Abstract
Coastal marsh plants are increasingly subject to physicochemical stressors under rising sea levels, and the maintenance of marsh ecological functions can depend on the ability of individual species and communities to tolerate or adapt to altered conditions. We conducted a greenhouse experiment to identify hydrology and salinity effects on growth of three common brackish marsh macrophytes of coastal Florida, USA: Distichlis spicata, Juncus roemerianus, and Spartina bakeri. The species were potted as monocultures and exposed to three salinities (0, 15, or 28 psu) and two hydrologic conditions (saturated, tidal) over 22 months. Final stem density of J. roemerianus and S. bakeri did not differ among treatments. In D. spicata, however, stem density was lowest at 28 psu and lower in tidal compared to saturated conditions. Mean stem height of all species was lowest at 28 psu. Aboveground biomass of J. roemerianus was not affected by the treatments, but in D. spicata and S. bakeri it was lowest at 28 psu. Results indicated that J. roemerianus was the most adaptable species and may, therefore, be more resilient to climate-change driven stressors. However, plant-plant interactions such as interspecific competition and facilitation can alter the response of individual species to environmental factors.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Coastal wetlands are vulnerable to stressors associated with rising sea levels, including increased depth and duration of flooding, increased salinity, and intensified erosive forces (Warren and Niering 1993; Morris et al. 2002; Day et al. 2008; Hughes et al. 2009). Intertidal wetlands have generally maintained their position during historical sea-level rise through surface and subsurface organic matter accumulation and mineral matter deposition. Climate change will accelerate the rate of sea-level rise in many regions, leading to changes in both soil- and surface-water salinities as saltwater inundates areas through tidal creeks, surface overflow, and/or groundwater flows. Wetland plants possess avoidance (e.g., salt exclusion and excretion) and tolerance (osmotic adjustment) strategies to adapt to changing salinities (reviewed in Mendelssohn and Batzer 2006). However, differential response of individual species or clones within a species to increased salinity can result in altered composition of a plant assemblage, which in turn may affect community physical structure. In addition to affecting the rate of sea-level rise, climate change has the potential to affect coastal plant communities through temperature alteration (Kirwan et al. 2009; Osland et al. 2013) and effects on the intensity and frequency of extreme weather events such as drought and tropical storms (Webster et al. 2005).
In south Florida, USA, salinity, hydrology, fire, and gradients of nutrient availability have been identified as key drivers affecting wetland plant community composition (Schmalzer et al. 1991; Childers et al. 2006; Troxler et al. 2014). Pearlstine et al. (2010) noted that increased temperature can have substantial impacts in subtropical areas such as south Florida, where many species are near the extremes of their temperature tolerance. Studies conducted in Florida have indicated a transition to more salt tolerant species in low marshes over the past few decades (Ross et al. 2014) and an increase in the coverage of mangrove forests in some areas as graminoid-dominated communities have decreased (Ross et al. 2000; Gaiser et al. 2006; Krauss et al. 2011; Raabe et al. 2012; Smith et al. 2013). When coastal development and infrastructure limit marsh migration landward as sea level rises, the persistence of marsh habitats is threatened (Watson et al. 2015).
Knowledge of individual plant species responses to environmental stressors can provide insights into potential impacts to plant community distributions over regional scales. We conducted a greenhouse study to evaluate how three common macrophytes of southwestern Florida brackish marshes respond to variation in hydrology and salinity. The species selected – Distichlis spicata (L.) Greene, Juncus roemerianus Scheele, and Spartina bakeri Merr. – can form large, near-monospecific stands. Both D. spicata and J. roemerianus are widely distributed along the northern Gulf of Mexico and south Atlantic coasts of the USA, whereas S. bakeri is limited to Florida, Georgia, South Carolina, and Texas (Natural Resources Conservation Service 2015). We hypothesized that the growth response of these species will vary with salinity level, and that the response will vary depending on hydrologic conditions. Specifically, we expected that growth suppression occurs at higher salinity levels, and that this effect is intensified with permanent soil saturation.
Methods
Plant Collection and Experimental Design
We collected plants in March 2009 from a brackish marsh located on Ten Thousand Islands National Wildlife Refuge (25°58′ N, 81°33′ W), near Naples in southwest Florida. The refuge includes brackish marsh, salt marsh, and mangrove forest habitats. The climate of this area is tropical to subtropical (reviewed in Lodge 2010). Average annual rainfall is between 127 and 152 cm; there is a distinct wet season from May to October, when about three-quarters of the total annual rainfall occurs, and a dry season from November to April. While winters are warm with average daytime temperatures frequently above 25 °C, freezing events can occur with the passage of cold fronts (Duever et al. 1994). South Florida is a partially inundated limestone platform with accumulating carbonate, organic, and siliceous sediments of biogenic origin (Wanless et al. 1994). Coastal salt marshes have peat soils that can be mixed with marls (calcitic mud) and silica sands (Davis 1946).
Ramets of Distichlis spicata, Juncus roemerianus, and Spartina bakeri were dug from the marsh using shovels; care was taken to avoid damaging roots and rhizomes. The soil organic surface layer was relatively shallow at the collection site, with a predominately-sand layer encountered at a depth of about 25 cm. The ramets were placed in buckets with roots submersed in shallow water and transported to an enclosed greenhouse facilities in Lafayette, Louisiana. Plastic buckets (30 cm diameter × 37 cm deep; 18.9 L) were prepared as potting containers by drilling small holes just below the bucket rim and near the bucket base to allow water circulation. The pots were filled first with a 12 cm layer of sand (mimicking the underlying sand layer noted in the field) followed by a soil mixture of two parts peat (sphagnum peat moss, Premier Horticulture Inc., Quakertown, Pennsylvania, USA) and one part sand by volume to within 3 cm of the pot rim. Soil was gently washed from the plant roots and the rhizomes were cut into sections with four to six active nodes. The sections were potted with rhizomes placed 2–3 cm below the sediment surface. Because the potting medium contained few nutrients, we added a commercial fertilizer at the manufacturer-recommended low dose to simulate oligotrophic conditions characteristic of the collection site. One slow-release fertilizer tablet (21 g, 20–10-5, N-P-K; Agriform Planting Tablets, Scotts Company, Marysville, Ohio, USA) was inserted about 3 cm deep into the soil midway between the plant and the pot edge. A 1-cm deep sand layer was placed on the sediment surface to prevent soil from being flushed from the pots during hydrologic manipulations. One pot of each species was placed in large fiberglass tanks (700 L), and fresh well-water was added to the tanks to reach the pot sediment surface. With additional pots used in a concurrent experiment (Howard et al. 2015), nine pots total were present in each treatment tank. Plants were allowed to acclimate to greenhouse conditions for four months in freshwater conditions before experimental treatments were applied.
A factorial block design with two hydrological conditions (saturated and tidal) and three salinities (0, 15, and 28 psu) was applied. Treatments were applied in the tanks described above, and each of the six treatment combinations was replicated four times (i.e., four blocks, each with six treatment tanks). Blocks were orientated north-south based on a probable east-west temperature gradient in the greenhouse generated by evaporative water-cooling pads along the west wall.
Treatments were initiated on 10 August 2009 (experiment day 1). The hydrologic regime at Ten Thousand Islands National Wildlife Refuge is variable, and is characterized by one dry and one wet season per year. A study indicated that water depths in brackish marshes on the refuge range from lows of about 25 cm below the marsh surface during the winter-spring dry season to highs of about 50 cm above the surface during the summer-fall wet season (Krauss et al. 2011). As a generalized composite treatment biased towards wetter conditions characteristic of most of the growing season, the tidal treatment consisted of two high water (15 cm above the soil surface) and two low water (5 cm below the soil surface) events over 24 h. The system was essentially closed (Fig. S1), with new water added to replace that lost to evapotranspiration. Tidal treatments were achieved using pumps and automated timers. Pumps were located in reservoirs adjacent to the treatment tanks; each treatment tank had a separate tidal reservoir and each replicate had a separate automated timer. When timers turned on, the tidal pumps ran for two hours beginning at 0800 and 2000 local daylight time. When the timers turned off, water gradually drained from the treatment tanks by gravity to the low-tide level where it remained until the start of the next tidal cycle. In saturated treatment tanks water was maintained within 1 cm of the sediment surface. Salinity treatments were initiated one day following establishment of the hydrologic treatments. We added commercial aquarium salt (Instant Ocean Sea Salt, Spectrum Brands, Blacksburg, Virginia, USA) to the tanks in equal increments daily over 10 days to reach the desired salinity level. A continuously-running submersible pump was placed in all tanks to maintain the salt in solution and avoid stagnation. Because we were concerned about possible micronutrient stress after 12 mo (yellowing of leaf tips was noted), a low-dose packet (110 g) of a granular fertilizer (Micromax Micronutrient, Scotts Company, Marysville, Ohio, USA) was added to supplement micronutrients; the packet was suspended in the water column of each tank on experiment days 373 (August 2010) and 457 (October 2010). To remove the accumulation of decaying leaf material in the tanks, the water was replaced in all treatment tanks and reservoirs on day 422 (October 2010). The experiment was concluded in July 2011, after 710 days (approximately 22 mo).
We measured tank water salinity, temperature and pH daily (YSI Model 63, Yellow Springs, Ohio, USA) during the 10-day period when salinity treatments were increased. We then adjusted salinity levels until relatively stable values (i.e., ± 0.5 psu of target salinity) were attained, which occurred on experiment day 22. Thereafter we adjusted water levels as needed and recorded tank water parameters twice weekly. Minor adjustments of tank water salinity were required throughout the experiment as salt was translocated into plant tissues or concentrated in soil interstitial water.
Data Collection
The number of live stems per pot (stem density; pot surface area of 0.062 m2) was measured in July 2009, prior to treatment application. Stem counts were also obtained in December 2009 (day 127), May 2010 (day 267), and at harvest in July 2011 (beginning day 710). The height of each live stem was also measured prior to treatment application in July 2009 and at harvest. During harvest all stems were clipped at the soil surface, and live and dead stems were separated. Belowground tissues were harvested by washing soil from the roots. Aboveground and belowground material was oven dried at 60 °C until a constant weight was reached for biomass determination.
Interstitial soil water in pots was collected at a depth of 15 cm below the soil surface using a tube and syringe in June 2010 (day 319) and June 2011 (day 675) for measurement of salinity and pH (YSI Model 63, Yellow Springs, Ohio, USA). Interstitial water was also collected August 2010 (day 379) for sulfide concentration and salinity measurement. The portion for sulfide analysis was stabilized with an antioxidant buffer, and measurements were obtained using a ion selective electrode (combination silver/sulfide, Orion Model 9616) and pH-mV meter (Corning Model 103). Reduction-oxidation potential (Eh) was also measured at this time by using a pH-mV meter and platinum electrodes linked to a calomel reference electrode. Platinum electrodes were inserted to a depth of 15 cm and allowed to stabilize at least 30 min before readings were recorded.
Statistical Analyses
All analyses of plant growth data were conducted separately by species, with hydrology and salinity as independent variables. Repeated measures two-way analysis of covariance (ANCOVA) was used to analyze treatment effects on stem density; stem density before treatments were applied was used as a covariate to control for variation in initial stem density. Data from December 2009 and May 2010 were included in these analyses. Final stem density and mean stem height were also analyzed by using two-way ANCOVA, with measurements before treatments used as covariates in the respective analyses. Two-way analysis of variance (ANOVA) was used to analyze aboveground and belowground biomass. To investigate if interstitial water characteristics varied by species, three-way ANOVA was applied to sulfide, Eh and salinity data collected in August 2010; hydrology, salinity, and species were the independent variables. Repeated measures three-way ANOVA was applied to examine salinity and pH data from June 2010 and 2011 across species.
SAS software (Proc Mixed, ver. 9.2, SAS Institute, Inc., Cary, North Carolina, USA) was used for all analyses. Data were checked for homogeneity and normality, and were transformed if assumptions were not met. Block was included as a main factor to account for variability due to location in the greenhouse. The alpha level used for analyses was 0.05. When significant main effects were identified, the Tukey-Kramer correction for least-means squares comparisons was applied based on the number of comparisons made.
Results
Stem Density over Time
We identified treatment effects on growth in all three species. All plants survived until harvest, with the exception of one D. spicata individual that died in the tidal-15 psu treatment. Variable means by treatment combinations are provided in Table S1. Stem density in D. spicata pots generally exceeded that of the other species over time in the saturated 0 and 15 psu treatments (Fig. 1). Repeated measures ANCOVA results indicated significant main factor or interaction effects on stem density (Table 1). Distichlis spicata stem density was greater in May 2010 than in December 2009 in the saturated-0 psu treatment only; density was equivalent at both times for all other treatment combinations. Salinity did not affect D. spicata stem density under tidal conditions at either time, but in saturated conditions density was lower at 28 psu compared to 0 psu in December 2009 and lower in both 28 and 15 psu compared to 0 psu in May 2010 (Fig. 1). Stem density in both J. roemerianus and S. bakeri was greater in May 2010 than in December 2009 regardless of hydrologic conditions or salinity. Stem density regardless of time or hydrology was lower in J. roemerianus at 28 and 15 psu compared to 0 psu, and in S. bakeri density was lower at 28 psu compared to 15 psu (Fig. 1).
Interstitial Water Characteristics
Some physico-chemical characteristics of the interstitial pot water/soil environment were affected by the treatments. Three-way ANOVA including species as an independent variable indicated that sulfide concentration in August 2010 was affected by two-way interactions: species and salinity (F4,34 = 21.11, P < 0.0001) and species and hydrology (F2,34 = 4.29, P = 0.0218). The highest mean sulfide concentration was 979 μM, and occurred in a S. bakeri pot in the tidal-15 psu treatment (Table 2). Sulfide concentration in S. bakeri pots was significantly greater than that in D. spicata and J. roemerianus pots in the 15 and 28 psu treatments, and in both hydrologic conditions. While concentration of this element was significantly different between all salinities in S. bakeri pots (highest at 15 psu), sulfide did not vary by salinity in pots of the other species. There were no treatment effects on interstitial water Eh (F5,34 = 1.00, P = 0.4314). Interstitial salinity levels generally followed the tank water regardless of species, and was affected by the interaction of species and hydrology (F2,34 = 3.73, P = 0.0343). Only S. bakeri pots showed a response; pot salinity for this species in August 2010 was lower in tidal (15.9 ± 4.5 psu) compared to saturated (20.1 ± 5.6 psu) conditions.
Repeated measures ANOVA examining interstitial soil water salinity resulted in two significant three-way interactions: species, hydrology, and time (F2,54 = 4.38, P = 0.0173); and species, hydrology, and salinity (F4,53 = 6.39, P = 0.0003). In the latter interaction, species differences were indicated by higher salinity in S. bakeri pots than in pots of the other species under saturated conditions at 15 and 28 psu (Table 3). While interstitial salinity did not vary by hydrology in D. spicata or J. roemerianus pots, it was lower in saturated compared to tidal conditions in S. bakeri pots at both 15 and 28 psu.
Final Plant Characteristics
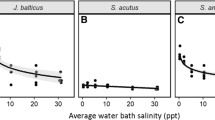
Growth of all species was reduced under some conditions at the conclusion of the 22 month experiment. Final stem density of J. roemerianus (F3,14 = 0.01, P = 0.9978) and S. bakeri (F3,14 = 0.13, P = 0.9403) was not affected by treatments (Fig.1), but D. spicata stem density varied with salinity (F2,13 = 7.29, P = 0.0075) and hydrology (F1,13 = 423.30, P = 0.0003). The number of D. spicata stems was lower at 28 psu (83.6 ± 11.4) compared to both 15 (131.6 ± 18.4) and 0 (134.6 ± 17.4) psu. It was also lower in tidal (90.7 ± 11.5) compared to saturated (139.1 ± 13.3) conditions. Salinity significantly affected final mean stem height for all species (P values were 0.0075 or lower), and height was always reduced at 28 psu (Fig. 2). Final mean height of J. roemerianus was also affected by hydrology (F1,9 = 22.87, P = 0.0010); it was lower under saturated (63.2 ± 2.8 cm) compared to tidal (73.9 ± 4.1 cm) conditions.
Aboveground biomass of D. spicata and S. bakeri was significantly affected by treatments (Table 4). It was lower at 28 psu compared to 0 and 15 psu for both species, and lower in tidal compared to saturated conditions for D. spicata (Fig. 3); aboveground biomass of J. roemerianus was not affected. Belowground biomass in all three species was affected by salinity (Table 4), and was lowest at 28 psu (Fig. 3). Similar to results for aboveground biomass, D. spicata belowground biomass was also lower in tidal compared to saturated conditions.
Significant biomass (mean ± standard error; aboveground or belowground, as indicated) results for a Distichlis spicata, by salinity (0, 15, 28 psu) and hydrology (saturated, tidal), b Juncus roemerianus, by salinity and c Spartina bakeri by salinity in a greenhouse experiment. Significantly different means are indicated by different letters
Discussion
Our study indicated that specific growth responses of D. spicata, J. roemerianus, and S. bakeri to the experimental treatments varied. While some growth parameters in all species were suppressed at the highest salinity, only D. spicata and J. roemerianus were affected by hydrologic conditions. In contrast to our expectations, we found little evidence of a synergistic effect between salinity and hydrology. At the conclusion of the experiment we found that growth of D. spicata was affected to the greatest degree, with decreased stem density, stem height, and biomass with increased salinity, as well as reduced biomass under tidal conditions. In comparison, S. bakeri final stem density did not vary with treatments; however, stem height and biomass of this species were reduced at the highest salinity. Growth of J. roemerianus was least affected by the treatments; its response was limited to decreased stem height and belowground biomass at the highest salinity, and decreased height in saturated conditions. The exception to a lack of interactive effects between hydrology (i.e., tidal or saturated conditions) and salinity was D. spicata stem density, which increased in saturated, 0 psu conditions only over time. Because stem density did not change in the other treatment combinations, it can be concluded that freshwater saturated conditions facilitated growth of D. spicata during this time.
Zonation of intertidal plant communities along elevational gradients has been described extensively in the literature (e.g., Mendelssohn and Seneca 1980; Niering and Warren 1980; Bertness 1991). This zonation is a result of flooding regimes, salinity, and resource competition (Emery et al. 2001; Pennings et al. 2005). The species included in our study are typically found in the high marsh zone (Eleuterius 1972; Montague and Wiegert 1990). Salinity and flooding tolerance of D. spicata and J. roemerianus have been described in previous studies, but there is little information on tolerance of S. bakeri to these stressors. Reduced growth in D. spicata was found in a greenhouse experiment with manipulated salinity and flooding depth; growth was lower at both 10 and 25 psu compared to freshwater conditions, and the two stress factors interacted to affect some growth measures (Howard and Rafferty 2006). Our results are consistent with those of a hydroponic study where D. spicata relative growth rate was reduced in solutions equivalent to 29 psu; this response, however, was identified only under low light levels (Kemp and Cunningham 1981). Similar findings from a study by Parrondo et al. (1978) indicated that D. spicata aboveground biomass was suppressed at 32 psu, but that no effects were apparent at 16 psu or lower. Our results also indicate that the vigor of D. spicata stands will be reduced if high marsh areas where it occurs become exposed to daily tides. A genetically distinct short-leaved form of J. roemerianus can persist in hypersaline marshes with salinity greater than 35 psu (Eleuterius 1989). High salinity tolerance in this species was also indicated in a greenhouse experiment where exposure to 30 psu for eight weeks did not affect J. roemerianus biomass (Touchette et al. 2009). We observed few effects when J. roemerianus was exposed to 28 psu for almost two years, and we found that this species was, in general, more tolerant of higher salinity than D. spicata and S. bakeri. Persistence of J. roemerianus was described by Brinson and Christian (1999) in an Atlantic coastal marsh undergoing subsidence; patches of this species were stable and resistant to replacement by other species, and where losses did occur they were associated with wrack disturbance and frequent tidal flooding. Spartina bakeri occurs in freshwater habitats (Flora of North America 2003) and may, therefore, have greater competitive ability compared to D. spicata and J. roemerianus, both of which are generally displaced by freshwater species in low salinity marshes. Greater competitive ability in S. bakeri was also indicated in a study documenting plant recovery following a fire, where S. bakeri growth was favored over that of J. roemerianus in mixed stands (Schmalzer et al. 1991). In our study the elevated interstitial water salinities compared to tank water salinities that was apparent for all species in 15 and 28 psu treatments suggests salt exclusion by roots. The fact that interstitial salinities in saturated treatments were significantly higher in S. bakeri pots suggests that this species was more effective than the others at salt exclusion under these conditions.
Reduced sulfur (sulfide) can be a phytotoxin (reviewed in Lamers et al. 2013), and may become an important limiting factor in wetlands exposed to rising sea levels. The mean Eh values recorded in our study were above −100 mV, the approximate value below which sulfate is reduced to sulfide (Megonigal and Rabenhorst 2013). Although we measured Eh just one time, the relatively high Eh values indicate that sulfate reduction probably occurred at a low rate. Sulfide has been implicated as an influence on plant community change in the south Florida, acting in concert with altered hydrology and phosphorus enrichment (Li et al. 2009; DeBusk et al. 2015); these studies focused on Cladium jamaicense and Typha domingensis, which are found in fresh to brackish marshes. Sulfide concentrations of 220 μM in hydroponic solutions reduced growth in C. jamaicense, but T. domingensis growth was not affected at concentrations up to 690 μM (Li et al. 2009). Although we found no existing information on the sulfide tolerance of the species in our study, data compiled from several studies indicated that sulfide concentration above 500 μM leads to decreased productivity in some salt marsh species (Lamers et al. 2013). In comparison, the highest recorded mean sulfide concentration in our study was 979 μM in S. bakeri pots in 15 psu treatments. Interestingly, we found no significant differences in growth of this species comparing 0 and 15 psu, but did find growth suppression in 28 psu treatments, with sulfide concentrations of 366 μM (saturated) and 462 μM (tidal). It therefore appears unlikely that sulfide concentrations generated in our study affected S. bakeri growth. Concentrations generally below 200 μM in D. spicata and J. roemerianus pots regardless of treatment also indicates that sulfide probably did not limit growth of these species in our study.
Controlled greenhouse studies can be difficult to relate to actual field settings. We were not able to simulate the slow increase in tidal range and salinity level, as would occur naturally with sea-level rise, in the greenhouse facility. A 10-day window over which salinity was raised to target levels was selected, but the tidal regime was established immediately; there was no gradual change from saturated conditions to a micro-tidal environment and, eventually, to the targeted tidal regime. No signs of flooding or salinity shock (e.g., yellowing, browning, or wilting of aboveground tissues) during the first two weeks of the experiment were evident for any of the species, and survival over the entire experiment was high (i.e., mortality occurred in just one pot). Although the pH levels in our soil mixture (Table 2) are low compared to near-neutral values commonly found in wetland soils (Ponnamperuma 1972), the values were similar to those recorded in Florida salt marsh peat soils (Davis 1946). We therefore believe it is reasonable to conclude that plant responses under the experimental conditions in our study can provide insight into their response in the field under similar salinity and hydrologic regimes.
The sustainability of coastal wetlands under rising sea levels will depend on their ability to maintain elevation within the intertidal zone. Vertical accretion involves accumulation of both inorganic and organic material, and the contribution of vegetation to soil elevation processes can be of primary importance in some regions (Cahoon et al. 2006; Nyman et al. 2006; McKee 2011; Baustian et al. 2012). Because growth of some marsh species is adversely affected by increased salinity and tidal inundation, plant species shifts driven by sea-level rise are probable (Spalding and Hester 2007; Fox et al. 2012), and replacement of high marsh species by low marsh species has been documented (Donnelly and Bertness 2001; Smith 2015). The ecosystem services provided by wetlands may be altered by such species shifts. For example, the composition of plant assemblages can affect carbon storage in wetlands (Elsey-Quirk et al. 2011; Comeaux et al. 2012). If landward migration of tidal wetlands cannot keep pace with sea-level rise or if geographic barriers or if human-produced infrastructure prevents migration, wetlands will be lost. We conclude that, of the three species studied, J. roemerianus was most able to adapt to increased salinity levels, and therefore may be the most resilient to environmental drivers that raise salinity. It should be noted, however, that plant-plant interactions, including competition and facilitation, may alter plant species response to physical and chemical stressors (Pennings et al. 2005).
References
Baustian JJ, Mendelssohn IA, Hester MW (2012) Vegetation’s importance in regulating surface elevation in a coastal marsh facing elevated rates of sea level rise. Glob Chang Biol 18:3377–3382
Bertness MD (1991) Zonation of Spartina patens and Spartina alterniflora in a New England salt marsh. Ecology 72:138–148
Brinson MM, Christian RR (1999) Stability of Juncus roemerianus patches in a salt marsh. Wetlands 19:65–70
Cahoon DR, Hensel PF, Spencer T, Reed DJ, McKee KL, Saintilan N (2006) Coastal wetland vulnerability to relative sea-level rise: wetland elevation trends and process controls. In: Verhoeven JTA, Beltman B, Bobbink R, Whigham DF (eds) Wetlands and natural resource management, vol 190. Ecological Studies, Spring, Berlin, pp. 271–292
Childers DL, Iwaniec D, Rondeau D, Rubio G, Verdon E, Madden CJ (2006) Response of sawgrass and spikerush to variation in hydrologic drivers in southern Everglades marshes. Hydrobiologia 569:273–292
Comeaux RS, Allison MA, Bianchi TS (2012) Mangrove expansion in the Gulf of Mexico with climate change: implications for wetland health and resistance to rising sea levels. Estuar Coast Shelf Sci 96:81–95
Davis JH Jr. (1946) The peat deposits of Florida: their occurrence, development and uses. Florida Geological Survey Bulletin No 30
Day JW, Christian RR, Boesch DM, Yanez-Aranciba A, Morris J, Twilley RA, Naylor L, Schaffer L, Stevenson C (2008) Consequences of climate change in the ecomorphology of coastal wetlands. Estuar Coasts 31:477–491
DeBusk TA, Dierberg FE, DeBusk WF, Jackson SD, Potts JA, Galloway SC, Sierer-Finn D, Gu B (2015) Sulfide concentration effects on Typha domingensis pers. (cattail) and Cladium jamaicense crantz (sawgrass) growth in Everglade marshes. Aquat Bot 124:78–84
Donnelly JP, Bertness MD (2001) Rapid shoreward encroachment of salt marsh cordgrass in response to sea-level rise. Proc Natl Acad Sci 98:14218–14223
Duever MJ, Meeder JF, Meeder LC, McCollum JM (1994) The climate of south Florida and its role in shaping the Everglades ecosystem. In: Davis SM, Ogden JC (eds) Everglades: the ecosystem and its restoration. St. Lucie Press, Delray Beach, pp. 225–248
Eleuterius LN (1972) The marshes of Mississippi. Castanea 37:153–168
Eleuterius LN (1989) Natural selection and genetic adaptation to hypersalinity in Juncus roemerianus Scheele. Aquat Bot 36:45–53
Elsey-Quirk T, Seliskar DM, Sommerfield CK, Gallagher JL (2011) Salt marsh carbon pool distribution in a mid-Atlantic lagoon, USA: sea level rise implications. Wetlands 31:87–99
Emery NC, Ewanchuk PJ, Bertness MD (2001) Competition and salt-marsh plant zonation: stress tolerators may be dominant competitors. Ecology 82:2471–2485
Flora of North America (2003) Magnoliophyta. In: Barkworth ME, Capels KM, Long S, Piep MB (eds) Commelinidae (in part): Poaceae, part 2, vol 25. Oxford University Press, New York
Fox L, Valiela I, Kinney EL (2012) Vegetation cover and elevation in long-term experimental nutrient-enrichment plots in Great Sippewissett salt marsh, Cape Cod, Massachusetts: implications for eutrophication and sea level rise. Estuar Coasts 35:445–458
Gaiser EE, Zafiris A, Ruiz PL, Tobias FAC, Scott MS (2006) Tracking rates of ecotone migration due to salt-water encroachment using fossil mollusks in coastal south Florida. Hydrobiologia 569:237–257
Howard RJ, Rafferty PS (2006) Clonal variation in response to salinity and flooding stress in four marsh macrophytes of the northern Gulf of Mexico, USA. Environ Exp Bot 56:301–313
Howard RJ, Krauss KW, Cormier N, Day RH, Biagas J, Allain L (2015) Plant-plant interactions in a subtropical mangrove-to-marsh transition zone: effects of environmental drivers. J Veg Sci 26:1198–1211. doi:10.1111/jvs.12309
Hughes ZJ, FitzGerald DM, Wilson CA, Pennings SC, Wieski K, Mahadevan A (2009) Rapid headwater erosion of marsh creeks in response to relative sea level rise. Geophys Res Lett 36:L03602
Kemp PR, Cunningham GL (1981) Light, temperature and salinity effects on growth, leaf anatomy and photosynthesis of Distichlis spicata (L.) Greene. Am J Bot 68:507–516
Kirwan ML, Guntenspergen GR, Morris JT (2009) Latitudinal trends in Spartina alterniflora productivity and the response of coastal marshes to global change. Glob Chang Biol 15:1982–1989
Krauss KW, From AS, Doyle TW, Doyle TJ, Barry MJ (2011) Sea-level rise and landscape change influence mangrove encroachment onto salt marsh in the Ten Thousand Islands region of Florida, USA. J Coast Conserv 15:629–638
Lamers LPM, Govers LL, Janssen ICJM, Geurts JJM, Van der Welle MEW, Van Katwijk MM, Van der Heide T, Roelofs JGM, Smolders AJP (2013) Sulfide as a soil phytotoxin – a review. Frontiers in Plant Science 4:A268
Li S, Mendelssohn IA, Chen H, Orem WH (2009) Does sulphate enrichment promote the expansion of Typha domingensis (cattail) in the Florida Everglades? Freshw Biol 54:1909–1923
Lodge TE (2010) The Everglades handbook: understanding the ecosystem, 3rd edn. CRC Press, Boca Raton
McKee KL (2011) Biophysical controls on accretion and elevation change in Caribbean mangrove ecosystems. Estuar Coast Shelf Sci 91:475–483
Megonigal JP, Rabenhorst M (2013) Reduction-oxidation potential and oxygen. In: DeLaune RD, Reddy KR, Richardson CJ, Megonigal JP (eds) Methods in biogeochemistry of wetlands. Soil Science Society of America, Madison, Wisconsin, pp. 71–85
Mendelssohn IA, Batzer DP (2006) Abiotic constraints for wetland plants and animals. In: Batzer DP, Sharitz RR (eds) Ecology of freshwater and estuarine wetlands. University of California Press, Berkeley, pp. 82–114
Mendelssohn IA, Seneca ED (1980) The influence of soil drainage on the growth of salt marsh cordgrass Spartina alterniflora in North Carolina. Estuar Coast Mar Sci 11:27–40
Montague CL, Wiegert RG (1990) Salt marshes. In: Meyers RL, Ewel JJ (eds) Ecosystems of Florida. University of Central Florida Press, Orlando, pp. 481–516
Morris JT, Sundareshwar PV, Nieitch CT, Kjerfve B, Cahoon DR (2002) Response of coastal wetlands to rising sea levels. Ecology 83:2869–2877
Natural Resources Conservation Service (2015) Plants database. http://plants.usda.gov. Accessed 20 Feb 2015
Niering WA, Warren RS (1980) Vegetation patterns and processes in New England salt marshes. Bioscience 30:301–307
Nyman JA, Walters RJ, DeLaune RD, Patrick Jr WH (2006) Marsh vertical accretion via vegetative growth. Estuar Coast Shelf Sci 69:370–380
Osland MJ, Enwright N, Day RH, Doyle TW (2013) Winter climate change and coastal wetland foundation species: salt marsh vs. mangrove forests in the southeastern United States. Glob Chang Biol 19:1482–1494
Parrondo RT, Gosselink JG, Hopkinson CS (1978) Effects of salinity and drainage on the growth of three salt marsh grasses. Bot Gaz 139:102–107
Pearlstine LD, Pearlstine EV, Aumen NG (2010) A review of the ecological consequences and management implications of climate change for the Everglades. J N Am Benthol Soc 29:1510–1526
Pennings SC, Grant M-B, Bertness MD (2005) Plant zonation in low-latitude salt marshes: disentangling the roles of flooding, salinity, and competition. J Ecol 93:159–167
Ponnamperuma FN (1972) The chemistry of submerged soils. Adv Agron 24:29–96
Raabe EA, Roy LC, McIvor CC (2012) Tampa Bay coastal wetlands: nineteenth to twentieth century marsh-to-mangrove conversion. Estuar Coasts 35:1145–1162
Ross ME, Meeder JF, Sah JP, Ruiz PL, Telesnicki GJ (2000) The southeast saline Everglades revisited: 50 years of coastal vegetation change. J Veg Sci 11:101–112
Ross MS, Sah JP, Meeder JF, Ruiz PL, Telesnicki G (2014) Compositional effects of sea-level rise in a patchy landscape: the dynamics of tree islands in the southeastern coastal Everglades. Wetlands 34S:S91–S100
Schmalzer PA, Hinkle CR, Mailander JL (1991) Changes in community composition and biomass in Juncus roemerianus Scheele and Spartina bakeri merr. Marshes one year after a fire. Wetlands 11:67–86
Smith SM (2015) Vegetation change in salt marshes of Cape Cod national seashore (Massachusetts, USA) between 1984 and 2013. Wetlands 35:127–136
Smith III TJ, Foster AM, Tiling-Range G, Jones JW (2013) Dynamics of mangrove-marsh ecotones in subtropical coastal wetlands: fire, sea-level rise, and water levels. Fire Ecology 9:66–77
Spalding EA, Hester MW (2007) Interactive effects of hydrology and salinity on oligohaline plant species productivity: implications of relative sea-level rise. Estuar Coasts 30:214–225
Touchette BW, Smith GA, Rhodes KL, Poole M (2009) Tolerance and avoidance: two contrasting physiological responses to salt stress in mature marsh halophytes Juncus roemerianus Scheele and Spartina alterniflora loisel. J Exp Mar Biol Ecol 380:106–112
Troxler TG, Childers DL, Madden CJ (2014) Drivers of decadal-scale change in southern Everglades wetland macrophyte communities of the coastal ecotone. Wetlands 34S:S81–S90
Wanless HR, Parkinson RW, Tedesco LP (1994) Sea level control on stability of Everglades wetlands. In: Davis SM, Ogden JC (eds) Everglades: the ecosystem and its restoration. St. Lucie Press, Delray Beach, pp. 199–223
Warren RS, Niering WA (1993) Vegetation change on a northeast tidal marsh: interaction of sea-level rise and marsh accretion. Ecology 74:96–103
Watson EB, Wigand C, Cencer M, Blount K (2015) Inundation and precipitation effects on growth and flowering of the high marsh species Juncus gerardii. Aquat Bot 121:52–56
Webster PJ, Holland GJ, Curry JA, Chang HR (2005) Changes in tropical cyclone number, duration, and intensity in a warming environment. Science 309:1844–1846
Acknowledgments
Funding for this study was provided by the U.S. Fish and Wildlife Service through Intra-Agency Agreement 401818 N511. We appreciate the assistance of Nicole Cormier, Andrew From, Sergio Merino, and Thomas Michot during data collection, and Darren Johnson for conducting statistical analyses. Constructive comments from Camille Stagg and two anonymous reviewers helped improve this manuscript. Any use of trade, firm, or product names is for descriptive purposes only and does not imply endorsement by the U.S. Government.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Howard, R.J., Biagas, J. & Allain, L. Growth of Common Brackish Marsh Macrophytes Under Altered Hydrologic and Salinity Regimes. Wetlands 36, 11–20 (2016). https://doi.org/10.1007/s13157-015-0711-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13157-015-0711-x