Abstract
This study reports the mapping, structural characteristics and distribution of mangrove species of the Andaman Islands. 79 mangrove patches were identified using Thiessen polygons of which, 53 accessible patches were surveyed during 2014–2015. Multiple linear transects were laid at each of the mangrove patches and data pertaining to diameter at breast height, basal area and height were recorded for all mangrove trees within a 10 × 10 m quadrat laid along the transects at 50 m intervals. Altogether, 178 quadrats were laid and 25 mangrove species representing 11 families and 14 genera were recorded. Of the 3073 mangrove plants enumerated in the present study, 96.06% were composed of trees (>4 m height), 1.99% of saplings (>1 m to ≤4 m height) and 1.95% of seedlings (≤1 m height). The number of species per quadrat ranged from 1 to 9 and majority of the quadrats had 2–3 species. Density and basal area of mangrove trees ranged from 1252 ha−1 to 2200 ha−1 and 30.8 m2/ha to 59.6 m2/ha, respectively. High diversity indices (Shannon-Wiener index ranged from 1.65 to 2.24; Simpson index from 0.74 to 0.85 and Pielou’s evenness index from 0.66 to 0.84) indicated greater diversity and even dispersion of mangroves in the Islands. The study also revealed that the mangrove forests of Andaman Islands were characterized by a mixed species composition. Most of the sites were represented by different combinations of dominant and co-dominant species, all from the family Rhizophoraceae. The present study highlights the need for conserving the mixed species mangrove forests of Andaman and Nicobar Islands and development of location-specific management plans for biodiversity conservation.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Mangrove ecosystem has an important role in ecological and socio-economic terms (Ronnback et al. 2007; Walters et al. 2008; Malik et al. 2015). They offer various livelihood services such as coastal defense, fisheries, carbon storage, tourism, water purification, timber and fuel (Spalding et al. 2014). Further, they protect the integrity of the coast by acting as coastal buffers against cyclones, storms, tsunamis and other waves and tidal damages (Alongi 2008). Research on mangroves has increased exponentially in the last 50 years (Lee et al. 2014). Human interactions with mangroves have also been increased in recent days coupled with natural disasters, which make them more vulnerable to anthropogenic and natural factors. As per recent estimate, mangroves have witnessed an annual loss between 0.16 and 0.39%, globally, due to rapid coastal development (Hamilton and Casey 2016) and mangrove cover has reportedly decreased in countries like China, Myanmar and Philippines, whereas, an increased mangrove cover was reported from India, Australia and New Zealand (Chaudhuri et al. 2015). Degradation and loss of these coastal buffering systems due to climate change and direct human impact negate the coastal protection they provide during extreme events and increase their vulnerability (Ellison 2015). Therefore, it is necessary to manage the mangrove forests through the integrated planning of coastal zone management (Yulianda et al. 2014).
India with its vast coastline of 8118 km has rich mangrove diversity and the ecosystem offers livelihood to several thousand people across India. Mangrove forests cover 4740 sq. km area along the Indian coastline of which 617 sq. km occur in the Andaman and Nicobar Islands (FSI 2015). The mangrove cover is larger and more widespread on the east coast of India than the west coast because of its distinctive geomorphological setting (Ragavan et al. 2016a). Andaman and Nicobar Islands (ANI) located in the Bay of Bengal is one of the most vulnerable coasts close to South east Asian countries which is experiencing frequent natural calamities such as earthquake, cyclones, storms, Tsunami etc. Mangroves of Andaman Islands are recognized as the best in the country in terms of density and growth (Dagar et al. 1991). In recent times, mangrove floristics of ANI have been studied by many (Roy 2003; Roy et al., 2009; Goutham-Bharathi et al. 2014; Ragavan et al. 2014; Ragavan et al. 2015a, b), and total of 38 true mangrove species belonging to 13 families and 19 genera have been known from ANI, which account for about 50% of the global true mangrove species (Ragavan et al. 2016b). In India, habitat destruction, over-exploitation, pollution and species introduction are identified as major causes of biodiversity loss (UNEP 2001) and the disturbance created by these factors determine the structural complexity and species composition (Sagar et al. 2003). Until recently, mangroves of the Islands had remained intact, but this situation is now changing (Spalding et al. 2010). Though not subject to human-driven land-use changes and urbanization threats, mangrove cover in the Islands continues to decline quite rapidly (Goutham-Bharathi et al. 2014).
Management of the fast-declining mangrove ecosystem requires a comprehensive understanding of the species composition and structural complexity (Dislich and Pivello 2002). Prior to forest management operations, biodiversity inventories were used to determine the nature and distribution of the species (Sagar et al. 2003). Though floristics and biodiversity of mangroves in the Islands have been well documented, little is known of the vegetation structure of the mangrove forests. The mangroves within the protected areas demarcated as per Wildlife (Protection) Act, 1972 and those within the identified tribal belts in the islands are under stringent regulatory regime. There is a need for developing management plans for conserving the mangroves outside such regulatory areas. Therefore, the present study was intended to examine the structural complexity and tree species composition of the mangrove forests of the Andaman Islands, with special focus on the areas, outside the protected areas. The study provides a comprehensive perspective of the mangroves of Andaman Islands and a framework for ascertaining their conservation significance. This would aid in formulating location-specific action plans as a part of the Island Coastal Zone Management Plans of Andaman Islands, a regulatory requisite under the Island Protection Zone (IPZ), 2011 notification of the Ministry of Environment, Forest and Climate Change, Government of India.
Materials and Methods
Study area
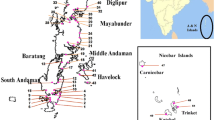
Mangroves in the ANI were mapped using satellite images at the scale of 1:50,000. Mangroves were differentiated from other coastal and as well as terrestrial vegetation based on their tones (red/pale red), texture (smooth), shape (irregular), location (in intertidal area and near high tidal area) and association (in warm waters on mudflat substrate and low energy coast) using satellite data. Mangrove patches lying outside the Marine Protected Areas / Tribal reserve areas were delineated based on the GIS point database using Thiessen polygons as described by Révész et al. (2003), and Biro et al. (2006), such that every polygon corresponding to each point represents a patch. A total of 79 patches were delineated representing the five regions of the Andaman Islands viz., North Andaman (Diglipur and Mayabunder), Middle Andaman (Baratang, Long Island and Rangat), South Andaman (Port Blair Range), Little Andaman (Hut Bay) and Ritchie’s Archipelago (Havelock and Neil), of which 53 patches were surveyed and rest of the patches were not accessible (Table 1). Each patch was given a code, “MGR-AN- 01”, representing the ecosystem, state and serial number of the patch from north to south, along with a corresponding local name. All the identified contiguous patches are shown in Fig. 1. The spatial extent of mangroves in each of the Island groups is shown in Table 2. The mangrove stands of ANI were classified based on the extent into three viz., small patches (≤5 km2); medium patches (5–10 km2) and large patches (≥10 km2).
Data collection
Field surveys were conducted during June 2014 to March 2015. In each patch, multiple linear transects (100 m) were laid perpendicular to the water front, with a minimum of 50 m interval. Quantitative data on mangrove vegetative structures were collected by laying quadrats (10 × 10 m) along the line transects. In each line transect, three quadrats were laid at 0, 50 and 100 m. Altogether, 178 quadrats were laid. Within each plot, all mangroves were identified to species level and counted according to three maturity categories as described by Menon (2006), viz., trees (>4 m height), saplings (>1 m to ≤4 m height) and seedlings (plant ≤1 m height). The measurements viz., tree height and girth at breast height (GBH) were noted for all trees and species-wise count data were collected for seedlings and saplings in each quadrat studied.
Data analysis
The vegetation data were quantitatively analysed for abundance, density and frequency (Curtis and Mclntosh 1950). The importance value index (IVI) for the tree species was determined as a sum of the relative frequency, relative density and relative dominance (Curtis 1959). In this study, the relative dominance was calculated based on the basal area of individual trees using GBH. Apart from this, various indices for assessing the health of the mangroves viz., Shannon-Wiener diversity index (H′) (Shannon and Weiner 1963), Margalef’s species richness (d) (Margalef 1978), Pielou’s evenness index (J′) (Pielou 1966), Simpson’s dominance index (D) (Simpson 1949), Complexity Index (I c ) (Holdridge 1967; Pool et al. 1977), Beta diversity (β-diversity) (Whittaker 1972) and Maturity Index Value (Nabi et al. 2012) were analyzed. Niche width was estimated to ascertain the adaptability of different mangrove species to tolerate conditions at the interface between different habitat types (Levins 1968). Similarity matrices between regions were constructed by using Bray-Curtis similarity index, based on the IVI of mangrove species. These matrices were used to display the similarity among the regions by means of hierarchical clustering (using group average method) and non-metric multidimensional scaling (nMDS). SIMPER analysis was performed to identify the mangrove species contributing for dissimilarity between regions. Cluster analysis, nMDS and SIMPER were performed using PRIMER v7 (Clarke and Gorley 2015). The floristic similarities between the different regions of Andaman Islands were examined based on the presence/absence of the species using the Sørensen (1948) Similarity Index, modified after Chao et al. (2006).
Results
Species composition
A total of 25 mangrove species belonging to 11 families and 14 genera were recorded in the Andaman Islands in the present study. Among the 11 families, number of species represented from the family Rhizophoraceae was the highest (8 species), followed by Lythraceae (4 species). Families of Avicennieaceae, Combretaceae, Euphorbiaceae and Meliaceae were represented by two species each, whereas Arecaceae, Leguminosae, Myrsinaceae, Rubiaceae and Sterculiaceae were represented by one species each. Number of mangrove species was found to be high in Middle Andaman (22 species), followed by South Andaman (15 species), Ritchie’s Archipelago (14 species), North Andaman (12 species) and Little Andaman (8 species). Three mangrove species, which are categorized as threatened species according to IUCN red list, viz., Phoenix paludosa (Near Threatened), Sonneratia ovata (Near Threatened) and Sonneratia griffithii (Critically Endangered) were found in the Andaman Islands. Of these, Phoenix paludosa was distributed in North, Middle and South Andaman Islands, whereas Sonneratia ovata and S. griffithii were found in Ritchie’s Archipelago and Middle Andaman, respectively (Table 3).
Structural features
The total tree density and stand basal area in Andaman Island were 1658/ha and 38.59 m2/ha, respectively. Of the 25 species recorded, IVI for Rhizophora apiculata was the highest and three genera viz., Rhizophora, Bruguiera and Ceriops together constituted 67% of the IVI (Fig. 2). This shows the dominance of family Rhizophoraceae in the Andaman Islands. The overall IVI contribution of threatened species viz., Sonneratia ovata (0.27%), Phoenix paludosa (1.0%) and Sonneratia griffithii was very low (2.19%). Apart from these species, 16 species were having <5% of IVI, thus indicating their rarity in Andaman Islands. Among the recorded species, Bruguiera gymnorhiza, Ceriops tagal, Heritiera littoralis, Rhizophora apiculata, R. mucronata and Xylocarpus granatum were widely distributed in all the five regions surveyed.
Density and basal area of mangrove trees in the surveyed regions of Andaman Islands ranged from 1252 ha−1 to 2200 ha−1 and from 30.8 m2/ha to 59.6 m2/ha, respectively (Table 3). As tree density increased, basal area occupied by trees also increased in the studied regions except for South Andaman, which showed an inverse relationship between them (Fig. 3). Dominance of individuals with low DBH size class (<40 cm) could be attributed to the low basal area and high density in South Andaman Island. Interestingly, in all the regions, the maximum tree density was contributed by mangrove species belonging to the family Rhizophoraceae viz., Rhizophora mucronata in North Andaman (444.4 ha−1) and Ritchie’s archipelago (537.5 ha−1); R. apiculata in Middle Andaman (322.2 ha−1) and South Andaman (818 ha−1); and Bruguiera gymnorhiza in Little Andaman (520.0 ha−1). Similarly, species of Rhizophoraceae family were observed to have attained the maximum basal areas in all regions as follows: Bruguiera gymnorhiza in North Andaman (12.12 m2/ha); Middle Andaman (7.74 m2/ha) and Little Andaman (40.39 m2/ha); R. apiculata in South Andaman (13.34 m2/ha); and R. mucronata in Ritchie’s Archipelago (12.58 m2/ha) (Table 3). Accordingly, IVI of these species were also the highest in their respective regions, which indicated their significant contribution to the overall community structure. On the basis of IVI, it was found that North Andaman and Ritchie’s archipelago were represented by Rhizophora community; Middle Andaman by Rhizophora-Ceriops community, South Andaman by Rhizophora-Bruguiera community and Little Andaman by Bruguiera-Rhizophora community (Table 4). The study revealed that the mangrove forests of the Island were characterized by a mixed species composition, all belonging to the family Rhizophoraceae. Shallower slopes in dominance-diversity curve (log normal) (Fig. 4) indicated higher evenness among the assemblages of mangrove species in all regions. Frequency of mangrove trees belonging to 20–40 cm GBH was the highest in all the regions except for Little Andaman, where the trees with <20 cm GBH had greater frequency (Fig.5). It is also evident that beyond 20–40 cm GBH class, the number of tree stands gradually decreased with the increase in GBH of trees. However, this pattern was not consistent for the mangroves in Little Andaman.
Niche width measures the degree of specialization of a species and its ability to exploit an environmental range in space and also to maintain its population in different environments (Kalakoti et al. 1987). The species with wider niches are considered to be more generalized (Smith 1980). Across all regions, the highest niche width was exhibited by the species Rhizophora apiculata (23.7) followed by R. mucronata (16.07) and Bruguiera gymnorhiza (13.93) suggesting their better adaptation to environmental conditions in all these regions (Table 5). Niche width of mangrove species in Little Andaman was found to be uniformly low. However, mangroves of Little Andaman had high complexity index (Ic = 507.48), which could be attributed to the presence of taller trees (11.1–15.3 m) and high stand basal areas (59.6 m2 ha−1). Structural complexities of mangroves increased gradually from North Andaman to Little Andaman, which was reflected in their I c values (Table 6). Maturity index value (MIV) also showed that mangrove stands in Little Andaman had comparatively higher maturity (MIV = 37.5) than the stands in other regions (Table 6). However, MIV values for all regions were far less than the maximum value of 100, indicating their low degree of maturity (MIV for Andaman Island = 11.0).
Variability among the patches
Among 53 patches studied, number of species recorded was maximum in Betapur (14 species) followed by Rangat Bay, Yerrata, Namunagar and Manjery (Table 7). Diversity index also was the highest for Betapur (H′ = 2.3). Total tree density was the highest in Aerial Bay whereas stand basal area was the highest in Long Island. The patch -specific information is summarized below.
North Andaman
Among the 9 patches surveyed in North Andaman, maximum number of species was recorded from Aerial Bay and Ramnagar (7 species each). Total tree density was high in Aerial Bay (1.03/m2), whereas stand basal area was high in Milangram (95.44 m2/ha) (Table 7). Tree density was the lowest in Shyam Nagar, followed by Milangram, Swarajgram, and Laximupur. However, the estimated stand basal areas of all the patches, except Shyam Nagar, in the North Andaman Islands, were greater than 25m2/ha, indicating the pristine nature of mangrove forests. Low stand basal area in Shyam Nagar (<10 m2/ha) suggested the disturbed nature of mangrove forests. Shyam Nagar is the only mangrove patch located in west coast of North Andaman Island, whereas all the other surveyed patches were located on the eastern coast. Thus, the study showed that the impact of the land upheaval in North Andaman following the 2004 catastrophic earth quake was more on the west coast than the east coast of Andaman.
Middle Andaman
Out of the 15 patches surveyed in Middle Andaman, mangrove species diversity was the maximum in Betapur (14 species), followed by Rangat Bay and Yerrata (Table 7). Tree density was the highest at Rangat Bay (0.75/m2) and the lowest at Wrafter’s Creek (0.14/m2). Except for three patches (Katan, Yerrata, and Rangat Bay), all the other patches showed stand basal area greater than 25m2/ha, which indicated the pristine nature of mangroves of Middle Andaman Island (Komiyama et al. 2008). Stand basal area was the highest at Long island (137.36m2/ha), followed by Panchawati (124.32 m2/ha) and Tugapur (119.62 m2/ha. Though mangrove patch of Panchawati had the second highest stand basal area, tree density was comparatively lower. This indicates the occurrence of trees with higher GBH size classes (>40 cm). For instance, S. griffithii, one of the critically endangered species, constitute 76.5% of the stand basal area in Panchwati, whereas it contributes only 6.67% to the total tree density of the islands and the mean GBH was 236 cm. Low stand basal area and higher tree density of mangrove patches of Yerrata, Rangat and Katan showed the dominance of adult individual trees with smaller GBH classes (<40 cm). High density and low basal area are quite common in the developing forest (Twilley 1995).
South Andaman
In South Andaman, a total of 17 patches were surveyed and the maximum number of species was recorded from Manjery and Naminagar (10 species each). Total tree density was high at Chidiyatapu I (1.03/m2) and stand basal area was high at Rutland (124.2 m2/ha) (Table 7). Most of the mangrove patches in South Andaman Islands had high tree density and low stand basal areas, which indicated the maturing potential and high abundance of adult individual trees with low GBH size class (20-40 cm). However, mangrove patches of Kodiyaghat and Collinpur were observed to have the lowest densities and high basal area suggesting that the mangrove forests in these places were mature and comprised of individuals of high GBH size class (>40 cm).
Ritchie’s Archipelago
In Ritchie’s Archipelago, out of the 12 patches delineated, ten patches were surveyed. The maximum number of species was recorded from Radhanagar (9 species), where the tree density was also the highest (0.33/m2). Stand basal area was the highest in Havelock beach (92.87 m2/ha) (Table 7). Tree density and basal area (<25m2/ha) were lower in Bharatpur, Sitapur and Laxmanpur, indicating low structural development of mangrove forest. In Neil Kendra, tree density was the lowest and basal area was comparatively higher, which indicated the dominance of mature trees. Comparatively, mangrove patches of Ritchie’s Archipelago were found to have low structural development.
Little Andaman
In Little Andaman, out of the six patches mapped, two patches were surveyed and the maximum number of species was recorded from V.K. Pur (6 species), where the tree density (0.24/m2) and stand basal area (73.04 m2/ha) were also high (Table 7). Bruguiera gymnorrhiza was the dominant species in V. K. Pur, whereas Rhizophora apiculata was the predominant mangrove species in Kalapathar.
Diversity pattern
The Shannon diversity index and Simpson index (1-D) for the whole ANI were estimated as 2.28 and 0.85, respectively. Values of β-diversity ranged from 0.8 to 3.1 in the studied regions of Andaman Islands (Table 6). Shannon-Wiener indices ranged from 1.65 to 2.24, indicating a high level of mangrove diversity in all regions of Andaman. Further, higher values of Simpson index (1-D = 0.74 to 0.85) and Pielou’s evenness index (‘J’ = 0.66 to 0.84) indicated the presence of high mangrove diversity and their even distribution in all regions. Among the regions, richness (d = 3.04) and diversity (H = 2.24; 1-D = 0.85) of mangrove species were the highest in Middle Andaman, which were similar to the overall richness and diversity observed for the Andaman Islands (d = 3.00; H = 2.28; 1-D = 0.85) (Table 6). Sørensen similarity indices between the survey regions were high (> 0.6; Table 9), indicating the close floristic affinities between the surveyed regions.
Based on the IVI of mangrove species, cluster analysis (CA) as well as nMDS ordination grouped the five regions of the Andaman Islands into three main groups: Cluster 1 composed of North Andaman, Middle Andaman and South Andaman; Cluster 2 and Cluster 3 having Ritchie’s Archipelago and Little Andaman respectively (Fig. 6). SIMPER analysis revealed a high level of dissimilarity of mangrove community structure between South Andaman and Little Andaman (60.44%), and the lowest level of dissimilarity between Middle Andaman and South Andaman (32.73%, Table 8). Dissimilarities of mangrove community structure among the studied regions were mainly contributed by Rhizophora apiculata (19.2 to 23.1%), R. mucronata (18.6 to 25.9%) and Bruguiera gymnorhiza (23.5 to 35.7%), all belonging to the family Rhizophoraceae.
Discussion
Species composition
The earliest reference on mangrove floristics of the ANI listed 27 species (Parkinson 1923). Subsequently, no significant change in mangrove floristics of ANI was reported till 1986. Singh et al. (1987) reported five new records viz., Bruguiera sexangula, Bruguiera cylindrica, Rhizophora stylosa, R. × lamarckii and Acrostichum speciosum from ANI. In recent times six new records viz., Sonneratia ovate , Sonneratia lanceolata, S. × urama, S. × gulngai, Rhizophora × annamalayana and Excoecaria indica and three new entities, viz., Rhizophora mucronata var. alokii, Acrostichum hybrid and Rhizophora × mohanii have also been reported from the ANI (Goutham-Bharathi et al. 2012; Ragavan et al. 2015a,b). Mangroves of the ANI, represented by 38 true mangrove species, account for approximately 50% of all mangrove species in the world (Ragavan et al. 2016a,b).
The reduction in the number of species reported in this study - could be attributed essentially to the differences in sampling locations (Whitmore 1990). The Andaman Islands are uniform in climatic and physiographic conditions and contribute to medium level of tree species diversity (Padalia et al. 2004). Among the five regions surveyed, except Ritchie’s Archipelago, all the other regions had estuarine type of mangroves, fringing the creeks to varying widths, whereas in Ritchie’s Archipelago mangroves were of coastal/fringe type. Generally, riverine mangroves develop the most structural complexity than fringe and basin forests and exhibit much higher value of complexity index than other habitats (Cintron et al. 1980). Coastal type mangroves are reported to have less diversity and zonation pattern (Utawale et al. 1973). Thus, mangroves in Ritchie’s Archipelago had shown low structural development, despite high species diversity than those of North Andaman and Little Andaman. The tree density in North Andaman was the lowest in the Andaman Islands. The low density and diversity in the North Andaman can be attributed to the land upheaval and easterly tilt of land caused by 2004 massive earthquake (Malik and Murty 2005; Ray and Acharyya 2007, 2011). Particularly, the uplift was more on the western side than on the eastern side of the Island - The uplift of land caused the significant reduction in mangrove area of North Andaman; however, its effects on individual species could not be assessed due to lack of patch-specific information. A change in the composition of mangrove stands of ANI following the land subsidence/upheaval was reported by Roy and Krishnan (2005). Coastal areas of South Andaman Islands were affected by subsidence. However, high tree density reported in this study supports the colonisation ability of mangroves in the submerged areas, which were inferred in earlier studies (Das et al. 2014).
Structural features
In total, 3073 mangrove plants were enumerated in 178 quadrats laid in the Andaman Islands in the present study, of which trees, saplings and seedlings accounted for 96.06%, 1.99% and 1.95% respectively. The low contribution of seedlings and saplings in the Andaman Islands could be attributed to the dense canopy and lack of canopy gap in surveyed sites. Very dense mangrove comprised 65% of the mangrove cover in the ANI (FSI 2015). Creation of canopy gap could be a key driver in the natural regeneration of the tropical mangrove, and in particular Rhizophora dominated mangroves (Kathiresan and Bingam 2001). The presence of dense canopy does not allow full penetration of solar radiation, which is a necessary factor for the growth of plants. Thus, the mangrove community in the Andaman Islands was found to be dominated by tree stands. Tree density in the Andaman Islands ranged from 0.05 to 0.5 individual/m2 and the tree density was 0.16 individual/m2, which was almost similar to the values reported for the mangrove forests in Thailand (Chasang 1984 and Macintosh et al. 2002) and Indonesia (Hinrichs et al. 2009) suggesting close floristic affinities of the Islands towards the Southeast Asian countries.
Most studies on the vegetative structure of the mangrove forests of the Andaman Islands (Mall et al. 1982, Dagar et al. 1991, Singh and Garge 1993) were from South Andaman, which is the most intensively studied region in Andaman (Goutham-Bharathi et al. 2014). According to Mall et al. (1982), the mangrove forests of South Andaman had a density of 0.11 individual/m2. Twenty-two years later, Padalia et al. (2004) reported a density of 0.067 individual/m2. According to the findings of Das et al. (2014), mangrove density in the Andaman Islands ranged from 3.07 to 5.69 individuals/m2. In the present study, the stand basal area in Andaman Islands ranged between 12.34 m2/ha and 295.5m2/ha, which is higher than that reported from Malaysia (1.5 to 49 m2/ha; Satyanarayana et al. 2010;) and Srilanka (48.25 to 27.1 m2/ha; Perara et al. 2013). Padalia et al. (2004) reported a stand basal area of 7.92 m2/ha from the Andaman Islands; this low basal area could be attributed to less sampling efforts. Pristine mangrove forests with minimal impacts have been shown to have a basal area of >25 m2 ha−1 (Komiyama et al. 2008; Kauffman et al. 2011); secondary forest has been found to have basal areas of around 15 m2 ha−1 (Komiyama et al. 2008; Cavalcanti et al. 2009), and disturbed forests show basal areas of <10 m2 ha−1 (Komiyama et al. 2008). In the present study, the observed stand basal areas of all the five surveyed regions were greater than 25 m2/ha, indicating that the mangroves in Andaman Islands are intact and pristine. High basal area can also be attributed to the low population density of species (Padalia et al. 2004). It was evident from this study that certain species with higher basal areas were reported with lower density of adult trees. This low tree density and high basal area are indicative of mature forests, with a good structural development (Cintron and Schaefer-Novelli 1983; Jimenez Lugo and Cintron 1985; Fromard et al. 1998).
Rarity in natural systems is common and is most often defined by two attributes: the species distribution and its abundance (Flather and Sieg 2007). Except for the colonizing/pioneering species of the family Rhizophoraceae, like Rhizophora apiculata, R. mucronata, Bruguiera gumnorhiza and Ceriops tagal, all the other true mangrove species encountered in the present study strongly exhibited a negative correlation between relative frequency and relative abundance. Therefore, species with restricted distribution should be studied extensively and species-specific conservation should be taken.
Higher population of Rhizophora apiculata, R. mucronata and Bruguiera gymnorhiza indicated their wide distribution/dominance in Andaman Islands, compared to another mangrove species. Population status of threatened species (Phoenix paludosa, Sonneratia griffithii and S. ovata) was <25 individuals/ha in the Andaman Islands, which emphasized the need of their conservation. Based on the overall stand basal area, it was evident that the mangrove stands in Andaman Islands were composed of uneven, aged, mixed mangrove forest with good structural development. Further, the frequency of occurrence was the highest for the 20–40 cm girth class, indicating that the mangrove community was still in growing phase, as also reported by earlier researchers (Balachandra 1988, Padalia et al. 2004). The low maturity index value (less than 100) also indicated the developing phase of Andaman mangroves. Complexity Index (Ic) is often used for quantitative description of the structural complexity of the tropical vegetation (Pool et al. 1977). These values for Andaman mangroves were far greater than reported in the earlier studies (Pool et al. 1977; Fromard et al. 1998; Amarasinghe and Balasubramaniam 1992), but were similar to the mangroves of Odisha (Upadhyay and Mishra 2014). The high complexity index values recorded in the present study could be attributed to dominance by riverine type of mangroves in Andaman Islands. In general, low β-diversity (<1) indicated low heterogeneity and values >5 shows high heterogeneity in forest structure (McCune and Grace 2002). The lowest β-diversity (<1.0) recorded in Ritchie’s Archipelago indicated the homogenous nature of mangroves. Other regions had relatively high β-diversity (≥2) indicating the extent of change in mangrove community composition in those regions. Overall β-diversity in Andaman Island (β w = 4.1) suggested a high level of heterogeneity between the regions in terms of mangrove communities.
Diversity pattern
Diversity pattern of mangrove species in the present study was found to be in agreement with the pattern described by Ragavan et al. (2015a,b). Cluster analysis and nMDS ordination clearly distinguished Little Andaman from the other studied regions in Andaman Islands. Though the clustering was explained by importance values of mangrove species, Little Andaman was found to have the lowest species richness (8 species), highest basal area (59.6 m2/ha), highest complexity index (507.4), highest maturity (37.5), lowest β-diversity (0.8) and low niche width of mangrove species (≤1.1).
Role of mangroves in coastal protection
Biological structures such as mangrove forests, salt marshes, seagrass beds and coral reefs attenuate waves and as a result provide coastal protection (Koch et al. 2009). Recently Yulianda et al. (2014) studied the influences of mangroves on the coastal conservation and found that density and thickness of mangroves growing on the seashore significantly contribute to maintaining the stability of the coast. It has also been known that coastlines with and without mangroves respond differently and mangrove-dominated coastal segments exhibit less erosion while non-vegetated segments or former mangrove areas incur substantial erosion (Thampanya et al. 2006). The protective role of mangrove forest against catastrophic events like cyclones, storms, tsunami etc., have been widely reported (Badola and Hussain 2005; Danielson et al. 2005; Kathiresan and Rajendran 2005; Iverson and Prasad 2007; Malik et al. 2015). However, few studies have reported the inefficiency of mangroves against long waves, including storm surges and tsunami (Kerr et al. 2006; Dahdough-Guebas, 2006; Yanagisawa et al. 2009). Recent research regarding the extent of coastal protection provided by mangroves includes observational studies, numerical modeling, and laboratory experiments (Marois and Mitsch 2015). Of these, observational studies have not provided conclusive results, whereas, numerical and physical models support the mitigating capabilities of mangroves for cyclone storm surges and small tsunamis (Hiraishi and Harada 2003; Yanagisawa et al. 2010; Zhang et al. 2012). The density and width of mangrove vegetation is expected to affect the ability of mangroves to reduce effects storm surge and tsunamis (Krauss et al. 2009; Zhang et al. 2012). Increased mangrove densities decreased the maximum water level, run-up height, and maximum flow speed (Gelfenbaum et al. 2007) and mangrove belts of several hundred meters wide are expected to reduce flood depth by 5 to 30%. Furthermore, the capacity of mangroves to reduce hazards may be limited by characteristics of the hazard, such as tsunami height or wind speeds during cyclones, which can also destroy mangroves. This also needs to be taken into account when planning coastal risk-reduction strategies, and is a further reason for ensuring a diverse approach to risk reduction. Studies on the economic valuation of mangroves have estimated coastal protection to be a major portion of their total value (Marois and Mitsch 2015). Chand et al. (2013) also reported that coastline protection as the most important benefits of mangroves followed by biodiversity and protection of natural resources in ANI. Rich species diversity and high structural complexity of Andaman mangroves observed in the present also indicate that mangroves play a greater role in protection of this island ecosystem. The structural complexity is one of the determining factors for the protective role of mangroves (Spalding et al. 2014), thus mangroves with high structural complexity play a significant role in coastal conversation and management (Bosire et al. 2008). The differences in the structural development may reflect the influence of variations in tidal range, salinity gradients, texture and stability of the soil, and contribution of nutrients (Delgado et al. 2001; Lovelock et al. 2005), which is specific to each habitat. But studies to explain the relation between these habitat characteristics and forest structure are limited in India. So such studies are needed for better understanding of various ecological services offered by Indian mangroves.
Mangroves are the only marginal ecosystems which share the resources with adjoining ecosystems (Vannucci 2001). Mangroves serve as an intermediate nursery habitat and may increase the survivorship and recruitment success of commercially valuable species harvested in other ecosystems, such as coral reefs and pelagic zones (Parrish 1989, Mumby et al. 2004; Honda et al. 2013) and also affect the productivity of adjacent habitat (e.g. coral reefs and seagrass; Nagelkerken et al. 2012). Andaman Islands are bestowed with various marine and coastal ecosystems (coral reefs, seagrass etc.,), which support rich floral and faunal diversity (Rajan et al. 2013). In many sites in Andaman, mangroves are located contiguous to areas with coral reefs and seagrasses. Thus, adverse effects on mangroves could lead to serious consequences of these adjoining fragile and important ecosystems.
Conclusion
Despite the wide ecological and socioeconomic values of mangroves, they are threatened globally due to various anthropogenic activities and global climate change. The primary driver of anthropogenic mangrove loss was found to be conversion of mangroves to aquaculture and agriculture (Thomas et al. 2017). The major factors which threatened Indian mangroves are deforestation for urbanization and aquaculture and agriculture expansion, reduction in freshwater flow, marine and coastal pollution, siltation, sedimentation and excessive salinity. Compared to other mangrove habitats, mangroves of ANI are free from these anthropogenic activities. However, geomorphological changes due to the massive earthquake and subsequent tsunami on 26th December 2004 have drastically affected the mangroves of ANI. About 54 km2 of mangroves were degraded between 2003 and 2013, and particularly between 2011 and 2013, 13 km2 of mangrove stands have been degraded (FSI 2015). Subsequently, during 2013–2015, it was found that the mangrove extent increased by 13km2.
Mangrove patches studied in Andaman Islands were dominated by the members of the family Rhizophoraceae and dominance of trees with 20–40 cm diameter size class and stand basal area greater than >25m2/ha in all surveyed regions are indicative of good structural development in mangroves of the Andaman Islands. The high diversity and structural values reported in the present study and recent increase of 13 km2 areas of mangroves in Andaman Islands (Forest Survey of India 2015) are indicative of high resilience potential and protective role of mangroves of Andaman Islands. The mangroves in Andaman Islands, in many locations, are in close proximity to the other coastal ecosystems like coral reefs and seagrasses, and in the light of the ecological interactions between these ecosystems and their associated marine biodiversity, preserving the luxuriant mangroves of these Islands assumes particular significance.
Conservation of mangroves is essential for sustaining and increasing the extent of mangroves in the islands, which are prone to many natural hazards. The ability of mangroves to reduce these coastal hazards, is influenced by the mangrove forest width and vegetation density. Generally, mangrove belts, which are several hundred meters wide and possess dense canopy are most effective in reducing hazards, and more specifically, predominance of the mangrove species with aerial roots would enable them to reduce wind and swell waves and also to withstand inundation by tsunamis. Thus, understanding the mangrove vegetation structure is always useful for their conservation and management. To safeguard the risk reduction values of mangroves, it is imperative to include mangroves restoration as a part of integrated coastal zone management plans (ICZMP).
The mangroves in India are conserved through multi-layered regulatory tools viz., Indian Forest Act, 1927; Forest (Conservation) Act, 1980; and the Island Protection Zone (IPZ), 2011 notification under the Environment (Protection) Act, 1986. The EPA, 1986 and the IPZ, 2011, thereunder, provide for identifying areas with high ecological significance and prepare location-specific integrated management plans. The Andaman and Nicobar Coastal Zone Management Authority (ANCZMA) established under the IPZ, 2011 notification and the Union Territory Forest Department are responsible for implementing the various provisions of the aforesaid regulatory frameworks. So far, the conservation regimes have laid emphasis on increasing the area of mangroves and most of the restoration/rehabilitation efforts were undertaken based on inadequate species-specific information. The contemporary mangrove conservation regime advocates “early detection and pre-emptive rehabilitation ”, for successful management. And to achieve this, location-specific and species-specific information on the mangrove stands are the prerequisites (Lewis et al. 2016). The spatial data collected in this study pertaining to the overall health of the ecosystems, dominant species, etc. would aid in preparing a science-based species-specific and location-specific management plan, within the existing legal framework for mangrove conservation in India. Thus, it would help to check degradation of existing mangroves and plan effective mangrove restoration/rehabilitation strategies. The current study is be a step towards this goal and would aid in location-specific conservation planning for proper management and rejuvenation of the mangroves in Andaman Islands.
References
Alongi DM (2008) Mangrove forests: resilience, protection from tsunamis, and responses to global climate change. Estuar Coast Shelf Sci 76:1–13
Amarasinghe MD, Balasubramaniam S (1992) Structural properties of two types of mangrove stands on the northwestern coast of Sri Lanka. Hydrobiology 247:17–27
Badola R, Hussain SA (2005) Valuing ecosystem functions: an empirical study on storm protection function of Bhitarkanika mangrove ecosystem, India. Environ Conserv 32(1):85–92
Balachandra L (1988) A comprehensive account of the mangrove vegetation of Andaman and Nicobar Islands. Ind For 114:741–751
Biró M, Révész A, Horváth F, Molnár Z (2006) Point based mapping of the actual vegetation of a large area in Hungary – description, usability and limitation of the method. Acta Bot Hungar 48:247–269
Bosire JO, Dahdouh-Guebas F, Walton M, Crona BI, Lewis RR, Field C, Kairo JG, Koedam N (2008) Functionality of restored mangroves: A review. Aquat Bot 89:251–259
Cavalcanti VF, Soares MLG, Estrada ECD, Chaves FO (2009) Evaluating mangrove conservation through the analysis of forest structure data. J Coast Res 56:390–394
Chand S, Srivastava RC, Krishnan P, Roy SD (2013) Valuation of mangrove services of Andaman and Nicobar Islands, India. Basic Res J Agric Sci Rev 2(6):130–137
Chao A, Chazdon RL, Colwell RK, Shen TJ (2006) Abundance-based similarity indices and their estimation when there are unseen species in samples. Biometrics 62:361–371
Chasang H (1984) Structure of a mangrove forest at Ko Yao Yai, Southern Thailand. In: Soepadmo E, Rao AN, Macintosh DJ (eds) Proceedings of the Asian symposium on mangrove environment research and management. Kuala Lumpur 1980. University of Malaya and UNESCO, Kuala Lumpur, pp 86–105
Chaudhuri P, Ghosh S, Bakshi M, Bhattacharyya S, Nath B (2015) A review of threats and vulnerabilities to mangrove habitats: with special emphasis on the east coast of India. J Earth Sci Clim Change 6:4
Cintron G, Schaefer-Novelli Y (1983) Introduction to mangrove ecology. Regional Office of Science and Technology. Latin America and UNESCO, Montevideo
Cintrón G, Lugo AE, Martínez R (1980) Structural and functional properties of mangrove forests. A symposium signalling the Completion of the ‘Flora of Panama’. University of Panama, Pananma city
Clarke KR, Gorley RN (2015) PRIMER v7: User Manual/Tutorial. PRIMER-E, Plymouth, p 296
Curtis JT, Mclntosh RP (1950) The interrelationships of certain analytic and synthetic Phyto-sociological characters. Ecology 31:434–455
Curtis JT (1959) The vegetation of Wisconsin, An ordination of plant communities. University of Wisconsin Press, Wisconsin
Dagar JC, Mongia AD, Bandhyopadhyay AK (1991) Mangroves of Andaman and Nicobar Islands. Oxford, and IBH, New Delhi, p 166
Dahdouh-Guebas F (2006) Coastal vegetation and the Asian tsunami. Science 311:37
Danielsen F, Sorensen MK, Olwig MF, Selvam V, Parish F, Burgess ND, Hirashi T, Karunagaran VM, Rasmussen MS, Hansen L, Quarto A, Suryadiputra N (2005) The Asian tsunami: A protective role for coastal vegetation. Science 310:643
Das AK, Jha DK, Prashanthi Devi M, Sahu BK, Vinithkumar NV, Kirubagaran R (2014) Post tsunami mangrove evaluation in coastal vicinity of Andaman Islands, India. J Coast Conserv 18:249–255
Delgado P, Hensel P, Jones JA, Day JD (2001) The Importance of propagule establishment and physical factors in mangrove distributional patterns in Costa Rican estuary. Aquat Bot 71:157–178
Dislich R, Pivello VR (2002) Tree structure and species composition changes in an urban tropical forest fragment (Sâo Paulo, Brazil) during a five year interval. Bol Bot Univ Sâo Paulo 20:1–11
Ellison JC (2015) Vulnerability assessment of mangroves to climate change and sea level rise impacts. Wetl Ecol Manag 23:115–137
Flather CH, Sieg CH (2007) Species rarity: definition, causes, and classification. In: Raphael M, Molina R (eds) Conservation of rare or little-known species. Island Press, Portland, pp 40–66
Fromard F, Puig H, Mougin E, Marty G, Betoulle JL, Cadamuro L (1998) Structure, above-ground biomass and dynamics of mangrove ecosystems: New data from French Guiana. Oecologia 115:39–53
FSI (Forest Survey of India) (2015) Status of forest report. Forest Survey of India, Dehra Dun, p 288
Gelfenbaum G, Vatvani D, Jaffe B, Dekker E (2007) Tsunami inundation and sediment transport in vicinity of coastal mangrove forest, in Proceedings of Coastal Sediments pp 1117–1128
Goutham-Bharathi MP, Kaliyamoorthy M, Roy SD, Krishnan P, George G, Murugan C (2012) Sonneratia ovata (Sonneratiaceae) -A New Distributional Record for India from Andaman and Nicobar Islands. Taiwania 57:406–409
Goutham-Bharathi MP, Dam Roy S, Krishnan P, Kaliyamoorthy M, Immanuel T (2014) Species diversity and distribution of mangroves in Andaman and Nicobar Islands India. Bot Mar 57:421–432
Hamilton SE, Casey D (2016) Creation of a high spatio-temporal resolution global database of continuous mangrove forest cover for the 21st century (CGMFC-21). Glob Ecol Biogeogr 25(6):729–738
Hinrichs S, Nordhaus I, Geist SJ (2009) Status, diversity and distribution patterns of mangrove vegetation in the Segara Anakan lagoon, Java, Indonesia. Reg Environ Chang 9:275–289
Hirashi T, Harada K (2003) Greenbelt tsunami protection in South–Pacific region. Rep Port Res Inst 42:1–23
Holdridge LR (1967) Life zone ecology. Tropical Science Center, San Jose
Honda K, Nakamura Y, Nakaoka M, Uy WH, Fortes MD (2013) Habitat use of fishes in coral reefs, seagrass beds and mangrove habitat in the, phillipines. PLoS One 8(8):e65735
Iverson L, Prasad A (2007) Using landscape analysis to assess and model tsunami damage in Aceh province, Sumatra. Landsc Ecol 22:323–331
Jiménez JA, Lugo AE, Cintron G (1985) Tree mortality in mangrove forests. Biotropica 17:177–185
Kalakoti BS, Pangty YPS, Saxena AK (1987) Quantitative analysis of high altitude vegetation in Kumaon Himalaya. J Indian Bot Soc 65:384–396
Kathiresan K, Bingam BL (2001) Biology of mangroves and mangrove ecosystems. Adv Mar Biol 40:81–251
Kathiresan K, Rajendran N (2005) Coastal mangrove forests mitigated tsunami. Estuar Coast Shelf Sci 65:601–606
Kauffman JB, Heider C, Cole TG, Dwire KA, Donato DC (2011) Ecosystem carbon stocks of Micronesian mangrove forests. Wetlands 31:343–352
Kerr AM, Baird AH, Campbell ST (2006) Comments on coastal mangrove forests mitigated tsunami by Kathiresan K and Rajendran N. Estuar Coast Shelf Sci 67(3):539–541
Koch EW, Barbier EB, Silliman BR, Reed DJ, Perillo GME, Hacker SD, Granek EF, Primavera JH, Muthiga N, Polasky S, Halpern BS, Kennedy CJ, Kappel CV, Wolanski E (2009) Non linearity in ecosystem services: temporal and spatial variability in coastal protection. Front Ecol Environ 7:29–37
Komiyama A, Ong JE, Poungparn S (2008) Allometry, biomass and productivity of mangrove forests: a review. Aquat Bot 89:128–137
Krauss K, Doyle T, Doyle T, Swarzenski C, From A, Day R, Corner W (2009) Water level observations in mangrove swamps during two hurricanes in Florida. Wetlands 29:142–149
Lee SY, Primavera JH, Dahdouh-Guebas MK, Bosire JO, Cannicci S, Diele K, Fromard F, Koedam N, Marchand C, Mendelssohn I, Mukherjee N, Record S (2014) Ecological role and services of tropical mangrove ecosystems: a reassessment. Glob Ecol Biogeogr 23:726–743
Levins R (1968) Evolution in changing environments. Princeton University Press, Princeton
Lewis RR, Milbrandt EC, Brown B, Krauss KW, Rovai AS, Beever JW, Flynn LL (2016) Stress in mangrove forests: early detection and preemptive rehabilitation are essential for future successful worldwide mangrove forests management. Mar Pollut Bull 109:764–771
Lovelock CE, Feller I, McKee KL, Thompson R (2005) Variation in mangrove forest structure and sediment características in Bocas del Toro, Panama. Caribb J Sci 41:456–464
Macintosh DJ, Ashton EC, Havanon S (2002) Mangrove rehabilitation and intertidal biodiversity: a study in the Ranong mangrove ecosystem, Thailand. Estuar Coast Shelf Sci 55:331–345
Malik JN, Murty CVR (2005) Landscape changes in Andaman and Nicobar Islands (India) due toMw 9.3 tsunamigenic Sumatra earthquake of 26 December 2004. Curr Sci 88:1384–1386
Malik M, Fensholt R, Mertz O (2015) Mangrove exploitation effects on biodiversity and ecosystem services. Biodivers Conserv 24:3543–3557
Mall LP, Billore SK, Amritphale AD (1982) Certain ecological observation on mangroves of Andaman Islands. Trop Ecol 23:225–233
McCune B, Grace JB, Urban DL (2002) Analysis of Ecological communities. MJM Software Design, Gleneden Beach
Margalef FR (1978) Information theory in ecology. Gen Syst 3:36–71. https://doi.org/10.1007/s11852-017-0539-z
Marois DE, Mitsch WJ (2015) Coastal protection from tsunamis and cyclones provided by mangrove wetlands- a review. Int J Biodivers Sci Ecosyst Serv Manage 11(1):71–83
Menon ARR (2006) Biodiversity characterization at landscape level using satellite remote sensing - Phase-II study. Research report No. 284. Kerala Forest Research Institute, Kerala, 30 pp
Mumby PJ, Edwards AJ, Arias-Gonzalez JE, Linderman KC, Blackwell PG, Gall A, Gorczynska MI, Harborne AR, Pescod CL, Renken H, Wabnitz CCC, Llewellyn G (2004) Mangroves enhance the biomass of coral reef fish communities in the Carribean. Nature 427:553–536
Nabi A, Rao PB, Prasad AVP (2012) Analysis of mangrove vegetation of Diviseema region, Krishna district, Andhra Pradesh. Inter J Plant, Anim Environ Sci 2:99–108
Nagelkerken I, Grol MGG, Mumby PJ (2012) Effects of marine reserves versus nursery habitat availability on structure of reef fish community. PLoS One 7:E36906
Padalia H, Chauhan N, Porwal MC, Roy PS (2004) Phytosociological observations on tree species diversity of Andaman Islands, India. Curr Sci 87:799–806
Parkinson CE (1923) A forest flora of the Andaman Islands. Bishen Singh and Mahendrapal Singh, Dehradun, 325 pp
Parrish JD (1989) Fish communities of interacting shallow-water habitats in tropical oceanic regions. Mar Ecol Prog Ser 58:143–160
Perera KARS, Amarasinghe MD, Somaratna S (2013) Vegetative structure and species distribution of mangroves along the soil salinity gradient in a microtidal estuary on the North-Western coast of Sri Lanka. Am J Mar Sci 1:7–15
Pielou EC (1966) The measurements of diversity in different types of biological collections. J Theor Bio 13:131–144
Pool DJ, Snedaker SC, Lugo AE (1977) Structure of Mangrove forests in Florida, Puerto Rico, Mexico and Costa Rica. Biotropica 9:195–212
Ragavan P, Saxena A, Mohan PM, Sachithanantham V, Coomar T (2014) Floral composition and taxonomy of mangroves of Andaman and Nicobar Islands. Ind J Geo-marine Sci 43(6):1037–1050
Ragavan P, Saxena A, Jayaraj RSC, Ravichandran K, Saravanan S (2015a) Rhizophora x mohanii: A putative hybrid between Rhizophora mucronata and Rhizophora stylosa from mangroves of the Andaman and Nicobar Islands, India. ISME/GLOMIS Electronic J 13:3–7
Ragavan P, Saxena A, Mohan PM, Ravichandran K, Jayaraj RSC, Saravanan S (2015b) Diversity, distribution and vegetative structure of mangroves of the Andaman and Nicobar Islands, India. J Coast Conserv 19:417–443
Ragavan P, Mohan PM, Saxena A, Jayaraj RSC, Ravichandran K, Saxena M (2016a) Mangrove floristics of the Andaman and Nicobar Islands: critical review and current scenario. Mar Biodivers:1–21. doi:https://doi.org/10.1007/s12526-016-0581-3
Ragavan P, Saxena A, Jayaraj JSC, Mohan PM, Ravichandran K, Saravanan S, Vijayaraghavan A (2016b) A review of mangrove floristics of India. Taiwania 61:224–242
Rajan PT, Sreeraj CR, Immanuel T (2013) Fishes of Andaman and Nicobar Islands: A Checklist. J Andaman Sci Assoc 19:47–87
Ray SK, Acharyya A (2007) 26 December 2004 earthquake: coseismic vertical ground movement in the Andaman Island. Geol Surv India Spec Publ 89:63–81
Ray SK, Acharyya A (2011) Coseismic uplift, slow plant mortality and ecological impact in North Andaman following the December 2004 (Mw> 9.2) earthquake. Curr Sci 101:218–222
Révész A, Horváth F, Czúcz B, Zs M, Biró M, Koczka K, Sipos F, Sipos K, Vajda Z, Papp O, Suskó Z (2003) A Nemzeti Ökológiai Hálózat vizsgálata a Duna-Tisza közén. Zárójelentés. MTA ÖBKI, Vácrátót
Ronnback P, Crona B, Ingwall L (2007) The return of ecosystem goods and services in replanted mangrove forests: Perspectives from local communities in Kenya. Environ Conserv 34:313–324
Roy SD (2003) A Compendium on Mangrove Biodiversity of Andaman & Nicobar Islands, Director, CARI Port Blair and Agro Ecosystem Director, NATP, 196 pp
Roy SD, Krishnan P (2005) Mangrove stands of Andaman vis-à-vis tsunami. Curr Sci 89:1800–1804
Roy SD, Krishnan P, George G, Kaliyamoorthy M, Goutham-Bharathi MP (2009) Mangroves of Andaman and Nicobar Islands. Central Agricultural Research Institute, Port Blair, 65 pp
Sagar R, Ragubanshi AS, Singh JS (2003) Tree species composition, dispersion and diversity along a disturbance gradient in a dry tropical forest region of India. Forest Ecol Manag 186:61–71
Satyanarayana B, Idris IF, Mohamad KA, Husain ML, Shazili NAM, Dahdouh-Guebas F (2010) Mangrove species distribution and abundance in relation to local environmental settings: a case study at Tumpat, Kelantan Delta, East coast of peninsular Malaysia. Bot Mar 53:79–88
Shannon CE, Weiner W (1963) The mathematical theory of communication. University of Illinois Press, Urbana
Simpson EH (1949) Measurement of diversity. Nature 163:688
Singh VP, Garge A, Pathak SM, Mall LP (1987) Pattern and process in mangrove forests of the Andaman Islands. Vegetatio 71:185–188
Singh VP, Garge A (1993) Ecology of mangrove swamps of the Andaman Islands. International Book Distributors, Dehradun, 181 pp
Smith PL (1980) Ecology and field biology. Harper and Row Publishers, New York
Spalding M, Kainuma M, Collins L (2010). World atlas of mangroves (version 1.1). A collaborative project of ITTO, ISME, FAO, UNEP-WCMC, UNESCO-MAB, UNU-INWEH and TNC. London (UK): Earthscan, London
Spalding M, McIvor A, Tonneijck FH, Tol S, van Eijk P (2014) Mangroves for coastal defense. Guidelines for coastal managers and policy makers. Published by Wetlands International and The Nature Conservancy, Cambridge
Sørensen T (1948) A method of establishing groups of equal amplitude in plant sociology based on similarity of species and its application to analyses of the vegetation on Danish commons. Kgl Danske Vidensk Selsk Biol Skr 5:1–34
Thampanya U, Vermaat JE, Sinsakul S, Panapitukkul N (2006) Coastal erosion and mangrove progradation of Southern Thailand. Estuar Coast Shelf Sci 68:75–85
Thomas N, Lucas R, Bunting P, Hardy A, Rosenqvist A, Simard M (2017) Distribution and drivers of global mangrove forest change, 1996-2010. PLosOne 12:e0179302
Twilley RR, Snedaker SC, Yanez-Arancibia A, Medina E (1995) Biodiversity and ecosystem functioning: ecosystem analysis. Mangrove systems. In: Heywood VH (ed) Global biodiversity assessment UNEP. Cambridge University Press, Cambridge, pp 387–392
UNEP (2001) India: State of the Environment – 2001. United Nations Environment Programme, Nairobi
Upadhyay VP, Mishra PK (2014) An ecological analysis of mangrove ecosystem of Odisha on the eastern coast of India. Proc Indian Natl Sci Acad 3:647–661
Utawale AG, Dwivedi SN, Singbal SYS (1973) Ecology of mangroves in Mandovi and Zuari estuaries and the interconnecting Cumbarja canal of Goa Ind. J Mar Sci 2:47–53
Vannucci M (2001) What is so special about mangroves? Braz J Biol 61:599–603
Walters BB, Ronnback P, Kovacs JM, Crona B, Hussain SA, Badola R, Primavera JH, Barbier E, Dahdouh-Guebas F (2008) Ethnobiology, socio-economics and management of mangrove forests: A review. Aquat Bot 89:220–236
Whitmore TC (1990) An Introduction to Tropical Rain Forests. Clarendon Press, Oxford
Whittaker RH (1972) Evolution and measurement of species diversity. Taxon 21:213–225
Yanagisawa H, Koshimura S, Goto K, Miyagi T, Imamura F, Ruangrassamee A, Tanavud C (2009) The reduction effect of mangrove forest on a tsunami based on field surveys at Pakarang Cape, Thailand and numerical analysis. Estuar Coast Shelf Sci 81:27–37
Yanagisawa H, Koshimura S, Miyagi T, Imamura F (2010) Tsunami damage reduction performance of a mangrove forest in Banda Aceh, Indonesia inferred from field data and a numerical model. J Geophys Res 115:1–11
Yulianda F, Wardiatno Y, Nurjaya IW, Herison A (2014) Coastal conservation strategy using mangrove ecology system approach. Asian J Scient Res 7:513–524
Zhang K, Liu H, Li Y, Xu H, Shen J, Rhome J, Smith Iii TJ (2012) The role of mangroves in attenuating storm surges. Estuar Coast Shelf Sci 102–103:11–2
Acknowledgements
The study was undertaken by ICAR-CIARI, Port Blair under the project entitled, “Determination of the Conservation Value of Mangroves of the Andaman”, which is a sub-component of the National Research Project, “Delineation of ecologically sensitive areas (ESA) and critically vulnerable coastal areas (CVCA) along the coast of India” sanctioned to NCSCM, Chennai by Society for Integrated Coastal Zone Management (SICOM), under the Ministry of Environment, Forest and Climate Change, Government of India. The authors thank Liju Thomas, Ganesh Basumatary, Aluri Swapna and Yuvraj for their immense help rendered in field data collection. The authors would also like to thank Dr. J. Joyson Joe Jeevamani and Dr. Yambem Tenjing Singh, Research Associates, NCSCM for their assistance in data analysis and Dr. R. Soundararajan, Senior Scientific Consultant, NCSCM for critically reviewing the manuscript.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kiruba-Sankar, R., Krishnan, P., Dam Roy, S. et al. Structural complexity and tree species composition of mangrove forests of the Andaman Islands, India. J Coast Conserv 22, 217–234 (2018). https://doi.org/10.1007/s11852-017-0588-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11852-017-0588-3