Abstract
Mangrove ecosystems are important sources of goods and services to people, supporting ecological, biological, social and economic values. Nevertheless the scale of human-impact on mangroves in many countries has increased dramatically over the past years. Understanding their structure and plant composition is decisive for a proper design of conservation and management strategies, therefore we assessed patterns of floristic composition and structure together with their spatial and temporal environmental variability. Sixty-five 100 m2 permanent plots were established across the study area in order to cover the full range of structural and floristic variation. We sampled interstitial water properties (salinity, total dissolved solid, pH, oxygen dissolved and temperature) during the dry and the wet seasons. We ran non-metric multidimensional scaling ordinations (NMDS) to discern both floristic composition and structural changes related to interstitial water. We also ran multiple linear regression analyses, to determine the relationship between interstitial water with stand structural variables. Results showed a floristic gradient related to salinity while structure indicated by stand density and tree sizes, were explained primarily by salinity along the whole year; oxygen dissolved and pH were also significant in both wet and dry season while temperature was only important in the dry season.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Mangrove forests dominate most tropical coastlines representing one of the most diverse and productive environments in the world. These ecosystems are widely recognized as an important source of goods and services to people, supporting ecological, biological, social and economic values. They protect coastlines from hurricane impacts, floods and sediment trapping (Ewel et al. 1998; Kathiresan and Bingham 2001; Moreno et al. 2002; Liu et al. 2008; Walters et al. 2008) and support estuarine and near-shore marine productivity, in part by providing critical habitat for juvenile fish and through the export of nutrient-rich water (Stringer et al. 2010). Mangroves however, also receive nutrient inputs through tides and fresh water, but also from bird rookeries (Reef et al. 2010). Similarly to other wetlands, mangroves are usually viewed as open access areas with common rights; therefore, these zones are prompted to conflicts which hinder their conservation (Walters et al. 2008).
By 2000, mangroves covered approximately 137,760 km2; although mostly distributed in tropical and subtropical regions of the world in 118 countries, they also extend into temperate regions where they reach their geographical limits. However, around 75% of world’s mangroves are found in just 15 countries (Giri et al. 2011; Thomas et al. 2017).
The scale of human-impact on mangroves in many countries has increased dramatically since the 1960s, (Macintosh and Ashton 2002); current research asserts that mangrove forests decreased globally by 1646 km2 between 2000 and 2012 corresponding to a total loss of 1.97% (Hamilton and Casey 2016). Countries showing relatively high amounts of mangrove loss include: Myanmar, Malaysia, Cambodia, Indonesia and Guatemala. Nonetheless, Myanmar represents the current hotspot for mangrove deforestation, with a rate more than four times higher than the global average (Hamilton and Casey 2016).
Mangroves are particularly vulnerable to human-induced exploitation due to their valuable wood and fisheries resources, and to the fact they are located in coastline and peri-urban areas growing adjacent or in close proximity to cities, constantly converted into other land uses (Macintosh and Ashton 2002; Okello et al. 2013; Bosire et al. 2014). This scenario has led to the protection of these valuable ecosystems (Moreno-Casasola and López 2009; Clare et al. 2011). However, despite the increasing efforts on mangrove ecosystem conservation (Kairo et al. 2001; Lewis 2005; Flores-Verdugo et al. 2006; Zaldívar-Jiménez et al. 2010), these are not sufficient to halt their loss and degradation (Clare et al. 2011; Rodríguez-Zúñiga et al. 2013; Valderrama et al. 2014). Climate change has induced new uncertainties in environmental stability, increasing the vulnerability of critical mangrove habitats; however there is controversy about the resilience of these ecosystems to climate changes in the scientific literature, particularly to sea level rise (Alongi 2002; Yáñez-Arancibia et al. 2010).
As the awareness of the importance of socio-economic approaches in conservation strategies has augmented, the result is an increase of thorough research in mangrove ecosystems (Walters et al. 2008; Martín and Montes 2010; He et al. 2015). However, it is difficult to establish useful guidelines in decision-making for their proper management because mangrove ecosystems exhibit a myriad of different floristic, structural, environmental and socio-economic circumstances (Ewel et al. 1998). Thus, it is essential to generate regional scientific knowledge, based on vegetation conditions and socio-economic uses so that stakeholders have proper tools to implement effective management policies (Martín and Montes 2010; Clare et al. 2011).
Mangroves exhibit spatial variation in the presence and abundance of plant species, tree sizes and total biomass production across intertidal zones. This phenomenon is termed “zonation” which is the joint result of the potential dispersion of seedlings, the response of species to environmental factors and to intraspecific competition (Lugo and Snedaker 1974; López and Ezcurra 2002). Regarding environmental factors, species zonation can be related to climatic factors such as rainfall and runoff, inundation period, water salinity (Yañez-Arancibia and Lara-Domínguez 1999), geomorphological settings (sediment and soil characteristics) (Pellegrini et al. 2009), biological components (bioturbation, supply of nutrients, herbivory) (Lee 1999; Feller and Sitnik 2002) and human activities (microclimatic variations, imbalance in the solar radiation, soil compaction) (Cavalcanti et al. 2015).
In addition, since water is the main component holding mangroves, water’s physicochemical characteristics and level fluctuation play an important role shaping vegetation structure and species composition (Stringer et al. 2010). Some of the most important interstitial parameters to consider in groundwater are interstitial salinity, to explain patterns of plant distribution and growth (Flores et al. 2007), and soil pH changes that modify chemicals and metals bioavailability and its absorption by plants (Hernández and Pastor 2008).
A number of studies (Feller et al. 1999; Lee 1999; Méndez-Alonzo et al. 2012; Proffitt and Travis 2014; Cavalcanti et al. 2015; Costa et al. 2015) have shown that mangrove forests structural characteristics are determined by the interface of different natural and anthropic factors operating at temporal and spatial scales. Hence, different environmental factors affect mangrove ecosystem leading to variations in vegetation structure and species composition, eventually creating patterns of spatial diversity (Twilley et al. 1996).
Mangrove forests in the Cuyutlan Lagoon, in Pacific West-Central Mexico, due to its closeness to peri-urban areas, its northern portion faces the most intensive human-induced impacts while the southern portion is more related to conservation activities. Therefore, in this paper, we investigate the structure and floristic composition of mangrove vegetation including the environmental settings of the Cuyutlan Lagoon. We sought to answer the following questions: (i) is there a gradient of structural and floristic complexity, according to anthropic activities in the study area? If so, (ii) are these structural and compositional differences related to the environmental conditions of the interstitial water? We hypothesize that: i. structural complexity decreases from southeast to northwest of the Cuyutlan Lagoon, likely related to anthropic impact as the southernmost part of the lagoon is the least disturbed; ii. interstitial water characteristics, particularly salinity, explain structural complexity and species composition of mangrove vegetation.
Materials and Methods
Study Area
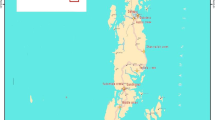
The present research was carried out in the Cuyutlan Lagoon, located in the state of Colima (18° 56′ - 19° 03’N, 104° 00′ - 104° 19’ W) (Contreras-Espinoza 1985), a large low relief coastal lagoon with ca. 7200 ha in Pacific West-Central Mexico, fringed by mangrove forests (Fig. 1a). The climate in this region is sub-wet and semidry-warm with summer rains. Average annual temperature is 26 °C, with a maximum of 28 °C and a minimum of 22 °C, annual precipitation ranges from 800 to 1200 mm (García 2004).
The Cuyutlan Lagoon is surrounded by mangrove vegetation in different conservation and successional status, with a diverse environmental heterogeneity with different land uses such as: sea salt production, agriculture, livestock, fishing and industrial activities (e.g. liquid gas storage, port operations, electricity production) (CEC 2016). The lagoon is connected, in its utmost northwestern portion, to the Pacific Ocean by a 250 m-wide mouth called Canal Tepalcates, and a 80 m-wide mouth called Canal Ventanas (Mellink and Riojas-López 2007; Torres and Quintanilla-Montoya 2014). The town of Manzanillo is also located at north of the Cuyutlan Lagoon, with a consequent lack of vegetation due to urbanization. The depth of the lagoon diminishes from northwestern to southeastern, with less than 1 m in its shallowest part. Water body’s salinity rises from west to east; occasionally the entrance of the Armeria River water, decreases salinity in the eastern end (Mellink and de la Riva 2005).
Study Plots and Forest Inventory
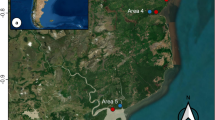
We identified five mangrove zones named hereafter: Zona Muelle (ZM); Zona Isla de los Pájaros (ZIP); Zona Salinera (ZS); Zona Caimanera (ZC) and Zona Campos (ZCP) (Fig. 1a, b). Zone selection was based on mangrove differential characteristics discerned by field visual inspections; these included floristic, structural, and anthropic. The zones mostly correspond to fringe mangrove forests, which have been persistently disturbed by the interplay of human activity, climate change and extreme events such as hurricanes, at least for the last 1300 years (Figueroa-Rangel et al. 2016).
In each mangrove zone, we implemented a stratified systematic sampling. In total, we established sixty-five 100 m2 permanent plots, 50 m away from each other, to cover the full range of environmental, structural and floristic composition. Sampling size varied according to the geographic extension of each zone as follows: ZM = seventeen plots; ZIP = eight plots; ZS = thirteen plots; ZC = seventeen plots and ZCP = ten plots (Fig. 1b).
Mangrove Structure
Mangrove structure was assessed in each 100 m2 permanent plot where all adult trees (trees ≥ 2.5 cm diameter at breast height (hereafter DBH) and ≥ 1.3 m tall) were tagged, enumerated, recorded and identified by species. Structural measurements included DBH (measured approximately 1.3 m above the ground) and canopy height measured with a Haga altimeter. For Rhizophora mangle individuals, DBH was measured approx. 30 cm above the highest rizophore. All adult trees were tagged with a numbered aluminium tag nailed on each stem above the DBH measurement point. Standing dead trees were not taken into account during permanent plot establishment. We also recorded DBH and height of juvenile trees (trees ˂ 2.5 cm DBH and higher than 1.3 m) in two 16 m2 sampling units; each located in the opposite corners of the 100 m2 plot. Juvenile trees were also tagged, enumerated, recorded and identified by species. Seedling abundance and heights (individuals <1.3 m height) were also measured in four 1 m2 sampling units, each located in the opposite corners of the 16 m2 plot. The total number of adult trees, saplings and seedlings were counted, botanically identified, and double-checked for inconsistencies. However, saplings and seedlings are beyond the scope of this paper. They are only described to indicate the overall vegetation sampling survey.
Interstitial Water
We sampled interstitial water properties twice a year, one in the dry season (April 2016) and another in the wet season (September 2016). Three random interstitial water samples were taken in each permanent plot at 30 to 40 cm depth using an acrylic tube attached to a syringe; in total, we took 145 interstitial water samples. In each water sample-point we recorded physiochemical variables such as: temperature, salinity, pH, oxygen dissolved (OD) and total dissolved solid (TDS) using a Multiparameter meter (Hanna HI 9828 model).
Statistical Analysis
In order to discern structural and floristic composition among zones, we computed stand density (number of individuals per plot ha−1), species density (individuals per species ha−1), mean diameter, DBH (cm), canopy height (average height of total individuals), total basal area (m2 ha−1), species basal area (m2 ha−1) and importance index values (IVI). We calculated IVI as the sum of relative density (number of trees per species/total number of trees), relative frequency (number of samples at which the species was counted /total number of samples) and relative basal area (basal area per species/total basal area all species) (Moore and Chapman 1986).
To compare mangrove structural and floristic composition among zones, we constructed box and whisker plots based on stand density, mean diameter, canopy height and total basal area by floristic zone (FZ; ZM; ZIP; ZS; ZC; and ZCP).
To discern both floristic composition and structural differences related to environmental conditions, we first ran non-metric multidimensional scaling (NMDS) ordinations. For floristic composition, we organised data into plot-by-species matrices with cells filled with the density values of each species. To discriminate structural differences, we built plot-by-structural variables matrices using stand density, mean diameter, canopy height and basal area data. In both cases, one for each of the sample seasons (dry and wet). We used the metaMDS function as implemented in the R “vegan” package and the Bray-Curtis dissimilarity as the metric distance (Oksanen et al. 2017).
Subsequently, we used the envfit function over the NMDS ordinations by fitting vectors representing the floristic zone and interstitial water variables (salinity, temperature, pH and OD; TDS resulted highly correlated to salinity and was not included in the analysis). With the stressplot function, we evaluated the goodness-of-fit between fitted vectors and ordination variables with an apriori p = 0.01 as a cutoff for statistical significance to minimize α inflation and potential problems with Bonferroni corrections (Oksanen et al. 2017).
In order to estimate the relationship of interstitial water variables (salinity, temperature, pH and OD) with structural variable (stand density, DBH, canopy height and basal area), multiple linear regression analyses were applied using each structural variable as a response factor; previously, generalized additive models and tree models were computed to investigate curvature and interactions respectively (Crawley 2015). All analyses were processed using R software, v. 3.4.3 (R Core Team 2017).
Results
Floristic Composition and Structural Variation by Floristic Zone
Floristic composition was represented by six woody species, in order of abundance: Laguncularia racemosa (L.) C.F. Gaertn., Rhizophora mangle L., Prosopis juliflora (Sw.) DC., Guazuma ulmifolia Lam., Terminalia catappa L., and Ficus insipida Willd. The first two species (L. racemosa and R. mangle) correspond to mangrove species, while the rest (P. juliflora, G. ulmifolia, T. catappa and F. insipida) are classified as mangrove-associated species in the study area. Species diversity was relatively uniform across the five floristic zones, albeit the ZCP was the less diverse with only two species (Table 1).
Mangrove stand structure was relatively similar between floristic zones, however the ZS had the narrowest diameter-class distribution, but at the same time it showed the highest density (Fig. 2). Despite the small differences in diameter-class distribution, the five floristic zones displayed a reverse-J-shaped diameter-class distribution (sensu Smith et al. 2014) declining monotonically as DBH increased, a pattern characteristic of immature uneven-aged stands (Fig. 2).
Taking into account the five floristic zones, the proportion in the number of individuals of R. mangle declined as mean diameter increased. Our result also showed that 78% of individuals of R. mangle occurred in DBH classes <10 cm. Conversely, 59% of the individuals of L. racemosa, including the non-mangrove associated species were present in classes <10 cm. This inequality was greater in the ZM (59% of L. racemosa <10 cm; 92% of R. mangle <10 cm; 67% of another species <10 cm) and the ZCP (49% of L. racemosa <10 cm; 96% of R. mangle <10 cm) (Fig. 2).
Laguncularia racemosa, the unique mangrove species with monopodial growth habit (sensu Tomlinson 1986) in our study plots, was the most dominant species across the study area. An interesting result was that in the ZCP zone, the utmost western zone close to the peri-urban area of Manzanillo, we only recorded individuals of R. mangle and L. racemosa. On the contrary, ZC was the richest zone in species composition presenting the six-species recorded in the study plots. IVI showed that L. racemosa was the dominant species in the five zones as compared to the others species involved in this study. The highest IVI for R. mangle occurred in ZIP (Table 1).
The box and whisker plots showed that structural variables differed across floristic zones (Fig. 3). The largest range on stand density corresponded to the ZM, while the ZS presented the narrowest. Mean diameter varied the most in the ZIP followed by ZC. Regarding canopy height, both ZIP and ZC, showed a large variation in the data, while the ZCP presented the narrowest. For basal area, the ZIP presented the highest range value.
The ZS had the highest stand density (4453.85 individuals ha−1); however, it showed the smallest mean diameter (8.66 cm) and one of the lowest basal area values (31.85 m2 ha−1). On the contrary, ZIP showed the lowest density (1287.5 individuals ha−1) altogether with the highest mean diameter (16.07 cm) but the lowest basal area (27.11 m2 ha−1) (Table 2).
Environmental-Floristic Variation Relationship
All zones presented a wide range of variability in salinity and OD. The ZS was the saltiest during both, wet and dry seasons; on the contrary, ZIP presented the lowest salinity values (Table 3). Oxygen dissolved was higher in the ZIP (6.43 mg/L in wet; 4.95 mg/L in dry season). However the lowest value during the wet season corresponded to ZC; there was no OD for the ZS during the dry season. Temperature and pH were relatively constant among zones with a mean temperature of 28.8 °C and pH of 6.9 in the wet season, and 27.90 °C and 6.91 in the dry season. (Table 3).
Non-metric multidimensional scaling ordinations resulted in two-dimensional solution, for both floristic composition (stress = 0.05) and for structural data (stress = 0.07) data (Fig. 4). For floristic composition data in the wet season, NMDS exhibited a cluster of sampling plots to the left of the diagram, near to L. racemosa while R. mangle was positioned over the right side (Fig. 4a). Mangrove-associated species (P. juliflora, G. ulmifolia, T. catappa and F. insipida) were distributed along axis 2. A rather similar pattern emerged for the dry season with L. racemosa and R. mangle along axis 1 and mangrove-associated species along axis 2 (Fig. 4b). For structural data, NMDS results showed most of the sampling plots highly dispersed over the diagram; structural variables were similarly scattered on the diagram with no clear gradient in any of the two axes. This pattern arose for both the wet (Fig. 4c) and the dry season (Fig. 4d).
Envfit function over the NMDS ordinations with floristic composition data for the wet season (a) and the dry season (b) (L.rac: Laguncularia racemosa; R.man: Rhizophora mangle; P.jul: Prosopis juliflora; G.ulm: Guazuma ulmifolia; F.ins: Ficus insipida; T.cat: Terminalia catappa); and for structural data for the wet (c) and the dry season (d); vectors represent interstitial water variables (salinity, temperature, pH, OD) and floristic zone. Numbers represent sampling plots
Envfit function showed that salinity was the strongest predictor driving the observed pattern of floristic composition for both, the wet (p = 0.02, r2 = 0.13) and the dry season (p < 0.001, r2 = 0.16) along axis 1 (Fig. 4a, b). For axis 2 none of the variables were statistically significant. Regarding structural data, envfit function showed salinity as a strong predictor on axis 1 (p < 0.001, r2 = 0.35) for both seasons. In axis 2, OD was significant for the wet season (p = 0.01, r2 = 0.14), while temperature was significant in the dry season (p = 0.04, r2 = 0.10) (Fig. 4a–b).
Multiple regression results showed that, different predictors (interstitial water variables) arose, depending on the structural variable used as a response factor, as well as on the season (Table 4). For DBH, salinity was the only significant (R2 = 0.11, p = 0.0055) predictor in the wet season, whilst pH, salinity and OD explained DBH in the dry season (R2 = 0.16, p = 0.0037). Salinity and pH, resulted significant for stand density in both seasons (wet: R2 = 0.28, p = 0.0001; dry R2 = 0.33, p < 0.0001). For canopy height values also fluctuated with the season; only pH for the wet season (R2 = 0.20, p = 0.0002), but salinity and OD in the dry season (R2 = 0.15, p = 0.0037). Finally, for basal area, pH and OD were significant in the wet season (R2 = 0.23, p = 0.0002) and temperature was only significant in the dry season (R2 = 0.10, p = 0.0077) (Table 4).
Discussion
Floristic Composition and Structural Variation
Two mangrove species (Laguncularia racemosa and Rhizophora mangle) were recorded in the present research, with L. racemosa exceeding R. mangle’s abundance across the geographical study area. These two species are among the four (R. mangle, L. racemosa, Avicennia germinans and Conocarpus erectus) reported for the Central Pacific Zone of Mexico (Acosta-Velázquez and Rodríguez 2007), where the Cuyutlan lagoon is located. The remaining four woody species, Prosopis juliflora, Guazuma ulmifolia, Terminalia catappa and Ficus insipida, corresponded to mangrove-associated species, often found at the landward edge of mangrove ecosystems globally (also called the ‘back mangrove’) (FAO 2007).
The five zones under study showed important variations in species diversity. While six species occurred in ZC, there were only three in ZIP and two in ZCP. The ZC is closer to the ZCP, with no important environmental differences; therefore, propagule dispersal ability should determine floristic similarities between zones (McKee 1995; Mason et al. 2013). However, the missing species in the ZCP correspond to mangrove-associated species, characterised by their lower stature; they are found at the landward edge of mangrove ecosystems (Doyle et al. 2009) where sea transfers its hydrologic influence to groundwater (Liu and Mou 2016). As the ZCP is a peri-urban zone, the increasing invasion by terrestrial edge woody species such as coconut, mango and plum crops (personal observations) might trigger the disappearance of the mangrove-associated species with the subsequent reduction of the mangrove fringe.
Regarding structure, values for stand density, DBH, canopy height and basal area were higher than those reported the Central Pacific Zone (Acosta-Velázquez and Rodríguez 2007). Structural development is often explained by high value in stand parameters such as number of species, basal area and canopy height (Corella et al. 2001). Therefore, the mangrove assemblage of the Cuyutlan Lagoon, despite its low species diversity, has higher structural complexity than similar regions with mangrove vegetation in Mexico (Acosta-Velázquez and Rodríguez 2007; Méndez et al. 2007).
ZC and ZCP are located in the northern part of the Cuyutlan Lagoon which maintain an intensive human activity through the development of high infrastructure (Valderrama-Landeros et al. 2017). The ZIP is located in the south on a small island, consequently influenced by water proximity and more isolated from the rest of the zones. The ZS, at the centre of the lagoon, is located over a flat environment in which water accumulation lead to evaporation producing high salinity. All the above-mentioned circumstances led us to hypothesize a lower forest complexity in the northern part. Contrary to our expectations, structural complexity of the five different mangrove zones of the Cuyutlan Lagoon, is not following a gradient from north to south or vice versa, as we hypothesized. Instead, our results showed that there is an assortment of structure and floristic composition along the vegetation mangrove, an effect of the multifaceted interaction of human and natural presses and pulses.
Frequency and intensity of tropical storms and hurricanes are increasing under climate warming conditions, affecting mangroves directly. Although the effect is evident in defoliation, litter production and tree mortality, there is also evidence of rapid recovery from this damage (Yáñez-Arancibia et al. 2010). Taking into account that commercial mangrove harvesting is not allowed in the study area, low values in diameter-size in both ZS and ZM could be explained by the relatively young stand structure observed in these floristic zones, likely as a consequence of heavy storms and hurricanes impacting vegetation over the last ~ 300 years (Figueroa-Rangel et al. 2016). After such events, mortality of adult individuals increases, which explains the high density for ZS; then the process promoted canopy gap development and the subsequent establishment of renewals (Manrow-Villalobos and Vilchez-Alvarado 2012), elucidating the small sizes in diameter for this zone. Additionally, although a number of individuals are old-growth trees, environmental conditions, particularly high salinity concentrations in the ground water, may be hindering their growth (Pinto-Nolla et al. 1995).
Diameter class distribution, mainly for mangrove species, showed that R. mangle individuals were more abundant in the categories <10 cm while L. racemosa showed individuals distributed along the whole range of diameters. These results were more evident in the ZM and the ZCP suggesting an apparent colonization of R. mangle. The same process that occurred in upland forests elsewhere (Kuuluvainen et al. 1998; Nanos et al. 2004).
Environmental Correlates
Data analyses revealed that, when using floristic composition data matrices in NMDS, a salinity gradient emerged along the Cuyutlan lagoon’s mangrove vegetation, for both the wet and the dry seasons. NMDS ordination also assembled sampling plots according to abundance of L. racemosa, which were closer to salinity than R. mangle; mangrove-associated species were dispersed with no apparent grouping. Conversely, with structural variables matrices, there were no grouping patterns and, although salinity was significant on axis 1 for both seasons, OD and temperature were important explaining variables as well. The same applied for multiple regression analyses since all interstitial water variables (pH included), in dissimilar level of significance, were important determining structure.
From these overall results, the second hypothesis is confirmed: salinity is the strongest interstitial water variable explaining both floristic composition and structure of mangrove vegetation, either using single (multiple linear regression) than multiple response variables (NMDS). Our findings provide empirical evidence that interstitial salinity may help to explain patterns of mangrove’s plant distribution in the Mexican Pacific (Flores et al. 2007). Ground water salinity, not only produces changes in mangrove and non-mangrove species physiology, it also induces changes in plant morphology because plants living in sites with high salinity endure both adaptive and selective pressures modifying their general structure (Pinto-Nolla et al. 1995). The low osmotic potential of saline soils sets constraints on the relationship of mangrove vegetation with water. The process induces physiological responses similar to those of terrestrial plants experiencing drought (Clough 1992). Salinity has another interesting effect; it constrains size inequality in mangrove habitats. As the salinity is greater, tree size and structural complexity decrease (Méndez-Alonzo et al. 2012). Our results sustain this fact as ZS plots located in the saltiest floristic zone, presented the lowest values for mean diameter, canopy height and basal area; this zone is located over a flat environment in which water accumulation lead to evaporation producing the highest salinity conditions in the study area.
Oxygen dissolved was also a significant variable determining structural distribution. Soil anoxic conditions seem to become an important variable for mangrove structure in the wet season, when heavy rains saturate the soil. Saturated soils in turn cause anaerobic conditions (Campos-Cascaredo and Moreno-Casasola 2009) and finally anoxic conditions can influence plant growth in three ways. Firstly, in order to satisfy root oxygen requirement, it must be a proper internal root gas transportation system. Secondly, the oxidation-reduction potential varies with anoxic conditions transforming essential elements, improving or restricting their availability. Finally, high degree of anaerobiosis can lead to the formation of H2S and another toxic substances such as acid-volatile sulphides, oils spills from ships and aromatic hydrocarbons which damage mangroves as they are phytotoxic and affect all stages of plant growth (Clough 1992; Michelato Ghizelini et al. 2012; Melo Queiroz et al. 2018). However, mangroves have adaptations to solve this lack of oxygen, allowing them to survive when the mean level of water is constant; but if floods persist for a long time, plants remain progressively stressed and eventually die (Yáñez-Arancibia et al. 2010). Although pH was not significant in the NMDS analysis, this variable was important explaining all variables related to structure in the multiple linear regressions. Soils with pH close to neutral allow Rhizophora plants to growth (Wakushima et al. 1994), since primary mineral soils nutrients (e.g. nitrogen, phosphorus and potassium), as well as secondary nutrients (e.g. sulfur, calcium and magnesium) are available at pH = 6.5; soils which are appropriate for seaward mangroves (Lugo and Snedaker 1974).
Salinity and OD variations could eventually modify the patterns of plant distribution in the lagoon; as an example, L. racemosa may be dominated by Rhizophora, as it is more tolerant to low oxygen availability and high rates of salinity (McKee 1995). A process already occurring with outbreaks in R. mangle in ZM and ZCP. Climate change effects through sea-level rise will also induce mangroves to move inland (Alongi 2008; Yáñez-Arancibia et al. 2014). However, as the adjacent inland areas of mangrove in the Cuyutlan Lagoon are occupied by anthropic development, mostly agriculture and livestock, landward migration would be constrained, causing a contraction of the mangrove fringe.
Considering temperature, during the dry season, which corresponds to months with the lowest minimum temperature of the year in our study area (Servicio Meteorológico Nacional 2010), the effect of temperature of the ground water becomes significant for the development of the mangrove forest. Specifically for growth rates in mangrove plants which have also been associated to temperature and humidity (Méndez-Alonzo et al. 2008). Although most of the mangrove ecosystems remains warm during the whole year, as they are tropical ecosystems (Alongi 2002), temperature oscillations might affect growth rates, possibly as a consequence of an overall reduction in growth potential, reduced photosynthetic carbon fixation, increased respiration, or a reduced capacity to achieve osmoregulation (Clough 1992).
Conclusions
Mangrove ecosystem of the Cuyutlan Lagoon is composed by vegetation with different patterns of spatial structure and floristic composition whose development is an effect of its location along the coast of the Mexican Pacific, a region with a critical interchange of human and natural drivers.
In terms of floristic composition, the ecosystem is very poor in plant diversity with only two mangrove species (Laguncularia racemosa and Rhizophora mangle) and four mangrove-associated species Prosopis juliflora, Guazuma ulmifolia, Terminalia catappa and Ficus insipida. On the contrary, there is a high structural complexity with an assortment of sizes (DBH and canopy height), abundances (stand density) and dominance (basal area). Using single and multiple response analysis, results revealed that salinity is the strongest interstitial water variable explaining both floristic composition and structure of mangrove vegetation. Other water variables such as pH, OD and temperature also regulate spatio-temporal changes in vegetation in the study area; spatially through the different structure of the zones along the lagoon; temporally, through environmental dissimilarities in the dry and the wet season. Interstitial salinity and temperature determined density and size of the trees. The soil’s anoxic condition in the heavy rains affected the mangrove forest structure as well. Salinity was also the main correlate explaining floristic composition. However, at present, human activities undertaken on the north of the lagoon, mainly infrastructure development, together with present and past natural events (hurricanes, tsunamis, storms and sea-level rise) are also affecting the functioning of this important coastal ecosystem.
References
Acosta-Velázquez J, Rodríguez MT (2007) Los manglares de México: estado actual y establecimiento de un programa de monitoreo a largo plazo: 1ra. etapa. Comisión Nacional para el Conocimiento y Uso de la Biodiversidad, México D. F
Alongi DM (2002) Present state and future of the world’s mangrove forests. Environmental Conservation 29:331–349. https://doi.org/10.1017/S0376892902000231
Alongi DM (2008) Mangrove forests: resilience, protection from tsunamis, and responses to global climate change. Estuarine, Coastal and Shelf Science 76:1–13. https://doi.org/10.1016/j.ecss.2007.08.024
Bosire JO, Kaino JJ, Olagoke O, Mwihaki LM, Ogendi GM, Kairo JG, Berger U, Macharia D (2014) Mangroves in peril: unprecedented degradation rates of peri-urban mangroves in Kenya. Biogeosciences 1:2623–2634
Campos-Cascaredo A, Moreno-Casasola P (2009) Suelos hidromórficos. In P. Moreno-Casasola and B. G. Warner (Eds.), Breviario Para describir, observar y manejar humedales. Serie Costa Sustentable no 1. RAMSAR, Instituto de Ecología a.C., CONANP, US fish and wildlife service, US State Department. Xalapa, Ver. México, pp 111–130
Cavalcanti ER, Domiciano J, Brandão JLS, Brito YC (2015) Space-time analysis of environmental changes and your reflection on the development of phenological of vegetation of mangrove. Journal of Agriculture and Environmental Sciences 4:245–253. https://doi.org/10.15640/jaes.v4n1a30
Clare S, Krogman N, Foote L, Lemphers N (2011) Where is the avoidance in the implementation of wetland law and policy? Wetlands Ecology and Management 19:165–182. https://doi.org/10.1007/s11273-011-9209-3
Clough BF (1992) Primary productivity and growth of mangrove forests. In: Robertson A, Alongi DM (eds) Tropical mangrove ecosystems, American G. Washington D.C., pp 225–249
Commission for Environmental Cooperation (2016) Wetlands in Manzanillo, factual record regarding submission SEM-09-002 Montreal, Canada: Commission for Environmental Cooperation. 96 pp.
Contreras-Espinoza F (1985) Las lagunas costeras mexicanas, 2nd edn. Centro de Ecodesarrollo, México DF
Corella F, Valdez JI, Cetina VM et al (2001) Estructura forestal de un bosque de mangles en Tabasco. Revista Ciencia Forestal en México 26:73–102
Costa P, Dórea A, Mariano-Neto E, Barros F (2015) Are there general spatial patterns of mangrove structure and composition along estuarine salinity gradients in Todos os Santos Bay? Estuarine, Coastal and Shelf Science 166:83–91. https://doi.org/10.1016/j.ecss.2015.08.014
Crawley MJ (2015) Statistics: an introduction using R, 2nd edn. John Wiley and Sons, Ltd., Chichester
Doyle TW, Krauss KW, Wells CJ (2009) Landscape analysis and pattern of hurricane impact and circulation on mangrove forests of the Everglades. Wetlands 29:44–53. https://doi.org/10.1672/07-233.1
Ewel KC, Twilley RR, Ong JE (1998) Different kinds of mangrove forests provide different goods and services. Global Ecology and Biogeography Letters 7:83–94. https://doi.org/10.2307/2997700
FAO (2007) The world’s mangroves 1980-2005. A thematic study prepared in the framework of the global Forest resources assessment 2005. FAO Forestry Paper 153:1–77
Feller IC, Sitnik M (2002) Mangrove ecology workshop manual. Smithsonian Institution, Washington DC
Feller IC, Whigham DF, O’Neill JP, McKee KL (1999) Effects of nutrient enrichment on within-stand cycling in a mangrove forest. Ecology 80:2193–2205
Figueroa-Rangel BL, Olvera-Vargas M, Vázquez-López JM, Willis KJ, Lozano-García S (2016) Modern and fossil pollen assemblages reveal forest taxonomic changes in the Mexican subtropics during the last 1300 years. Review of Palaeobotany and Palynology 231:1–13. https://doi.org/10.1016/j.revpalbo.2016.04.007
Flores F, Moreno P, Agraz CM, et al (2007) La topografía y el hidroperíodo: dos factores que condicionan la restauración de los humedales costeros. Boletín de la Sociedad Botánica de México Suplemento:33–47
Flores-Verdugo F, Agraz-Hernández C, Benítez-Pardo D (2006) Creación y restauración de ecosistemas de manglar: principios básicos. Gobierno Municipal de Jalapa, Instituto de Ecología AC, Xalapa, México, pp 1093–1110
García E (2004) Modificaciones al sistema de clasificación climática de Köppen. Instituto de Geografía. Universidad Nacional Autónoma de México, Mexico DF
Giri C, Ochieng E, Tieszen LL, Zhu Z, Singh A, Loveland T, Masek J, Duke N (2011) Status and distribution of mangrove forests of the world using earth observation satellite data. Global Ecology and Biogeography 20(1):154–159. https://doi.org/10.1111/j.1466-8238.2010.00584.x
Hamilton SE, Casey D (2016) Creation of a high spatio-temporal resolution global database of continuous mangrove forest cover for the 21st century (CGMFC-21). Global Ecology and Biogeography 25:729–738. https://doi.org/10.1111/geb.12449
He J, Moffette F, Fournier R, Revéret JP, Théau J, Dupras J, Boyer JP, Varin M (2015) Meta-analysis for the transfer of economic benefits of ecosystem services provided by wetlands within two watersheds in Quebec, Canada. Wetlands Ecology and Management 23:707–725. https://doi.org/10.1007/s11273-015-9414-6
Hernández AJ, Pastor J (2008) La restauración ecológica de ecosistemas degradados: marcos conceptuales y metodologías para la acción. Contaminación de Suelos. Tecnologías para su Recuperación, CIEMAT, Madrid, pp 61–82
Kairo JG, Dahdouh-Guebas F, Bosire J, Koedam N (2001) Restoration and management of mangrove systems - a lesson for and from the east African region. South African Journal of Botany 67:383–389. https://doi.org/10.1016/S0254-6299(15)31153-4
Kathiresan K, Bingham BL (2001) Biology of mangroves and mangrove ecosystems. Advances in Marine Biology 40:81–251. https://doi.org/10.1016/S0065-2881(01)40003-4
Kuuluvainen T, Järvinen E, Hokkanen TJ et al (1998) Structural heterogeneity and spatial autocorrelation in a natural mature Pinus sylvestris dominated forest. Ecography 21:159–174
Lee SY (1999) Tropical mangrove ecology: physical and biotic factors influencing ecosystem structure and function. Austral Ecology 24:355–366. https://doi.org/10.1046/j.1442-9993.1999.00984.x
Lewis RR (2005) Ecological engineering for successful management and restoration of mangrove forests. Ecological Engineering 24:403–418. https://doi.org/10.1016/j.ecoleng.2004.10.003
Liu Q, Mou X (2016) Interactions between surface water and groundwater: key processes in ecological restoration of degraded coastal wetlands caused by reclamation. Wetlands 36:95–102. https://doi.org/10.1007/s13157-014-0582-6
Liu K, Li X, Shi X, Wang S (2008) Monitoring mangrove forest changes using remote sensing and GIS data with decision-tree learning. Wetlands 28:336–346. https://doi.org/10.1672/06-91.1
López J, Ezcurra E (2002) Los manglares de México: una revisión. Madera y Bosques Número especial:27–51
Lugo AE, Snedaker SC (1974) The ecology of mangroves. Annual Review of Ecology, Evolution, and Systematics 5:39–64
Macintosh DJ, Ashton EC (2002) A review of mangrove biodiversity conservation and management. University of Aarhus, Denmark
Manrow-Villalobos M, Vilchez-Alvarado B (2012) Estructura, composición florística, biomasa y carbono arriba del suelo en los manglares Laguna de Gandoca y Estero Moín, Limón, Costa Rica. Revista Forestal Mesoamericana Kurú 9:1–18
Martín B, Montes C (2010) Funciones y servicios de los ecosistemas: una herramienta para la gestión de los espacios naturales. Guía científica de Urdaibai. UNESCO, Dirección de Biodiversidad y Participación Ambiental del Gobierno Vasco, pp 13–32
Mason NWH, Wiser SK, Richardson SJ, Thorsen MJ, Holdaway RJ, Dray S, Thomson FJ, Carswell FE (2013) Functional traits reveal processes driving natural afforestation at large spatial scales. PLoS One 8:e75219. https://doi.org/10.1371/journal.pone.0075219
McKee KL (1995) Seedling recruitment patterns in a Belizean mangrove forest: effects of establishment ability and physico-chemical factors. Oecologia 101:448–460. https://doi.org/10.1007/BF00329423
Mellink E, de la Riva G (2005) Non-breeding waterbirds at Laguna de Cuyutlan and its associated wetlands, Colima, Mexico. Journal of Field Ornithology 76:158–167
Mellink E, Riojas-López M (2007) Modificaciones estructurales artificiales de Laguna Cuyutlán, Colima, México. Revista Geografica 142:131–142
Melo Queiroz H, Nuto Nóbrega G, Otero XL, Osório Ferreira T (2018) Are acid volatile sulfides (AVS) important trace metals sinks in semi-arid mangroves? Marine Pollution Bulletin 126:318–322. https://doi.org/10.1016/j.marpolbul.2017.11.020
Méndez AP, López-Portillo J, Hernández-Santana JR et al (2007) The mangrove communities in the arroyo Seco deltaic fan, Jalisco, Mexico, and their relation with the geomorphic and physical–geographic zonation. Catena 70:127–142. https://doi.org/10.1016/j.catena.2006.05.010
Méndez-Alonzo R, López-Portillo J, Rivera-Monroy VH (2008) Latitudinal variation in leaf and tree traits of the mangrove Avicennia germinans (Avicenniaceae) in the central region of the Gulf of Mexico. Biotropica 40:449–456. https://doi.org/10.1111/j.1744-7429.2008.00397.x
Méndez-Alonzo R, Hernández-Trejo H, López-Portillo J (2012) Salinity constrains size inequality and allometry in two contrasting mangrove habitats in the Gulf of Mexico. Journal of Tropical Ecology 28:171–179. https://doi.org/10.1017/S0266467412000016
Michelato Ghizelini A, Mendonça-Hagler LC, Macrae A (2012) Microbial diversity in brazilian mangrove sediments – a mini review. Brazilian Journal of Microbiology 43:1242–1254. https://doi.org/10.1590/S1517-83822012000400002
Moore PD, Chapman SB (1986) Methods in plant ecology. Blackwell Scientific, Michigan
Moreno E, Peña AG, Del Carmen M, et al (2002) Los manglares de Tabasco, una reserva natural de carbono. Madera y Bosques Número especial:115–128
Moreno-Casasola P, López H (2009) Muestreo y análisis de la vegetación de humedales. In: Moreno-Casasola P, Warner BG (eds) Breviario para describir, observar y manejar humedales, Serie Cost. RAMSAR, Instituto de Ecología A.C., CONANP, US Fish and Wildlife Service, US State Department, México, p 407
Nanos N, González-Martínez SC, Bravo F (2004) Studying within-stand structure and dynamics with geostatistical and molecular marker tools. Forest Ecology and Management 189:223–240
Okello JA, Schmitz N, Kairo JG, Beeckman H, Dahdouh-Guebas F, Koedam N (2013) Self-sustenance potential of peri-urban mangroves: a case of Mtwapa creek Kenya. Journal of environmental science and. Water Resources 2(8):277–289
Oksanen J, Blanchet FG, Friendly M, et al (2017) Package “vegan”. Community Ecology Package, pp 1–292
Pellegrini JAC, Soares MLG, Chaves FO et al (2009) A method for the classification of mangrove forests and sensitivity/vulnerability analysis. Journal of Coastal Research 56:443–447
Pinto-Nolla F, Naranjo-González G, Hernández-Camacho J (1995) Influencia del habitat en la morfometria y morfologia del “mangle salado” Avicennia germinans en el litoral Caribe Colombiano. Revista de la Academia Colombiana de Ciencias Exactas, Físicas y Naturales 19:481–498
Proffitt CE, Travis S (2014) Red mangrove life history variables along latitudinal and anthropogenic stress gradients. Ecology and Evolution 4:2352–2359. https://doi.org/10.1002/ece3.1095
R Core Team (2017) R: a language and environment for statistical computing version 3.4.3 ed. R Foundation for Statistical Computing, Vienna
Reef R, Feller IC, Lovelock CE (2010) Nutrition of mangroves. Tree Physiology 30:1148–1160. https://doi.org/10.1093/treephys/tpq048
Rodríguez-Zúñiga MT, Troche-Souza C, Vázquez-Lule AD, et al (2013) Manglares de México. Extensión, distribución y monitoreo. Comisión Nacional para el Conocimiento y Uso de la Biodiversidad, México DF
Servicio Meteorológico Nacional (2010) Proyecto bases de datos climatológicos. Comisión Nacional del Agua. México. Available via CONAGUA. https://smn.conagua.gob.mx/es/informacion-climatologica-ver-estado?estado=col
Smith DM, Larson BC, Kelty MJ, Ashton PNS (2014) The practice of silviculture. John Wiley & Sons Inc, New York
Stringer CE, Rains MC, Kruse S, Whigham D (2010) Controls on water levels and salinity in a barrier island mangrove, Indian River lagoon, Florida. Wetlands 30:725–734. https://doi.org/10.1007/s13157-010-0072-4
Thomas T, Lucas R, Bunting P, Hardy A, Rosenqvist A, Simard A (2017) Distribution and drivers of global mangrove forest change, 1996–2010. PLoS One 12(6):e0179302. https://doi.org/10.1371/journal.pone.0179302
Tomlinson PB (1986) The botany of mangroves. Cambridge University Press, Cambridge
Torres J, Quintanilla-Montoya AL (2014) Alteraciones antrópicas: historia de la Laguna de Cuyutlán, Colima. Investigación ambiental, ciencia y política pública 6:29–42
Twilley RR, Snedaker SC, Yáñez-Arancibia A, Medina E (1996) Biodiversity and ecosystem processes in tropical estuaries: perspectives of mangrove ecosystems. In: Mooney HA, Cushman JH, Medina E (eds) Functional roles of biodiversity: a global perspective. John Wiley & Sons, Chichester, pp 327–370
Valderrama L, Troche C, Rodriguez MT, Marquez D, Vázquez B, Velázquez S, Vázquez A, Cruz MI, Ressl R (2014) Evaluation of mangrove cover changes in Mexico during the 1970–2005 period. Wetlands 34:747–758. https://doi.org/10.1007/s13157-014-0539-9
Valderrama-Landeros LH, Rodríguez-Zúñiga MT, Troche-Souza C, et al (2017) Manglares de México: actualización y exploración de los datos del sistema de monitoreo 1970/1980–2015. Comisión Nacional para el Conocimiento y Uso de la Biodiversidad, México DF
Wakushima S, Kuraish S, Sakurai K (1994) Soil salinity and pH in japanese mangrove forests and growth of cultivated mangove plants in different soil conditions. Journal of Plant Research 107:39–46
Walters BB, Rönnbäck P, Kovacs JM, Crona B, Hussain SA, Badola R, Primavera JH, Barbier E, Dahdouh-Guebas F (2008) Ethnobiology, socio-economics and management of mangrove forests: a review. Aquatic Botany 89:220–236. https://doi.org/10.1016/j.aquabot.2008.02.009
Yañez-Arancibia A, Lara-Domínguez AL (1999) Ecosistemas de Manglar en América Tropical. Instituto de Ecología A.C. México; UICN/ORMA Costa Rica; NOAA/NMFS Silver Spring MO USA
Yáñez-Arancibia A, Day JW, Twilley RR, Day RH (2010) Los manglares frente al cambio climático ¿Tropicalización global del Golfo de México? In: Yáñez-Arancibia A (ed) Impactos del cambio climático sobre la zona costera. Instituto de Ecología A. C., Texas Sea Grant Program, Instituto Nacional de Ecología, México, pp 91–126
Yáñez-Arancibia A, Day JW, Sánchez-Gil P, Day JN, Lane RR, Zárate-Lomelí D, Vásquez HA, Rojas-Galaviz JL, Ramírez-Gordillo J (2014) Ecosystem functioning: the basis for restoration and management of a tropical coastal lagoon, Pacific coast of Mexico. Ecological Engineering 65:88–100. https://doi.org/10.1016/j.ecoleng.2013.03.007
Zaldívar-Jiménez MA, Herrera-Silveira JA, Teutli-Hernández C et al (2010) Conceptual framework for mangrove restoration in the Yucatán peninsula. Ecological Restoration 28:333–342
Acknowledgements
We thank to the Cuyutlan Ecological Centre “El Tortugario” for their help during the field sampling survey and their logistic support. M. Sc. José Guadalupe Morales Arias also provided valuable help during fieldwork. This study was supported by the Mexican National Council for Science and Technology (Conacyt) through a Doctorate Scholarship to the first author. The comments of two anonymous reviewers substantially improve the content of this paper.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Torres-Fernández del Campo, J., Olvera-Vargas, M., Figueroa-Rangel, B.L. et al. Patterns of Spatial Diversity and Structure of Mangrove Vegetation in Pacific West-Central Mexico. Wetlands 38, 919–931 (2018). https://doi.org/10.1007/s13157-018-1041-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13157-018-1041-6