Abstract
Agricultural activities are major sources of non-point pollutants causing eutrophication. Vegetated constructed wetlands are used as a best management practice for sequestration of nutrients from agricultural runoff. However, plants release nutrients back into the system as they decompose after senescence, affecting the nutrient removal efficiency of a constructed wetland. This information is important for a focused selection of plants and for improving the effectiveness of a constructed wetland. A greenhouse experiment was conducted to study the release of phosphorus by common freshwater macrophytes - Juncus effusus, Carex lurida and Dichanthelium acuminatum var. acuminatum during plant decomposition. Microcosms with the mixed culture of these three species showed higher phosphorus retention rates compared to monoculture microcosms. Results indicate that plant species differ in their nutrient removal efficiencies when grown in the mixed culture compared to monoculture treatments, indicating that nutrient removal efficiencies vary with plant species composition. Thus, plant species may play an important role in determining the phosphorus removal rates of vegetated constructed wetlands.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Nutrient fluxes to coastal areas have risen in recent decades because of high concentrations of nutrients in agricultural runoff deposited into surface waters (Howarth et al. 2011). Due to their nutrient retention characteristics, plants are used to solve eutrophication problems of freshwater bodies (Aoi and Hayashi 1996) and to remove pollutants in constructed wetlands (Delgado et al. 1993; Maine et al. 2004). Despite the advances in understanding eutrophication, it still remains one of the foremost problems in protecting freshwater and coastal marine ecosystems (Schindler 2006). Agricultural runoff is a major source of non-point pollution contributing to eutrophication (Carpenter et al. 1998). Phosphorus inputs to freshwater can accelerate eutrophication; hence, control of P inputs and their subsequent transport in runoff is critical to reduce eutrophication (Sharpley et al. 1997). One of several best management practices recommended for the removal of nutrients and pesticides from agricultural runoff is the use of constructed wetlands (Cooper et al. 2004; Bouldin et al. 2005).
Constructed wetlands are engineered systems that have been designed and constructed to utilize the natural processes involving wetland vegetation, soils, and their associated microbial assemblages to assist in treating wastewater (Hammer and Bastian 1989). Removal of phosphate in wetlands is known to be mediated by precipitation, adsorption, plant uptake and microbial assimilation (Moshiri 1993; Kadlec and Knight 1996; Reddy et al. 1999). Phosphorus storage in vegetation can range from short- to long-term, depending on type of vegetation, litter decomposition rates, leaching of P from detrital tissue, and translocation of P from above- to belowground biomass (Garver et al. 1988). Phosphorus uptake by wetland macrophytes is usually highest during the beginning of the growing season (in most regions during the early spring) before maximum growth rate is attained (Boyd 1969). The concentration of phosphorus in plant tissue of these macrophytes varies among species, sites and by season (Brix and Schierup 1989). Phosphorus storage in aboveground biomass of emergent wetland macrophytes is usually short-term with a large amount of P being released during the decomposition of litter (Vymazal 1996). The aboveground portions of wetland macrophytes return P to the water, while belowground portions returns P to the soil (Reddy et al. 1999).
Wetland plants are capable of retaining nutrients in high concentrations, but decomposition of macrophytes may lead to the release of the retained nutrients and, thus, become another source of nutrients to the water column or any part of the system (Kröger et al. 2007). Hence, while considering the nutrient retention capacity of a vegetated constructed wetland, it is important to take into consideration the amount of nutrients released back into a system following plant senescence (through leaching and decomposition) in order to estimate the actual effectiveness of the system. In most mitigation studies, this concept is not taken into consideration while calculating the net retention rate of the system and, hence, might lead to the failure of the system to mitigate nutrients efficiently (Kröger et al. 2007). Therefore, it is important to know the phosphorus release rate of plants during decomposition for planning an efficient constructed wetland system. The main objective of this study was to examine the release of phosphorus by Juncus effusus, Carex lurida and Dichanthelium acuminatum var. acuminatum following plant senescence in constructed wetland systems.
Materials and Methods
A greenhouse experiment was conducted to study the phosphorus retention rates of Juncus effusus (J. effusus), Carex lurida (C. lurida) and Dichanthelium acuminatum var. acuminatum (D. acuminatum) by studying the amount of phosphorus released following plant senescence. These species are native macrophytes used in constructed wetlands belonging to the Juncaceae, Cyperaceae, and Poaceae families, respectively (USDA 2007; Zazo et al. 2008; Brisson and Chazarenc 2009; White et al. 2012). The experiments were conducted in the greenhouse at the University of Mississippi Field Station (UMFS) as described previously (Menon et al. 2013). In brief, 16 plastic barrels (volume = 105 l) were filled with sediment (mixture of sand and clay) taken from unused ponds at the UMFS, Abbeville, MS, USA and were filled with groundwater to 10 cm depth above the sediment surface. After 2 weeks, young plant ramets were collected from UMFS wetlands and planted into the barrels in June (hereafter called mesocosms) as described previously (Menon et al. 2013). Each vegetated mesocosm was planted with 33 individual plant ramets, with four replicate mesocosms for each of the monocultures (four each per plant species) and four replicate mesocosms with the mixed culture (11 plants from each species). The mesocosms were maintained until the end of the experiment without replantation.
Monocultures and the mixed culture of all the three plants, C. lurida, J. effusus, D. acuminatum, were dosed with a total of 108 l of 2.5 mg/L of phosphorus over a period from June to November (each dose consisted of 18 l of 2.5 mg/l) in 2008 and 2009 as described earlier in a previous experiment to study the phosphorus retention capacity of the above plants (Menon and Holland 2013). The dose was added six times each year and the last dose was added on November 22nd (both in 2008 and 2009). The aboveground tissue of 12 plant stems (three from each replicate mesocosm) of each species from monoculture and the mixed culture was clipped above the sediment surface and placed into each litter bag (mesh size: 5 mm mesh diameter) in December for both years. Four replicate litter bags were set up for each species from monocultures (each bag with 12 plants) and for plants from the mixed cultures (each bag with 12 plants, 4 each from three species). All the bags were weighed using a weighing scale before they were placed in the tubs. There was no difference in the weights between the litterbags and they all weighed approximately 10 g. The litter bags were weighted using a small pebble and placed in 18 l plastic tubs (hereafter called microcosms) filled up to the 18 L mark with ground water from the University of Mississippi Field Station (Fig. 1). Baseline phosphorus concentration of UMFS ground water was 0.002 mg P/L. A control treatment with only UMFS ground water and litterbags was also established in order to determine if the phosphorus concentration in the water fluctuated over the course of the experiment.
The experiments were initiated on 1st December in both 2008 and 2009. Water samples were collected from the tubs beginning on 15 December in each experiment, and thereafter samples were collected on the 15th day of each month through March of the following year. In total, samples were collected four times for each experiment. The water column was mixed before sampling and a single sample was collected at 10 cm depth from the water surface. Water samples (250 ml) collected were analyzed for total phosphorus. Immediately before the decomposition experiment, the total phosphorus concentration in a subsample (1 g) of the aboveground plant tissue from each plant stem placed in the litter bags was also determined. The total phosphorus in water and plant samples were determined using a Dionex ion chromatograph and the ammonium per sulfate digestion method (Murphy and Riley 1962; American Public Health Association APHA 1998).
The total phosphorus concentration values were all normalized to per gram of plant tissue or to the number of plants to avoid the variation in the biomass due to difference in height and in the number of plants. Difference between the total phosphorus in the water column in the treatment tubs were statistically analyzed using repeated analysis of variance and Tukey’s posthoc test. The percent rate of phosphorus released into the water column in each treatment was calculated as the ratio of average TP in water to TP in initial plant biomass multiplied by 100. The differences in the percent rate of phosphorus released in 2008 and 2009 for all treatments were analyzed using repeated analysis of variance and Tukey’s posthoc test. The mean percentage of P released for all treatments for both years were compared using a one way analysis of variance.
Results
All the treatment microcosms had a floating film towards the end of the experiment in both years. The chemistry of the film was not determined due to time constraints. The total phosphorus (TP) content in the aboveground tissue in December 2008, before the start of the decomposition experiment, indicates J. effusus and C. lurida had higher concentrations of TP compared to D. acuminatum in both monoculture and the mixed culture treatments (Table 1). In 2008, the total phosphorus concentrations in the water showed a significant treatment by time interactive effect and a difference in the amount of phosphorus released by the different treatments over time (F = 12.19, p < 0.001, Fig. 2a). The total phosphorus concentration in the water column was highest in January for J. effusus and C. lurida and then decreased over time. Water column total phosphorus concentration in the J. effusus treatment was significantly greater (Tukey’s HSD test, p < 0.05) than that in other treatments in December 2008. Also, the total phosphorus concentration in the water column for the mixed treatment was significantly lower than other treatments in January 2009 (Tukey’s HSD test, p < 0.05). Moreover, D. acuminatum showed significantly higher phosphorus concentrations from all the treatments in February 2009 and March 2009 (Tukey’s HSD test, p < 0.05).
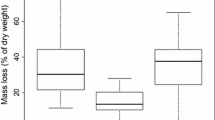
The total phosphorus release rate (mean of the difference between final and initial concentrations) in 2008 was highest in D. acuminatum treatments; lowest release rates were found for mixed and C. lurida treatments (Table 2). However, the temporal patterns in the percent rate of P released varied among the treatments in 2008 (Fig. 2b). While the highest percent rate of P was released in December for J. effusus and C. lurida, it was the highest in February for D. acuminatum and the mixed treatment. In addition, for J. effusus the release rates did not differ significantly between the months of January, February and March (p > 0.05). This pattern was not observed for the other treatments. D. acuminatum and the mixed treatment exhibited similar temporal pattern but the rates were significantly lower for the mixed treatment compared to D. acuminatum (p < 0.05). Interaction effects of the percent P release rates with respect to treatment (species) and time were significant (F = 23.52, p < 0.0001). The percent P release rates differed significantly between all the treatments except J. effusus and the mixed treatment (p > 0.05).
Repeated measures ANOVA results for 2009 indicates that there was a significant difference in the amount of phosphorus released into the water column between the different treatments over time (F = 4.7, p <0.05, Fig. 3a). TP in the water column for C. lurida was significantly lower in December while it differed significantly in January for J. effusus and C. lurida (Tukey’s HSD test, p < 0.05). However, there was no difference in the TP in the water column in February and March for all the treatments. C. lurida had the highest TP concentration in the tissue before the experiment began (Table 1) and released relatively lower rates of P into the water column (Table 2). Apparently C. lurida and the mixed culture treatment released similar amounts of P into the water column in both experiments. Moreover, J. effusus showed the least amount of TP release (mean) from its aboveground plant tissue (Table 2). A temporal variation in the percent rate of P released was found for all treatments except for the mixed culture treatment (Fig. 3b). The pattern of the temporal variation was similar for J. effusus and C. lurida. Unlike the 2008 experiment, the highest percent rate of P was released in March for all the treatments in the 2009 experiment. Interaction effects of the percent P release rates with respect to treatment (species) and time were significant (F = 4.3, p < 0.0001). Similar to the 2008 experiment, the percent P release rates differed significantly between all the treatments except J. effusus and the mixed culture treatment (p > 0.05).
A significant difference was found in the mean percent P released by J. effusus and D. acuminatum in both the experiments (p < 0.05, Table 2). The rates were lower in the second experiment compared to the first experiment for J. effusus and D. acuminatum. The percent rates for C. lurida and the mixed culture treatment did not differ significantly between the two experiments. The control treatment indicated that the TP concentrations of the background (UMFS ground water) did not change significantly throughout the course of the experiment (p = 0.824).
Discussion
Wetland plants are an integral part of constructed wetland systems, but there is little quantitative information available comparing their growth characteristics and treatment performance in constructed wetland systems (Tanner 1996; Vymazal 2007). Selection of plants is a crucial factor for the efficient functioning of constructed wetlands since storage in plant biomass is a saturable process, meaning plants release nutrients back into the ecosystem during decomposition. By using a microcosm study, we were able to examine the differential release rates of phosphorus due to decomposition of aboveground biomass of three wetland plants that are commonly used in constructed wetlands. Our results indicate that the plant species differed in the amount of total phosphorus released into the system. These variations in the amount of phosphorus released suggest that plant species exhibit different nutrient removal efficiencies.
Wetland plants are known to differ in their capacity to assimilate nutrients from wastewater (Kadlec and Knight 1996). For instance, the nutrient uptake capacity of Phragmites australis was higher than that of Cyperus papyrus (Brix and Schierup 1989). However, Richardson and Marshall (1986) found that plant dieback resulted in a 5-fold increase in P flux in the water column. Kröger et al. (2007) studied the leaching of nutrients after plant senescence of Leersia oryzoides and found that it retained N and P during the growing season but released the nutrients back into the water column after plant senescence, which would create eutrophic conditions in receiving waters during the winter months (December to January). Therefore, while it is important to consider how well certain plant species can retain the nutrients initially from wastewater, it is equally important to study the retention rates during decomposition (Tyler et al. 2012). However, the release of accumulated nutrients by emergent wetland macrophytes during decomposition has received much less attention compared to the ability of plants to sequester nutrients (Boyd and Hess 1970; Vymazal 2007). In this study, we found a difference in the TP concentrations between different time points and also found that the plant species differ in their responses (release of phosphorus) over time. This could be due to the difference in the decomposition rates between the treatments which could be due to the differences in the plant tissue structures and morphology. D. acuminatum has round hollow stems while C. lurida have triangular stems and J. effusus has round stout soft stems (USDA 2007).
In the 2008 experiment, the percent P released decreased as the experiment progressed for all treatments except J. effusus. However, in the 2009 experiment, this trend was reversed for all treatments. This complimentary trend could be due to the fact that the plants harvested in the 2009 experiment stayed longer in the mesocosms before they were harvested. This could have led to the development of secondary tissue structures or lignin deposits in the cell walls, thereby delaying decomposition rates in the 2009 experiment. Although C. lurida was the most efficient at retaining phosphorus among monoculture treatments in 2008, J. effusus was the most efficient in 2009 (Table 2).
Moreover, compared to monoculture treatments, the mixed culture treatment released lower amounts of phosphorus in both 2008 and 2009. The effectiveness of the mixed culture treatment over monoculture treatments in retention of phosphorus may be due to more diverse plant structures holding phosphorus, which could have led to a difference in the surface area available to store phosphorus. There was also a year effect on the phosphorus release rates among all the treatments and this could be due to the difference in the phosphorous concentration in the plant tissues at the start of the experiment or due to differential decomposition. The allocation and storage of nutrients in the aboveground and belowground tissue varies with plant species (Kao et al. 2003). Differences in release rates between treatments may therefore be due to species differences in nutrient storage capacity. However, we did not measure final plant biomass, examine phosphorus storage in belowground tissues, or determine storage differences between stems and leaves. A difference in bacterial communities in the phyllosphere or on other aboveground tissues of the species studied may have resulted in the differences in phosphorus utilization that were identified among the treatments. Therefore, there is a need for further studies examining the link between tissue structural differences and nutrient storage and release, as well as the effect of microbial community structure on phosphorus release during decomposition.
It is important to study nutrient immobilization by different plant species as they differ in their morphology and physiology. In conclusion, our results lead us to recommend the use of J. effusus and C. lurida (either grown as monoculture or mixed culture) as plants that could be used for the efficient removal of phosphorus in constructed wetlands due to the low phosphorus release rates during decomposition. However, since D. acuminatum released almost 20 % of the phosphorus initially retained in the plant tissue, this may not be a suitable plant for use in constructed wetlands designed for phosphorus retention. This information indicates that a focused selection of plants can improve the effectiveness of constructed wetlands. The results obtained from this research are useful for designing efficient constructed wetlands and also contribute information to the database on the phosphorus retention capacity of different plants. Thus, plant species used may play an important role in determining the phosphorus removal rate of constructed wetlands.
References
American Public Health Association (APHA) (1998) Standard methods for the examination of water and wastewater, 20th edn. American Public Health Association, Washington
Aoi T, Hayashi T (1996) Nutrient removal by water lettuce (Pisitia stratiotes). Water Science and Technology 34:407–412
Bouldin J, Farris J, Moore M, Smith S, Stephens W, Cooper C (2005) Evaluated fate and effects of atrazine and lambda‐cyhalothrin in vegetated and unvegetated microcosms. Environmental Toxicology 20:487–498
Boyd CE (1969) Production, mineral nutrient absorption, and biochemical assimilation by Justicia americana and Alternanthera philoxeroides. Archives of Hydrobiology 66:139–160
Boyd CE, Hess LW (1970) Factors Influencing shoot production and mineral nutrient levels in Typha latifolia. Ecology 51:296–300
Brisson J, Chazarenc F (2009) Maximizing pollutant removal in constructed wetlands: should we pay more attention to macrophyte species selection? Science of the Total Environment 407:3923–3930
Brix H, Schierup HH (1989) The use of aquatic macrophytes in water-pollution control. Ambio 18:100–107
Carpenter SR, Caraco NF, Correll DL, Howarth RW, Sharpley AN, Smith VH (1998) Nonpoint pollution of surface waters with phosphorus and nitrogen. Ecological Applications 8:559–568
Cooper C, Moore M, Bennett E, Smith S Jr, Farris J, Milam C, Shields F Jr (2004) Innovative uses of vegetated drainage ditches for reducing agricultural runoff. Water Science and Technology 49:117–123
Delgado M, Bigeriego M, Guardiola E (1993) Uptake of Zn, Cr and Cd by water hyacinths. Water Research 27:269–272
Garver EG, Dubbe DR, Pratt DC (1988) Seasonal patterns in accumulation and partitioning of biomass and macronutrients in Typha spp. Aquatic Botany 32:115–127
Hammer DA, Bastian RK (1989) Wetlands ecosystems: natural water purifiers. In: Hammer DA (ed) Constructed wetlands for wastewater treatment. Lewis publishers, Chelsea, pp 5–19
Howarth R, Chan F, Conley DJ, Garnier J, Doney SC, Marino R, Billen G (2011) Coupled biogeochemical cycles: eutrophication and hypoxia in temperate estuaries and coastal marine ecosystems. Frontiers in Ecology and the Environment 9:18–26
Kadlec RH, Knight RL (1996) Treatment wetlands. CRC. Baca Raton, Florida
Kao JT, Titus JE, Zhu WX (2003) Differential nitrogen and phosphorus retention by five wetland plant species. Wetlands 23:979–987
Kröger R, Holland MM, Moore M, Cooper C (2007) Plant senescence: A mechanism for nutrient release in temperate agricultural wetlands. Environmental Pollution 146:114–119
Maine MA, NL S é, Lagger SC (2004) Chromium bioaccumulation: comparison of the capacity of two floating aquatic macrophytes. Water Research 38:1494–1501
Menon R, Holland MM (2013) Phosphorus retention by Juncus effusus, Carex lurida and Dichanthelium acuminatum var. acuminatum in freshwater wetlands. Water, Air, Soil Pollution 224:1602.
Menon R, Jackson CR, Holland MM (2013) The Influence of Vegetation on Microbial Enzyme Activity and Bacterial Community Structure in Freshwater Constructed Wetland Sediments. Wetlands:1–14.
Moshiri G (1993) Constructed wetlands for water quality improvement. Lewis Publishers, Boca Raton
Murphy R, Riley JP (1962) A modified single solution method for the determination of phosphate in natural waters. Analytica Chimica Acta 27:31–36
Reddy KR, O’Connor GA, Schelske CL (1999) Phosphorus biogeochemistry in subtropical ecosystems. CRC, Boca Raton
Richardson CJ, Marshall PE (1986) Processes controlling movement, storage, and export of phosphorus in a fen peatland. Ecological Monographs 56:279–302
Schindler DW (2006) Recent advances in the understanding and management of eutrophication. Limnology and Oceanography 51:356–363
Sharpley A, S Rekolainen, H Tunney, O Carton, P Brookes and A Johnston (1997) Phosphorus in agriculture and its environmental implications. p. 1–53. Phosphorus loss from soil to water. Proceedings of a workshop, Wexford, Irish Republic, 29–31 September 1995. CAB International
Tanner CC (1996) Plants for constructed wetland treatment systems - A comparison of the growth and nutrient uptake of eight emergent species. Ecological Engineering 7:59–83
Tyler H, Moore M, Locke M (2012) Potential for phosphate mitigation from agricultural runoff by three aquatic macrophytes. Water, Air, & Soil Pollution 223:4557–4564
USDA and NRCS (2007) The PLANTS Database (http://plants.usda.gov). National Plant Data Center, Baton Rouge, Lousiana.
Vymazal J (1996) Constructed wetlands for wastewater treatment in the Czech Republic the first 5 years experience. Water Science and Technology 34:159–164
Vymazal J (2007) Removal of nutrients in various types of constructed wetlands. Science of the Total Environment 380:48–65
White SA, Taylor MD, Damrel DZ (2012) Floral colonization of a free-water surface constructed wetland System in Grady County, Georgia. Castanea 77:159–171
Zazo JA, Paull JS, Jaffe PR (2008) Influence of plants on the reduction of hexavalent chromium in wetland sediments. Environmental Pollution 156:29–35
Acknowledgement
Support for this work was provided by USDA Agricultural Research Service Cooperative Agreement No. 58-6408-6052 and a Ralph Powe Award from The University of Mississippi Field Station. Thanks to Drs. Charlie Cooper, Colin Jackson, Matt Moore, and Robbie Kröger for design suggestions and to Forrest Briggs, Tara Davis, and Clint Helms for logistical support.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Menon, R., Holland, M.M. Phosphorus Release due to Decomposition of Wetland Plants. Wetlands 34, 1191–1196 (2014). https://doi.org/10.1007/s13157-014-0578-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13157-014-0578-2