Abstract
Heavy metal pollution in the surficial sediments derived from the estuary in Daliao River and Yingkou Bay is investigated to assess environmental quality, pollution level, bioavailability and toxicity. The ranges of Pb, Co, Zn and Cu concentrations in the surficial sediments are: 16.57–39.18, 3.61–16.02, 16.53–39.18, 2.77–43.80 mg/kg. Results of the geoaccumulation index (I geo) show that the pollution levels of four metals are in the “unpolluted” class except for Pb in 15 sampling sites. The pollution level of the study area assessed by pollution load index (PLI) shows that except for the moderately polluted region of sites 1, 2, 3, 8, 12 and 13, other sites belong to unpolluted state. The sequence of pollution extent of different heavy metals is: Pb > Zn > Co > Cu. At all sampling sites, the grades of potential ecological risk of Co, Cu, Pb and Zn are “light”. The order of potential ecological risk is: Pb > Co > Cu > Zn. Sequential extraction of the metals indicates that the states of Pb, Cu, Co and Zn in the sediment are relatively stable at most sites of the estuary in Daliao River and Yingkou Bay, which means that there is a low source of pollution arriving in this area. While only at several sites, Co, Pb and Zn are labile, which are considered as anthropogenically originated.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Sediments are often the sinks of metal contaminants in an aquatic system (Ridgway and Shimmield 2002). However, some of the sediment-bound metals may be released back to water with the change of environmental conditions and impose adverse effects on living organisms.
The chemical forms of heavy metals redound to understand the potential and actual environmental impacts of contaminated sediments. Among pollutants present in the environment, heavy metals have been of interest due to their toxicity, persistence and prevalence (Shriadah 1999; Tam and Wong 2000; Nasr et al. 2006). Heavy metals are among the most widespread of various pollutants originating from anthropogenic activities (Audry et al. 2004). They are distinct from organic substances, as they do not degrade and can only migrate and accumulate in different components of a natural ecosystem. Heavy metals can endanger public health by being incorporated into the food chain (Gladyshev et al. 2001; Shrivastava et al. 2003). A few researches have been done to investigate the contamination of sediments by metals and organic compounds in Liaodong bay (Guo et al. 2006; Lin et al. 2009). This research investigated the metal concentration, distribution, speciation, contamination source and assessed metal pollution in surficial sediment of the estuary in Daliao River and Yingkou Bay, which is an important region of Liaodong Bay (Northeast China).
Direct determination of specific chemical forms is generally impractical due to various binding phases of metals and their structural properties (Amany and Ahmed 1999; Gianpiero et al. 2007; Edward et al. 2005). Alternatively, sequential extraction procedure, which defines the metal activity by the analytical schemes, becomes preferred (Gonzalez et al. 2000). It was developed first by Tessier, Campbell and Bisson (Tessier et al. 1980) and afterwards modified in different ways (Campanella et al. 1995). In this paper, the six-step sequential extraction scheme proposed by Han Fengxiang and Amos Banin (Han and Amos 1997) was chosen as the experimental method.
YingKou Bay lies in the eastern part of Liaodong coast between longitude 40°18′34.7″ and 41°34′52.2″ and Latitude 122°03′02.9″ to 122°51′35.6″. Liao River system is constituted by four main rivers: Hun River, Taizi River, Daliao River, Liao River and their branches. Daliao River lies in the middle and downstream of Liao River system, which is the main waterhead in the Liaoning Province. The area of Liao River system reaches 27,309 km2, including big cities such as Shenyang, Fushun, Benxi and Anshan. Yingkou Bay is the inner bay of Bo Sea and Liao River system that flow into this bay. Yingkou Bay becomes a big cesspool and the water quality is class IV. The main pollution is organic contamination such as COD (386 thousand tons/year) and NH3-N (46.9 thousand tons/year) (Wu et al. 2011; Mu and Luan 2004). The type of Yingkou Bay belongs to silt-muddy plain and strong corrosion coast. Liaohe delta belongs to one part of the hollow of Bo Sea and has endured strong subsidence since the movement of the Himalaya Mountains. The thickness of accumulated sediment adds 1,000–2,000 m, which is constituted by incompact deposit layer of Quaternary period and the bedrock is buried deeply (Wang and Li 1983; Song et al. 1997). The rock bodies are susceptible to erosion as favored by both climate and tectonic factors. Tectonism includes the presence of numerous faults, which have been intermittently active during Quaternary period. This leads to sediment compaction with simultaneous subsidence of the basin. This unstable tectonic setting of the basin of sedimentation is further aided by the prevailing wet, hot and warm climate with high relative humidity and temperature in summer. The sediment load shows a great seasonal variation in discharge. Strong tidal currents lead to a network of deeply scoured tidal channels overlapping the tidal plain across the wash plain. Therefore, Liaohe delta is the wash plain due to the fill of rivers. The status of the land uses in Liaohe delta is: grassland, cultivated land, woodland, building-sites land, Yan, unused land and water bodies (Chang et al. 2002; Fan 2009). Liao River system and the estuary are shown in (Fig. 1, ESM only).
This paper reports on the concentrations and speciation of four metals (Co, Cu, Pb, Zn) in the surficial sediments of estuary in Daliao River and Yingkou Bay. The main aims are: (1) to determine total concentrations deposited in the surficial sediments to determine their variability and origin; (2) to assess the degree of pollution in the surficial sediments by index of geoaccumulation, pollution load index, potential ecological risk index and enrichment factor; and (3) to investigate the mobility and availability of heavy metals in sediments using a six-stage sequential chemical extraction technique.
Materials and methods
Sample collection and pre-treatment
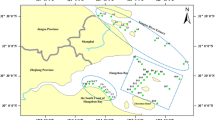
The sampling location of the estuary in Daliao River and Yingkou Bay is shown in Fig. 1. Coastal surficial sediment samples are gathered from 35 sites in Daliao River and Yingkou Bay including five sampling areas: I, downstream area of Daliao River (1, 2, 3, 4); II, estuary in Daliao River (5, 6, 7); III, inner bay (8–16); IV, seawater interjunction of inside bay and outside bay (17–25); V, outside bay (26–35). The sampling areas are divided into five regions from the downstream area of Daliao River to the outside bay to investigate the variation rules of the distribution of heavy metals along estuary in Daliao River and Yingkou Bay. The surface sediment samples (15–20 cm) were collected from 35 sampling sites during August 2007, using an Ekman grab sampler according to the method (Inmaculada et al. 2010; Ahmed and Soraya 2007). Sediment samples were immediately isolated from the atmosphere inside a plastic bag to prevent any oxidation reaction in the sedimentary material and were placed in an icebox until analysis. The physical and chemical properties including: pH, EC (conductance), temperature of the overlying water, TOC of the surficial sediments were determined according to standard methods (Essien et al. 2009; Wajid et al. 2008; Alessandra et al. 2008).
Reagents and apparatus
All chemicals used are of analytical grade from Bosichuangqi Reagents and Apparatus Company. All solutions were prepared using double-distilled water (DDW). All calibration and reagent solutions were stored in vessels made of boronsilica glass of the highest quality.
Sediment treatment and analysis
After collection, surficial sediment samples were dried at room temperature, ground to pass through a 63-μm sieve and preserved (Bryan and Langston 1992; Langston and Spence 1994). After digesting with a mixture of concentrated HNO3–HF–HClO4 (GB 17378.5 1998), the sample was analyzed to determine the concentrations of Co, Cu, Pb and Zn by inductively coupled plasma mass spectrometry (ICP-MS, X Series II). The chemical distributions of metals (Co, Cu, Pb, Zn) are determined by the six-step sequential extraction scheme proposed by Han Fengxiang and Amos Banin (Han and Amos 1997). The method is described as the following procedure, which is shown in Table 1 (ESM only). Blank and certified reference materials [So-4(619), So-1(F48)], supplied by Canadian Certified Reference Material Project, Canada (CCRMP; Li et al. 1995) were analyzed concurrently with sediment samples to ensure consistency and accuracy of recoveries over metal analyses. The determined results of total concentration obtained in these experiments corresponded with those obtained for the certified materials, and the recoveries of the six-step fractionation with respect to the total metal concentrations are described in Table 2 (ESM only). The description of those reference materials has been given by Potts et al. (1992).
Results and discussion
The character of sediment and overlying water
Physicochemical parameters of surficial sediments and the overlying water (temperature, EC, pH, dissolved oxygen) are displayed in Table 1. The TOC values in the analyzed sediment ranged from 1.32 to 2.76% with the average of 2.29%. The TOC level is reduced approximately in this order: I > II > III > IV > V, which reflects the mud containing high organic matter in the river and the export of it from the estuary to the bay. Organic carbon has the significant role in the geochemical cycle of elements that accumulate in sediments; it might be used as an index of depositional environment and sedimentary processes (Seralathan et al. 1993).
Total metal concentration in surficial sediment
Table 2 represents the total content of four metals in 35 sampling sites. The mean concentration of Co is 10.17 mg/kg; the maximum at site 3 is 16.02 mg/kg and the minimum at site 23 is 3.61 mg/kg. The average concentration of Cu is 20.03 mg/kg; the maximum at site 12 is 43.80 mg/kg and the minimum at site 23 is 2.77 mg/kg. The mean concentration of Pb is 26.62 mg/kg; the maximum at site 3 is 39.18 mg/kg and the minimum at site 25 is 16.57 mg/kg. The mean concentration of Zn is 69.38 mg/kg; the maximum at site 3 is 125.93 mg/kg; the minimum at site 23 is 16.53 mg/kg. The concentration of Co that increased at the five different regions is in the order: I > II > III > V > IV; Cu is in the order: I > II > III > V > IV; Pb is in the order: I > II> V > III > IV; Zn is in the order: I > III> V > II > IV. Estuarine sediments may serve as effective traps of river metals (De et al. 1985). The amount of metallic concentration accumulated in residual phase may be attributed to geopogen source, while the potentially labile phases may be considered as anthropogenically originated.
Comparison to other water bodies
To evaluate the pollution level of heavy metals in surficial sediments of the estuary in Daliao River and Yingkou Bay, the content of heavy metals in surficial sediments from other water bodies have been described in Table 3 (Satyanarayana et al. 1994; Santosh et al. 2004; Muthu Raj and Jayaprakash 2008; Yang 2007; Zhou et al. 2004). It reveals that the level of heavy metals in the surficial sediments of the study area is low compared with other sea areas basically. The content of Co is lower than that of Viskhapatnam Coast, India, Zhujang estuary, PRC, Tees estuary, UK, higher than Bay of Bengal, India, but is similar to that of Diamond Harbor, India, and Changjang estuary, PRC. The content of Cu is lower than that of Viskhapantnam Coast, India, Bay of Bengal, India, Zhujang estuary, PRC and Tees estuary, UK, but is similar to Diamond Harbor, India, and Changjang estuary, PRC. The content of Pb is lower than that of Viskhapantnam Coast, India, Bay of Bengal, India, Zhujang estuary, PRC and Tees estuary, UK, higher than that of Diamond Harbor, India, but is similar to Changjang estuary, PRC. The content of Zn is lower than that of Viskhapatnam Coast, India, Bay of Bengal, India, Zhujang estuary, PRC and Tees estuary, UK, higher than Diamond Harbor, India, but is similar to Changjang estuary, PRC.
Methods for estimating pollution impact
Index of geoaccumulation
The geoaccumulation Index (I geo) is put forward by Müller for the quantification of metal contamination in sediments (Müller 1969) and widely applied by many researchers (Muthu Raj and Jayaprakash 2008; Essien et al. 2009; Getaneh and Alemayehu 2006; Krishna and Govil 2008). It is expressed as the following:
where C n is the measured sedimentary concentration for metal n; B n is the background value (average shale) for the metal n; 1.5 is in order to allow content fluctuations of a given substance in the environment as well as very small anthropogenic influences. The average shale values for different metals are taken from Wedepohl (1995), which are shown in Table 3 (ESM only). Müller has distinguished six classes of the geoaccumulation index (Table 4, ESM only). The geoaccumulation index (I geo) of the four metals is shown in Table 4. The most of I geo of the four kinds of metals of surficial sediment are below 0, which indicated the “unpolluted” class. However, the pollution class of Pb is “unpolluted to moderately polluted” class at sites 1, 2, 3, 4, 5, 6, 8, 11, 12, 13, 26, 28, 30, 31, 35. The sequence of the pollution class of different heavy metals is: Pb > Zn > Co > Cu. The sequence of pollution level in 35 samples is: II > I = V > III > IV.
Pollution load index (PLI)
Pollution load index was put forward by Tomlinson et al. (1980) to understand the contamination state of heavy metals, which was constituted by many kinds of heavy metals in the assessment region and was calculated by using the heavy metal data and average natural background. PLI can reflect the extent of contribution to pollution of every kind of heavy metals and the changing extent in time and space.
The contamination factor was calculated (CF = metal concentration in sediment/background concentration for that metal); then PLI was computed from the following equation:
where PLI is pollution load index, CF is contamination factor, n is the number of metals investigated. The assessment result is given in Table 5. The result of the assessment shows that except for the moderately polluted region of sites 1, 2, 3, 8, 12 and 13, other sites belong to “unpolluted state”. The sequence of pollution extent of different heavy metals is: Pb > Zn > Co > Cu. The PLIZone is 0.60 and it shows that the research area belongs to “unpolluted state” on the whole. The sequence of the pollution extent of different sampling area is: I > II> III > V > IV. The maximum of PLI is at sites 3 and 12, while the lowest is at site 23.
Assessment of potential ecological risk contamination of heavy metals
Potential ecological risk index (RI) is introduced to assess the contamination degree of heavy metals in the sediment. RI was founded by Hankanson (1980). The equations for calculating the RI apply the following equations, according to the toxicity of heavy metals and response of the environment:
where \( C_{f}^{{{\mathbf{i}}i}} \) is the single-element pollution factor, \( C^{i} \) is the concentration of the element in samples, and \( C_{n}^{i} \) is a reference value for the element which is considered to average value of global shale. In this study, \( C^{i} \) is the measured concentration value for the element. \( E_{r}^{i} \) is the potential ecological risk index of an individual element. \( T_{r}^{i} \) is the toxic factor of an individual element (Table 5, ESM only; Essien et al. 2009). RI represents the sensitivity of the biological risk caused by the toxic substance and illustrates the potential ecological risk caused by the overall contamination. Based on the single-element, pollution factor (\( C_{n}^{i} \)) and its grades, the ecological risk of four kinds of elements and the value is shown in Table 6. Grade standards for potential ecological risk are shown in (Table 6, ESM only; Hankanson 1980; Li et al. 2008). The results show that the ecological risk of four kinds of elements in surficial sediments is low. At all sampling sites, the grades of potential ecological risk of Co, Cu, Pb and Zn are “light”. The order of potential ecological risk is: Pb > Co > Cu > Zn. The order of RI shows that the pollution extent is: I > II> III > V > IV. Potential ecological risk index (RI) shows the maximum is at site 23 and the minimum is at site 3.
Enrichment factor
To assess the relative contribution of natural and anthropogenic metal input to sediments, it is necessary to have some ideas of what natural concentrations are expected to be in sediments of a given region. To assess metal inputs to sediments in this region, it is necessary to standardize their content in relation to the reference element, which is assumed to have little variability of occurrence and is present in trace concentration in the examined environment. The usual reference elements are Sc, Mn, Al, Fe and Ti (Krzysztof et al. 1997). A reference element is characterized by the low occurrence variability. Scandium (Sc) is chosen as the geochemical normalizer because of its conservative nature during diagenesis. Sc has little variability of occurrence and presents in trace concentration in Daliao River and Yingkou Bay. (Fan 2009; Krzysztof et al. 1997; Tomza et al. 1982; Buat-Menard 1979). EF has also been used as an indication of degree of pollution or environmental contamination or both. The EF is defined as:
where (C x /C Sc) sample is the ratio of the concentration of an element x in mg/kg in the analyzed sample and (C x /C Sc) is the same ratio in the crustal material (natural background level). The classes of enrichment factor are listed in Table 7 (ESM only).
Table 7 presents the mean EF value of the metals with respect to the natural background concentration (Table 3, ESM only). The enrichment factor of Co at sites 4, 6, 7, 8, 19, 23, 24, 27, 32, 33 and 35 is “minor enrichment”, while at other sites it is “no enrichment”. The enrichment factor of Cu at sites 6, 8, 12, 33 and 35 is “minor enrichment”, while at other sites it is “minor enrichment”. The enrichment factor of Pb at sites 1, 2, 3, 6, 7, 8, 10, 11, 12, 13, 14, 15, 16, 17, 19, 20, 21, 26, 28, 29, 30, 31, 32, 34 is “minor enrichment”; at sites 4, 5, 9, 18, 22, 24, 25, 27, 33 and 35 it is “moderate enrichment”, but at site 23 it is “moderately severe enrichment”. The enrichment factor of Zn at sites 1, 2, 3, 4, 5, 7, 8, 10, 11, 12, 13, 14, 15, 16, 17, 18, 19, 21, 23, 24, 26, 27, 28, 30, 31, 32, 33, 34 and 35 is “minor enrichment”; at sites 6, 9, 20, 22, 25 and 29 there is “no enrichment”.
Therefore, this study reveals EF value for Pb is “minor enrichment” to “moderate enrichment” at most sites and regions; for Zn it is “minor enrichment” at most sites and regions; for Cu and Co it is “minor enrichment” at most sites and regions. The enrichment factor of “minor enrichment”, “moderate enrichment” and “severe enrichment” means the metals have originated from non-crustal sources, i.e. anthropogenic pollution. The enrichment factor of “no enrichment” indicates no enrichment and the metals in sediment originate from crustal source.
Metals speciation in sediments
To study the fraction of sedimentary metals among the geochemical phases and to assess their long-term potential emission in the surrounding environment, selective chemical extraction is widely used (Jones and Turki 1997). Exchangeable fraction is considered to be the most mobilized and bioavailable phase in the soil and sediment, followed by the carbonate phase (Tessier et al. 1980; Ahumada et al. 1999; Howari and Banat 2001). Easily reducible oxides and reducible oxides exit as nodules, concretions and cement between particles or as a coating on particles. These oxides are excellent scavengers for trace metals and can be mobilized under the reducing and acidic conditions (Tessier et al. 1980; Banat et al. 2005). The organic phase is a relatively stable phase in nature, but it can be mobilized under strong oxidizing conditions due to degradation of organic matter, which can result in the release of the soluble metal (Tessier et al. 1980). The residual solid should contain mainly primary and secondary minerals, which may hold trace metals within their crystal structure. The metal in the residuals fraction cannot be easily released to the environment as the metal is bound to crystal lattice (Ahumada et al. 1999; Howari and Banat 2001; Huang et al. 2007). Therefore, the sequence of the labile/stable extent of the six fractions is: EXC (more labile) > CARB (intermediately labile) > ERO, OM, RO (less labile and stable) > RES (more stable). Assuming that mobility is related to solubility, the metal solubility decreases in the following order: EXC > CARB > ERO > OM > RO > RES. The speciation of four heavy metals is shown in Figs. 2, 3, 4, 5. The exchangeable fraction in the sediment is able to retain heavy metals in the following order (mean value): Cu (3.89%) > Zn (3.72%) > Pb (2.98%) > Co (2.04%). The carbonate fraction in the sediment is able to retain heavy metals in the following order: Pb (19.21%) > Co (11.30%) > Zn (13.14%) > Cu (3.40%). The easily reducible oxides fraction in the sediment is able to retain heavy metals in the following order: Pb (8.17%) > Co (7.77%) > Zn (5.86%) > Cu (3.18%). The organic matter fraction in the sediments is able to retain heavy metals in the following order: Co (14.14%) > Pb (9.27%) > Zn (8.22%) > Cu (6.85%). The reducible oxides in the sediment are able to retain heavy metals in the following order: Cu (29.85%) > Pb (27.29%) > Zn (19.31%) > Co (18.07%). The residual fraction in the sediment is able to retain heavy metals in the following order: Cu (52.83%) > Zn (0.49.75%) > Co (46.68%) > Pb (33.08%). The residual form is named an “inert phase” and corresponds to the part of the metals which cannot be mobilized. The high percentages of heavy metals were found in residual phase, which cannot be easily released in the aquatic environment as they are bound to the crystal lattice of the minerals in the sediments (Savvides et al. 1995). Based on the residual fraction, the solubility and mobility of the metals may decrease in the following order: Pb (fraction, 33.08%; concentration, 8.80 mg/kg) > Co (fraction, 46.68%; concentration, 4.75 mg/kg) > Zn (fraction, 0.49.75%; concentration, 34.52 mg/kg) > Cu (fraction, 0.52.83%; concentration, 10.58 mg/kg). Metals present in this fraction are a measure of the degree of environmental pollution: the higher the metals present in this fraction, the lower the degree of pollution (Ahumada et al. 1999; Howari and Banat 2001).
Co fraction
The percentage of Co associated with different fractions is in the following order (mean value): RES (46.68%) > RO (18.07%) > OM (14.14%) > CARB (11.30%) > ERO (7.77%) > EXC (2.04%). The RO, OM and CARB fractions are reasonably high. At all sites, Co is mostly concentrated in the RES (17.66–85.35%) fraction with the average value of 46.68%. At site I, it is found that EXC (0.37%) and ERO (4.22%) fractions are absent; at site II, it is found that EXC (0.71%) and RES (8.33%) fractions are absent; at site III, it is found that EXC (0.37%) fraction is absent; at site IV, it is found that EXC (2.97%) fraction is absent; at site V, it is found that EXC (3.78%) and ERO (7.12%) fractions are absent. These indicate that Co is regarded as “more stable” in the sediment of the total area. Metals in this fraction are the lower degree of pollution in the sediment. In addition, the different chemical forms of Co can affect the solubility and mobility of the metal in sediments. The different distribution patterns of Co in different regions may be related to Co sources and sediment characteristics. At all sites, the organic fraction is relatively high (>10%). It shows that the sediments of those sites are influenced by organic sewage. The main reasons are the serious organic contamination in the study region and the sedimentation (Chapman 1988). Textural characteristics, major elements and total organic carbon in sediments were marked as the causative factors of sedimentary pollution in Yingkou Bay. Agricultural run-off, sewage outfall and the redistribution of contaminated sediments from multifarious industries are all potential contemporary sources of contamination of organic fraction of Co in sediment. This conclusion is consistent with the researches (Chapman 1988; Himer et al. 1990; Luoma and Phillips 1988).
Cu fraction
Generally, Cu associated with different fractions followed the order (mean value): RES (52.83%) > RO (29.85%) > OM (6.85%) > EXC (3.89%) > CARB (3.40%) > ERO (3.18%). Cu is dominated by RES and RO fractions, and other fractions are relatively low. From the speciation data obtained, it appears that Cu basically exists in the RES (average value, 52.83%) fraction, which consisted of primary and secondary minerals essentially. Therefore, the high Cu concentration (52.83%) in RES fraction indicates that sediment is relatively unpolluted. At site I, it is found that EXC (2.08%), ERO (1.54%) and OM (6.75%) fractions are absent; at site II, it is found that EXC (2.21%), CARB (3.36%), ERO (3.89%) and OM (4.06%) fractions are absent; at site III, it is found that EXC (0.95%), CARB (3.02%), ERO (2.61%) and OM (2.70%) fractions are absent; at site IV, it is found that EXC (3.35%), CARB (2.78%), ERO (5.11%) and OM (9.33%) fractions are absent; at site V, it is found that EXC (8.25%), CARB (1.51%), ERO (2.41%) and OM (9.22%) fractions are absent. These indicate that it should contain mainly primary and secondary minerals, which may hold trace metals within their crystal structure. Metals in this fraction indicate the low degree of pollution. The high percentage of Cu in residual fraction indicates the sediment is relatively unpolluted and originates from geochemical background rather than anthropogenic source.
Pb fraction
The percentage of Pb associated with different fractions is in the following order (average value): RES (33.08%) > RO (27.29%) > CARB (19.21%) > OM (9.27%) > ERO (8.17%) > EXC (2.98%). The speciation pattern of Pb for all sites indicates that Pb is bounded to RES (average value, 33.08%) followed by RO (mean value, 27.29%) and CARB (19.21%). For site I, it is found that EXC (0.24%), CARB (0.06%), ERO (0.00%) and RO (2.49%) fractions are absent; for site II, it is found that CARB (0.64%), ERO (4.83%) and RO (0.64%) fractions are absent; for site III, it is found that EXC (1.11%), CARB (0.00%), ERO (0.00%), OM (0.7.87%) and RO (0.00%) fractions are absent; for site IV, it is found that CARB (0.30%), RO (1.33%), OM (7.12%) and ERO (8.15%) fractions are absent; for site V, it is found that EXC (3.34%), CARB (6.73%), ERO (0.00%) and RO (2.67%) are absent. These indicate that Pb at most sites of the sediment occurs in highly resistant minerals and therefore is unlikely to be released to porewater through dissociation. The high percentage of Pb in RES fraction indicates that the sediment is relatively unpolluted. The percentage Pb in organic fraction at site I, II and V is relatively high (>10%), which shows that these sites are influenced by organic sewage due to the same reason in Sect. “Co fraction”. At site III and IV, Pb originates from geochemical background rather than anthropogenic source.
Zn fraction
Generally, Zn associated with different fraction follows the order (mean value): RES (49.75%) > RO (19.31%) > CARB (13.14%) > OM (8.22%) > ERO (5.42%) > EXC (3.72%). The RO and CARB fraction are reasonably high. Therefore, the result indicates that the sediment is relatively polluted. At site I, it is found that EXC (0.74%), CARB (9.49%), OM (2.40%), ERO (2.40%) and OM (9.72%) are absent; for site II, it is found that EXC (4.31%), ERO (6.20%) and OM (9.33%) are absent; for site III, it is found that EXC (1.22%) and OM (5.57%) are absent; for site IV, it is found that EXC (0.00%), ERO (5.60%) and OM (6.81%) are absent, for site V, it is found that ERO (2.29%) is absent.
Conclusions
The reported metal concentrations may serve as a basis for future investigations in Daliao River and Yingkou Bay. The ranges of Pb, Co, Zn and Cu concentrations in surficial sediment are: 16.57–39.18, 3.61–16.02, 16.53–39.18, 2.77–43.80 mg/kg. In the downstream area of Daliao River (site I) and estuary in Daliao River (site II), higher concentrations of the four metals were detected, which indicate the main pollution source of metals from Daliao River; while at the other three sites (site III, IV, V), the concentrations of the four metals were low, which indicate that dilution, diffusion and self-purification capacity engendered by tide process have some influences on the metal pollution. The variation of heavy metals in sediment in different sites may also be due to absorption, sedimentation and flocculation dynamics that take place in the estuary and the bay. On the whole, the level of total concentration of four metals in surficial sediment is lower than or similar to other reference coastal areas.
Four kinds of assessment methods: the geoaccumulation index (I geo), pollution load index (PLI), potential ecological risk index (RI) and enrichment factor (EF) are used to illustrate the pollution level. Results of the geoaccumulation index (I geo) show that the pollution level of four metals is in the “unpolluted” class except for Pb at 15 sampling sites. The pollution level of the study area assessed by pollution load index (PLI) shows that except for the moderately polluted region of sites 1, 2, 3, 8, 12 and 13, other sites belong to the unpolluted state. The sequence of pollution extent of different heavy metals is: Pb > Zn > Co > Cu. At all sampling sites, the grades of potential ecological risk of Co, Cu, Pb and Zn are “light”. The order of potential ecological risk is: Pb > Co > Cu > Zn.
The sequential extraction scheme is used to understand the mobility of heavy metals in the surficial sediment in the research area. Sequential extraction of the metals indicates that Co is predominately present in the RES fraction followed by the RO, OM fraction; Cu is predominately present in the RES fraction followed by the RO fraction; Pb predominates in the RES fraction followed by RO and CARB fraction; Zn predominates in the RES fraction followed by RO and CARB fraction, which indicates the sediment is relatively unpolluted at most sampling sites and originates from geochemical background rather than anthropogenic source. The solubility and mobility of the metals may decrease in the following order: Pb > Co > Zn > Cu.
In the present study, Pb, Co, Zn and Cu are stable under normal conditions. The organic fraction of Co is relatively high (>10%), which shows that the sediment of those sites is influenced by organic sewage. At most sites of Daliao River and Yingkou Bay, Pb, Co, Zn and Cu are relatively stable, which means that there is low source of pollution arriving at this area. While only at several sites, Co, Pb and Zn are labile, which are related to the source of pollution from the plating plant, tannery, steelworks and paper mill. Higher total concentrations and percentages of metals in non-residual fractions indicate the anthropogenic inputs to the sediment in the surrounding area.
References
Administration of Quality Supervision, GB 17378.5 (1998) Environmental quality standard for marine sediment. Standard Press of China, Beijing (in Chinese)
Ahmed AA, Soraya B (2007) Determination of heavy metals (Cd, Cr, Cu, Fe, Ni, Pb, Zn) by ICP-OES and their speciation in Algerian Mediterranean Sea sediments after a five-stage sequential extraction procedure. Environ Monit Assess 135:265–280
Ahumada I, Mendoza J, Navarrete E, Ascar L (1999) Sequential extraction of heavy metals in soils irrigated with wastewater. Commun Soil Sci Plant Anal 30:1507–1519
Alessandra A, Rossella G, Loredana M (2008) Sediment concentrations of trace metals in the Berre Lagoon (France): an assessment of contamination. Arch Environ Contam Toxicol 54:372–385
Amany GT, Ahmed AS (1999) Heavy metal concentrations in surficial sediments from Wadi El Natrun saline lakes, Egypt. Int J Salt Lake Res 8:75–92
Audry S, Schäfer J, Blanc G, Jouanneau J (2004) Fifty-year sedimentary record of heavy metal pollution (Cd, Zn, Cu, Pb) in the Lot River reservoir (France). Environ Pollut 132:413–426
Banat KM, Howari FM, Al-Hamad AA (2005) Heavy metals in urban soils of central Jordan: should we worry about their environmental risks? Environ Res 97:258–273
Bryan GW, Langston WJ (1992) Bioavailability, accumulation and effects of heavy metals in sediments with special reference to United Kingdom estuaries: a review. Environ Pollut 76:89–131
Buat-Menard P (1979) Influence de la relombée atmosphérique sur la chimie des métaux en trace dans la matière en suspension de ľ Atlantique Nord. Thesis, University of Paris VI, pp 434
Campanella L, Dorazio D, Petronio BM, Pietrantonio E (1995) Proposal for a metal speciation study in sediments. Anal Chim Acta 309:387–393
Chang Y, Jia ZB, Zhao ZJ, Fang ZG, Jia YX (2002) The oscillation trend and mechanism of the COD. Acta Sci Nat 38(4):535–542 (in Chinese)
Chapman PM (1988) Marine sediment toxicity tests. In: Lichtenberg JL, Winter JA, Weber CI, Fradkin L (eds) Chemical and biological characterization of municipal sludges, sediments, dredge spoil and drilling muds ASTM STP, 976. American Society for Testing and Materials, Philadelphia, pp 391–402
De AK, Sen AK, Karim M, Stockton RA (1985) Pollution profile of the Damodar river sediments. Environ Int 11:453–457
Edward DB, Ian RP, Darry WH (2005) Trace metal distribution and enrichment in benthic, estuarine sediments: Southport Broadwater, Australia. Environ Geochem Health 27:369–383
Essien JP, Antai SP, Olajire AA (2009) Distribution, seasonal variations and ecotoxicological significance of heavy metals in sediments of crossriver estuary mangrove swamp. Water Air Soil Pollut 197:91–105
Fan YH (2009) The pollution characteristics and bioavailability of heavy metals in the Daliao River water system. PhD thesis, Beijing Normal University, PRC (in Chinese)
Getaneh W, Alemayehu T (2006) Metal contamination of the environment by placer and primary gold mining in the Adola region of southern Ethiopia. Environ Geol 50:339–352
Gianpiero A, Igino C, Sergio P, Pierluigi B, Edoardo R (2007) Metal pollution assessment of surface sediments along a new gas pipeline in the Niger Delta (Nigeria). Environ Monit Assess 125:291–299
Gladyshev MI, Gribovskaya IV, Ivanova EA, Moskvichova AV, Muchkina Ya E, Chuprov SM (2001) Metal concentrations in the ecosystem and around recreational and fish-breeding pond Bugach. Water Resour 28:288–296
Gonzalez A, Rodriguez MT, Sanchez JCJ, de La Rosa FJB (2000) Assessment of metals in sediments in a tributary of Guadalquivir River (Spain): heavy metal partitioning water–sediments interaction. Water Air Soil Pollut 121:11–29
Guo W, He MC, Yang ZF, Lin CY (2006) Distribution of polycyclic aromatic hydrocarbons in water, suspended particulate matter and sediment from Daliao River catchment, China. In: The 3nd China international symposium on persistent toxic substances, Oct 22–25, Beijing
Han FX, Amos B (1997) Long-term transformations and redistribution of potentially toxic heavy metals in arid-zone soils incubated: I. Under saturated conditions. Water Air Soil Pollut 95:399–423
Hankanson L (1980) An ecological risk index for aquatic pollution control―a sedimentological approach. Water Res 14:975–1001
Himer AV, Kristsotakis K, Tobschall HJ (1990) Metal–organic association in sediments-1. Comparison of unpolluted recent and ancient sediments and sediments affected by anthropogenic pollution. Appl Geochem 5:491–505
Howari FM, Banat KM (2001) Assessment of Fe, Cd, Hg and Pb in the Jordan and Yarmouk river sediments in relation to their physicochemical properties and sequential extraction characterization. Water Air Soil Pollut 132(1–2):43–59
Huang JM, Huang RQ, Jiao JJ, Chen KP (2007) Speciation and mobility of heavy metals in mud in coastal reclamation areas in Shenzhen, China. Environ Geol 53:221–228
Inmaculada DV, Luis CP, Francisco JR (2010) Sediment resuspension in two adjacent shallow coastal lakes: controlling factors and consequences on phosphate dynamics. Aquat Sci 72:21–31
Jones B, Turki A (1997) Distribution and speciation of heavy metals in superficial sediments from the Tees estuary, north-east England. Mar Pollut Bull 34(10):768–779
Krishna AK, Govil PK (2008) Assessment of heavy metal contamination in soils around Manali industrial area, Chennai, Southern India. Environ Geol 54:1465–1472
Krzysztof L, Jan C, Jacek P, Danuta W, Jerzy K (1997) Use of enrichment, and concentration factors together with geoaccumulation indexes to evaluate the content of Cd, Cu and Ni in the Rybnik water reservoir in Poland. Water Air Soil Pollut 93:347–365
Langston WJ, Spence SK (1994) Metal analysis. In: Calow P (ed) Handbook of ecotoxicology, vol 2, Ch 4. Blackwell, Oxford, pp 45–78
Li XD, Barry JC, Michael HR, Iain T (1995) Sequential extraction of soils for multielement analysis by ICP-AES. Chem Geol 124:109–123
Li J, Zhang Y, Gong XY, Yao HL, Wang XQ, Li J, Qi SH (2008) Pollution and assessment of heavy metals in surface sediments of Xinghua Bay, Fujian Province. Environ Sci Technol 31(1):125–128 (in Chinese)
Lin C, He MC, Ying ZF (2009) Contents, chemical forms, and contamination of trace metals in the sediments from the Daliao river system, its estuary and Songhua river. In: International conference on environmental pollution and ecological remediation in industrial regions. April 24–26, Shanghai, China
Luoma SN, Phillips DJH (1988) Distribution, variability and impacts of trace elements in San Francisco Bay. Mar Pollut Bull 19(9):413–425
Mu SL, Luan TX (2004) The pollution trend and ecological assessment of surface water in Liaoning Province. Water Conserv Hydroelectr 22(6):56–58 (in Chinese)
Müller G (1969) Index of geoaccumulation in sediments of the Rhine River. Geo J 2:108–118
Muthu Raj S, Jayaprakash M (2008) Distribution and enrichment of trace metals in marine sediments of Bay of Bengal, off Ennore, south-east coast of India. Environ Geol 56:207–217
Nasr SM, Okbah MA, Kasem SM (2006) Environmental assessment of heavy metal pollution in bottom sediments of Aden Port, Yemen. Int J Oceans Oceanogr 1:99–109
Potts PJ, Tindle AG, Webb PC (1992) Geochemical reference material compositions. Whittes, Caithness
Ridgway J, Shimmield G (2002) Estuaries as repositories of historical contamination and their impact on shelf seas. Estuar Coast Shelf Sci 55:903–928
Santosh KS, Stanislav FB, Asokkumar B, Mahua S, Halka B (2004) Levels of elements in the surficial estuarine sediments of the Hugli River, northeast India and their environmental implications. Environ Int 30:1089–1098
Satyanarayana D, Panigrahy PK, Sahu SD (1994) Metal pollution in harbour and coastal sediments of Visakapatnam, east coast of India. India J Mar Sci 23:52–54
Savvides C, Papadopoulos A, Haralambous KJ, Loizidou M (1995) Sea sediments contaminated with heavy metals: metal speciation and removal. Water Sci Technol 32:65–73
Seralathan P, Meenakshikutty NR, Asaref KV, Padmalal D (1993) Sediment and organic carbon distribution in the Cochin harbor area. Indian J Mar Sci 22:225–252
Shriadah MMA (1999) Heavy metals in mangrove sediments of the United Arab Emirates shoreline (Arabian Gulf). Water Air Soil Pollut 116:523–534
Shrivastava P, Saxena A, Swarup A (2003) Heavy metal pollution in a sewage-fed lake of Bhopal (MP), India. Lakes Reserv Res Manag 8:1–4
Song YY, Zhan YW, Wang YG (1997) Modern sedimentation character of the estuary in the north Liaodong bay. Acta Oceanol Sin 19(5):145–149 (in Chinese)
Tam NFY, Wong YS (2000) Spatial variation of heavy metals in surface sediments of Hong Kong mangrove swamps. Environ Pollut 110:195–205
Tessier A, Campbell PGC, Bisson M (1980) Trace metal speciation in the Yamaska and St. Francois rivers (Quebec). Can J Earth Sci 17:90–105
Tomlinson DL, Wilson JG, Harris CR, Jeffrey DW (1980) Problems in the assessment of heavy metals levels in estuaries and the formation of a pollution index. Helgol Mar Res 33(1–4):566–575
Tomza U, Maenhaut W, Cafmeyer J (1982) Trace elements in atmospheric aerosols at Katowice, Poland. In: Hemphill DD (ed) Trace substances in environmental health-XVI. University of Missouri, Columbia, pp 105–115
Wajid R, Akif Z, Nayyara N, Mohsan N (2008) Heavy metal pollution assessment in various industries of Pakistan. Environ Geol 55:353–358
Wang Q, Li F (1983) The changes of marine-continental conditions in the west coast of the Bohai gulf during quaternary. Mar Geol Quat Geol 3(4):83–89 (in Chinese)
Wedepohl KH (1995) The composition of the continental crust. Geochim Cosmochim Acta 59:1217–1232
Wu ZH, He MC, Lin CY, Fan YH (2011) Distribution and speciation of four heavy metals (Cd, Cr, Mn, Ni) in the surficial sediments from estuary in Daliao River and Yingkou Bay. Environ Earth Sci 63(1):163–175
Yang YQ (2007) The distribution, speciation and the potential ecological risk assessment of heavy metals in the Pearl River estuary and Adjacent Shelf. PhD thesis, School of the Chinese Academy of Sciences, PRC, pp 8
Zhou HY, Peng XT, Pan JM (2004) Distribution, source and enrichment of some chemical elements in sediments of the Pearl River Estuary, China. Cont Shelf Res 24:1857–1875
Acknowledgments
The author acknowledges the financial support from the National Key Basic Research and Development Plan of China (973 Program, 2004CB418502).
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Wu, Z., He, M. & Lin, C. Environmental impacts of heavy metals (Co, Cu, Pb, Zn) in surficial sediments of estuary in Daliao River and Yingkou Bay (northeast China): concentration level and chemical fraction. Environ Earth Sci 66, 2417–2430 (2012). https://doi.org/10.1007/s12665-011-1466-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12665-011-1466-1