Abstract
Surface soils (0–20 cm) were collected at five sampling sites along a 250-m sampling zone perpendicular to a tidal creek in the T. chinensis wetland of the Yellow River Delta of China in June, August and November of 2007 and April of 2008. Soils were assessed for the spatial and temporal distributions of metals (As, Ni, Cr, Zn, Pb, Cd and Cu) and the potential risk to the T. chinensis wetland. Our results showed that the mean contents for all metals exceeded the background values of the Yellow River Delta, except for Pb and Zn. Arsenic content exceeded the probable effect level values at all sampling sites during the four periods. A multivariate analysis indicated that As, Cd, Pb and Zn were derived from the same origin, Cr and Ni mainly originated from parent rocks, whereas Cu and Pb have another same origin. The geoaccumulation index (I geo ) indicated that there was no Cu, Pb or Cr pollution at five sampling sites during all sampling periods. The average toxic units (TU) of metals in this region followed the order As > Ni > Cr > Zn > Pb > Cd > Cu. Three metals contributed more to the ∑TUs (sum of the toxic units) than other metals, as follows: As (40 %), Ni (20 %) and Cr (20 %).
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Coastal wetlands are ecosystems and interaction ecotones that are located between terrestrial and aquatic systems. By functioning as a natural filter, coastal wetlands can remove chemicals and pollutants from the water (Qin and Mitsch 2009). Wetland soils serve as a source, sink and transfer for chemical pollutants, including organic compounds and metals, because of the physico-chemical processes of adsorption, ligand exchange and sedimentation (Reddy and DeLaune 2008; Lau and Chu 2000). The environmental behavior of metals in wetland soils primarily depends on their specific chemical forms and on their binding state, which influence their bioavailability, mobility, and toxicity to organisms (Passos et al. 2010; Wang and André 2009). However, accelerated physico-chemical processes such as the decalcification, desalinization, and the oxidation of reduced iron, manganese and sulfur compounds that occur in wetland soils may affect the stabilization of trace metals (Giani et al. 2003; Jacob and Otte 2003). Moreover, metal pollution is persistent and bioaccumulates in ecosystems (Wu et al. 2012; Gao and Chen 2012). Metals, immobilized in the soils, can be accumulated in aboveground plant tissues which can enlarge food chain contamination. Finally, litter containing metals, often with increasing concentrations during decomposition, can be taken up by detritus feeders or can be transported to the estuary (Teuchies et al. 2012). Meanwhile, if plentiful amounts of metals are accumulated in plants, it will adversely affect the absorption and transport of essential elements, disturb the metabolism and have an impact on growth and reproduction, which can further affect the productivity of wetlands (Xu and Shi 2000). Moreover, environmental changes such as those affecting global climate and environmental pollution can also lead to the degradation of wetlands (Liu et al. 2014). Therefore, a better understanding of dynamic changes in metals can contribute to wetland conservation and management and human health.
Over the past few decades, increasing metal contamination has attracted more public concerns because of urbanization and reclamation activities (e.g., agriculture, aquaculture, harbor construction and traffic) in coastal regions (Li et al. 2014), such as in the Pearl River Delta (Bai et al. 2011a), the Niger Delta (Olawoyin et al. 2012) and the Mekong River Delta (Cenci and Martin 2004). Bai et al. (2011a) posited that wetland reclamation might increase metal contents in wetland soils. Abdallah (2011) noted that agriculture and aquaculture could also elevate metal (e.g., Cd) in lagoon sediments. Previous studies also demonstrated that traffic could bring a high potential risk of metal pollution in roadside soils through deposition and road runoff (Bai et al. 2009; Chen et al. 2001). More recently, Bai et al. (2012) reported that the flow-sediment regulation regime also elevated metal levels.
The Yellow River Delta (YRD) is one of the most active regions of land-ocean interactions and is under huge pressure from enormous environmental deterioration originating from agriculture, aquaculture, and road and harbor construction. Some studies regarding heavy metal pollution and land-use effects have been performed in the natural coastal wetlands in this region (Bai et al. 2011a, 2012). However, little is known about the spatial and temporal variation of metals in the coastal salt marshes. Therefore, the primary objectives of this study are (1) to investigate seasonal changes in the distribution of selected metals (As, Cd, Cr, Ni, Cu, Pb, and Zn) in marsh soils of the T. chinensis wetland in the YRD, (2) to identify their sources using multivariate analysis, and (3) to evaluate the ecotoxicity and potential ecological risks from these metals.
Materials and Methods
Site Description
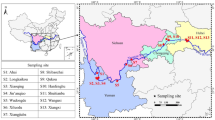
This study was conducted in the Yellow River Delta (YRD) (36°55′–38°16′N, 117°31′–119°18′E) (Fig. 1), which is in Eastern China on the coast of the Bohai Sea. It has a warm temperate continental monsoon climate with distinct seasons. The average temperature is 12.1 °C with 196 frost-free days. The annual evaporation is 1962 mm and the annual average rainfall is 552 mm, with approximately 70 % of the precipitation falling from June to August. Intrazonal tidal soil and salt soil are the dominant soil types in the study area (Zhang et al. 2013). The Yellow River Delta is one of the most integrated, widest, and youngest wetland ecosystems in China (Jiang et al. 2012). The coastal wetlands, which are strongly affected by societal and economic development, are an ecologically fragile area that is threatened by wetland drainage for conversion to farmland, channel construction and road reclamation. Moreover, there has been a flow-sediment regulation scheme from June to July of each year since 2002 by the Yellow River Conservancy Commission to control the discharge of water and sediments from the Xiaolangdi Reservoir and scour the lower reaches (Bai et al. 2012).
Sample Collection and Analysis
Five sampling sites (Sites A, B, C, D and E) were selected along a 250-m sampling zone perpendicular to a tidal creek in June, August, and November of 2007 and April of 2008. Soil samples in the top 20 cm depth were collected with three replicates at each of the five sampling sites. All soil samples were then placed in polyethylene bags and brought to the laboratory. All collected samples were air-dried at room temperature for 3 weeks and sieved through a 2-mm nylon sieve to remove coarse debris and then ground using a pestle and mortar until all particles could pass through a 0.149-mm nylon sieve.
The soil samples were digested with a HClO4-HNO3-HF mixture in Teflon tubes. Approximately 0.10 g of dried soil were transferred to an extraction vessel with 3 ml HNO3, 1 ml HClO4 and 1 ml HF and digested at a temperature of 160 °C for 6 h. The residue was then dissolved in 1 ml of 4 M HCl, diluted to 10 ml with deionized water and analyzed for As, Cd, Cr, Cu, Ni, Pb, Zn, P and S. The digested sample solutions were analyzed by inductively coupled plasma atomic emission spectrometry (ICP/AES). Quality assurance and quality control were assessed with duplicates, method blanks and standard reference materials (GBW07401) from the Chinese Academy of Measurement Sciences (NSMMC 2010) for each batch of samples (with one blank and one standard for each 10 samples). The recoveries of samples spiked with standards ranged from 95 to 105 %. Total nitrogen (TN) was measured using the Elemental Analyzer (Vario EI, Elementar Co., Germany). Soil organic matter (SOM) was measured by using dichromate oxidation (Walkley and Black 1934). Soil pH was measured in 1:5 soil/water (m/v) suspensions with a Hach pH meter (Hach company, Loveland, CO, USA). Salinity was determined in the supernatant of 1:5 soil-water mixtures using a salinity meter (VWR Scientific, West Chester, Pennsylvania, USA). Soil particle size was analyzed using a Laser Particle Size Analyzer (Microtrac Inc., USA).
Geoaccumulation Index
The geoaccumulation index (Igeo) was first proposed by Müller (1979) to assess the degree of soil metal contamination. Igeo values are defined by the following formula:
Where Cn is the measured concentration of metal (n) and Bn is the geochemical background concentration of this metal. There is a 1.5 background matrix correction factor that includes possible variations in the background values from lithogenic effects (Müller 1979). In the present study, the background values were obtained on the basis of mean values for the environmental background concentration of loess materials in the Yellow River (CNEMC 1990) (Table 1). The geoaccumulation indexes were classified into seven classes as follows: (i) unpolluted (Igeo ≤ 0), (ii) unpolluted to moderately polluted (0 < Igeo ≤ 1), (iii) moderately polluted (1 < Igeo ≤ 2), (iv) moderately to strongly polluted (2 < Igeo ≤ 3), (v) strongly polluted (3 < Igeo ≤ 4), (vi) strongly to extremely polluted (4 < Igeo ≤ 5), and (vii) extremely polluted (Igeo > 5).
Ecotoxicity Assessment
The probable effect level (PEL) and the threshold effect level (TEL) were applied to assess the ecological risks from metals (MacDonald et al. 2000). The PEL represents the concentration above which adverse effects are expected to occur frequently, and the TEL represents the concentrations above which the toxic effects of long-term exposure to these contaminants may be expected (Smith et al. 1996). The toxic unit (TU), which is defined as the ratio of the determined content to the probable effect level (PEL), was used to assess the ecological toxicity. Pedersen et al. (1998) proposed that the potential acute toxicity of the contaminants in a sample can be estimated as the sum of the toxic units (∑TUs). The PEL and TEL values for all studied metals are listed in Table 1.
Statistical Analysis
Pearson correlation coefficients were performed to identify the relationships among different metals. The relationships between concentrations of the investigated metals and selected soil properties (TP, TN, TS, SOM, pH, salinity, sand, clay and slit) were also examined. PCA is one of the most commonly used multivariate statistical techniques in environmental studies to simplify the interpretation of complex systems (Ravindra et al. 2008). A complex data set is simplified by creating several new variables or factors, each representing a cluster of interrelated variables within the dataset. Before performing PCA, Kaiser-Meyer-Olkin (KMO) and Bartlett’s sphericity tests were used to examine the validity of PCA. ANOVA was implemented to test the significant differences of seven metals during the four seasons, respectively. Differences were considered significant when P < 0.05. The coefficient of variation was calculated by the ratio of standard deviation and average. A statistical analysis was performed with SPSS 13.0 and Origin 8.0 software packages.
Results
The Mean Concentrations of Metals Over Four Sampling Periods
Figure 2a summarizes the average contents of total As, Cd, Cr, Cu, Ni, Pb and Zn in the top 20 cm of soil from T. chinensis wetlands during the four sampling periods. As shown in Fig. 2a, the mean values of As, Cd and Zn exhibited the highest level in August, while the lowest in November. The average Cr and Ni levels were the highest in November and the lowest in June. Moreover, the concentration of Cu and Pb have no obvious change in the four sampling periods. Meanwhile, the Cr and Ni values exhibited significant differences between June and the other months, and the Cd and Zn levels showed significant differences among four sampling periods except for November and April. However, no significant differences in the Cu and Pb were observed among the four sampling periods. From Fig. 2b, we can see that the coefficients of variation for all metals were less than 0.3 over the four sampling periods except for Zn (0.33) in November. The mean contents of all metals in the study periods exceeded their background values in the Yellow River Delta, except for Pb in April and November and Zn in April. In particular, the mean levels of As and Cd were approximately 3.91 and 12.21 times higher than the background values for As and Cd (Table 1). In comparison with other larger deltas throughout the world, the As, Cd and Cr levels in this study were higher, whereas there were lower levels for Cu, Ni, Pb and Zn (Table 2).
Spatial and Seasonal Variation in Metals Over Four Sampling Periods
The spatial and temporal distributions of heavy metals in the top 20 cm of soils are illustrated in Fig. 3. We observed that the concentrations of Cu and Pb showed no obvious fluctuations along the sampling zone over the four sampling periods. When compared to the TEL-PEL sediment quality guidelines, the concentrations of Cu and Pb during the four sampling periods were lower than the TEL value at 100 % of all sampling sites, while As showed values higher than the PEL at 100 % of all sampling sites during the four sampling periods. Meanwhile, the concentration of Cd and Zn showed an obvious fluctuations in August along the sampling sites. The concentration of Cd in all sampling sites in June and August were higher than their TEL value. However, with the exception of Sites A and B in August, the concentration of Zn were all less than their TEL value. On the other hand, the concentration of Ni fell in the range between TEL and PEL in June along the sampling sites except for Site A. Moreover, the concentration of Ni showed a trend of decline along the sampling sites in June. Amongst the four sampling periods, the concentration of Cr mostly fell in the range between TEL and PEL along the sampling sites.
Principal Component Analysis
The 15 variables in this study were summarized by three principal components. The three principal components (PC1, PC2 and PC3) were extracted with eigenvalues > 1 when applying varimax rotation, accounting for the majority (82.90 %) of the total variances (Table 3). The first principal component (PC 1), with a variance of 41.3 %, was highly correlated with As, Cd, Pb, Zn, pH, TP, salinity and silt. PC 2 explained 28.5 % of the total variance with significant loadings on Cr and Ni. PC 3 explained 13.2 % of the total variance with significant loadings on Cu, Pb and TN.
Assessment of Metal Pollution Using I geo
Temporal changes in the I geo of the metals are illustrated in Fig. 4. According to the Müller scale (Müller 1979), all of the sampling sites had I geo values for Cr, Cu, Ni and Pb that were below zero during the four sampling periods. Moreover, the I geo values for Zn were grouped into Class II (0 < Igeo ≤ 1) in August at Site A and Site B. However, both As and Cd showed a higher contamination level at all five sampling sites in the four sampling periods. The I geo values of As at all sampling sites were grouped into Class II (0 < Igeo ≤ 1) in November and April and Class III (1 < I geo < 2) in August in five sampling sites. All of the I geo values of Cd were grouped into Class III (1 < I geo < 2) in November and April, Class IV (2 < I geo < 3) in June and August at Site A, Site D and Site E, and Class V (3 < I geo <4) in August at Site B and Site C. Generally, the higher I geo values of these heavy metals were observed at sampling sites in August rather than other sampling periods.
The Ecological Toxicity of Metals in Surface Soils
Figure 5 shows the TU and the sum of the TUs (∑TUs) of all metals in the top 20 cm of soils at five sampling sites during four different sampling periods. The average TU value of metals in this region followed the order As > Ni > Cr > Zn > Pb > Cd > Cu. As, Ni and Cr made higher contributions to the ∑TUs (i.e., 40.3 ± 7.2, 22.3 ± 4.6, and 19.7 ± 4.6 %, respectively) than the other metals at all sampling sites. However, Cu contribution to the ∑TUs was the lowest (2.6 ± 0.4 %). The sum of ∑TUs for all metals in August showed relatively higher levels when compared with other sampling periods. The ∑TU values in this study soil were generally all greater than 4, except the values from Sites C, D and E in June and Sites A and B in April. The ∑TU value at Site B in August even exceeded 6.
Relations Between Metals and Soil Properties
A correlation analysis was performed on the basis of all of the measured data in four sampling periods to determine the relation between metals and physico-chemical properties. As shown in Table 4, there were significant correlations among As, Cd and Zn. Significant correlations were also observed between Cr and Ni, and between Cu and Zn. With the exception of Cu, all metals showed significant correlations with P. Moreover, pH and salinity were significantly and positively correlated with As, Cd and Pb. Meanwhile, the data showed that positive correlation exists between Pb and Zn, As and Cd. However, no significant correlation was observed between metals, SOM and S in this study. Additionally, total N had no significant correlation with metals except for Pb. As, Cd and Zn concentrations were significantly affected by slit in this studied area.
Discussion
The Spatial and Seasonal Dynamics of Metals
The coefficients of variation for all the metals in our study (<0.3) fell in the medium spatial variation range defined by Cambardella et al. (1994). Most metals showed the highest mean concentration in November, such as Cr, Cu, Ni, and Pb. In general, most of the metals were significantly higher in winter, which is due to climatic variation during summer and winter. Soil organic matter content is also the most dominant factor controlling the concentration and retention of these elements in the soils. High organic matter plays an important role in the soil structure, water retention, cation exchange and formation of complexes (Yahaya et al. 2009). The phenomena of high metal concentration in soils during winter were also explained by Niskavaara et al. (1997), where they observed that during late autumn and winter the debris from the dying vegetation is accumulated on the soils, increasing the concentration of all these components. Similar to our findings, Iqbal and Shah (2011) reported that most of the precipitation was observed in summer which partially removes the soluble metal concentrations from the soil, whereas winter mostly remained dry thereby accumulating the deposited metal contents in the soil. However, Cd, As and Zn showed the highest concentrations in Summer, which were associated with the flow-sediment regulation in July. Because the increased input and retention capacity during the flow-sediment regulation can lead to increased precipitation and deposition of particulate metals to the surface soil (Bai et al. 2012). Additionally, microbial activity in the soil will be greater in the summer but it is unclear whether this would increase metal levels by accumulation or decrease them by mobilization. Moreover, contrasted to Pb, Cr had obvious fluctuation along the sampling sites from A to E, which might be related to salinity gradient along the sampling sites from A to E. Du Laing et al. (2009) reported that soluble Cr can be enhanced with increasing salinity, whereas the salinity do not significantly affect Pb mobility. Some researchers have reported that environmental factors affecting variability in metal concentrations of surface soil layers can be further complicated by interrelationships with anthropogenic inputs (Bibby and Webster-Brown 2005; Duquesne et al. 2006). If anthropogenic inputs were relatively constant, temporal variation would be dictated by in situ environmental factors. However, if anthropogenic inputs were sporadic and/or extreme, contaminant input would determine both spatial and seasonal distribution (Boyes and Elliott 2006; Shepherd et al. 2006).
The mean contents of Cu, Pb, Ni and Zn in the soils from the Yellow River Delta were generally lower relative to other larger deltas around the world (e.g., the Pearl River Delta, Yangtze River Delta, Niger Delta, and the Red River Delta, Table 2). This implied that metal pollution in YRD is less serious than in deltas of other major rivers, probably due to the lower degree of industrialization in the Yellow River Detla. However, comparing the concentrations obtained from this research with those obtained during the 1990s, metal pollution aggravated in this delta in the last decade, which mainly due to the intensive human activities, the rapid development of petrol oil industries, irrigated agriculture and sediment movements resulting from the flow-sediment regulation of Xiaoliangdi Reservoir.
Correlation Between Metals and Selected Soil Properties
Soil properties play an important role in the mobility and bioavailability of metals, thus influencing their distribution in soils (Khan et al. 2008). Although some researchers have demonstrated that soil organic matter can act as a major sink for metals due to its strong capacity for complexing metallic contaminants (Bai et al. 2010), metals were not significantly correlated with soil organic matter in this study. This is in agreement with the conclusions concluded by Fitz and Wenzel (2002), that no evidence were observed that soil organic matter contributed to the sorption of significant amounts of As in soils. Moreover, total P was closely related to Pb and Zn in this study soil because the phosphate can reduce Pb or Zn mobility by ionic exchange and the precipitation of newly formed minerals with low solubility and bioaccessibility (Scheckel and Ryan 2003; Kumpiene et al. 2008). Additionally, concentrations of As, Cd and Pb were significantly correlated with soil pH. Numerous studies have demonstrated the importance of pH in determining the fate of metals in soils, Lead et al. (1999) has been observed that a small shift in pH can produce a sharp increase in particulate metal concentrations, and further, that Pb and Cu are more strongly bound to oxy-hydroxide surfaces than other metals, e.g. Ni and Zn (Forstner 1987). What’s more, the narrow range of pH (7.78–8.98) measured in the sampled topsoils would contribute to the lowest mobility of some metals because of subalkaline environment (Kumpiene et al. 2008), which could result in their accumulations in soils. Meanwhile, the CaCO3 in calcareous soils could lead to Cu, Zn and Pb precipitation as CuCO3, ZnCO3 and PbCO3 (De la Fuente et al. 2008), which were their dominated forms in alkaline soils and all of them were difficult to be assimilated by plants (wang et al. 2007). It is reasonable to see a significant positive correlation between salinity and most metals (Che et al. 2010). What’s more, slit had positive correlation with As, Cd and Zn, which can be interpreted that the specific surface area of soil increases as the particles become finer, leading to an increase in the effective contact area between adsorbent and adsorbate, this result in the adsorption of large amounts of metals (Shim et al. 2003).
Possible Sources Based on Principal Component Analysis
The significantly positive correlations among these metals using pearson’s correlation matrix suggested, to some extent, a common source (Al-Khashman and Shawabkeh 2006). However, the significant correlation is not always expected to be a common source (Ham and Pazira 2011). It implies that the single correlation analysis may not be enough for source identification of metals and it should be conducted together with other analysis tools. Therefore, PCA was performed on these metals, TP, TN, TS, SOM, pH, salinity and particle size. KMO and Bartlett’s results were 0.81 and 1674.31 (p < 0.01), indicating that PCA may be useful in dimensionality reductions. Generally, the component total of >0.60 is regarded as a major contributor while <0.30 as a minor contributor. In this study, As, Cd, Pb and Zn were grouped into PC 1, indicating that they might be derived from common sources. Heavy applications of agrochemicals and fertilizers in the Yellow River drainage plain in addition to the oilfield pollution in this region might be important sources of these metals (Li et al. 2006; Bai et al. 2011b; Krishna et al. 2011). Tang et al. (2010) observed that higher As and Cd contents in the seawater of the Yellow River Estuary were primarily affected by inputs from the Yellow River. Some studies also indicated that Zn in the Yellow River Delta primarily originates from anthropogenic activities in the upper stream region (Han 2009; Song et al. 2013). Cr and Ni (grouped into PC 2) belong to the siderophile elements, and they are the primary rock-forming elements (Okbah et al. 2014). It is easy for them to enter into iron magnesium silicate minerals, because of their similar ionic radius. These elements association are considered to represent the lithology of the study area, indicating a lithogenic origin from the loess materials of the Loess Plateau (Bai et al. 2012) and a natural input; i.e., they are derived from terrigenous detritus material transported by surface runoff (Krishna et al. 2011). Cu and Pb were grouped into PC 3, indicating that they might originate from another common source such as tidal seawater. Additionally, conventional onshore crude oil extraction came from venting and flaring operations, and volatilization caused air emissions of toxic heavy metals such as lead (Khoo and Tan 2006). Meanwhile, atmospheric deposition was also a source of metals in Yellow River Delta (Luo et al. 2010). As shown in Table 3, significant correlations among these metals (e.g., As, Pb and Zn, Cr and Ni) further implied that they had common sources, which is consistent with the result of the principal component analysis. Additionally, Pb and Zn were significantly correlated with soil P and N, which also implied that they might have a similar origin (e.g., agricultural runoff and intensive fertilizer application).
Contamination and Toxicity Assessments of Metals in Wetland Soils
Possible enrichment of metals in wetlands soils was evaluated using geoaccumulation (Igeo) (Müller 1979). The contamination level is assessed by comparing the present concentration with preindustrial levels. The geoaccumulation index (Igeo) of target elements indicated that there was no Cu, Pb and Cr pollution at five sampling sites for all sampling periods in the study area, that As was present at unpolluted to moderate pollution levels in November and April and moderate to strong pollution level in June and August, whereas Cd was present at a moderate pollution level in November and April, and moderate or moderate to strong pollution levels in June and August. The high concentrations of Cd and As from June to August might be related to the sediment movements resulting from the implementation of flow-sediment regulations in the Xiaolangdi Reservoir in July (Bai et al. 2012).
The TU approach is mostly used to test the response addition model for chemical mixtures by comparing the likely relative toxicity induced by elements (Horvat et al. 2007). The toxicity assessment of the selected samples showed that As, Ni and Cr had higher contribution rates to the sum of the TUs. Cd made a lower toxicity contribution despite its higher pollution level on the basis of the Igeo assessment. As a result of the much higher PEL value of Cd, this assessment would certainly underestimate their toxicity when using a TU approach. Therefore, a complementary approach including standard soil criteria and diffident assessment methods should be considered to provide a more accurate and comprehensive assessment of the risk from metals to the environment. What’s more, the higher TUs of metals were clearly observed in August, indicating that the flow-sediment regulation might increase the ecological risks in this region (Bai et al. 2012). Based on the analysis results of Igeo and TUs, arsenic contamination was generally serious in this region with higher As levels exceeding the PEL value. Moreover, Kupchella and Hyland (1986) presented that the substance containing As might be transformed by the addition of carbon and hydrogen as a methyl group (CH3) resulting in methylarsines—which is much more toxic to living things than the unmethylated forms. Therefore, arsenic should be taken into consideration especially separately from other metals in this study area.
Conclusions
The present study illustrated obvious spatial and seasonal variations of the selected metals contents in surface soil samples. The results showed that most metals have the highest mean concentration in November, such as Cr, Cu, Ni, and Pb. However, Cd, As and Zn showed the highest concentrations in Summer, which were particularly associated with the flow-sediment regulation in July. Moreover, contrasted to Pb and Cu, Cr, As and Ni had obvious fluctuation along the sampling sites from A to E, which might be related to salinity gradient along the sampling sites from A to E. Slit, pH and salinity had obvious effects on the concentration of metals. PCA and person’s correlation matrix analysis showed that As, Cd, Pb and Zn originate from heavy applications of agrochemicals, oilfield pollution and tidal seawater, while Cr and Ni might originate from parent rocks. Additionally, Pb and Zn were significantly correlated with soil P and N, which also implied that they might have a similar origin (e.g., agricultural runoff and intensive fertilizer application). Although soil metals showed low contamination in this region, it is necessary to control As pollution to protect ecological security. These results could be used as a contribution to the knowledge and rational management of the Yellow River Delta.
References
Abdallah MAM (2011) Ecological risk assessment of heavy metals from the surficial sediments of a shallow coastal lagoon, Egypt. Environ Technol 32:979–988
Al-Khashman OA, Shawabkeh RA (2006) Metals distribution in soils around the cement factory in southern Jordan. Environ Pollut 140:387–394
Bai JH, Cui BS, Wang QG, Gao HF, Ding QY (2009) Assessment of heavy metal contamination of roadside soils in Southwest China. Stoch Environ Res Risk Assess 23:341–347
Bai JH, Cui BS, Yang ZF, Xu XF, Gao HF, Ding QY (2010) Heavy metal contamination of cultivated wetland soils along a typical plateau lake from southwest China. Environ Earth Sci 59:1781–1788
Bai JH, Xiao R, Cui BS et al (2011a) Assessment of heavy metal pollution in wetland soils from the young and old reclaimed regions in the pearl river estuary, South China. Environ Pollut 159:817–824
Bai JH, Huang LB, Deng HY et al (2011b) Contamination characteristics of heavy metals in wetland soils along a tidal ditch of the yellow river estuary, China. Stoch Environ Res Risk Asses s25:671–676
Bai JH, Xiao R, Zhang KJ, Gao HF (2012) Arsenic and heavy metal pollution in wetland soils from tidal freshwater and salt marshes before and after the flow-sediment regulation regime in the yellow river delta, China. J Hydrol 450:244–253
Bibby RL, Webster-Brown JG (2005) Characterisation of urban catchment suspended particulate matter (Auckland region, New Zealand); a comparison with non-urban SPM. Sci Total Environ 343:177–197
Boyes S, Elliott M (2006) Organic matter and nutrient inputs to the humber estuary, England. Mar Pollut Bull 53:136–143
Cambardella CA, Moorman TB, Parkin TB et al (1994) Field-scale variability of soil properties in central Iowa soils. Soil Sci Soc Am J 58:1501–1511
Cenci RM, Martin JM (2004) Concentration and fate of trace metals in mekong river delta. Sci Total Environ 332:167–182
Che GQ, Zeng GM, Du CY, Huang DL, Tang L, Wang L et al (2010) Transfer of heavy metals from compost to red soil and groundwater under simulated rainfall conditions. J Hazard Mater 181:211–216
Chen ZR, Kostaschuk R, Yang M (2001) Heavy metals on tidal flats in the Yangtze Estuary, China. Environ Geol 40:742–749
China National Environmental Monitoring Center (CNEMC) (1990) Chinese elemental background values for soils. Chin Environ Sci, Beijing (in Chinese)
De la Fuente C, Clemente R, Bernal MP (2008) Changes in metal speciation and pH in olive processing waste and sulphur-treated contaminated soil. Ecotoxicol Environ Saf 70:207–215
Du Laing G, Rinklebe J et al (2009) Trace metal behaviour in estuarine and riverine floodplain soils and sediments: A review. Sci Total Environ 13:3972–3985
Duquesne S, Newton LC, Giusti L, Marriott SB, Stark HJ, Bird DJ (2006) Evidence for declining levels of heavy metals in the severn estuary and bristol channel, UK and their spatial distribution in sediments. Environ Pollut 143:187–196
Fitz WJ, Wenzel WW (2002) Arsenic transformations in the soil-rhizosphere-plant system: fundamentals and potential application to phytoremediation. J Biotechnol 99:259–278
Forstner U (1987) Sediment-associated contaminants-an overview of scientific bases for remedial options. Hydrobiologia 149:221–246
Gao XL, Chen TAC (2012) Heavy metal pollution status in surface sediments of the coastal Bohai Bay. Water Res 46:1901–1911
Giani L, Ahrens V, Duntze O, Irmer SK (2003) Geo-pedogenese mariner rohmarschen spiekeroogs. J Plant Nutr Soil Sci 166:370–378
Ham A, Pazira E (2011) Heavy metals assessment and identification of their sources in agricultural soils of Southern Tehran, Tran. Environ Monit Assess 176:677–691
Han X (2009) Quantitative Assessment of Environmental Impacts of Petroleum Development in the Yellow River Delta. Ocean University of China, Qingdao (in Chinese)
Hang XS, Wang HY, Zhou JM et al (2009) Risk assessment of potentially toxic element pollution in soils and rice (Oryza Sativa) in a typical area of the Yangze River Delta. Environ Pollut 157:2542–2549
Horvat T, Vidaković-Cifrek Ž, Oreščanin V et al (2007) Toxicity assessment of heavy metal mixtures by Lemna minor L. Sci Total Environ 384:229–238
Hu BQ, Li J, Zhao JT et al (2013) Heavy metals in surface sediments of the Liaodong Bay, Bohai Sea: distribution, contamination, and sources. Environ Monit Assess 185:5071–5083
Iqbal J, Shah MH (2011) Distribution, correlation and risk assessment of selected metals in urban soils from Islamabad, Pakistan. J Hazard Mater 192:887–898
Jacob DL, Otte ML (2003) Conflicting processes in the wetland plant rhizosphere: metal retention or mobilization? Water Air Soil Pollut 3:91–104
Jiang D, Fu X, Wang K (2012) Vegetation dynamics and their response to freshwater inflow and climate variables in the yellow river delta China. Quat Int. doi:10.1016/ j.2012. 10.059
Khan S, Cao Q, Zheng YM et al (2008) Health risks of heavy metals in contaminated soils and food crops irrigated with wastewater in Beijing, China. Environ Pollut 152:686–692
Khoo HH, Tan RBH (2006) Environmental impact evaluation of conventional fossil fuel production (oil and natural gas) and enhanced resource recovery with potential CO2 sequestration. Energy Fuel 20:1914–1924
Krishna AK, Mohan KR, Murthy NN (2011) A multivariate statistical approach for monitoring of heavy metals in sediments: a case study from Wailpalli Watershed, Nalgonda District, Andhra Pradesh, India. Res J Environ Earth Sci 3:103–113
Kumpiene J, Lagerkvist A, Maurice C (2008) Stabilization of As, Cr, Cu, Pb and Zn in soil using amendments-a review. Waste Manag 28:215–225
Kupchella CE, Hyland MC (1986) Environmental science. Allyn and Bacon Inc., Newton, p 405
Lau SSS, Chu LM (2000) The significance of sediment contamination in a coastal wetland, Hong Kong, China. Water Res 34:379–386
Lead JR, Hamilton-Taylor J, Davison W, Harper M (1999) Trace metal sorption by natural particles and coarse colloids. Geochim Cosmochim Acta 63:1661–1670
Li YD, Wang YB, Gou X, Su YB, Wang G (2006) Risk assessment of heavy metals in soils and vegetables around non-ferrous metals mining and smelting sites, Baiyin, China. J Environ Sci 18:1124–1134
Li Y, Zhang HB, Chen XB et al (2014) Distribution of heavy metals in soils of the yellow river delta: concentrations in different soil horizons and source identification. J Soils Sediments. doi:10.1007/s11368-014-0861-0
Liu JL, Wu H, Feng JX, Li ZJ, Lin GH (2014) Heavy metal contamination and ecological risk assessments in the sediments and zoobenthos of selected mangrove ecosystems, South China. Catena 119:136–142
Luo W, Lu YL, Wang TY, Hu WY et al (2010) Ecological risk assessment of arsenic and metals in sediments of coastal areas of northern Bohai and Yellow Seas, China. A J Hum Environ 39:367–375
MacDonald DD, Ingersoll CG, Berger TA (2000) Development and evaluation of consensus-based sediment quality guidelines for freshwater ecosystems. Arch Environ Contam Toxicol 39:20–31
Müller G (1979) Schwermetalle in den sedimenten des Rheins-Veranderungen seit. Umschau 79:778–783
Niskavaara H, Reimann C, Chekushin V et al (1997) Seasonal variability of total and easily leachable element contents in topsoils (0-5 cm) from eight catchments in the European Arctic (Finland, Norway and Russia). Environ Pollut 96:261–274
NSMMC (National Standard Material Management Committee) (2010) Standard material directory of P.R.C. Chinese Measuring Press, Beijing
Okbah MA, Nasr SM, Soliman NF, Khairy MA (2014) Distribution and contamination status of trace metals in the mediterranean coastal sediments, Egypt. Soil Sediment Contam 23:656–676
Olawoyin R, Oyewole SA, Grayson RL (2012) Potential risk effect from elevated levels of soil heavy metals on human health in the Niger delta. Ecotoxicol Environ Saf 85:120–130
Passos EA, Alves JC, Santos DIS et al (2010) Assessment of trace metals contamination in estuarine sediments using asequential extraction technique and principal component analysis. Microchem J 96:50–57
Pedersen F, Bjørnestad E, Andersen HV et al (1998) Characterization of sediments from copenhagen harbour by use of biotests. Water Sci Technol 37:233–240
Phuong NM, Kang YM, Sakurai K et al (2010) Levels and chemical forms of heavy metals in soils from Red River Delta, Vietnam. Water Air Soil Pollut 207:319–332
Qin P, Mitsch WJ (2009) Wetland restoration and ecological engineering: international conference of wetland restoration and ecological engineering. Ecol Eng 35:437–441
Ravindra K, Sokhi R, Van Grieken R (2008) Atmospheric polycyclic aromatic hydrocarbons: source attribution, emission factors and regulation. Atmos Environ 42:2895–2921
Reddy KR, DeLaune RD (2008) Biogeochemistry of Wetlands: Science and Applications, 349 1st Ed. CRC Press, Boca Raton
Scheckel KG, Ryan JA (2003) In vitro formation of pyromorphite via reaction of Pb sources with soft-drink phosphoric acid. Sci Total Environ 302:253–265
Shepherd KA, Ellis PA, Rivett MO (2006) Integrated understanding of urban land, groundwater, baseflow and surface-water quality-The City of Birmingham. UK Sci Total Environ 360:180–195
Shim YS, Kim YK, Kong SH et al (2003) The adsorption characteristics of heavy metals by various particle sizes of MSWI bottom ash. Waste Manage 23:851–857
Smith SL, MacDonald DD, Keenleyside KA, Ingersoll CG, Field LJ (1996) A preliminary evaluation of sediment quality assessment values for freshwater ecosystems. J Great Lakes Res 22:624–638
Song S, Li FD, Li J, Liu Q (2013) Distribution and contamination risk assessment of dissolved trace metals in surface waters in the yellow river delta. Hum Ecol Risk Assess 19:1514–1529
Tang A, Liu R, Ling M, Xu LM, Wang JY (2010) Distribution characteristics and controlling factors of soluble heavy metals in the yellow river estuary and adjacent sea. Procedia Environ Sci 2:1193–1198
Teuchies J, Jacobs S, Oosterlee et al (2012) Role of plants in metal cycling in a tidal wetland: implications for phytoremediation. Sci Total Environ 445:146–154
Walkley A, Black IA (1934) An examination of the Degtjareff method for determining soil organic matter, and a proposed modification of the chromic acid titration method. Soil Sci 377:29–38
Wang F, André T (2009) Zero-valent sulfur and metal speciation in sediment pore waters of freshwater lakes. Environ Sci Technol 43:7252–7257
Wang HQ, Liu XH, Li GX (2007) Soil environment. Higher Education Press, Beijing, p 72 (in Chinese)
Wu ZH, He MC, Lin CY (2012) Environmental impacts of heavy metals (Co, Cu, Pb, Zn) in surficial sediments of estuary in daliao river and yingkou bay (northeast China):concentration level and chemical fraction. Environ Earth Sci 66:2417–2430
Xu Q, Shi G (2000) The toxic effects of single Cd and interaction of Cd with Zn on some physiological index of [Oenanthe javanica (Blume) DC]. J Nanjing Normal Univ (Nat Sci) 23:97–100 (In Chinese)
Yahaya MI, Mohammad S, Abdullahi BK (2009) Seasonal variations of heavy metals concentration in abattoir dumping site soil in Nigeria. J Appl Sci Environ Manag 13:9–13
Zhang LH, Song LP, Zhang LW et al (2013) Seasonal dynamics in nitrous oxide emissions under different types of vegetation in saline-alkaline of the yellow river delta, China and implications for eco-restoring coastal wetland. Ecol Eng 61:82–89
Acknowledgments
This study was financially supported by the National Basic Research Program of China (no. 2013CB430406), the National Science Foundation for Innovative Research (no. 51121003), the National Natural Science Foundation (nos. 51179006 and 51379012) and the Fok Ying Tung Educational Foundation (132009). The authors acknowledge all colleagues for their contribution in the field works.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Lu, Q., Bai, J., Gao, Z. et al. Spatial and Seasonal Distribution and Risk Assessments for Metals in a Tamarix Chinensis Wetland, China. Wetlands 36 (Suppl 1), 125–136 (2016). https://doi.org/10.1007/s13157-014-0598-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13157-014-0598-y