Abstract
Macrophytes and bacteria are key drivers of nitrogen removal in constructed wetlands. Through mesocosm experiments with vegetated submerged beds and free water surface wetlands in various operational modes, wetland configurations, and system layouts, this study developed empirical models for non-destructive estimation of plant biomass growth and associated nitrogen assimilation and explored the combined effects of multiple factors that influence microbial nitrogen removal. The above-ground biomass of individual plants was a power function of plant height for both Cyperus alternifolius and Typha angustifolia. Below- to above-ground biomass ratio was 0.38 for C. alternifolius and 2.73 for T. angustifolia. Because of greater tolerance to ammonia stress, C. alternifolius and C. papyrus grew faster than T. angustifolia. There were no significant effects of wetland type, vegetation, and plant species on microbial nitrogen removal. Microbial nitrogen removal was inhibited by free ammonia at 13.3–16.2 mg N/L. Denitrification and anammox were suppressed at dissolved oxygen greater than 1.9 mg/L. Microbial removal of ammonia in vegetated submerged beds was sensitive mainly to dissolved oxygen, pH, and influent ammonia concentration, while in free water surface wetlands, it was sensitive to influent ammonia concentration, pH, and temperature.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
A variety of ammonia-rich wastewater is generated at low biodegradable organic carbon concentrations, such as mature landfill leachate, anaerobic digestate, and livestock wastewater. Constructed wetlands have proven to be effective in removing nitrogen from wastewater, where macrophytes and bacteria are considered the key drivers of nitrogen removal (Vymazal 2013; Kadlec and Wallace 2009). Ammonia at low concentrations can be taken up by plants as a nutrient, but at high concentrations, it can cause stress (Britto and Kronzucker 2002). The relative contribution of nitrogen assimilation into plant biomass to overall nitrogen removal in constructed wetlands has been reported in a wide range in the literature (Kadlec and Wallace 2009; Vymazal 2007). It can vary with plant species, growth stage, and ammonium concentration. Quantifying the relative contribution of plant uptake of nitrogen and the factors that influence it can guide design and management of constructed wetlands. Besides contaminant removal, wetland plants may be harvested for beneficial uses such as production of bioenergy and animal feed additives (Berry et al. 2017; Dragoni et al. 2017; Tanaka et al. 2016). Non-destructive estimation of plant biomass growth will facilitate management of plants in constructed wetlands.
Microbial removal of ammonia in constructed wetlands involves nitrification, denitrification, and anammox (Kadlec and Wallace 2009; Vymazal 2007). Nitrification is a two-step aerobic process that oxidizes ammonia to nitrite (partial nitrification or nitritation) and nitrite to nitrate. Denitrification is a multi-step anaerobic process that reduces nitrate to nitrite and finally to nitrogen gas. Anaerobic ammonia oxidizing (anammox) bacteria can use nitrite to oxidize ammonia to nitrogen gas despite converting 7.4–11% of ammonia- and nitrite-nitrogen to nitrate-nitrogen (Li and Tao 2017; Lotti et al. 2014). In single reactors, denitrifying and anammox bacteria may occur in anoxic micro-niches, such as the deep layer of biofilms, while nitritation takes place in aerobic micro-niches, such as the outer layer of biofilms (Daverey et al. 2015; Almstrand et al. 2014). Therefore, a combination of nitritation, anammox, and denitrification in single reactors becomes an appealing design consideration for ecologically engineered treatment wetlands. The simultaneous nitritation, anammox, and denitrification (SNAD) process needs less oxygen than the conventional nitrification-denitrification process. Both denitrification and nitritation may produce nitrite for anammox. Denitrification is also needed to avoid accumulation of nitrate produced by anammox and complete nitrification and remove organic carbon present more or less in various ammonia-rich wastewaters. SNAD has been found in different treatment systems, including constructed wetlands (Li and Tao 2017; Pang et al. 2015; Zhi et al. 2015; Tao et al. 2012; Kumar and Lin 2010; Chiemchaisri et al. 2009). A few studies (Li and Tao 2017; Zhi et al. 2015; Tao et al. 2012; Chiemchaisri et al. 2009) have greatly enhanced SNAD in single-constructed wetlands by employing cost-effective approaches to manipulate the factors influencing SNAD and investigated the effects of individual parameters such as dissolved oxygen (DO) concentration, pH, temperature, influent ammonia concentration, and free ammonia concentration. Actually, nitrogen removal by SNAD in supplementation of the simultaneous nitrification-denitrification process is the net effect of multiple operational and design parameters.
The first objective of this study was to develop empirical models for non-destructive estimation of plant biomass growth and associated nitrogen assimilation, based on several years of mesocosm experiments with vegetated submerged beds and free water surface wetlands in various operational modes, wetland configurations, and system layouts. The second objective of this study was to explore the combined effects of multiple factors that influence microbial nitrogen removal through multiple linear regression and sensitivity analysis. The experimental and statistical analyses provide valuable guidance to enhance nitrogen removal in constructed wetlands.
Materials and methods
Configuration and layout of constructed wetlands
Four mesocosms were set up and operated differently each year from 2011 to 2017 in a greenhouse in Syracuse, New York, USA (Fig. 1). The mesocosms were rectangular wooden tanks lined with black pond liners. Each mesocosm was 42 cm wide, 45 cm long, and 53 cm tall. Two of them were vegetated submerged bed (VSB) wetlands and the other two free water surface (FWS) wetlands. The VSB wetlands were packed with marble chips (effective size d10 = 0.81 cm; porosity 0.41), having a 40-cm saturated layer and a 5-cm unsaturated layer. Once a year, approximately 0.25 L of electric arc furnace slag (d10 = 0.7 cm) was mixed in each VSB to buffer water pH (Wen et al. 2013). Two modifications were made to evaluate the effects of climate and medium type: (1) a submersible quartz heater (0.32 m long) set at 28 °C was buried in the center of VSB1 in 2011 to mimic a tropical climate, and (2) marble chips in VSB2 were replaced by rubber mulch (d10 = 1.6 cm; porosity 0.53) in 2012. Marble chips and rubber mulch are commercially available landscaping products, made of marble rocks and tire scraps, respectively. Marble chips are composed mainly of calcium carbonate and can release alkalinity at pH 7 and below (Wen et al. 2013). The rooting substrate at the bottom 20 cm of each FWS mesocosm was sandy loam. Approximately 0.5 L of electric arc furnace slag was cast each year on the rooting substrate of each FWS wetland. Prior to the monitoring period of each year, all the plants were taken out and immature plants were evenly transplanted to the mesocosms (Fig. 1), including common wetland plants Typha angustifolia (narrow-leaf cattail), Phragmites australis (common reed), Cyperus alternifolius (umbrella sedge), and Cyperus papyrus.
Initially and after medium replacement, each mesocosm was seeded with 3 L of primarily treated sewage and 2 L of anaerobically digested dairy manure having approximately 41 g/L of volatile solids and 1.8 g/L of total ammonia nitrogen (TAN). The mesocosms were operated by batch for a hydraulic residence time of 7 days, with two FWS-VSB or VSB-FWS series (Fig. 1). There were two exceptions: (1) the FWS-VSB series were operated by continuous feeding in 2017, and (2) all of the four mesocosms were operated independently in 2015. The influent (25 L per batch) was made by diluting the filtrate of anaerobically digested dairy manure. Although constructed wetlands can be used to treat such wastewater as landfill leachate, livestock wastewater, and anaerobic digestate at high ammonia concentrations (Li and Tao 2017; Vymazal 2013; Wen et al. 2013), the dilution ratios in this study were chosen to attain moderate ammonia concentrations that have been treated in full-scale constructed wetlands (Rozema et al. 2016; Kadlec and Wallace 2009; Vymazal 2007).
Field measurement and water quality analysis
Three to 4 weeks after seeding each year, the wetlands were monitored for 6–9 weeks (Table 1). Measurements were taken weekly in situ for water temperature, pH, and DO, using portable meters. Effluent volume in batch operation or flow rate in continuous operation was determined with a graduated cylinder. Inflow rate in continuous operation was set with a peristaltic pump. Influent and effluent samples were collected along with in situ measurements. Concentrations of TAN, nitrite, and nitrate were determined with a QuikChem 8500 series flow injection analyzer (LaChat Instruments, Loveland, CO) using the phenolate method for ammonia and hydrazine reduction method for nitrate and nitrite. Concentration of total inorganic nitrogen (TIN) was calculated as summation of TAN, nitrite nitrogen, and nitrate nitrogen. Concentration of free ammonia was calculated as a function of temperature, pH, and TAN concentration (Ukwuani and Tao 2016). Additionally, influent was analyzed occasionally for total Kjeldahl nitrogen (TKN), 5-d biochemical oxygen demand (BOD5), orthophosphate phosphorus (OrthoP), and alkalinity, following the Standard Methods (APHA et al. 2012). A YSI ProODO optical DO meter (YSI Incorporated, Yellow Springs, Ohio) was used to track initial and diurnal changes of DO and temperature in VSB1 and FWS2 during three batches in 2015.
Assessment of nitrogen removal performance and mechanisms
Because the wetlands were operated in different modes (batch and continuous feeding), laid out differently (in parallel and series), and fed with influent at different ammonia concentrations, it would be misleading to compare removal efficiencies among the individual wetlands or across the years. Rather, mass removal rate was used to evaluate nitrogen removal performance, evaluate the relative contributions of plant uptake and microbial removal, and identify factors influencing microbial removal and plant uptake.
Wetland treatment performance was assessed in terms of areal mass removal rates for TAN and TIN. Overall nitrogen removal rate minus plant assimilation rate of nitrogen was considered to be the removal rate by microbial conversions since adsorption to the packing materials, volatilization, and sediment accretion were negligible as justified by earlier studies for similar constructed wetlands (Li and Tao 2017; Tao et al. 2012). Multiple linear regressions were performed with a backward elimination approach to identify the factors influencing microbial nitrogen removal. One-way analysis of variance (ANOVA) was used to compare the differences between mesocosms. A p value ≤ 0.05 indicated a significant difference between any pair of means. Kinetics for ammonia removal at two different temperatures was investigated by taking samples and measurements in the two batch-operated VSB wetlands over 7 days in April 2012.
Determination of plant growth and nitrogen uptake
Each C. alternifolius plant has a leafless triangular stem with umbrella spokes and bracts at the top of the stem (Fig. A1). After transplanting to the mesocosms in 2014, 261 remaining C. alternifolius plants were measured for stem height and above-ground dry weight. After transplanting in 2015, 265 remaining C. alternifolius plants were measured for stem height and above-ground dry weight. The above-ground tissues were cut 1 cm above the ground with stainless steel scissors and air-dried for 8 days. The datasets of 2014 and 2015 were plotted in Microsoft Excel to generate a relationship between above-ground biomass and stem height by regression analysis. In 2017, total height and above-ground dry weight were measured for 83 C. alternifolius plants cut before the monitoring period and 202 after the monitoring period. A relationship between total height and above-ground dry weight was developed with the 2017 measurements. The ratio of below- to above-ground biomass was determined for C. alternifolius in 2017 and for C. papyrus in 2012 by drying the plants taken out of the mesocosms. Similarly, T. angustifolia was taken out of VSB2 after the monitoring period in 2015 to develop a regression equation between above-ground biomass and total height and determine the ratio of below- to above-ground biomass.
Plant stems were counted in the mesocosms while total and stem heights were measured on site two to three times during each monitoring period. Above-ground biomass on each plant monitoring date was then estimated with the regression equations at the measured plant stem number and heights. The growth rate of above-ground plant biomass in each mesocosm was estimated with Eq. 1 for each monitoring period. Nitrogen contents in above- and below-ground tissues were determined as TAN after Kjeldahl digestion of dry samples in triplicate for both C. alternifolius and T. angustifolia in 2015. Total nitrogen assimilation rate by both above- and below-ground tissues was estimated with Eq. 2.
where Up = rate of plant assimilation for nitrogen, g N/m2/day; Na, Nb = nitrogen content of above- and below-ground tissues, g N/g (dry weight); ΔMa = specific increase rate of above-ground plant biomass between two consecutive measuring dates, g (dw)/m2/day; R = ratio of below- to above-ground dry weight; As = surface area of a mesocosm, m2; D = number of days between the two measuring dates, day; and ∑Ma,1, ∑Ma,2 = total above-ground plant biomass in a mesocosm on the two measuring dates, g.
Results and discussion
Operational conditions
The influent had high ammonia concentrations despite the variations over the years (Table 1). Influent nitrogen was primarily in ammonia, with negligible nitrite and nitrate and less than 5% in organic nitrogen. The influent had very low BOD/N ratios, suggesting high potential for nitrogen removal in the constructed wetlands, with negligible carbonaceous biochemical oxygen demand. The pH values and orthophosphate concentrations were high. Stripping pretreatment of the wastewater in 2014 resulted in the higher influent pH value. Because pH was buffered by dissolution of furnace slag and marble chips in the mesocosms, the variations in influent pH and alkalinity should not have a crucial impact on water pH in the mesocosms.
Diurnal variations of water temperature in the VSB and FWS wetlands were small, within 2.5 °C (Fig. A2a,b). Although influent DO was increased during influent preparation and feeding, wetland DO concentration decreased sharply and stabilized in 6–9 h (Fig. A2c). Electric arc furnace slag is able to quickly buffer pH (Wen et al. 2013). Weekly measurements, therefore, are presented in Figs. 2 and 3 and Table 2 for effluent DO, pH, and temperature, respectively, to represent the operational conditions.
Biomass yield and nitrogen uptake by wetland plants
Table 3 summarizes plant density, average height, and above-ground biomass growth rate. In general, C. alternifolius and C. papyrus grew faster in FWS wetlands (6.6–33.1 g (dw)/m2/day) than VSB wetlands (0.42–4.4 g (dw)/m2/day). T. angustifolia in the VSB wetlands grew slower (0.9–2.9 g (dw)/m2/day) than the Cyperus species, possibly because Cyperus species are more tolerant to ammonia stress than Typha (Tao et al. 2015). A TAN concentration exceeding 147–236 mg N/L exerts stress on C. alternifolius growth (Tao et al. 2015). Cattail growth begins to be negatively affected at TAN concentrations greater than 46–82 mg/L (Tao et al. 2012; Hill et al. 1997) and is significantly affected at TAN concentrations exceeding 160–264 mg/L (Tao et al. 2012; Clarke and Baldwin 2002). When influent and effluent TAN concentrations were 81–154 mg/L in 2014 and 2015, cattails in the VSB wetlands showed signs of stress in the form of yellow/brown leaves. Possibly because of ammonia stress, P. australis in the FWS wetlands was infested severely by aphids in 2011 and 2012 and its biomass growth was not quantified.
The above-ground biomass of individual C. alternifolius plants was found to be a power function of plant height based on the measurements of the harvested plants in 2014, 2015, and 2017 (Figs. 4 and Fig. A3). The smaller R2 value in 2014 and 2015 could be attributed to the errors associated with cutting stems from roots and measuring stem height. Although the 2014/2015 and 2017 equations have different exponents and constants, their biomass estimates at the measured total and stem heights in 2014 and 2015 differed only by approximately 19%. The above-ground dry weight of individual C. alternifolius and C. papyrus plants was hence estimated with the regression equation developed with 2017 measurements, which covered both short and tall plants and had a higher R2 value. The below- to above-ground ratio of C. alternifolius was determined to be 0.38 ± 0.05 in 2017.
Above-ground biomass of T. angustifolia was a power function of total height (Fig. 5), which was similar to a power function for T. latifolia (Tao et al. 2012). Below- to above-ground biomass ratio of T. angustifolia was 2.73, which was higher than that of T. latifolia, 2.09 as reported by Tao et al. (2012). Nevertheless, both of the Typha species had much higher below- to above-ground ratios than C. alternifolius. This is because cattail is characterized by long lateral tubes and thick roots (Fig. A4). Because several cattail stems could be connected to a lateral tube, the below- to above-ground ratio was determined as the ratio of the total below-ground to the total above-ground dry weights.
Nitrogen content of C. alternifolius was determined to be 34.3 mg N/g above-ground dry weight and 14.3 mg N/g below-ground dry weight. Based on the below- to above-ground biomass ratio, the nitrogen content of total C. alternifolius biomass was estimated to be 28.8 mg N/g (dw), which is close to that of C. papyrus (26.0 mg N/g) as reported by Tao et al. (2012). Therefore, nitrogen assimilation rates for both Cyperus species were estimated with the nitrogen content determined in this study with C. alternifolius samples. Nitrogen content of T. angustifolia was determined to be 14.0 mg N/g above-ground and 25.9 mg N/g below-ground.
The total nitrogen assimilation rates by above- and below-ground tissues are presented in Fig. 6. In summary, nitrogen assimilation rate was 0.27–0.94 g/m2/day for C. alternifolius and 0.56–0.65 g/m2/day for C. papyrus in the FWS wetlands, while being 0.11–0.25 g/m2/day for C. alternifolius, 0.02 g/m2/day for C. papyrus, and 0.07–0.25 g/m2/day for T. angustifolia in the VSB wetlands. The higher nitrogen assimilation rates in the FWS wetlands were associated with their higher plant biomass growth rates. These power functions of plant biomass, below- to above-ground plant biomass ratios, and nitrogen content in the wetland plants support non-destructive methods to estimate plant assimilation rate for nitrogen, thus providing a convenient tool to guide wetland vegetation management.
Ammonia removal rate and influencing factors
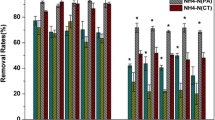
TAN and TIN removal rates varied in the ranges of 0.19–2.02 and 0.17–2.10 g/m2/day, respectively, over the 6 years with different wetland configurations, operational modes, and system layouts (Figs. 1 and 6). Plant assimilation of nitrogen contributed 2.8–30.8% to the overall TIN removal in the VSB wetlands and 16.6–78.6% in the FWS wetlands as shown in Fig. 6. In a similar FWS wetland at influent TAN concentrations (90–264 mg/L) also similar to the present study, Tao et al. (2012) estimated that nitrogen assimilation by T. latifolia and C. prolifer in FWS wetlands accounted for 4–25% of TIN removal rates. The difference in contributions of plant uptake to overall nitrogen removal between the present study and Tao et al. (2012) confirmed that C. alternifolius is more tolerant to ammonia stress than T. latifolia and C. prolifer. Tao et al. (2015) found that C. alternifolius tolerated long periods of exposure to ammonia concentrations up to 219 mg/L. T. latifolia and C. prolifer could be stressed by ammonia as low as 46–76 mg/L (Tao et al. 2012). Therefore, selection of plant species for constructed wetlands treating ammonia-rich wastewater has to consider the tolerance of wetland species to ammonia in order to benefit not only nitrogen removal but also the health of wetland ecosystems.
When ammonium is plentiful, wetland plants prefer ammonium over nitrate as a nitrogen source for biomass growth (Jampeetong et al. 2013; Kadlec and Wallace 2009; Vymazal 2007). Therefore, subtracting plant nitrogen assimilation rate from an overall TAN or TIN removal rate could approximate the TAN or TIN removal rate by microbial processes in the mesocosms that had high concentrations of ammonia (Table 1) and little nitrate (Table A1). Although the overall TAN and TIN removal rates were significantly lower in VSB2 than the other three mesocosms in 2015 (ANOVA p = 0.05, 0.03) due to the lower nitrogen assimilation rate of plants in VSB2, there were insignificant differences across the four mesocosms in the microbial TAN and TIN removal rates (p = 0.14, 0.10). The parallel operation in 2015, hence, indicated insignificant differences in microbial nitrogen removal between the two wetland types. Although plants may release exudates to support denitrification, heterotrophic denitrification and autotrophic anammox can play complementary roles in complete nitrogen removal, depending on availability of organic carbon and nitrate (Du et al. 2017; Li and Tao 2017). Side-by-side comparisons of the vegetated versus non-vegetated VSB mesocosms and those with different plant species in 2013–2017 indicated insignificant effects of vegetation on microbial nitrogen removal and insignificant differences between plant species (ANOVA p = 0.10–0.89). Therefore, the microbial communities were likely similar in species composition over the six study periods and possibly similar to those communities dominated by aerobic ammonia-oxidizing bacteria, heterotrophic denitrifying bacteria, and anammox bacteria in similarly inoculated constructed wetlands treating dairy wastewater. The same research group as this study used fluorescence in situ hybridization and transmission electron microscopy to identify the microbial community compositions in those similarly operated constructed wetlands (Li and Tao 2017; Wen et al. 2013; He et al. 2012). Nevertheless, the activities of each functional group of microorganisms could have varied with the operational conditions such as pH value and DO concentration under different operational modes and wetland configurations.
Linear regression confirmed that microbial TAN and TIN removal rates were affected by various factors as shown in Eqs. 3–6, with R2 = 0.95–0.96 and p = 0.001 for FWS wetlands and R2 = 0.66–0.67 and p = 0.05 for VSB wetlands. These regression equations can be used to guide design and improve operation of constructed wetlands for TAN and TIN removal. Cautions, however, must be taken when applying to the conditions outside their boundaries of pH, DO concentration, temperature, and free ammonia concentration.
with 6.1 < pH < 8.3, 0.2 < DO < 4.2, 14 < T < 25, 0.03 < FA < 14, and 60 < Ci < 390.
with 0.2 < DO < 2.2, 16 < T < 30, 0.2 < FA < 17, and 102 < Ci < 212.
where RTAN and RTIN = microbial removal rate of TAN and TIN, g/m2/day; pH = effluent pH; DO = effluent DO concentration, mg/L; T = effluent temperature, oC; FA = effluent concentration of free ammonia, mg N/L; and Ci = influent TAN or TIN concentration, mg/L.
The positive temperature effect was experimentally displayed by the significant differences in TAN and TIN removal rates between the heated VSB1 and unheated VSB2 in 2011 (ANOVA p < 0.001). Kinetic study with a 7-day batch operation of these two VSB wetlands showed that both TAN and TIN mass reduction followed the first-order reaction kinetics, with a removal rate constant of 0.165/day in VSB1 at water temperature of 27.3 ± 1.5 °C and 0.061/day in VSB2 at 22.3 ± 2.0 °C (Fig. 7). It is noteworthy that the strong temperature effect in the VSB wetlands reveals potentially greater microbial nitrogen removal under less varying, higher temperature such as in tropical climates relative to that under varying, lower temperature such as in a temperate climate.
Equations 3–6 indicate a positive relationship of microbial N removal rate with influent nitrogen concentration. Mass removal rate of constructed wetlands generally increases with increasing loading rate, or concentration at a given influent flow rate or volume, because of increased substrate availability to microorganisms (Vymazal 2013; Tao et al. 2007). VSB2 in 2012 was packed with rubber mulch having a greater porosity than marble chips in VSB1, which explained that VSB2 had a significantly higher ammonia removal rate (ANOVA p = 0.01) because of a higher areal mass loading rate at the same water depth and a larger influent volume. When the FWS wetlands were in the second stage of the wetland series in 2011–2014, the microbial removal rates in the FWS wetlands were smaller (Fig. 6). In contrast, the microbial removal rates in the FWS wetlands were larger in 2017 when they were the first stage of treatment and in 2015 when all the mesocosms were in parallel. A positive relationship of microbial N removal rate with influent nitrogen concentration suggests that too much dilution of a concentrated wastewater may actually result in decreased TAN and TIN removal rates. In real applications, dilution of concentrated wastewater can be attained by effluent recirculation or mingling with lightly contaminated water such as storm water runoff. Dilution ratio should be determined based on the estimated final influent concentration.
The TAN removal rates were generally similar to the TIN removal rates, indicating little accumulation of nitrite and nitrate. Therefore, microbial nitrogen removal could be attributed to SNAD and simultaneous nitrification-denitrification. Traditionally, biological nitrogen removal is interpreted by nitrification and denitrification processes, whereas recent studies (Li and Tao 2017; Zhi et al. 2015; Tao et al. 2012; Chiemchaisri et al. 2009) have found that anammox and SNAD could play a crucial role in ammonia removal in constructed wetlands. Controlling pH, DO, and free ammonia concentration is an important strategy to enhance SNAD (Li and Tao 2017; Zhi et al. 2015; Wen et al. 2013; Tao et al. 2012). Both nitritation in the SNAD process and nitrification in the simultaneous nitrification-denitrification process consume alkalinity. Marble chips and furnace slag in the mesocosms maintained water pH at different levels (Fig. 3). The pH values of 6.8–8.5 in the VSB wetlands were in the range suitable for SNAD, explaining the absence of pH from Eqs. 5 and 6. Nitrification-denitrification possibly played a minor role in the VSB wetlands where there was limited availability of organic carbon for the heterotrophic denitrifying bacteria. Rather, exudates released by the fast-growing plants in the FWS wetlands could promote nitrification-denitrification over SNAD. Twenty-five percent of the average pH values in the FWS wetlands were at 8.1–8.3, which were above the pH range of 7.0–7.5 favorable for denitrification (Metcalf and Eddy / AECOM 2014), explaining the negative effect of pH on microbial nitrogen removal as shown in Eqs. 3 and 4.
Free ammonia concentration varied between 0.03 and 16.2 mg N/L across the mesocosms over the years (Fig. 8), largely because of the different pH values. A free ammonia concentration of 0.2–1.0 mg N/L can be high enough to favor nitritation over nitrite oxidation (Miot and Pagilla 2010; Chung et al. 2006), whereas anammox can be inhibited by free ammonia at greater than 4.5–25 mg N/L (Li and Tao 2017; Fernández et al. 2012; Jaroszynski et al. 2011). Therefore, free ammonia had a negative effect on microbial removal in Eqs. 3–6. It appeared that microbial nitrogen removal was inhibited by free ammonia at concentrations of 13.3–16.2 mg N/L in the VSB wetlands in 2013 compared with the lower free ammonia concentrations in the VSB wetlands in the other years. The FWS wetlands in 2017 had high nitrogen removal rates despite the high free ammonia concentrations, which might be attributed to the high temperature.
As Li and Tao (2017) found, constructed wetlands can have the two microbial treatment processes complemented as operating conditions vary. To take advantage of SNAD, nitrite oxidation has to be suppressed since nitrite-oxidizing bacteria compete with aerobic ammonia-oxidizing bacteria for oxygen and with anammox bacteria for nitrite. Nitrite oxidation is suppressed, and SNAD is promoted to reach high nitrogen removal rates at DO 0.8–1.7 mg/L in the bulk water of constructed wetlands (Li and Tao 2017). The VSB wetlands had DO at 0.3–2.2 mg/L, thus having a positive effect as shown in Eqs. 5 and 6. The FWS wetlands had higher DO concentrations, averaging at 2.0 mg/L, thus having a negative effect in Eqs. 3 and 4. Moreover, the anaerobic micro-niches for anoxic denitrification and anammox can be limited when the bulk water has a higher DO concentration. It appeared that the microbial nitrogen removal rates were lower and TIN removal rates were lower than TAN removal rates when DO concentration was above 1.9 mg/L as demonstrated by FWS wetlands in 2012–2014 (Figs. 2 and 6), indicating suppressed denitrification and anammox at DO greater than 1.9 mg/L.
Sensitivity of microbial nitrogen removal to influencing factors
The yearly varied operational modes, wetland configurations, and system layouts made the regression Eqs. 3–6 effective and applicable for diverse conditions. As the effects of multiple influencing factors are mixed, however, it is challenging to directly utilize Eqs. 3–6 to determine or assess design and operational parameters. Comparing the sensitivity of nitrogen removal to each influencing factor can better guide wetland design. Using Eqs. 3 and 5, the responses of microbial TAN removal rate to the change of an influencing factor were computed at the averages of the other factors for three influent TAN concentrations (Table 4). The changes of pH, DO, and temperature were analyzed at the minimum, average, and maximum of the mesocosm measurements in individual monitoring periods. The influent TAN concentrations were analyzed at 60, 200, and 400 mg/L, being close to their lowest, average, and highest measurements in the study periods. A TAN concentration of 60 mg/L represents secondary treated sewage (Metcalf and Eddy / AECOM 2014). The TAN concentration of 400 mg/L represents the highest one-time reversible inhibition concentration for ammonia-tolerant C. alternifolius (Tao et al. 2015).
The changes of microbial TAN removal rate in Table 4 revealed that (1) for the VSB wetlands, temperature was the least sensitive factor; (2) for the VSB wetlands, DO was the most sensitive factor at influent TAN concentrations from 60 to 200 mg/L while DO and pH were the most sensitive factors at influent TAN concentrations from 200 to 400 mg/L; (3) for the FWS wetlands, DO was the least sensitive factor; and (4) for the FWS wetlands, influent TAN concentration and pH were the most sensitive factors. VSB wetlands have an unsaturated layer that reduces heat loss from water, resulting in temperature being the least sensitive factor. Meanwhile, VSB wetlands have smaller water surface in contact with air and surface reaeration is dominated by slow molecular diffusion, resulting in DO concentration being a limiting factor. The FWS wetlands were less limited by DO because of surface reaeration through both molecular and turbulent diffusion. Coincidently, the highest microbial TAN removal rate estimated for FWS wetlands, 5.05 g/m2/day, appeared at the highest influent ammonia concentration (400 mg/L), average DO concentration (1.5 mg/L), and the lowest pH.
Conclusions
The rate of nitrogen assimilation into plant biomass could vary substantially with ammonia concentration and plant species. Ammonia-tolerant C. alternifolius and C. papyrus grew faster than T. angustifolia in the wetlands at less inhibitory ammonia concentrations. Above-ground biomass of individual plants was a power function of plant height for both C. alternifolius and T. angustifolia. C. alternifolius had a small below- to above-ground biomass ratio (0.38) and higher nitrogen content in the above-ground than below-ground tissues. T. angustifolia had a large below- to above-ground biomass ratio (2.73) and lower nitrogen content in above-ground than below-ground tissues. Therefore, Cyperus species are preferred for ammonia removal in constructed wetlands by plant uptake of nitrogen and harvesting above-ground tissues.
Microbial nitrogen removal in the mesocosms was attributed to SNAD and simultaneous nitrification-denitrification. There were no significant effects of wetland type, vegetation presence, and plant species on microbial nitrogen removal. Microbial TAN removal rate correlated linearly with multiple operational parameters including influent ammonia concentration, DO, pH, free ammonia concentration, and temperature. Microbial TAN removal is sensitive mainly to DO, pH, and influent ammonia concentration in VSB wetlands, while sensitive to influent ammonia concentration, pH, and temperature in FWS wetlands.
References
Almstrand R, Persson F, Daims H, Ekenberg M, Christensson M, Wilén B-M, Sörensson F, Hermansson M (2014) Three-dimensional stratification of bacterial biofilm populations in a moving bed biofilm reactor for nitritation-anammox. Int J Mol Sci 15:2191–2206
American Public Works Association (APHA), American Water Works Association, Water Environment Federation (2012) Standard methods for the examination of water and wastewater, 22nd edn. American Public Works Association, Washington, DC
Berry P, Yassin F, Grosshans R, Lindenschmidt K-E (2017) Surface water retention systems for cattail production as a biofuel. J Environ Manag 203:500–509
Britto DT, Kronzucker HJ (2002) NH4 + toxicity in higher plants: a critical review. J Plant Physiol 159:567–584
Chiemchaisri C, Chiemchaisri W, Junsod J, Threedeach S, Wicranarachchi PN (2009) Leachate treatment and greenhouse gas emission in subsurface horizontal flow constructed wetland. Bioresour Technol 100:3808–3814
Chung J, Shim H, Park S (2006) Optimization of free ammonia concentration for nitrite accumulation in shortcut biological nitrogen removal process. Bioprocess Biosyst Eng 28:275–282
Clarke E, Baldwin AH (2002) Responses of wetland plants to ammonia and water level. Ecol Eng 18:257–264
Daverey A, Chen Y-C, Dutta K, Huang Y-T, Lin JG (2015) Start-up of simultaneous partial nitrification, anammox and denitrification (SNAD) process in sequencing batch biofilm reactor using novel biomass carriers. Bioresour Technol 190:480–486
Dragoni F, Giannini V, Ragaglini G, Bonari E, Silvestri N (2017) Effect of harvest time and frequency on biomass quality and biomethane potential of common reed (Phragmites australis) under paludiculture conditions. Bioenergy Research 10:1066–1078
Du R, Cao S, Li B, Niu M, Wang S, Peng Y (2017) Performance and microbial community analysis of a novel DEAMOX based on partial-denitrification and anammox treating ammonia and nitrate wastewaters. Water Res 108:46–56
Fernández I, Dosta J, Fajardo C, Campos JL, Mosquera-Corral A, Méndez R (2012) Short- and long-term effects of ammonium and nitrite on the anammox process. J Environ Manag 95:170–174
He Y, Tao W, Wang Z, Shayya W (2012) Effects of pH and seasonal temperature variation on simultaneous partial nitrification and anammox in free-water surface wetlands. J Environ Manag 110:103–109
Hill DT, Payne VWE, Rogers JW, Kown SR (1997) Ammonia effects on the biomass production of five constructed wetland plant species. Bioresour Technol 62:109–113
Jampeetong A, Konnerup D, Piwpuan N, Brix H (2013) Interactive effects of nitrogen form and pH on growth, morphology, N uptake and mineral contents of Coix lacryma-jobi L. Aquat Bot 111:144–149
Jaroszynski LW, Cicek N, Sparling R, Oleszkiewicz JA (2011) Importance of the operating pH in maintaining the stability of anoxic ammonium oxidation (Anammox) activity in moving bed biofilm reactors. Bioresour Technol 102:7051–7056
Kadlec R, Wallace S (2009) Treatment wetlands. CRC Press, Boca Raton, FL
Kumar M, Lin J (2010) Co-existence of anammox and denitrification for simultaneous nitrogen and carbon removal—strategies and issues. J Hazard Mater 178:1–9
Li H, Tao W (2017) Efficient ammonia removal in recirculating vertical flow constructed wetlands: complementary roles of anammox and denitrification in simultaneous nitritation anammox and denitrification process. Chem Eng J 317:972–979
Lotti T, Kleerebezem R, Lubello C, van Loosdrecht MCM (2014) Physiological and kinetic characterization of a suspended cell anammox culture. Water Res 60:1–14
Metcalf & Eddy / AECOM (2014) Wastewater engineering: treatment and resource recovery, fifth ed. In: McGraw Hill higher education. NY, New York
Miot A, Pagilla KR (2010) Control of partial nitritation of centrate in a sequencing batch reactor. Water Environ Res 82:819–829
Pang Y, Zhang Y, Yan X, Ji G (2015) Cold temperature effects on long-term nitrogen transformation pathway in a tidal flow constructed wetland. Environ Sci Technol 49:13550–13557
Rozema ER, VanderZaag AC, Wood JD, Drizo A, Zheng Y, Madani A, Gordon RJ (2016) Constructed wetlands for agricultural wastewater treatment in northeastern North America: a review. Water 8(5):173. https://doi.org/10.3390/w8050173
Tanaka TST, Irbis C, Kumagai H, Inamura T (2016) Timing of harvest of Phragmites australis (CAV.) Trin. ex Steudel affects subsequent canopy structure and nutritive value of roughage in subtropical highland. J Environ Manag 166:420–428
Tao W, Hall KJ, Duff SJB (2007) Microbial biomass and heterotrophic production of surface flow mesocosm wetlands treating woodwaste leachate: responses to hydraulic and organic loading and relations with mass reduction. Ecol Eng 31:132–139
Tao W, Han J, Li H (2015) Investigation into ammonia stress on Cyperus alternifolius and its impact on nutrient removal in microcosm experiments. J Environ Manag 163:254–261
Tao W, Wen J, Han Y, Huchzermeier M (2012) Nitrogen removal in constructed wetlands using nitrification-denitrification and nitritation-anammox: effects of influent nitrogen concentrations. Water Environ Res 84:2099–2105
Ukwuani A, Tao W (2016) Developing a vacuum thermal stripping—acid absorption process for ammonia recovery from anaerobic digester effluent. Water Res 106:108–115
Vymazal J (2013) The use of hybrid constructed wetlands for wastewater treatment with special attention to nitrogen removal: a review of a recent development. Water Res 47:4795–4811
Vymazal J (2007) Removal of nutrients in various types of constructed wetlands. Sci Total Environ 380:48–65
Wen J, Tao W, Wang Z, Pei Y (2013) Enhancing simultaneous nitritation and anammox in recirculating biofilters: effects of unsaturated zone depth and alkalinity dissolution of packing materials. J Hazard Mater 244–245:671–680
Zhi W, Yuan L, Ji G, He C (2015) Enhanced long-term nitrogen removal and its quantitative molecular mechanism in tidal flow constructed wetlands. Environ Sci Technol 49:4575–4583
Acknowledgements
It is acknowledged that teaching assistants and many students in the Ecological Engineering for Water Quality classes from fall 2011 to spring 2017 operated and monitored the wetland treatment systems. Sean Matus completed additional measurements of plants in 2015. It is acknowledged that James Carmody proofread this revised manuscript.
Funding
SUNY ESF provided Graduate Assistantships to the teaching assistants and the ERE teaching funds provided partial support of laboratory expenses.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The author declare that they have no conflict of interest.
Additional information
Responsible editor: Diane Purchase
Electronic supplementary material
ESM 1
(PDF 903 kb)
Rights and permissions
About this article
Cite this article
Tao, W. Microbial removal and plant uptake of nitrogen in constructed wetlands: mesocosm tests on influencing factors. Environ Sci Pollut Res 25, 36425–36437 (2018). https://doi.org/10.1007/s11356-018-3543-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-018-3543-4




 = marble chips,
= marble chips,  = rubber mulch,
= rubber mulch,  = water,
= water,  = pump head;
= pump head;  = C. alternifolius,
= C. alternifolius,  = C. papyrus,
= C. papyrus,  = P. australis, and
= P. australis, and  = T. angustifolia
= T. angustifolia





