Abstract
High arsenic (As) levels occur naturally in geothermal areas, potentially polluting downstream wetland ecosystems. The study was to determine the distribution of As among aqueous, solid, and plant phases in the Guandu Wetland of Taiwan. Chemical compounds (As, Fe, Mn, TOC, SO4 2-, and FeS2) and isotopic compositions (δ34S) in water and soil samples were analyzed to characterize the As distribution. The sequential extraction of As and total As in plant samples wasanalyzed to estimate the bioconcentration factor (BCF) and translocation factor (TF; defined as the ratio of metal concentration in the shoots to those in the roots) of As in Kandelia obovata in aqueous and solid phases. The As concentrations in plants (23.69 mg/kg) were higher than in the surrounding water (0.0018 mg/L) and soils (17.24 mg/kg). Kandelia obovata have high As bioavailability and low TF, causing easy adaptation to grow in As-contaminated wetland ecosystems. BCFplants/water (13657.92) was higher than BCFplants/soil (1.38). The uptake and bioaccumulation of As in Kandelia obovata are significant; therefore, Kandelia obovata is an As accumulator. The uptake As by the Kandelia obovata plant might depend on the oxidation of As-contained FeS2 in the aerial roots and/or adsorption of As on root surface.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Arsenic (As) is a toxic metalloid and human carcinogen in the natural environment (Smedley and Kinniburgh 2002). High As levels occur naturally in geothermal areas because of volcanic activity, resulting in pollution of groundwater, geothermal spring water, downstream wetlands, and estuary ecosystems (Lièvremont et al. 2009).
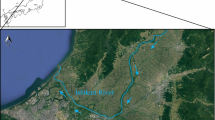
The Guandu Wetland is located in the estuary of the Tanshui and Keelung Rivers and is widely affected by tidal conditions (Fig. 1). The tidal estuarine wetland is flooded twice a day; therefore, seawater mixing with fresh water results in variations of salinity, sulfate concentrations, pH, and redox conditions, which may cause As release and retention reactions. Most metal sulfides provide a potential sink for As in anoxic sediments (Dellwig et al. 2002), especially in coastal area where sediment material were easily deposited in the marine formation. The biogeochemical reactions in anaerobic environment may govern sulfate/sulfide cycling in sedimentary marine formations (Thamdrup et al. 1993; Canfield and Thamdrup 1996). Numerous studies have indicated that isotopic techniques are useful for understanding the influence of sulfur cycling on As mobility in the geochemical environment (Lipfert et al. 2007; Mukherjee and Fryar 2008; Kao et al. 2011). However, both the accumulation and impact of As on ecosystems in the wetland are not yet adequately distinguished.
Guandu Wetland is a tidal estuary natural preserve which has Kandelia obovata, Phragmites communis and Cyperus malaccensis mangrove species in the benthic-mud areas. Kandelia obovata is the most dominant mangrove species in Guandu Wetland (Hsueh and Lee 2000). Kandelia obovata has three physiological mechanisms: (1) Aeration roots transfer oxygen to the roots, thus Kandelia obovata can survive in anaerobic wetlands (Kadlec and Knight 1996). (2) Viviparous seedlings help disseminate and reproduce Kandelia obovata (Chou et al. 1987). (3) The salt tolerance of Kandelia obovata enables survival in the estuary ecosystem (Mitsch and Gosselink 2000). Kandelia obovata has been widely studied and was mainly focused on the accumulation of heavy metals, effect of heavy metals on plant growth, and effects of root exudates on heavy metal toxicity (Lu et al. 2007; Xie et al. 2012). As heavy metal transported to the aerial part in Kandelia obovat, the stem acts as a cation exchange column which can effectively reduce the amount of heavy metal accumulation in leaves (Hardiman and Jacoby 1984). The high content of heavy metal in roots is an important tolerance mechanism of Kandelia obovata.
Variations of salinity, sulfate concentrations, pH, and redox conditions in wetland system may potentially affect As release and retention reactions. Hence the distribution and mobility of As among aqueous, solid, and plant phases are worth for investigation. The purpose of this study was to discriminate the distribution of As among aqueous, solid, and plant phases in the Guandu Wetland, Taiwan. Chemical parameters, As species, ferrous iron species and sulfur isotopic compositions (i.e., δ34S[SO4] and δ18O[SO4]), were analyzed. The bioaccumulation and translocation capacity of the major wetland plant, Kandelia obovata, was quantitatively assessed to illustrate the uptake of As in the wetland environment. Results of this study will provide valuable information to improve our understanding of the transfer pathways of As in mangrove ecosystem in the region.
Materials and Methods
Study Area
The Guandu Wetland is located in the southwestern part of the Guandu Plain in Taipei, Taiwan, which is downstream of the Beitou geothermal spring (Fig. 1). In 1960s, many geothermal fields at Beitou have been developed to generate energy from the steam and hot water reservoirs of Tatun Volcano Group (Song et al. 2000) which lies between two major thrust faults, the Chinshan Fault and the Kanchiao Fault (Lai et al. 2010, 2011). In geothermal spring water at Beitou, Taipei City, Taiwan, the As concentration is as high as 4.32 mg/L(Chen et al. 2007), exceeding the ground water contamination standard of 0.25 mg/L and potentially resulting in bioaccumulation of As in the downstream wetland ecosystem. The As-rich spring water flows to Huang Gang Creek, discharging a high-As flux to the regional Guandu Plain. Long-term irrigation with high As contents has caused the average As content of surface (0–15 cm) and subsurface (15–30 cm) soil in the Guandu Plain to be 145 and 143 mg/kg, respectively, substantially higher than the soil contamination standard of 60 mg/kg (Chiang et al. 2010). High As contents may accumulate downstream of the Guandu Wetland and may influence aqueous, solid, and plant phases in the Guandu Wetland.
Because the Guandu Wetland is near the mouth of the Keelung and Tanshui Rivers, it has a semi-diurnal tidal regime with a tidal amplitude of approximately 1–2 m; and although it is only 10 km away from the Tanshui River estuary, the wetland area is widely affected by tidal fluctuations. Tidal seawater, which can intrude into the upper estuary approximately 25 km from the river mouth, mixes with the river water during high tide, but mixes only partially during low tide (Liu et al. 2001).
The mangrove ecosystems are particularly abundant in the Guandu Wetland, and the Kandelia obovata is one of the most dominant plant species found in this area. Mangroves are capable of absorbing and accumulating pollutant toxins in both roots and aerial parts. As a result, the pollutants are transferred to the detrital food chain in the wetland ecosystems. The pathway of toxins transfer in mangroves via detrital food chain introduces As into the detritus feeding communities and thus resulting in input of As into the detrital and coastal food chains. Similarly, the deposit feeders, such as fiddler crabs, which are exposed to high As sediment may transfer the toxin into the food chains. As may be further transferred and biomagnified at higher trophic levels in the wetland ecosystem. It is thus important to know the As distribution in the aqueous, sediment, and mangrove phases to formulate effective management plan in the Guandu Wetland.
Water, Soil and Plant Sampling
The surface water and soil samples in this study were collected from inland sites and 5 randomly selected sites (S1, S2, S5, S7, and S9). Core samples were collected at the inner (depth of 70 cm, S2) and outer sites (depth of 85 cm, S5) (Fig. 1). Porewater samples with a vertical interval of 5 cm were extracted using a Rhizon sampler (microporous polymer, <0.2 μm pore size). The sediment samples with an interval of 5 cm were air-dried (overnight) to facilitate analyzing the chemical compounds. All water samples were stored in polyethylene containers, maintained at 4 °C, and sent to the laboratory within 24 h. All soil samples were stored in N2-purged plastic bags, dried at room temperature, and homogenized using 100-mesh sieves in an anaerobic glove box. A split sample was removed from the glove box in an oven at 50 °C for 72 h.
Plant samples were taken from the same location as the surface water, and the soil samples (S1, S2, S5, S7, and S9) were collected in 3 replicates. Samples of Kandelia obovata were roughly washed with tap water and then rinsed with deionized water. The various plant tissues, such as live roots, stems, leaves, and seedlings, were separated and delivered to the laboratory within 24 h. The samples were dried in the oven at 50 °C for 48 h, ground into powders, and passed through 100-mesh sieves (Abedin et al. 2002).
Chemical Compounds of Water, Soil and Plant Analysis
The dissolved oxygen (DO; Thermo Fisher Scientific Star-A 2235), redox potential (Eh; Mettler Toledo Inlab 501 Redox), and pH (Mettler InLab Routine; Calibration Buffer Solution 4, 7 and 10) of the water samples were measured in situ. Water samples were filtered through 0.2 μm membrane filters (Advantec; mixed cellulose ester) and acidified with a few drops of 3 M HNO3 to a pH of about 2 (APHA 1998). The Alkalinity (Alk) and total organic carbon (TOC), and NO3 -, NH4 +, SO4 2-, HS-, Cl-, Ca2+, Mg2+, Na+, K+, As, Fe, and Mn concentrations were analyzed. Alkalinity and TOC were measured using the titration and high-temperature combustion methods, respectively (APHA 1998). Ions such as SO4 2-, Cl-, NH4 + and HS- were determined using an ion chromatograph (IC) (DIONEX ICS-900), and Cl- was determined using AgNO3 titration. Dissolved metal ions, including Na+, Mg2+, Ca2+, K+, Fe and Mn, were measured using an inductively coupled plasma-optical emission spectrometer (ICP-OES) (Perkin Elmer Optima 7300DV ICP-OES) (APHA 1998).
The preservative procedure of As and Fe species followed that outlined by Wang et al. (2011). After pumping, all groundwater samples were filtered through a 0.2 μm pore membrane filter to prevent microbial activity and remove suspended particles. In order to pre-treat groundwater samples by the complexation of Fe, acidification of samples had been proposed to prevent the effects of Fe precipitation on As speciation (McCleskey et al. 2004). Ferrous (Fe2+) concentrations of the samples were measured colorimetrically using a ferrozine method (Lovley and Phillips 1987). The difference between the concentrations of total Fe and Fe2+ was considered the Fe3+ concentration. The arsenic species were separated using an anion column (Phenomenex Nucleosil, 10 μm, 250 mm × 4.6 mm) connected to a high-performance liquid chromatography (HPLC) (Perkin Elmer Series 200 HPLC Pump), which was interfaced to an electro-thermal atomic absorption spectrometer (AAS) (Perkin Elmer AAnalyst 200 AAS) and a hydride generation (HG) system (Perkin Elmer FIAS 100) (Huang et al. 2003). The variances of duplicate measurements were less than 10 %; recoveries of check and spike samples were between 85 % and 115 %, respectively.
The soil samples were air-dried, digested in aqua regia, and filtered to determine the metal ion concentrations such as Fe and Mn, using ICP-MS. Sulfate and total organic carbon contents were analyzed by turbidimetric method and Walkley-Black method, respectively. To determine the total As concentration, 30 % H2O2 and 9.6 M HCl were added to the soil samples to remove organic matter; the soil samples were then filtered. The AAS and HG systems (Perkin Elmer FIAS100) were used, in which a mixture of 0.5 % NaBH4, 0.25 % NaOH, and 1 M HCl was used to reduce arsenic to arsine (EPA, NIEA S310.62C). All the metal concentrations of solid phases are given on dry matter basis.
The digestion procedure of As in the plant followed that outlined by Tang and Miller (1991). The plant samples, which were various Kandelia obovata tissues, required the addition of 25 ml of HNO3 and were allowed to stand overnight. The plant samples were then supplemented with 30 % H2O2 and heated to 120 °C for 3–4 h to remove organic matter. At room temperature the mixtures were filtered (0.2 μm pore membrane filter), and the total As was determined using an electro-thermal atomic absorption spectrometer (AAS) and a hydride generation (HG) system, as described previously. The standard reference material used was tomato leaves (NIST1573a; As = 0.112 mg/kg) and the extraction recovered 72 % to 106 % of the total As.
Sulfur Isotope Analysis
Porewater samples were filtered with 0.2 μm membrane filters and acidified with 1 M HCl to maintain the solution pH < 2; 10 % BaCl2 was then added to the samples to produce BaSO4 precipitation. The samples were then filtered and dried (Yanagisawa and Sakai 1983). The δ34S[SO4] was analyzed in the isotope laboratory at the University of Arizona using a continuous-flow gas-ratio mass spectrometer (Thermo Scientific Delta PlusXL) (Supporting material (a)).
Before analyzing the sulfur isotope, chrome-reducible sulfide (CRS; FeS2-S) had to be extracted from the sediment samples. Both the sediment samples and a small beaker containing a 15-ml 3 % alkaline zinc (Zn) acetate solution were placed in a bottle. The cap of the bottle was tightened and the solution was flushed with nitrogen for 30 s. After flushing, 15 ml of 6 N HCl (anaerobic acid) and 15 ml of an anaerobic Cr(II) solution were added to the bottle and left to stand for 48 h. An alkaline Zn trap was retrieved to analyze ZnS and measure CRS using iodine titration of ZnS precipitation (Hsieh and Shieh 1997). The δ34S[FeS2] were analyzed using ZnS powder in the isotope laboratory at the University of Arizona by the Thermo Scientific Delta PlusXL (Supporting material (b)).
Calculation of Translocation Factor and Bioconcentration Factor
The translocation factor (TF) is defined as the ratio of metal concentration in the shoots to those in the roots (Cui et al. 2007; Li et al. 2007; Malik et al. 2010)
where Asshoots and Asroots are the As concentrations (mg/kg) accumulated in the shoots and roots, respectively. TF > 1 indicates that the plant translocates metals effectively from the roots to the shoots (Baker and Brooks 1989).
The bioconcentration factor (BCF) is defined as the ratio of metal concentrations in the roots to those in the soil or water, and is determined using Eq. (2) (Abdul and Thomas 2009)
where Asplants and Asenvironment are As concentrations (mg/kg) in the plants and in the environment (soil or water), respectively, BCF > 1 indicates that the plant is a metal accumulator.
Results and Discussion
Aqueous and Solid Phases As in Surface Water and Soil
Table 1 listed 18 analyzed chemical compound concentrations in the surface water samples (S1-S20). The average of DO and Eh in the surface water were 4.86 mg/L and +79.25 mV, respectively, indicating that the oxidative condition in surface water is a major redox state. The significant effect of tidal seawater and infiltration of rainfalls in wetland resulted in high EC, DO, and Eh values in surface water. The range of As, Fe, and Mn concentrations in the surface water were from 0.0039 to 0.0011 (mean: 0.0024 mg/L), 0.06 to 0.42 (mean: 0.10 mg/L), and 0.09 to 0.36 mg/L (mean: 0.18 mg/L), respectively. Notably, As concentrations in the surface water were lower than that outlined in the drinking water standard (0.01 mg/L) of the Environmental Protection Administration (EPA) of Taiwan. According to the classifications of the Piper diagram (Fig. 2) in the Guandu Wetland, Type I represented the carbonate/temporary hardness, Type II represented the alkali carbonate, Type III represented the non-carbonate/permanent hardness, and Type IV represented saline. The Piper diagram indicated that the principal water type in the Guandu Wetland was Type IV (N = 20). The result showed that the chemical compositions of surface water in the Guandu Wetland were mainly controlled by mixing of seawater. Hence the occurrences of low heavy metal concentration in surface water were strongly affected by dilution of tidal seawater. The range of As, Fe, and Mn concentration in the surface soil was from 10.1 to 19.1 (mean: 16.03 mg/kg), 29000 to 46900 (mean: 40725 mg/kg), and 259 to 625 mg/kg (mean: 409 mg/kg). The As concentration in surface soil was higher than general soil content 5–10 mg/kg (mean: 7.2 mg/kg) (Table 2) (Boyle and Jonasson 1973). The As concentration in the surface soil was also lower than the 60 mg/kg soil contamination standard of the Environmental Protection Administration (EPA) of Taiwan. A contents in surface water and topsoil are below the permissible environmental contamination guidelines and possess no environmental risks. However As concentrations in surface water of this study are lower than those in other As contaminated wetland system suggesting that As may be absorbed on the surface of the Fe/Mn (hydr)oxide in oxidative condition of nature wetland system (Zheng et al. 2004; Gonzalez et al. 2006).
Profiles of Aqueous and Solid Phases in Porewater and Sediment
Figure 3 showed the spatial variation of pH, Eh, and EC which were measured in porewaters. The Eh of porewaters decreased from the surface to a deep depth, whereas pH and EC increased. Infiltration of rainfalls and surface water flows diluted the EC values of pore water in the shallow layer, but had a mild effect in deep layer. Permeated oxidative species (e.g., DO, NO3 --N, and SO4 2-) were also increased in the shallow layer (Table 1). The low concentration of aqueous As in the shallow layer may be caused by the adsorption of aqueous As on the amorphous Fe (hydr)oxides (Fig. 4) (McArthur et al. 2001; Nickson et al. 2000), leading to the high solid As concentrations in depth of 5 cm (S2, 23.8 mg/kg) and 15 cm (S5, 21.6 mg/kg) (Fig. 5).
Because aqueous As(V) concentrations were very low or close to zero, we herein only show the As (III) concentration profile. High aqueous As(III) concentrations were found in 45–55 cm deep (up to 125.35 μg/L) at S2 and up to 26.39 μg/L in 70 cm deep at S5. Aqueous Fe(II) concentrations in porewater of S2 and S5 ranged from 0.01 to 22.15 mg/L and 0 to 6.27 mg/L, respectively (Fig. 4). The vertical redox gradient of S2 and S5 were moving from the oxidizing to the reducing condition along with depth. Redox-related processes in wetlands were largely controlled by the reduction of FeOOH in the presence of organic matter (OM) during bacteria respiration, serving as electron donors (Bauer et al. 2008; Hossain et al. 2012).
High aqueous As concentrations occurred in the transition zone of the shallow and deep layer and was accompanied with the low concentrations of solid-phase As; this may be caused by the reductive dissolution of Fe oxides (Figs. 4 and 5).
Under the reducing conditions in the deep layer, aqueous As concentrations might be constrained by precipitated sulfide minerals in the sediment (Fig. 6), which are the product derived from sulfate reduction. The sulfur isotopes fractionation factors (ε = δ34S[FeS2]-δ34S[SO4]) can be used to evaluate the sulfur cycling and microbial processes. Sulfate reducing bacteria (SRB) preferred the lighter sulfur isotopes of sulfate (Kaplan and Rittenberg 1964), and thus, increased in δ34S[SO4] with depth represents sulfate reduction in deep layer and easy adsorption of As on the pyrite surface. Figure 6 shows that the fractionation factor decreased with depth, but pyrite and δ34S increased with depth. Hence in the deep layer, aqueous As can be constrained by the formation of FeS2 in sediment during bacterial sulfate reduction that is governed by the relative enrichment of the δ34S[SO4] and S isotope fractionation factor (ε) values, in accordance with elevated As and FeS2 concentrations in sediments (Figs. 5 and 6). In contrast, aqueous As was liberated due to oxidation where solid FeS2 was dissolved, resulting the in the low solid FeS2 concentrations, low sulfur fractionation factor, and positive Eh values in the shallow oxidizing-layer (Van Stempvoort and Krouse 1994; Lipfert et al. 2007). The sulfide oxidation processes may liberate As but low aqueous As concentrations were found in shallow oxidizing-layer (Table 1), suggesting other geochemical processes or As transfer pathways such as uptake by plants may be involved.
Arsenic Uptake in Kandelia Obovata
The range of As concentrations in the plant (Kandelia obovata) was 20.21–28.33 mg/kg (mean: 23.69 mg/kg), and the results indicated that Kandelia obovata had a higher As concentration than did the surrounding water (mean: 0.0018 mg/L) and soil (mean: 17.24 mg/kg) (Table 3). Kandelia obovata thrives in anaerobic wetlands because its aeration roots transfer oxygen to its roots (Kadlec and Knight 1996), thereby forming iron oxide (iron plaque) around the roots which can adsorb As on the surface. The formed iron plaque on the roots of Kandelia obovata can prevent the uptake of As and preserve the root (Kadlec and Knight 1996; Meharg 2004; Fitz and Wenzel 2002; Liu et al. 2004a). The mean As concentration in Kandelia obovata decreased from the roots (19.74 mg/kg) to the stems (1.76 mg/kg), leaves (1.71 mg/kg), and seedlings (0.48 mg/kg) (Table 3), suggesting that As accumulated mostly in the roots. These results were also consistent with those of rice and fern roots, on which iron plaque was also present (Williams et al. 2007; Casado et al. 2007; Zandsalimi et al. 2011). Therefore, Kandelia obovata may still continue to survive in polluted areas, because its tolerance to and adsorption of As are more effective than those of other plants.
In this study, TFshoots/roots = 0.199 indicated that Kandelia obovata accumulated As in the roots but did not transfer to the shoots (Table 4). TFstems/roots = 0.088, TFleaves/roots = 0.088, and TFseedlings/roots = 0.024 indicated that the As concentrations in the roots transferring to the stems were similar to those of the leaves but are larger than those of the seedlings (Table 3). As a result, all translocation factors of Kandelia obovata less than 1 indicated that the transformation of As in various plant tissues was extremely low, thereby facilitating adaptation when growing in an As-contaminated wetland ecosystem.
Notably, some previous studies have indicated that the translocation factors of trace metals (such as Al, Cu, Ni, Fe, Pb, and V) in plants (e.g., Malva parviflora, Suaeda aegyptiaca, Chrozophora tinctoria, Fagonia bruguieri, Gynandriris sisyrinchium, and Ducrosia anethifolia) were greater than 1; these plants were also the trace metal accumulators (Abdul and Thomas 2009). In addition, the translocation factors of the Cretan brake fern and Chinese brake fern were 1.00–2.61 and 0.17–3.98, respectively (Wei and Chen 2006).
Arsenic Bioconcentration Factors in the Plant
In this study, the average BCFplant/soil of Kandelia obovata was 1.49; the results show that accumulation and uptake of heavy metals by Kandelia obovata was greater than that of other plants. Because BCFplants/soil > 1, Kandelia obovata can be considered an As accumulator (Ma et al. 2001). Cao and Ma (2004) applied the BCFplants/soil to evaluate the As bioaccumulation in carrots and lettuce, grown on chromate copper arsenate (CCA)-contaminated soil. The BCFplants/soil is 0.10–1.61.The range of BCFplants/soil in As-polluted mining soils is 0.0001–0.019 according to different tolerant plants (Casado et al. 2007). The BCFplants/soil of Kandelia candel on Cu, Zn, Pb, Cd, and Ni is 2.79, 2.77, 3.03, 4.20, and 4.97, respectively (Chiu and Chou 1991).
By contrast, BCFplants/water can reflect the accumulation of As in plants more accurately because only a small portion of the total soil As can easily be uptaken by plant roots. A small fraction (0 %–2 %) of the exchangeable solid phase As appeared to have been adsorbed in the wetland and were extracted by NaNO3 (Chen 2010), indicating that the uptake of chemical compounds in plants from sediments was difficult. The average BCFplant/water of Kandelia obovata was 13657.92, and it was greater than BCFplant/soil in this study (Table 5). The result shows that the uptake of As in Kandelia obovata was significant in water because of the low As concentration in the water, which might be removed either by tidal effects or plant uptake. Moreover, approximately 60 %–90 % of the extracted As contents were incorporated in the amorphous and crystalline metal oxides, and the sulfate reduction simultaneously reduced the As mobility (Chen 2012). Only 0.2 % proportions of exchangeable phase were found in shallow layer (Chen 2012), suggesting that the plants uptake of As from soil was mainly from amorphous metal oxides. Under oxidation condition of shallow layer, the aqueous As concentrations with high proportions of sulfides phase were higher than those of in low proportions of sulfides phase. The oxidation of As-bearing sulfide hence may thus mobilize As in the shallow layer. Hence, the uptake mechanism of Kandelia obovata might depend on the oxidation of As-contained FeS2 in the aerial roots and/or adsorption of As in root surface. Notably, the iron plaque forming in the root surface had strong affinity for adsorbing As. (Chen et al. 1980; Liu et al. 2004b). It will be interesting to analyze the roots with and without plaques in future studies.
Conclusions
The study discriminates the distribution and mobility of As among aqueous, solid, and plant phases and assesses bioaccumulation and translocation capacity of As in plant in the Guandu Wetland, Taiwan. The vertical redox profile of core samples S2 and S5 showed two distinct oxidizing and reducing zones. As adsorbed on the surface of Fe oxides and Fe sulfide in the shallow oxidizing layer, and in the deep reducing layer, respectively. High aqueous As occurred in the transition zone of the shallow and deep layer, which may be resulted in the reductive dissolution of Fe oxides. According to results of S isotopic fractionation, As can be constrained by the formation of FeS2 during bacterial sulfate reduction in deep layer. In contrast, aqueous As was liberated due to FeS2 oxidation in the shallow layer. Arsenic is mostly accumulated in Kandelia obovata roots, and the accumulation and translocation capacity of As are higher than those of in other plants. Kandelia obovata is an As accumulator. The uptake mechanism of the plant might depend on the oxidation of As-contained FeS2 in the aerial roots and/or adsorption of As in root surface. The transfer pathway of As in mangroves among aqueous, solid, and plant phases provides information on As uptake by Kandelia obovata in the Guandu Wetland. Furthermore, the results provide the ecological basis for future research in the development of biomonitoring tools and management plan for tidal mangrove plain.
References
Abdul HBO, Thomas BV (2009) Translocation and bioaccumulation of trace metals in desert plants of Kuwait Governorates. Research Journal of Environmental Sciences 3:581–587
Abedin MJ, Cotter-Howells J, Meharg AA (2002) Arsenic uptake and accumulation in rice (Oriza sativa L.) irrigated with contaminated water. Plant and Soil 240:311–319
APHA (1998) Standard methods for the examination of water and water waste, 20th edn. American Public Health Association, Washington, DC
Baker AJM, Brooks RR (1989) Terrestrial higher plants which hyper accumulate metallic elements-a review of their distribution, ecology and phytochemistry. Biorecovery 1:81–126
Bauer M, Fulda B, Blodau C (2008) Groundwater derived arsenic in high carbonate wetland soils: sources, sinks, and mobility. Science of the Total Environment 401:109–120
Boyle RW, Jonasson IR (1973) The geochemistry of arsenic and its use as an indicator element in geochemical prospecting. Journal of Geochemical Exploration 2:251–296
Canfield DE, Thamdrup B (1996) Fate of elemental sulfur in an intertidal sediment. FEMS Microbiology Ecology 19:95–103
Cao X, Ma LQ (2004) Effects of compost and phosphate on plant arsenic accumulation from soils near pressure treated wood. Environmental Pollution 132:435–442
Casado M, Anawar HM, Garcia-Sanchez A (2007) Arsenic bioavailability in polluted mining soils and uptake by tolerant plants (El Cabaco mine, Spain). Bulletin of Environmental Contamination and Toxicology 79:29–35
Chen CC, Dixon JB, Turner FT (1980) Iron coatings on rice roots: morphology and models of development. Soil Science Society of America Journal 44:1113–1119
Chen PT, Hsiao JC, Chao YT (2007) The assessment of characteristics for arsenic contamination problems in the Datun geothermal spring water. Geosciences Assembly (TGA), H1–4A-09(May 2007) (in Chinese)
Chen YC (2010) Distribution and geochemical cycling of arsenic in Guandu wetland. Master Thesis. Taiwan: Graduate Institute of Applied Geology. National Central University, Taiwan. (in Chinese)
Chen YY (2012) Distribution and accumulation of Arsenic among solid, aqueous and plant phases in the Guandu wetland of Taiwan. Master Thesis. Bioenvironmental system engineering college of bioresources and agriculture. National Taiwan University, Taiwan. (in Chinese)
Chiang KY, Lin KC, Lin SC, Chang TK, Wang MK (2010) Arsenic and lead (beudantite) contamination of agricultural rice soils in the Guandu Plain of northern Taiwan. Journal of Hazardous Materials 181:1066–1071
Chiu CY, Chou CH (1991) The distribution and influence of heavy metals in mangrove forests of the Tamshui estuary in Taiwan. Soil Science & Plant Nutrition 37:659–669
Chou CH, Chang FJ, Huang YH (1987) Review of the mangrove ecosystems. Society of Wildlife and Nature:23–58 (in Chinese)
Cui S, Zhou Q, Chao L (2007) Potential hyperaccumulation of Pb, Zn, Cu and Cd in endurant plants distributed in an old smeltery, northeast China. Environmental Geology 51:1043–1048
Dellwig O, Böttcher ME, Lipinski M, Brumsack HJ (2002) Trace metals in Holocene coastal peats and their relation to pyrite formation (NW Germany). Chemical Geology 182:423–442
Fitz WJ, Wenzel WW (2002) Arsenic transformations in the soil- rhizosphere- plant system: fundamentals and potential application to phytoremediation. Journal of Biotechnology 99:259–278
Gonzalez ZI, Krachler M, Cheburkin AK, Shotyk W (2006) Spatial distribution of natural enrichments of arsenic, selenium, and uranium in a minerotrophic peatland, Gola di Lago, Canton Ticino, Switzerland. Environmental Science and Technology 40:6568–6574
Hardiman R, Jacoby B (1984) Absorption and translocation of Cd in bush beans (Phaseolus vulgaris). Physiologia Plantarum 61:670–674
Hossain M, Williams PN, Mestrot A, Norton GJ, Deacon CM, Meharg AA (2012) Spatial heterogeneity and kinetic regulation of arsenic dynamics in mangrove sediments: The Sundarbans, Bangladesh. Environmental Science and Technology 46:8645–8652
Hsieh YP, Shieh YN (1997) Analysis of reduced inorganic sulfur by diffusion methods: improved apparatus and evaluation for sulfur isotopic studies. Chemical Geology 137:255–261
Hsueh ML, Lee HH (2000) Diversity and distribution of the mangrove forests in Taiwan. Wetlands Ecology and Management 8:233–242
Huang YK, Lin KH, Chen HW (2003) Arsenic species contents at aquaculture farm and in farmed mouthbreeder (Oreochromis mossambicus) in blackfoot disease hyperendemic areas. Food and Chemical Toxicology 41:1491–1500
Kao YH, Liu CW, Wang SW, Wang PL, Wang CH, Maji SK (2011) Biogeochemical cycling of arsenic in coastal salinized aquifers: evidence from sulfur isotope study. Science of the Total Environment 409:4818–4830
Kaplan IR, Rittenberg SC (1964) Microbial fractionation of sulphur isotopes. Journal of General Microbiology 34:195–212
Kadlec RH, Knight RL (1996) Treatment Wetlands. Lewis-CRC Press, Boca Raton
Lai YM, Lin YJ, Song SR (2010) Topography and Volcanology of the Huangtsuishan Volcano Subgroup, Northern Taiwan. Terrestrial Atmospheric and Oceanic Sciences 21:599–609
Li MS, Luo YP, Su ZY (2007) Heavy metal concentration in soils and plant accumulation in a restored manganese mineland in Guangxi, South China. Environmental Pollution 147:168–175
Lièvremont D, Bertin PN, Lett MC (2009) Arsenic in contaminated waters: biogeochemical cycle, microbial metabolism and biotreatment processes. Biochimie 91:1229–1237
Lipfert G, Sidle WC, Reeve AS, Ayuso RA, Boyce AJ (2007) High arsenic concentrations and enriched sulfur and oxygen isotopes in a fractured-bedrock ground-water system. Chemical Geology 242:385–399
Liu WC, Hsu MH, Kuo AY, Kuo JT (2001) The influence of river discharge on salinity intrusion in the Tanshui estuary, Taiwan. Journal of Coastal Research 17:544–552
Liu CM, Song SR, Chen YL, Tsao S (2011) Characteristics and origins of hot springs in the Tatun Volcano Group. Terrestrial Atmospheric and Oceanic Sciences 22:475–489
Liu WJ, Zhu YG, Smith A, Smith SE (2004a) Do phosphorus nutrition and iron plaque alter arsenate (As) uptake by rice seedlings in hydroponic culture? New Phytologist 162:481–488
Liu WJ, Zhu YG, Smith FA, Smith SE (2004b) Do iron plaque and genotypes affect arsenate uptake and translocation by rice seedlings (Oryza sativa L.) grown in solution culture? Journal of Experimental Botany 55:1707–1713
Lovley DR, Phillips EJP (1987) Rapid assay for microbially reducible ferric iron in aquatic sediments. Applied and Environmental Microbiology 53:1536–1540
Lu HL, Yan CL, Liu JC (2007) Low-molecular-weight organic acids exuded by Mangrove (Kandelia candel (L.) Druce) roots and their effect on cadmium species change in the rhizosphere. Environmental and Experimental Botany 61:159–166
Ma LQ, Komar KM, Tu C, Zhang W, Cai Y, Kenelly ED (2001) A fern that hyperaccumulates arsenic. Nature 409:579–582
McArthur JM, Ravenscroft P, Safiullah S, Thirlwall MF (2001) Arsenic in groundwater: testing pollution mechanisms for aquifers in Bangladesh. Water Resource Research 37:109–117
McCleskey RB, Nordstrom DK, Maest AS (2004) Preservation of water samples for arsenic(III/V) determinations: an evaluation of the literature and new analytical results. Applied Geochemistry 19:995–1009
Meharg AA (2004) Arsenic in rices understanding a new disaster for South-East Asia. Trends in Plant Science 9:415–417
Mitsch WJ, Gosselink JG (2000) Wetlands. Wiley, New York
Malik RN, Husain SZ, Nazir I (2010) Heavy metal contamination and accumulation in soil and wild plant species from industrial area of Islamabad, Pakistan. Pakistan Journal of Botany 42:123–127
Nickson RT, McArthur JM, Ravenscroft P, Burgess WG, Ahmed KM (2000) Mechanism of arsenic release to groundwater, Bangladesh and West Bengal. Applied Geochemistry 15:403–413
Mukherjee A, Fryar AE (2008) Deeper groundwater chemistry and geochemical modeling of the arsenic affected western Bengal basin, West Bengal, India. Applied Geochemistry 23:863–894
Smedley PL, Kinniburgh DG (2002) A review of the source, behavior and distribution of arsenic in natural waters. Applied Geochemistry 17:517–568
Song SR, Tsao S, Lo HJ (2000) Characteristics of the Tatun volcanic eruptions, north Taiwan: implications for a cauldron formation and volcanic evolution. Journal of the Geological Society of China 43:361–378
Tang T, Miller DM (1991) Growth and tissue composition of rice grown in soil treated with inorganic copper, nickel, and arsenic. Communications in Soil Science and Plant Analysis 22:2037–2045
Thamdrup B, Finster K, Hansen W, Bak F (1993) Bacterial disproportionation of elemental sulfur coupled to chemical reduction of iron and manganese. Applied Environmental Microbiology 59:101–108
Yanagisawa F, Sakai H (1983) Preparation of SO2 for sulphur isotope ratio measurements by the thermal decomposition of BaSO4-V2O5-SiO2 mixtures. Analytical Chemistry 55:985–987
van Stempvoort DR, Krouse HR (1994) Controls of δ18O in sulfate. In: Alpers CN, Blowes DW, (eds) Environmental geochemistry of sulfide oxidation, 550. ACS Symposium Series; American Chemical Society Publications. p.446-480
Wang SW, Liu CW, Lu KL, Chang YP, Chang TW (2011) Distribution of inorganic As species in groundwater samples with the presence of Fe. Water Quality, Exposure and Health 2:181–192
Wei CY, Chen TB (2006) Arsenic accumulation by two brake ferns growing on an arsenic mine and their potential in phytoremediation. Chemosphere 63:1048–1053
Williams PN, Villada A, Deacon C, Raab A, Figuerola J, Green AJ, Feldmann J, Meharg AA (2007) Greatly enhanced arsenic shoot assimilation in rice leads to elevated grain levels compared to wheat & barley. Environmental Science & Technology 41:6854–6859
Xie XY, Weiss DJ, Weng BS, Liu JC, Lu HL, Yan CL (2012) The short-term effect of cadmium on low molecular weight organic acid and amino acid exudation from mangrove (Kandelia obovata (S., L.) Yong) roots. Environmental Science and Pollution Research 19:1–12
Zandsalimi S, Karimi N, Kohandel A (2011) Arsenic in soil, vegetation and water of a contaminated region. International journal of Environmental Science and Technology 8:331–338
Zheng Y, Stute M, van Geen A, Gavrieli I, Dhar R, Simpson HJ, Schlosser P, Ahmed KM (2004) Redox control of arsenic mobilization in Bangladesh groundwater. Applied Geochemistry 19:201–214
Acknowledgments
The authors would like to thank the National Science Council of the Republic of China, Taiwan, for financially supporting this research under Contract No. NSC-98-2313-B-002-053-MY3.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(PDF 99 kb)
Rights and permissions
About this article
Cite this article
Liu, CW., Chen, YY., Kao, YH. et al. Bioaccumulation and Translocation of Arsenic in the Ecosystem of the Guandu Wetland, Taiwan. Wetlands 34, 129–140 (2014). https://doi.org/10.1007/s13157-013-0491-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13157-013-0491-0