Abstract
The distribution of heavy metals (Cu and Zn) and their geochemical speciation (exchangeable fraction, carbonate fraction, Fe/Mn oxide fraction, organic fraction and residual fraction) in wetland soils of Poyang Lake were examined by modified Tessier sequential extraction methods. The results showed that the Cu and Zn concentrations in the wetland soils were 6.83–342.54 mg·kg−1 and 34.39–195.36 mg·kg−1, respectively. The highest heavy metal concentrations were found in the wetland soils located in the lower reaches of mines and metal smelters. The metal speciation analysis showed that Cu and Zn were mainly linked to the residual fraction in all samples which account for 65.64% and 68.14%, respectively. The content of the exchangeable fraction (Cu 1.00%, Zn 3.60%) and carbonate fraction (Cu 2.09%, Zn 1.53%) was generally lower. Assessments of pollution levels revealed that Cu and Zn were much higher than their background value and heavy metal pollution in the wetland soils located in the lower reaches of mines and metal smelters was often more severe. The sites Duchang and Hukou were least polluted by Cu and Zn.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Wetlands play an important role in retaining pollutants and protecting water quality of rivers or/and lakes (Mitsch and Gosselink 2000; Knox et al. 2017; Melly et al. 2017; Cui et al. 2016). Wetlands have long been recognized as an important sink for heavy metals due to a variety of physical, chemical, and biological processes which involve sedimentation, settling, adsorption, precipitation, and absorption and induced changes in biogeochemical cycles by plants and bacteria (Lu et al. 2016; Garg 2015; Oyuela Leguizamo et al. 2017). They are potential sources for the surrounding communities providing a wealth of ecological, social and economic functions to the countries in the region (Matagi et al. 1998; Jiang et al. 2016). Poyang Lake is the largest freshwater lake in China and one of the most important wetlands in the world. It receives a large quantity of water coming from the Ganjiang River, Fuhe River, Xinjiang River, Raohe River and Xiushui River, together with several smaller tributaries such as Xihe River, Zhangtian River and Tongjing River etc., then releases the incoming water into the Yangtze River through a narrow passage regulated at Hukou. These inputs are the most significant contributors of industrial wastewater and domestic sewage to the lake. At the upstream of these rivers especially at the upstream of Raohe River, many non-ferrous metal mines have been exploited for decades, such as Dexing and Yongping copper mines, Yinshan lead-zinc mine and Huaqiao gold mine. In addition, the Guixi metallurgical refinery, are located next to the Poyang Lake. Mining activities and metal smelting have been known to be the main heavy metal sources (Rodríguez et al. 2009; Li et al. 2014; Xiao et al. 2017; Kwon et al. 2017; Hamiani et al. 2010). The exploration and extraction of the vast mineral resources in the surrounding areas of the Poyang Lake catchment has produced a large amount of waste water and residue containing heavy metals dumping into Poyang Lake (Chen et al. 2016; Wang et al. 2017). The results from the determination of heavy metals in water and sediments reflect the impacts of industrial activities and urban development (Zhang et al. 2012; He et al. 1997). Cu, Pb, and Zn levels in sediments from Poyang Lake averaged 30.20 mg·kg−1, 68.18 mg·kg−1, and 75.56 mg·kg−1, respectively, values that are significantly higher than their background concentrations of 4.75, 17.57, and 45.75 mg·kg−1, respectively (Zhang et al. 2012).
Although copper and zinc are the essential minerals for humans, overdosing on these beneficial elements can also cause health problems (Gale et al. 2004; Yu et al. 2016). They cannot degrade, and continuously deposited and incorporated into water, sediments, and aquatic organisms (Linnik and Zubenko 2000). The toxicity of the metal particularly depends on their chemical forms rather than on their total contents (Liu et al. 2007), and therefore, measurements of total metal concentrations in soils or sediments may not reveal useful information when assessing the impact and estimation of environmental effects of metals such as bioavailability, mobility, and toxicity (Tüzen 2003; Ahlf et al. 2009) because it is the chemical species that determines the mobility and bioavailability of the metal in soil and sediment to other environmental compartments, such as water and plants, when physical and chemical properties of soil and sediment are favorable (Yang et al. 2014; Zhang et al. 2017). The study of heavy metals in water and sediments in the Poyang Lake watershed has gained considerable interest recently (Luo et al. 2008; Yuan et al. 2011; Zhang et al. 2012). However, few reports have focused on the speciation analysis of heavy metals in the wetland soils of Poyang Lake. It is extremely significant to analyze the heavy metal speciation and to find out the distribution in different speciation.
Heavy metals in soils can be classified as adsorptive and exchangeable species, bond to carbonate phases, bond to Fe/Mn oxide, bond to organic matter and sulfides, and detrital or lattice metals. Tessier et al. (1979) set up the five-step sequential extraction method to separate five metal fractions in soil and sediment samples. The main objective of the present study were: (1) to investigate the Cu and Zn concentrations and distributions in the wetland soils from Poyang Lake (China); (2) to analysis the different geochemical fractions of Cu and Zn in the wetland soils; (3) to assess the level of selected metals pollution in the wetland soils using the geoaccumulation index (Igeo).
Material and Methods
Study Area
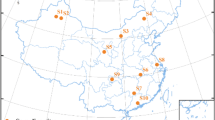
Poyang Lake is located in the north part of Jiangxi province, lying on the south bank of the middle and lower reaches of Yangtze River (Fig.1). The Lake, with an area of 3583 km2 and a volume of 27.6 km3, is about 173 km in length from north to south and 74 km width from east to west (Xu et al. 2001). Poyang Lake receives annual mean precipitation of 1636 mm, typical of the subtropical humid monsoon climate. Temperatures are highly seasonal, with June–August average of 27.3 °C and December–February average of 7.1 °C, and annual average of 17.6 °C. The annual potential evapotranspiration is 1049 mm·year−1, with the highest rates during May to September. The lake is divided into two parts by the Songmenshan Mountain in the middle of the lake. The northern part is the water channel joining the Yangtze River, with the length of 40 km and the width of 3-5 km (the narrowest point is 2.8 km or so). The southern part is the main lake, with the length of 133 km and the furthest width of 74 km.
Sampling sites of wetland soils taken from Poyang Lake. Notes: 1-the entrance of Ganjiang River south branch 2-the entrance of Xinjiang River 3- the entrance of Fuhe River 4-Kangshan 5-Caijiawan 6-the entrance of Raohe River 7-Longkougang 8-Laoyemiao 9-Zhouxi 10-Duchang 11-Hukou 12-Xingzi 13-Wucheng 14- the entrance of Xiushui River 15- the entrance of Ganjiang River north branch
The surrounding Poyang Lake area is a typical water-land transition zone and represents a unique lake ecosystem in the world. Its seasonal variations in inundation regimes along with water level fluctuations generate a unique landscape of marshlands in winter and flood plains in summer, which are dominantly controlled by the water balance between the Yangtze River and five major tributaries (Ganjiang River, Fuhe River, Xinjiang River, Raohe River and Xiushui River) (Shankman et al. 2006; Hui et al. 2008). During the wet seasons, water recharges from Jiangxi basin and the Yangtze River increase the lake coverage to the peak of approximately 4000 km2, whereas during the low-water dry months, the lake becomes a complex assembly of hydrological distinct rivers and shallow waters interspersed with meadows (Jia et al. 2013). This unique hydrologic rhythm in the Poyang Lake area has formed many types of wetlands and provides a suitable habitat to breed rich biodiversity.
Sample Collection and Pre-Treatment
Fifteen soil samples were collected from the top 20 cm at various locations in each quadrant (Fig. 1) to offer a composite sample using a manual soil auger in September, 2013. There was a sampling point at the each main branch flow into the Poyang Lake, and about every 10 km in the main channel set a sampling point. The soils were placed in polythene bags, stored in cooled boxes and transported to the research laboratory. Soil samples were air-dried at room temperature and sieved through a 2-mm nylon sieve to remove coarse debris. The fresh soils were oven dried at 105 °C for 24 h and weighed for soil moisture (Ministry of agriculture, animal husbandry and fishery, People’s Republic of China 1987).
Soil organic matter (SOM) was estimated by measuring the loss of weight on ignition at 550 °C (Díaz-de Alba et al. 2011). Soil pH was measured using a pH meter (soil: water =1: 5). The dry samples were ground with a pestle and mortar until all particles passed a 200 mesh nylon sieve and used for determining chemical properties of soils.
Ultrapure water with 18 MΩ·cm (supplied from a Millipore Milli-Q system) resistivity was used for extraction, solution preparation, and rinsing during the analytical studies. All chemicals and standard solutions used in the experiments were guarantee reagent grade. All glassware and plastic containers were cleaned using a nitric acid (20%, v/v) bath overnight and rinsed with ultrapure water before they were used for analyses.
The Sequential Extraction Procedure
Chemical fractionation of metals in the soil samples was completed using the modified Tessier five-step sequential extraction procedure described by Akcay et al. (2003). A detailed description of this procedure is provided below.
The first fraction (F1) that contains exchangeable species of the trace metals is obtained by the following procedure: each soil sample is extracted at room temperature for an hour with 10 ml of magnesium chloride solution (1 M MgCl2) adjusted to pH 7.0 with ammoniac with continuous agitation.
The second fraction (F2) contains the species bound to carbonates. The residue from the first extraction is leached for 5 h at room temperature with 10 ml of NaOAc (1 M) adjusted to pH 5.0 with acetic acid (HOAc) plus agitation.
The residue from the second extraction is extracted with 20 ml NH2OH·HCl (0.004 M) dissolved in 25% (v/v) HOAc heated for 6 h at 95 °C with occasional agitation. This third fraction (F3) includes trace metal species bound to Fe and Mn oxides (third fraction).
3 ml of 0.02 M HNO3 and 5 ml of 30% H2O2 adjusted to pH 2.0 with HNO3 are added to the residue from the third extraction. Samples were heated at 85 °C for 2 h with occasional agitation. A second 3 ml aliquot of 30% H2O2 adjusted to pH 2.0 with HNO3 is then added and the sample is heated again to 85 °C for 3 h with intermittent agitation. After cooling, 5 ml of 3.2 M NH4OAc in 20% (v/v) HNO3 is added and the sample is diluted to 20 ml and agitated continuously for 30 min. The addition of NH4OAc prevents adsorption of the extracted metals onto the oxidized sediment. The fourth extract (F4) thus involves the trace metal species bound to organic matter (fourth fraction).
Finally, the residue from the fourth extraction was digested with a mixture in a ratio of 3:1:1:1, of HNO3: HF: H2O2: HCl for 3 h in a water-bath. The residue (F5) dissolved entirely and it was diluted with 100 ml ultra-pure water.
After each successive extraction, separation was performed by centrifuging the sample at 5000 rpm for 30 min. The supernatant was then removed with a micropipette and analyzed for trace metals, whereas the residue was once again washed in 10 ml of ultra-pure water, after centrifugation for an hour, this second supernatant is discarded. Concentrations of Cu and Zn in the digests acquired at each stage were determined using a flame atomic absorption spectrometer (PerkinElmer, AA800). The calibration line method was used to quantify selected metals and the digests were appropriately diluted whenever required (Radojevic and Bashkin 1999). All the measurements were made in triplicates. The calculated relative standard deviations (RSD) of total contents for Cu and Zn were below 10%.
Determination of Metal Concentrations
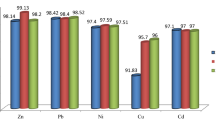
The total heavy metal concentrations of the prepared soils (0.5 g) were determined by an atomic absorption spectrometer fitted with flame or graphite furnace atomization (PerkinElmer, AA800) after microwave digestion (SEM, Mars5) in a mixture of HNO3 (6 ml), HCl (2 ml), HF (2 ml) and H2O2 (2 ml). Laboratory blanks were analyzed for the same elements to control for heavy metal contamination during the digestion process. Reagent blanks were prepared in the same manner as the samples and the blanks were used to correct instrument readings. Quality control was provided by a duplicate analysis of standard reference materials (GBW08301) from the Chinese Academy of Measurement Sciences. And it was also employed in the chemical extraction. The recoveries of heavy metals in the certified samples ranged from 95 to 105%.
Internal Check Recovery
An internal check was performed on the sequential extraction by comparing the total amount of metal extracted by different reagents during the sequential extraction procedure with the results of the total metal concentration (Nemati et al. 2011). The recovery of the sequential extraction method was calculated as follows:
The results shown in Table 1 indicate that the sums of the five fractions are in good agreement with the total digestion results at all sites, with satisfactory recoveries (99.48–103.02%). In addition, the modified Tessier five-step sequential extraction procedure is reliable and can be repeated.
Geoaccumulation Index (Igeo)
The index of geoaccumulation (Igeo) enables the assessment of contamination by comparing the current and pre-industrial concentrations of the metals in earth crust (Muller 1969). It can also be applied to the assessment of soil Contamination (Iqbal and Shah 2011). It is computed using the following mathematical formula: \( {\mathrm{I}}_{\mathrm{geo}}={ \log}_2\left(\frac{C_n}{1.5{B}_n}\right) \), where Cn is the measured concentration of the metal in the soil samples and Bn is the geochemical background value in earth crust. The factor 1.5 is introduced to minimize the effect of possible variations in the background values which may be attributed to geogenic variations. The Igeo values were interpreted as: Igeo ≤ 0 - practically uncontaminated, 0 < Igeo ≤ 1 - uncontaminated to moderately contaminated, 1 < Igeo ≤ 2 - moderately contaminated, 2 < Igeo ≤ 3 - moderately to heavily contaminated, 3 < Igeo ≤ 4 - heavily contaminated, 4 < Igeo ≤ 5 - heavily to extremely contaminated and Igeo > 5 - extremely contaminated (Muller 1969).
Statistical Analysis
Correlation relationships between heavy metals and physicochemical characterization was performed using the software packages of Sigmaplot 6.0 and SPSS statistics 17.0. Differences were considered significant when p < 0.05.
Results
Physicochemical Characterization of Wetland Soils
Soil physicochemical characterization such as pH, SOM and moisture can impact the accumulation of heavy metals in wetland soils (Bai et al. 2012; Yao et al. 2016), so it is essential to analyze their levels and distributions in different soils. The pH, SOM and moisture of wetland soils at various sites were summarized in Table 1. The pH values ranged from 4.9 to 8.4, with the highest value observed in Hukou (site 11) and the lowest value in the entrance of Ganjiang River north branch (site 15). The pH values in the wetland soils of the main channel showed an increasing trend from upstream to the outlet of the Lake (range 5.6–8.4). Alkaline soils were found in the northern part of Poyang Lake with pH values ranging from 7.3–8.4 (i.e., within the normal range of lake waters), which suggests that lake water intrusion impacts on the pH conditions of the soils. The acidic soils appeared associated to the others remaining sampling sites. The moisture content ranged from 6.0% to 24.3% with mean values of 17.9%, the highest content appeared in Hukou and the lowest content was found in Duchang. The lower soil moisture in Duchang is due to the sandy soil with low water retention capability. The organic matter contents varied between 1.51% and 6.76%, with a mean value of 4.70%, obtaining the highest percentages in soils from Hukou site and the entrance of Xinjiang River. The studied soils contained high organic matter contents that could decrease the metal availability by complexing.
Total Metal Concentrations
Total heavy metal concentration in the soil reflect natural differences in soil genesis and properties and the degree of contamination. The concentrations of selected metals at fifteen sampling sites are listed in Table 1. The total heavy metal contents in the Poyang Lake wetland soils ranged from 34.39 to 195.36 mg·kg−1 Zn; 6.839 to 342.54 mg·kg−1 Cu. The entrance of the Raohe River was the most contaminated area by Zn and the most contaminated by Cu was in Caijiawan. The Duchang and Hukou sites were the least contaminated by Cu and Zn. The non-parametric Spearman correlation analysis in this study showed significantly negative correlation between these heavy metals and pH (p < 0.01; Table 2), suggesting that the metal concentrations will increase with the decrease of pH value. There were significant correlations among the Zn and Cu (p < 0.01), implying that the Zn and Cu shared the same source of contamination. However, no significant correlations between these heavy metals and soil moisture were identified. SOM exhibited a positive linear relationship with Cu (p < 0.01). But Zn was not significantly correlated with SOM, which might be due to lower SOM levels in the study area.
Metal Speciation
Chemical speciation differentiates metals of natural origin and/or derived from anthropogenic sources. Metal fractionation is of critical importance to their potential toxicity and mobility (Islam et al. 2015; Pejman et al. 2017). Results of speciation of heavy metals in different sampling sites are shown in Fig. 2. The percentage of metal extracted was calculated from the ratio between the concentration of the element in each fraction, and the sum of concentrations in all fractions. Orders of different fractions of heavy metals in wetland soils of Poyang Lake were:
The highest portion of Cu was obtained in residual fraction at all sites expect the Longkouguang site where Cu was obtained mainly in the organic fraction followed by the residual fraction. The highest residual fraction of Cu was found in the Xiushui River site. The lowest residual fraction of Cu was found at Xinjiang River site with 45.82%. The lowest levels of Cu occurred in exchangeable fractions with an average of 1.00%, followed by carbonates fraction (2.09%).
The lowest levels Zn occurred in carbonates fractions with an average of 1.53%, followed by exchangeable fraction (3.60%). The highest levels Zn occurred in residual fraction with a range from 49.06% to 90.76%, followed by Fe/Mn oxide fraction (average 18.51%). The lowest residual fraction of Zn was found in the Xinjiang River site with 49.09%.
Geoaccumulation Index (Igeo)
Igeo is the quantitative measure of the pollution index in soils. Any increase in the current levels is viewed to be anthropogenic. The background of Cu and Zn in the Poyang Lake are 4.75 mg·kg−1 and 45.75 mg·kg−1 respectively (Zhang et al. 1988). As shown in Fig.3, the Igeo values for the metals revealed that the studied soils were characterized as extremely contaminated at the site 5 (Caijiawan), heavily contaminated at sites 3, 4, 6 and 8, moderately to heavily contaminated at sites 1, 2, 9 and 12–15, moderately contaminated at site 7, uncontaminated to moderately contaminated at site 11, and practically uncontaminated at site 10 by Cu. The soils were practically uncontaminated at sites 10–11, uncontaminated to moderately contaminated at most sites (1, 2, 7, 9, 12, 14, 15), and moderately contaminated at sites 3–6, 8 and 13 by Zn.
Discussion
Correlation Analysis
The soil pH is known to play an important role in controlling the mobilization–immobilization processes of heavy metals (Huang et al. 2017). The lower pH may promote the remobilization of the metals depending on the buffering capacity of the existing alkalinity (Batchman et al. 2001; Chen and Lin 2001). The negative correlation between pH and heavy metal concentration demonstrated it. Stability of Cu in soil is strongly pH dependent – the mobility increases with decreasing pH (Kumpiene et al. 2008). Kumpiene et al. (2008) also concluded that leaching of Cu, Zn, and Pb was strongly pH dependent, with lowest mobility being around neutral to slightly alkaline conditions. Their mobility is usually the lowest in these soils with slightly alkaline pH (Bai et al. 2011; Rennert et al. 2010) and the lowest mobility would favor metal accumulation in the soil (Kashem and Singh 2001).
Soil organic matter can act as a major sink for heavy metals due to its strong complexing capacity for metallic contaminants (Bai et al. 2011; Laing et al. 2009). Association of the metals with SOM can be explained by the high affinity of the metals for the humic substances that comprise much of this material. This is due to the formation of the stable metal complexes and in soluble metal sulfides that are important sinks for trace metals in the soils (Passos et al. 2010). There was no significant correlation between Zn and SOM, which suggested that SOM was not considered to represent the main carrier of Zn in the Poyang Lake wetland soils. Cu exhibited significantly correlated with SOM (p < 0.01). This result as supported by Bai et al. (2011) and Laing et al. (2009). They reported that SOM can keep Cu mobility low in soil by chemisorption.
Metal Speciation
The residual fraction is bound with mineral lattice and can only be released during the weathering process, so it is basically biological unavailable because the weathering process is much longer than the life period (Teasdale et al. 2003). The metal present in the detrital and primary mineral phases indicated that soils were relatively unpolluted and that the metals were derived mainly from geogenic origin (Davutluoglu et al. 2010). In the residual fraction, metals are bound to silicates and are therefore unavailable to the aquatic system (Tüzen 2003). The relative content of a metal in the residual phase can be construed as a measure of the contribution of natural sources, and also of the degree of contamination of the fluvial system, with a higher percentage indicative of lower levels of pollution (Singh et al. 2005). High levels of heavy metals (Cu: 65.64% and Zn: 68.14%) in residual phase reveals that these metals are relatively insensitive to any change of surrounding conditions in wetlands of Poyang Lake.
The Fe/Mn oxide phase has been proved to be sensitive to anthropogenic inputs (Modak et al. 1992). Fe/Mn oxides fraction is difficult to release due to strong ionic bonding. However, if the Eh in soil decreased, it may deoxidize and cause secondary pollution. The Fe/Mn oxides fraction of Zn is significant higher than Cu suggested the Zn is more likely to be affected by human activities.
Exchangeable fraction refers to the metals directly adsorbed on soil. The heavy metals bound to exchangeable fractions were absorbed in clay and humus, which are sensitive to environmental changes, easy to migrate and transform, and have high bioavailability and toxicity. This fraction is usually used to represent the environmentally available components (Anju and Banerjee 2010). A high percentage of clay and organics in the soil phase may possibly act as an adsorbent, retaining metals through ion exchange processes with a net result of increased levels of trace metals in the exchangeable phase. So a low percentage of organics may result in the decreased levels of trace metals in the exchangeable phase. The results of this article just confirmed it. The SOM in the wetlands soil of Poyang Lake was obvious low, and the levels of Cu and Zn in the exchangeable phase was also low.
The heavy metals bound to carbonate fractions co-precipitated with carbonate minerals and are easily released under acidic conditions. The fractions introduced by man’s activity include the exchangeable and bound to carbonates fractions. These are considered to be weakly bonded metals which may equilibrate with the aqueous phase and thus become more rapidly bioavailable (Salomons and Förstner 1980; Pardo et al. 1990). Level of bioavailability (exchangeable + carbonate-bound) (Sundaray et al. 2011) and non bioavailability fractions of heavy metals in each soil samples of studied area are presented in Table 1. The bioavailable fraction represents the fraction that when the right pH and redox conditions are favorable, the metal will be soluble and can be taken up by aquatic biota causing environmental toxicity (Sundaray et al. 2011). The higher percent of exchangeable and carbonate fractions the soils contain, the easier heavy metals can be released into water and the higher bioavailability heavy metals have. The average concentration in the bioavailable fraction of Cu and Zn was very low (1.44 mg·kg−1 and 6.20 mg·kg−1, respectively) in the wetland soils of Poyang Lake. It was indicated that the Cu and Zn were not easy to release into water and have lower bioavailability in the study area. Generally, the percentage of the bioavailable metals in soils in present study followed the order: Zn > Cu. Results of this study showed that Zn was more mobile which would render them more dangerous for the studied area. The concentration in the bioavailable fraction is a serious environmental concern. The speciation studies of Cu and Zn in these wetland soils indicated that there was very low risk of metal contamination (from the percent distribution of the fractions).
Conclusions
The modified five-step sequential extraction procedure was used to investigate the chemical speciation and distribution patterns of copper and zinc in the wetland soils of the Poyang Lake, China. The highest content copper and zinc of concern, came from the copper mine and lead-zinc mine exploitation, but its main chemical fraction was the residue, which had little effect on environment and humans. The area of least contaminated by copper and zinc was found at the Duchang and Hukou sites. The content of the exchangeable fraction and carbonate fraction was generally low. The wetland soil of the northern part of Poyang Lake was alkaline soil (pH 7.26–8.45). Igeo revealed that most of the sampling sites were heavily contaminated and moderately to heavily contaminated by Cu. And most of the sampling sites were uncontaminated to moderately contaminated and moderately contaminated by Zn. Overall, sites 10 and 11 were least polluted by Cu and Zn. The effectiveness of the modified Tessier five-step sequential extraction procedure for heavy metal chemical species analysis was demonstrated. The important and reliable information obtained describe the heavy metal chemical fractionation and their pollution level of wetland soils in Poyang Lake. It has been shown that the mining activities surrounding the Poyang Lake are affecting this ecosystem. The research results provided useful information to local managers and decision makers. The results provided a strong data support for the prevention and control of heavy metal pollution in the wetland soil of Poyang Lake.
References
Ahlf W, Drost W, Heise S (2009) Incorporation of metal bioavailability into regulatory frameworks-metal exposure in water and sediment. J Soils Sediments 9:411–419
Akcay H, Oguz A, Karapire C (2003) Study of heavy metal pollution and speciation in Buyak Menderes and Gediz river sediments. Water Res 37:813–822
Anju M, Banerjee DK (2010) Comparison of two sequential extraction procedures for heavy metal partitioning in mine tailings. Chemosphere 78(11):1393–1402
Bai JH, Xiao R, Cui BS, Zhang KJ, Wang QG, Liu XH, Gao HF, Huang LB (2011) Assessment of heavy metal pollution in wetland soils from the young and old reclaimed regions in the Pearl River estuary, South China. Environ Pollut 159:817–824
Bai JH, Xiao R, Zhang KJ, Gao HF (2012) Arsenic and heavy metal pollution in wetland soils from tidal freshwater and salt marshes before and after the flow-sediment regulation regime in the Yellow River Delta, China. J Hydrol 450-451:244–253
Batchman TM, Friese K, Zachmann DW (2001) Redox and pH conditions in the water column and in the sediments of an acidic mining lake. J Geochem Explor 73:75–86
Chen SY, Lin JG (2001) Bioleaching of heavy metals from sediment: significance of pH. Chemosphere 44:1093–1102
Chen H, Chen R, Teng Y, Wu J (2016) Contamination characteristics, ecological risk and source identification of trace metals in sediments of the Le'an river (China). Ecotoxicol Environ Saf 125:85–92
Cui BS, He Q, Gu BH, Bai JH, Liu XH (2016) China’s coastal wetlands: understanding environmental changes and human impacts for management and conservation. Wetlands 36(Suppl 1):S1–S9
Davutluoglu IO, Seckın G, Kalat DG, Yılmaz T, Ersu CB (2010) Speciation and implications of heavy metal content in surface sediments of Akyatan lagoon –Turkey. Desalination 260:199–210
Díaz-de Alba M, Galindo-Riano MD, Casanueva-Marenco MJ, García-Vargas M, Kosore CM (2011) Assessment of the metal pollution, potential toxicity and speciation of sediment from Algeciras Bay (south of Spain) using chemometric tools. J Hazard Mater 190:177–187
Gale NL, Adams CD, Wixson BG, Loftin KA, Huang YW (2004) Lead, zinc, copper, and cadmium in fish and sediments from the big river and Flat River creek of Missouri's old lead belt. Environ Geochem Health 26:37–49
Garg J (2015) Wetland assessment, monitoring and management in India using geospatial techniques. J Environ Manag 148:112–123
Hamiani OE, Khalil HE, Lounate K, Sirguey C, Hafidi M, Bitton G, Schwartz C, Boularbah A (2010) Toxicity assessment of garden soils in the vicinity of mining areas in southern Morocco. J Hazard Mater 177:755–761
He M, Wang Z, Tang H (1997) Spatial and temporal patterns of acidity and heavy metals in predicting the potential for ecological impact on the le an river polluted by acid mine drainage. Sci Total Environ 206(1):67–77
Huang J, Yuan F, Zeng G, Li X, Gu Y, Shi L, Liu W, Shi Y (2017) Influence of pH on heavy metal speciation and removal from wastewater using micellar-enhanced ultrafiltration. Chemosphere 173:199–206
Hui FM, Xu B, Huang HB, Yu Q, Gong P (2008) Modelling spatial-temporal change of Poyang Lake using multi temporal landsat imagery. Int J Remote Sens 29:5767–5784
Iqbal J, Shah MH (2011) Distribution, correlation and risk assessment of selected metals in urban soils from Islamabad, Pakistan. J Hazard Mater 192:887–898
Islam MS, Ahmed MK, Raknuzzaman M, Mamun MHA, Islam MK (2015) Heavy metal pollution in surface water and sediment: a preliminary assessment of an urban river in a developing country. Ecol Indic 48:282–291
Jia YF, Jiao SW, Zhang YM, Zhou Y, Lei GC, Liu GH (2013) Diet shift and its impact on foraging behavior of Siberian crane (Grus leucogeranus) in Poyang Lake. PLoS One 8(6):1–9
Jiang HB, Wen Y, Zou LF, Wang ZQ, He CG, Zou CL (2016) The effects of a wetland restoration project on the Siberian crane (Grus leucogeranus) population and stopover habitat in momoge national nature reserve, china. Ecology Engineering 96:170–177
Kashem MA, Singh BR (2001) Metal availability in contaminated soils: II. Uptake of cd, Ni and Zn in rice plants grown under flooded culture with organic matter addition. Nutr Cycl Agroecosyst 61(3):257–266
Knox SH, Dronova I, Sturtevant C, Oikawa PY, Matthes JH, Verfaillie J, Baldocchi D (2017) Using digital camera and Landsat imagery with eddy covariance data to model gross primary production in restored wetlands. Agric For Meteorol 237–238:233–245
Kumpiene J, Lagerkvist A, Maurice C (2008) Stabilization of as, Cr, cu, Pb and Zn in soil using amendments—a review. Waste Manag 28:215–225
Kwon JC, Nejad ZD, Jung MC (2017) Arsenic and heavy metals in paddy soil and polished rice contaminated by mining activities in Korea. Catena 148:92–100
Laing DG, Rinklebe J, Vandecasteele B, Meers E, Tack FMG (2009) Trace metal behaviour in estuarine and riverine floodplain soils and sediments: a review. Sci Total Environ 407:3972–3985
Li Z, Ma Z, van der Kuijp TJ, Yuan Z, Huang L (2014) A review of soil heavy metal pollution from mines in China: pollution and health risk assessment. Sci Total Environ 468:843–853
Linnik PM, Zubenko IB (2000) Role of bottom sediments in the secondary pollution of aquatic environments by heavy metal compounds. Lakes Reserv Res Manag 5:11–21
Liu YS, Ma LL, Li YQ, Zheng LT (2007) Evolution of heavy metal speciation during the aerobic composting process of sewage sludge. Chemosphere 67(5):1025–1032
Lu QQ, Bai JH, Gao ZQ, Zhao QQ, Wang JJ (2016) Spatial and seasonal distribution and risk assessments for metals in a Tamarix chinensis wetland, China. Wetlands 36:125–136
Luo MB, LQ J, Cao WP, Wang ML (2008) Study of heavy metal speciation in branch sediments of Poyang Lake. J Environ Sci 20(2):161–166
Matagi SV, Swai D, Mugabe R (1998) A review of heavy metal removal mechanisms in wetlands. Afr J Trop Hydrobiol Fish 8:23–35
Melly BL, Schael DM, Gama PT (2017) Perched wetlands: an explanation to wetland formation in semi-arid areas. J Arid Environ 141:34–39
Ministry of agriculture, animal husbandry and fishery, People's Republic of China (1987) GB 7172–87, method for the determination of soil water content. China Standard Press, Beijing
Mitsch WJ, Gosselink JG (2000) Wetlands. John Wiley & Sons, New York
Modak DP, Singh KP, Chandra H, Ray PK (1992) Mobile and bound forms of trace metals in sediments of the lower Ganges. Water Res 26(11):1541–1548
Muller G (1969) Index of geoaccumulation in sediments of the Rhine River. The Journal of Geology 2:108–118
Nemati K, Abu Bakar NK, Radzi Abas M, Sobhanzadeh E (2011) Speciation of heavy metals by modified BCR sequential extraction procedure in different depths of sediments from Sungai Buloh, Selangor, Malaysia. J Hazard Mater 192:402–410
Oyuela Leguizamo MA, Fernández Gómez WD, Sarmiento MCG (2017) Native herbaceous plant species with potential use in phytoremediation of heavy metals, spotlight on wetlands — a review. Chemosphere 168:1230–1247
Pardo R, Barrado E, Perez L, Vega M (1990) Determination and association of heavy metals in sediments of the Pisucrga, river. Water Res 24(3):373–379
Passos EA, Alves JC, Santos IS, Alves JP, Garcia CAB, Costa ACS (2010) Assessment of trace metals contamination in estuarine sediments using a sequential extraction technique and principal component analysis. Microchem J 96:50–57
Pejman A, Nabi Bidhendi G, Ardestani M, Saeedi M, Baghvand A (2017) Fractionation of heavy metals in sediments and assessment of their availability risk: a case study in the northwestern of Persian gulf. Mar Pollut Bull 114(2):881–887
Radojevic M, Bashkin VM (1999) Practical environmental analysis. Royal Society of Chemistry, Cambridge
Rennert T, Meibner S, Rinklebe J, Totsche KU (2010) Dissolved inorganic contaminants in a floodplain soil: comparison of in situ soil solutions and laboratory methods. Water Air Soil Pollut 209:489–500
Rodríguez L, Ruiz E, Alonso-Azcárate J, Rincón J (2009) Heavy metal distribution and chemical speciation in tailings and soils around a Pb-Zn mine in Spain. J Environ Manag 90:1106–1116
Salomons W, Förstner U (1980) Trace metal analysis on polluted sediments. Part II: evaluation of environmental impact. Environmental Technology Letter 1:506–517
Shankman D, Keim BD, Song J (2006) Flood frequency in China's Poyang Lake region: trends and teleconnections. Int J Climatol 26:1255–1266
Singh KP, Mohan D, Singh VK, Malik A (2005) Studies on distribution and fractionation of heavy metals in Gomti river sediments-a tributary of the Ganges, India. J Hydrol 312:14–27
Sundaray SK, Nayak BB, Lin S, Bhatta D (2011) Geochemical speciation and risk assessment of heavy metals in the river estuarine sediments-case study: Mahanadi basin, India. J Hazard Mater 186:1837–1846
Teasdale PR, Apte SC, Ford PW, Batley GE, Koehnken L (2003) Geochemical cycling and speciation of copper in waters and sediments of Macquarie harbour, western Tasmania. Estuar Coast Shelf Sci 57:475–487
Tessier A, Campbell PGC, Bisson M (1979) Sequential extraction procedure for the speciation of particulate trace metals. Anal Chem 51:844–851
Tüzen M (2003) Determination of trace metals in the river Yeşilırmak sediments in Tokat, Turkey using sequential extraction procedure. Microchem J 74:105–110
Wang H, Zhao Y, Liang D, Deng Y, Pang Y (2017) 30+ year evolution of cu in the surface sediment of Lake Poyang, China. Chemosphere 168:1604–1612
Xiao R, Wang S, Li RH, Wang JJ, Zhang ZQ (2017) Soil heavy metal contamination and health risks associated with artisanal gold mining in Tongguan, Shaanxi, China. Ecotoxicol Environ Saf 141:17–24
Xu D, Xiong M, Zhang J (2001) Analysis on hydrologic characteristics of Poyang Lake. Yangtze River 32(2):21–27 (in Chinese with English abstract)
Yang J, Chen L, Liu LZ, Shi WL, Meng XZ (2014) Comprehensive risk assessment of heavy metals in lake sediment from public parks in Shanghai. Ecotoxicol Environ Saf 102:129–135
Yao XY, Xiao R, Ma ZW, Xie Y, Zhang MX, Yu FH (2016) Distribution and contamination assessment of heavy metals in soils from tidal flat, oil exploitation zone and restored wetland in the Yellow River estuary. Wetlands 36(1):153–165
Yu YX, Wang H, Li Q, Wang B, Yan ZH, Ding AZ (2016) Exposure risk of rural residents to copper in the Le’an River Basin, Jiangxi Province, China. Sci Total Environ 548–549:402–407
Yuan GL, Liu C, Chen L, Yang Z (2011) Inputting history of heavy metals into the inland lake recorded in sediment profiles: Poyang lake in China. J Hazard Mater 185:336–345
Zhang B, Lu ZG, Zhu HF (1988) Studies on Poyang Lake. Shanghai Science and Technology Press, Shanghai, p 40
Zhang DW, Wei YH, Zhang L, Lin GG, Chen YW, Tu TT (2012) Distribution of heavy metals in water, suspended particulate matter and sediment of Poyang lake, China. Fresenius Environ Bull 21:1910–1919
Zhang C, Shan B, Tang W, Dong L, Zhang W, Pei Y (2017) Heavy metal concentrations and speciation in riverine sediments and the risks posed in three urban belts in the Haihe Basin. Ecotoxicol Environ Saf 139:263–271
Acknowledgements
The research work Supported by the National Natural Science Foundation of China (NO. 41663004), the Open Project Program of the Key Laboratory of Poyang Lake Environment and Resource Utilization Ministry of Education, Nanchang University (NO.PYH2015-02), the Open Project Program of State Key Laboratory of Food Science and Technology, Nanchang University (No. SKLF-KF-201619) and the Natural Science Foundation of Jiangxi Province (NO.20114BAB213023).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Wang, M., Hu, K., Zhang, D. et al. Speciation and Spatial Distribution of Heavy Metals (cu and Zn) in Wetland Soils of Poyang Lake (China) in Wet Seasons. Wetlands 39 (Suppl 1), 89–98 (2019). https://doi.org/10.1007/s13157-017-0917-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13157-017-0917-1