetland, Dodla et al. (2008) artificially treated sediment with increased concentrations of NO3 to simulate anthropogenic input and found that DEA rates increased from 0.005 to 0.4425 and from 0.0075 to 0.4225 (g N m−2 d−1), respectively. The wetlands we sampled generally had DEA rates between 0.01 and 0.2 g N m−2 d−1, while a select group of sites were an order of magnitude higher at around 1.0 g N m−2 d−1. The wetlands in this study with higher DEA rates tended to be adjacent to active agricultural row crops, dense urban residential land use or golf courses with little natural buffer between those activities and the wetland to remove nutrients loads from runoff (See maps in Online Resource 1). Along with potentially higher nutrient loads, sites with higher DEA rates also tended to have relatively wet soils compared to others which would likely lead to anoxic subsurface conditions necessary for denitrification (Ullah and Faulkner 2006). These results support our hypotheses that higher denitrification rates would be measured in freshwater wetlands experiencing generally wetter soil conditions and higher available nutrient concentration regimes. Biochemical scale factors, such as soil moisture, have been shown to be as important as the position of sites within the landscape for controlling potential denitrification rates (Ullah and Faulkner 2006).
Not all sites with high percentages of agricultural or urban land in their drainage basins had elevated DEA rates, suggesting that local site characteristics may play a more influential role than upstream drainage basin land use in this area. Local conditions may influence current DEA rates while antecedent conditions or upstream loading history may influence microbial community structure and function including their ability to process nitrogen inputs via denitrification. It could be that chronic increases in nutrient loading from human activities, such as fertilizer application to urban and agricultural lands in our studied wetlands with relatively high DEA rates, may alter the quality and delivery rates of organic matter inputs from upstream plant production enough to affect wetland soil microbial community structure or function (Marschner et al. 2003; Chu et al. 2007), but estimates of chronic nutrient or organic matter loading per watershed were not generated during this study. Our results do, however, suggest that DEA is controlled by a mix of site-specific characteristics and landscape scale upstream land use, but more work will be needed to tease out the interdependencies among these factors.
Inter-site variability in DEA rates was large within our wetlands (Table 2) resulting in no meaningful differences among wetland types, although sites with relatively higher percentages of agricultural and or urban land in their drainage basins tended to have higher DEA rates than natural wetlands. Some of this variability may stem from specific differences in human use of the land within the broad watershed land use categories, and assumed differences in nitrogen and organic matter loads we defined here (e.g., agricultural, urban and natural). Our wetlands had upstream agricultural activities that ranged from areas of pasture to row crops. These type of differences in upstream agricultural practices can influence denitrification rates in wetlands (Xue et al. 1999; Poe et al. 2003; Tanner et al. 2005; Ullah and Faulkner 2006) and streams (Inwood et al. 2005). Upstream urban areas of our wetlands included deep incised drainage ditches adjacent to residential single-family homes, overflow areas downstream of created lakes bordered by houses, and retention ponds receiving runoff from upstream golf course communities. Wetland denitrification rates respond to urban land use differences such as these (Gale et al. 1993; Mallin et al. 2002; Lewis et al. 2002; Kohler et al. 2004). The variability in DEA rates among sites within each land cover gradient could be reduced by focusing future sampling efforts within more specific land use conditions only identifiable during site visits. Additional intra-site sampling may also better control for the large intra-site variability found during this work leading to better separation of factors responsible for inter-site variability.
Intra-site variability reduced our ability to separate average DEA rates along gradients of explanatory variables. Microtopographic related differences in water content, organic matter content, and nutrient availability can increase DEA variability over short spatial scales (Bruland et al. 2006). In a waste water treatment wetland, Kjellin et al. (2007) found that intra-site variability in soil nitrogen and water residence times explained differences in DEA rates. Spatial variability in DEA rates and autocorrelation with wetland soil characteristics is influenced by the scale of analyses and wetland development history which make extrapolations from our point measurements to field scales unjustifiable (Orr et al. 2014). Temporal changes in environmental conditions may also have increased our within site variability (Wigand et al. 2004). Intra-site variability made comparisons of DEA rates to explanatory variables less clear due to lack of sampling power and/or the course characterization of upstream landscapes into one of just three categories.
Several soil attributes trended with elevated or low DEA rates. Consistent with other studies, our high DEA rates were found in soils with high organic matter content (Seitzinger 1994; White and Reddy 1999; Lin et al. 2007; Lane et al. 2015), high percentages of carbon and nitrogen (Gale et al. 1993; Lin et al. 2007; Lu et al. 2009), and soil extractable nitrate (White and Reddy 1999; Dodla et al. 2008; Lane et al. 2015). Dodla et al. (2008) measured increased potential denitrification rates two to six times higher as soil extractable nitrate concentrations increased from 2 to 10 mg NO3-N l−1 and identified a positive correlation between organic carbon and potential denitrification rates. Soil attributes serve as a good indicator for estimating whether a wetland will have generally high or low DEA rates and may provide useful information for future research site selection. Soil attribute or condition assessments might also help managers select sites with higher potential functional rates for wetland creation or restoration to mitigate increased nutrient loading downstream of urban and agricultural lands. Changes in soil attributes could also serve as an indicator of changes in potential ecosystem denitrification function at restored sites.
Porewater nitrate concentrations did not correlate with DEA rates and as such may not be the best indicator of the biological community’s capacity to remove nitrogen since reduced concentrations may be found in both low DEA wetlands and high DEA wetlands. Low porewater nitrate concentrations could be indicative of an area receiving little nitrate loading or an area receiving larger loads but with a microbial community acclimated to this nitrate regime that is processing the more available porewater nitrate as fast as it is loaded (Jordan et al. 2007; Ligi et al. 2014). Nutrient loading data would have allowed us to replace the spot measurement of pore water nitrate with an explanatory variable more independent of the response variable. Microbial communities may not be able to react to rapidly changing porewater nitrate concentrations, but their potential denitrification capacity should relate to generally higher or lower chronic nitrogen loads that might be reflected by soil attributes.
Soil attributes are not as dynamic as porewater nutrient concentrations and so might serve as predictive indicators of the status of the soil microbial community and general levels of nutrient availability for denitrification (Enwall et al. 2005; Dodla et al. 2008). Previous studies have shown potential denitrification to be positively correlated with soil nitrate and organic carbon (Robertson et al. 1988; Davidsson and Leonardson 1997; Ullah et al. 2005; Lin et al. 2007). Several soil characteristics and one landscape factor correlated positively with DEA rates in our wetlands (Step-wise multiple regression p < 0.01). Soil extractable nitrate, percent nitrogen, and percent agricultural land in upstream basin combined to explain a majority (r2 = 0.77) of the variability in average DEA rates among sites. Percent nitrogen was strongly correlated with percent organic matter and percent carbon and either of these might serve as substitutes in the regression model. Also, percent agricultural land has a strong negative relationship with percent natural land in the study area so one should be careful to not interpret model results as causality. The bi-modal grouping of results left our model lacking data for wetlands with mid-range soil nitrate and organic matter content. Our a-priori selection of wetland sites along gradients of agricultural, urban, and natural land using GIS data and our need to find upstream drainage basins with clearly dominant land types to increase our resulting model’s range of applicability limited our ability to find similar wetland conditions within each group. It is clear from our results that sites even with similar percentages of our broadly categorized land uses can behave functionally differently from each other, and this is probably a result of nuances in sites’ upstream land use, local differences in soil attributes, as well as topographic and hydrologic differences.
Near surface water inundation of the soil fosters the setup of an anaerobic layer that should be conducive to denitrification, and past inundation history does affect the magnitude of nitrate removal via denitrification (Venterink et al. 2002). The wetlands with the highest DEA rates in this study tended to be relatively wet with near surface or standing water regularly present. Mangrove systems were the exception to this, being regularly flooded by the tide but having lower DEA rates common in these marine influenced systems (Rivera-Monroy and Twilley 1996; Corredor et al. 1999). It is still unclear, however, how the dynamics of wetland inundation affect microbial community size or structure (Ishida et al. 2006; Mentzer et al. 2006). Regular pulsing of soil water depth may increase the volume of soil where denitrifiers are in close proximity to nitrifiers, thereby allowing the microbial community responsible for denitrification to expand their presence within the soil column (Tanner et al. 1999). A larger community of denitrifiers, when given the non-limiting conditions of the DEA incubations, may have higher potential denitrification rates than a community restricted to a narrow band of soil.
It remains challenging to determine the ultimate drivers of denitrification capable microbial communities at different scales (Enwall et al. 2010; Bru et al. 2011) and further work will be needed before we can make confident recommendations to managers on how to consider the different scale factors controlling the important wetland function of denitrification in managed lands. Denitrifying communities have been shown to respond to combinations of inherent and manageable soil properties at the field scale (Enwall et al. 2010). While there seems to be a general consensus that local scale soil characteristics influence denitrification rates in wetlands it is unclear if changing upstream landscapes and resulting organic matter and nutrient loads cause chronic shifts in denitrifying community structure as wetland conditions change or if the microbial community can adjust their functional response without a change in microbial community structure (Balser et al. 2006). Microbial process rates related to nitrogen transformation may be more related to microbial community structure than environmental conditions (Balser and Firestone 2005). Adaptation of the microbial community to a wider temporal range of environmental conditions may allow existing community structures to better adjust their denitrification related functions to levels appropriate for a given landscape, while a microbial community adapted to a consistently narrow range of environmental conditions would require more long-term community structure changes to respond to acute changes or pulses in nitrogen availability.
Our results confirm that while local scale soil conditions, such as soil extractable nitrate, percent soil nitrogen, and possibly soil water content, may be dominant indicators for estimating denitrification potential in freshwater wetlands, landscape scale characteristics, such as the relative percentage of agricultural land use in upstream areas, also contribute some additional explanatory power for estimating denitrification potentials and may be the ultimate driver of soil characteristics and microbial community structure. Correlation-based approaches to predicting denitrification potentials can only take you so far, however. A better understanding of how microbial biogeochemistry is affected by local environmental factors and microbial community structure (e.g., elucidated using genomics etc.) might lead to improved mechanistically based models that link wetland and upstream land use characteristics to spatial and temporal variation in net nitrogen processing rates. Recent advances in environmental genomics should help inform this topic by providing a new set of tools to answer unresolved research questions about microbial structure and function (Wallenstein et al. 2006; Nocker et al. 2007).
References
Alongi D, Trott L, Wattayakorn G, Clough B (2002) Below-ground nitrogen cycling in relation to net canopy production in mangrove forests of southern Thailand. Marine Biology, Springer-Verlag 140:855–864
Anderson B, Bartlett K, Frolking S, Hayhoe K, Jenkins J, Salas W (2010) Methand and nitrous oxide emissions from natural sources. EPA 430-R-10-001. US EPA, Washington, DC
APHA (1998) Standard methods for the examination of water and waste water, 20th edn. American Public Health Association, Washington, DC
Balser TC, Firestone MK (2005) Linking microbial community composition and soil processes in a California annual grassland and mixed-conifer forest. Biogeochemistry, Kluwer Academic Publishers 73:395–415
Balser TC, McMahon KD, Bart D, Bronson D, Coyle DR, Craig N, Flores-Mangual ML, Forshay K, Jones SE, Kent AE, Shade AL (2006) Bridging the gap between micro - and macro-scale perspectives on the role of microbial communities in global change ecology. Plant and Soil, Kluwer Academic Publishers 289:59–70
Boon PI, Sorrell BK (1991) Biogeochemistry of billabong sediments. I. the effect of macrophytes. Freshwater Biology, Blackwell Publishing Ltd 26:209–226
Bru D, Ramette A, Saby NPA, Dequiedt S, Ranjard L, Jolivet C, Arrouays D, Philippot L (2011) Determinants of the distribution of nitrogen-cycling microbial communities at the landscape scale. The ISME Journal, International Society for Microbial Ecology 5:532–542
Bruland G, Richardson C, Whalen S (2006) Spatial variability of denitrification potential and related soil properties in created, restored, and paired natural wetlands. Wetlands, Springer Netherlands 26:1042–1056
Brush GS (2009) Historical land use, nitrogen, and coastal eutrophication: a paleoecological perspective. Estuaries and Coasts 32:18–28
Chiu C-Y, Lee S-C, Chen T-H, Tian G (2004) Denitrification associated N loss in mangrove soil. Nutrient Cycling in Agroecosystems. Netherlands, Kluwer Academic Publishers 69:185–189
Chu H, Lin X, Fujii T, Morimoto S, Yagi K, Hu J, Zhang J (2007) Soil microbial biomass, dehydrogenase activity, bacterial community structure in response to long-term fertilizer management. Soil Biology and Biochemistry, Elsevier 39:2971–2976
Comer PJ, Goodin K, Tomaino A, Hammerson G, Kittel G, Menard S, Nordman C, Pyne M, Reid M, Sneddon L, Snow K (2005) Biodiversity values of geographically isolated wetlands in the United States. NatureServe, Arlington
Corredor JE, Morell JM, Bauza J (1999) Atmospheric nitrous oxide fluxes from mangrove sediments. Marine Pollution Bulletin. Great Britain, Pergamon 38:473–478
Cowardin LM, Carter V, Golet FC, T. LaRoe E (1979) Classification of wetlands and deepwater habitats of the United States. FWS/OBS-79/31. Washington, D.C., US Department of the Interior and US Fish and Wildlife Service
Davidson EA, Seitzinger S (2006) The enigma of progress in denitrification research. Ecological Applications, Ecological Society of America 16:2057–2063
Davidsson T, Leonardson L (1997) Seasonal dynamics of denitrification activity in two water meadows. Hydrobiologia. Belgium, Kluwer Academic Publishers 364:189–198
Dodla SK, Wang JJ, DeLaune RD, Cook RL (2008) Denitrification potential and its relation to organic carbon quality in three coastal wetland soils. Science of the Total Environment, Elsevier 407:471–480
Enwall K, Philippot L, Hallin S (2005) Activity and composition of the denitrifying bacterial community respond differently to long-term fertilization. Applied and Environmental Microbiology, American Society for Microbiology 71:8335–8343
Enwall K, Throbäck IN, Stenberg M, Söderström M, Hallin S (2010) Soil resources influence spatial patterns of denitrifying communities at scales compatible with land management. Applied and Environmental Microbiology, American Society for Microbiology 76:2243–2250
Gale PM, Dévai I, Reddy KR, Graetz DA (1993) Denitrification potential of soils from constructed and natural wetlands. Ecological Engineering. Amsterdam, Elsevier Science Publishers B.V 2:119–130
Greening H, Janicki A (2006) Toward reversal of eutrophic conditions in a subtropical estuary: water quality and seagrass response to nitrogen loading reductions in Tampa Bay, Florida, USA. Environmental Management, Springer-Verlag 38:163–178
Greening HS, Cross LM, Sherwood ET (2011) A multiscale approach to seagrass recovery in Tampa Bay, Florida. Ecological Restoration 29:82–93
Groffman P, Holland E, Myrold D, Robertson G, XiaoMing Z, Coleman D, Bledsoe C, Sollins P (1999) Denitrification. Standard soil methods for long-term ecological research. 272–288
Groffman PM, Butterbach-Bahl K, Fulweller RW, Gold AJ, Morse JL, Stander EK, Tague C, Tonitto C, Vidon P (2009) Challenges to incorporating spatially and temporally explicit phenomena (hotspots and hot moments) in denitrification models. Biogeochemistry 93(1):49–77
Hanson GC, Groffman PM, Gold AJ (1994) Symptoms of nitrogen saturation in a riparian wetland. Ecological Applications, Ecological Society of America 4:750–756
Hoos AB, McMahon G (2009) Spatial analysis of instream nitrogen loads and factors controlling nitrogen delivery to streams in the southeastern United States using spatially referenced regression on watershed attributes (SPARROW) and regional classification frameworks. Hydrological Processes, John Wiley & Sons, Ltd 23:2275–2294
Howarth RW (2008) Coastal nitrogen pollution: a review of sources and trends globally and regionally. Harmful Algae 8:14–20
Inwood SE, Tank JL, Bernot MJ (2005) Patterns of denitrification associated with land use in 9 midwestern headwater streams. Journal of the North American Benthological Society, The Society for Freshwater Science 24:227–245
Ishida CK, Kelly JJ, Gray KA (2006) Effects of variable hydroperiods and water level fluctuations on denitrification capacity, nitrate removal, and benthic-microbial community structure in constructed wetlands. Ecological Engineering, Elsevier 28:363–373
Jordan S, Stoffer J, Nestlerode J (2011) Wetlands as sinks for reactive nitrogen at continental and global scales: a meta-analysis. Ecosystems, Springer-Verlag 14:144–155
Jordan TE, Andrews MP, Szuch RP, Whigham DF, Weller DE, Jacobs AD (2007) Comparing functional assessments of wetlands to measurements of soil characteristics and nitrogen processing. Wetlands, Springer Netherlands 27:479–497
Kjellin J, Hallin S, Wörman A (2007) Spatial variations in denitrification activity in wetland sediments explained by hydrology and denitrifying community structure. Water Research, Elsevier 41:4710–4720
Kohler EA, Poole VL, Reicher ZJ, Turco RF (2004) Nutrient, metal, and pesticide removal during storm and nonstorm events by a constructed wetland on an urban golf course. Ecological Engineering, Elsevier 23:285–298
Kristensen E, Jensen MH, Banta GT, Hansen K, Holmer M, King GM (1998) Transformation and transport of inorganic nitrogen in sediments of a southeast Asian mangrove forest. Aquatic Microbial Ecology 15:165–175
Lane CR, Autrey BC, Jicha T, Lehto L, Elonen C, Seifert-Monson L (2015) Denitrification potential in geographically isolated wetlands of North Carolina and Florida, USA. Wetlands 35(3):459–471
Lee RY, Joye SB (2006) Seasonal patterns of nitrogen fixation and denitrification in oceanic mangrove habitats. Marine Ecology Progress Series 307:127–141
Lewis MA, Boustany RG, Dantin DD, Quarles RL, Moore JC, Stanley RS (2002) Effects of a coastal golf complex on water quality, periphyton, and seagrass. Ecotoxicol Environ Saf 53:154–162
Ligi T, Truu M, Truu J, Nõlvak H, Kaasik A, Mitsch WJ, Mander Ü (2014) Effects of soil chemical characteristics and water regime on denitrification genes (nirS, nirK, and nosZ) abundances in a created riverine wetland complex. Ecological Engineering, Elsevier 72:47–55
Lin Y-F, Jing S-R, Lee D-Y, Chang Y-F, Shih K-C (2007) Nitrate removal and denitrification affected by soil characteristics in nitrate treatment wetlands. Journal of Environmental Science and Health, Part A, Taylor & Francis 42:471–479
Lindau CW, DeLaune RD, Jiraporncharoen S, Manajuti D (1991) Nitrous oxide and dinitrogen emissions from Panicum hemitomon S. freshwater marsh soils following addition of N-15 labelled ammonium and nitrate. Journal of Freshwater Ecology, Taylor & Francis 6:191–198
Lu S, Hu H, Sun Y, Yang J (2009) Effect of carbon source on the denitrification in constructed wetlands. Journal of Environmental Sciences 21:1036–1043
Mallin MA, Ensign SH, Wheeler TL, Mayes DB (2002) Pollutant removal efficacy of three wet detention ponds. Journal of Environmental Quality. Madison, WI, American Society of Agronomy, Crop Science Society of America, and Soil Science Society 31:654–660
Marschner P, Kandeler E, Marschner B (2003) Structure and function of the soil microbial community in a long-term fertilizer experiment. Soil Biology and Biochemistry, Pergamon 35:453–461
McClain ME, Boyer EW, Dent LC, Gergel SE, Grimm NB, Groffman PM, Hart SC, Harvey JW, Johnston CA, Mayorga E, McDowell WH, Pinay G (2003) Biogeochemical hot spots and hot moments at the interface of terrestrial and aquatic ecosystems. Ecosystems 6(4):301–312
McKee KL, Mendelssohn IA, Hester MW (1988) Reexamination of pore water sulfide concentrations and redox potentials near the aerial roots of Rhizophora mangle and Avicennia germinans. American Journal of Botany 75:1352–1359
Mentzer JL, Goodman RM, Balser TC (2006) Microbial response over time to hydrologic and fertilization treatments in a simulated wet prairie. Plant and Soil, Kluwer Academic Publishers 284:85–100
Nedwell DB, Blackburn TH, Wiebe WJ (1994) Dynamic nature of the turnover of organic carbon, nitrogen and Sulphur in the sediments of a Jamaican mangrove forest. Marine Ecology Progess Series 110:223–231
Nocker A, Burr M, Camper AK (2007) Genotypic microbial community profiling: a critical technical review. Microbial Ecology 54(2):276–289
Orr CH, Predick KI, Stanley EH, Rogers KL (2014) Spatial autocorrelation of denitrification in a restored and a natural floodplain. Wetlands, Springer Netherlands 34:89–100
Pinay G, Roques L, Fabre A (1993) Spatial and temporal patterns of denitrification in a riparian Forest. Journal of Applied Ecology, British Ecological Society 30:581–591
Poe AC, Piehler MF, Thompson SP, Paerl HW (2003) Denitrification in a constructed wetland receiving agricultural runoff. Wetlands, Springer Netherlands 23:817–826
Poor ND, Raymond Pribble J, Schwede DB (2013) Application of watershed deposition tool to estimate from CMAQ simulations the atmospheric deposition of nitrogen to Tampa Bay and its watershed. Journal of the Air & Waste Management Association 63(1):100–114. https://doi.org/10.1080/10962247.2012.739109
Racchetti E, Bartoli M, Soana E, Longhi D, Christian RR, Pinardi M, Viaroli P (2011) Influence of hydrological connectivity of riverine wetlands on nitrogen removal via denitrification. Biogeochemistry, Springer Netherlands 103:335–354
Reddy KR, DeLaune RD (2008) Biogeochemistry of wetlands: science and applications. CRC Press, Boca Raton
Rheinhardt RD, Brinson MM (2002) An evaluation of North Carolina Department of Transportation wetland mitigation sites: selected case studies. Technical Report: FHWA/NC/2002–009, CTE/NCDOT Joint Environmental Research Program
Rivera-Monroy VH, Twilley RR (1996) The relative role of denitrification and immobilization in the fate of inorganic nitrogen in mangrove sediments (Terminos lagoon, Mexico). Limnology and Oceanography, Association for the Sciences of Limnology and Oceanography, Inc 41:284–296
Robertson LA, van Niel EWJ, Torremans RAM, Kuenen JG (1988) Simultaneous nitrification and denitrification in aerobic Chemostat cultures of Thiosphaera pantotropha. Appl Environ Microbiol 54:2812–2818
Russell M, Rogers J, Jordan S, Dantin D, Harvey J, Nestlerode J, Alvarez F (2011) Prioritization of ecosystem services research: Tampa Bay demonstration project. Journal of Coastal Conservation, Springer Netherlands 15:647–658
Seitzinger S, Harrison JA, Böhlke JK, Bouwman AF, Lowrance R, Peterson B, Tobias C, Drecht GV (2006) Denitrification across landscapes and waterscapes: a synthesis. Ecological Applications, Ecological Society of America 16:2064–2090
Seitzinger SP (1994) Linkages between organic matter mineralization and denitrification in eight riparian wetlands. Biogeochemistry. Netherlands, Kluwer Academic Publishers 25:19–39
Sherwood ET, Greening HS (2014) Potential impacts and management implications of climate change on Tampa Bay estuary critical coastal habitats. Environmenal Management, Springer 53(2)
Sherwood ET, Greening AS, Janicki AJ, Karlen DJ (2015) Tampa Bay estuary: monitoring long-term recovery through regional partnerships. Regional Studies in Marine Science. https://doi.org/10.1016/j.rsma.2015.05.005l
Smith MS, Tiedje JM (1979) Phases of denitrification following oxygen depletion in soil. Soil Biology and Biochemistry. Great Britain, Pergamon Press, Ltd 11:261–267
Tanner CC, D'Eugenio J, McBride GB, Sukias JPS, Thompson K (1999) Effect of water level fluctuation on nitrogen removal from constructed wetland mesocosms. Ecological Engineering, Elsevier 12:67–92
Tanner CC, Nguyen ML, Sukias JPS (2005) Nutrient removal by a constructed wetland treating subsurface drainage from grazed dairy pasture. Agriculture, Ecosystems and Environment, Elsevier 105:145–162
Team C (2013) R: A language and environment for statistical computing. R Foundation for Staistical Computing. Vienna, Austria
Ullah S, Breitenbeck GA, Faulkner SP (2005) Denitrification and N2O emission from forested and cultivated alluvial clay soil. Biogeochemistry, Kluwer Academic Publishers 73:499–513
Ullah S, Faulkner SP (2006) Denitrification potential of different land-use types in an agricultural watershed, lower Mississippi valley. Ecological Engineering, Elsevier 28:131–140
USEPA (1979) Methods for chemical analysis of water and wastes. EPA/600/4–79/020. Washington, DC, US EPA
Venterink HO, Davidsson TE, Kiehl K, Leonardson L (2002) Impact of drying and re-wetting on N, P and K dynamics in a wetland soil. Plant and Soil. Netherlands, Kluwer Academic Publishers 243:119–130
Wallenstein MD, Myrold DD, Firestone M, Voytek M (2006) Environmental controls on denitrifying communities and denitrification rates: insights from molecualr methods. Ecological Applications 16(6):2143–2152
White JR, Reddy KR (1999) Influence of nitrate and phosphorus loading on denitrifying enzyme activity in Everglades wetland soils. Soil Science Society of America Journal. Madison, WI, Soil Science Society 63:1945–1954
Wigand C, McKinney RA, Chintala MM, Charpentier MA, Groffman PM (2004) Denitrification enzyme activity of fringe salt marshes in New England (USA). Journal of Environmental Quality. Madison, WI, American Society of Agronomy, Crop Science Society of America, and Soil Science Society 33:1144–1151
Xian G, Crane M (2005) Assessments of urban growth in the Tampa Bay watershed using remote sensing data. Remote Sensing of Environment, Elsevier 97:203–215
Xian G, Crane M, Su J (2007) An analysis of urban development and its environmental impact on the Tampa Bay watershed. Journal of Environmental Management 85:965–976
Xue Y, Kovacic DA, David MB, Gentry LE, Mulvaney RL, Lindau CW (1999) In situ measurements of denitrification in constructed wetlands. Journal of Environmental Quality. Madison, WI, American Society of Agronomy, Crop Science Society of America, and Soil Science Society of America 28:263–269
Author information
Authors and Affiliations
Corresponding author
Additional information
The views expressed in this (article/chapter/paper) are those of the author[s] and do not necessarily reflect the views or policies of the U.S. Environmental Protection Agency.
Rights and permissions
About this article
Cite this article
Russell, M., Fulford, R., Murphy, K. et al. Relative Importance of Landscape Versus Local Wetland Characteristics for Estimating Wetland Denitrification Potential. Wetlands 39, 127–137 (2019). https://doi.org/10.1007/s13157-018-1078-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13157-018-1078-6