Abstract
Nitrogen (N) addition can affect soil organic matter (SOM) decomposition by enhancing soil enzyme activity, increasing labile organic carbon (C) fractions and decreasing litter C/N ratio. This study was designed to investigate the short-term effect of N addition on litter and soil chemical and biological properties in Calamagrostis angustifolia freshwater marshes. A field experiment was conducted in which soil was treated with N addition of 0 (N0), 12 (N1), and 24 (N2) g N m−2 year−1, respectively. Above-ground biomass increased by 40 % and 32 % for N1 and N2 treatment, respectively. Added N increased litter C and N concentrations as well as β-glucosidase and invertase activities. However, litter C/N ratio decreased with N addition. N1 treatment increased microbial biomass carbon (MBC) in both topsoil and subsoil, but no changes in dissolved organic carbon (DOC) were observed. Total N concentrations in subsoil increased both in N1 and N2 treatments; NH4 +-N decreased in the N1 treatment, while NO3 −-N increased in the N2 treatment in topsoil. N2 treatment increased β-glucosidase, invertase, and urease activities in both soil layers. The differences in litter and soil characteristics caused by N addition suggest that N addition has the capacity to increase soil C transformation rates in marshland.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Nitrogen (N) is a key element controlling the species composition, diversity, dynamics, and functioning of many terrestrial, freshwater, and marine ecosystems (Vitousek et al. 1997). Anthropogenic emissions of N in the past several decades have resulted in a global increase in atmospheric deposition that threatens the structure and function of N-limited ecosystems (Galloway et al. 2002). In China, anthropogenic reactive N emissions to the atmosphere have increased dramatically due to rapid agricultural, industrial and urban development (Liu et al. 2011a). According to Galloway et al. (1996), China’s contribution to the global total N emissions will increase from about 20 % in 1990 to about 25 % in 2020.
Nitrogen addition can induce alterations to a wide range of litter and soil characteristics (Smaill et al. 2008). Greater N availability leads to changes in nutrient uptake and photosynthetic efficiency by plants, ultimately controlling the quantity and biochemistry of litter inputs to the soil and altering substrates that can be leached or microbially decomposed (Smemo et al. 2006). The decomposition rate of litter is controlled by litter quality. Many studies have shown that litter with higher N content, microbial enzyme activity and lower C/N ratios decomposes faster and that increased rates of litter decomposition would speed up the rates of nutrient cycling and enhance soil fertility (Saiya-Cork et al. 2002; Kim et al. 2004; Zhang et al. 2012). The effects of N addition on litter decomposition differ depending on the stage of decomposition. Nitrogen addition stimulates the initial litter decomposition; however, chronic N addition generally has either no effect or negative effect on decomposition rates (Magill and Aber 1998; Fang et al. 2007).
Nitrogen addition can significantly impact soil carbon (C) and N dynamics (Salinas-Garcia et al. 1997). Fontaine et al. (2007) and Huang et al. (2011) have shown that the effect of N addition on soil organic matter (SOM) decomposition depends on the chemical composition of SOM and the availability of labile C substrates. Changes in soil organic C may be revealed by variations in soil labile organic carbon (LOC) fractions in short-term experiments (Chen et al. 2012). Thus, it is important to investigate how N addition influences soil LOC fractions. Elevated N addition can also alter the N cycle of terrestrial ecosystems, especially soil N transformations. Studies have shown marked effects of both short- and long-term N addition, which resulted in increase of net N mineralization and nitrification rates, and also shown evidence of increased rates of leaching of NO3 − ions and soil acidification (Carroll et al. 2003; Vestgarden et al. 2003; Chen and Hogberg 2006).
Microbial enzymes play important roles in the biochemical functioning of soils, including SOM formation and degradation, nutrient cycling, and decomposition of litter (Acosta-Martínez et al. 2007; Tripathi et al. 2007; Guo et al. 2011). Nitrogen addition is able to directly affect microbial production of soil enzymes, and may have indirect effects on the activities of soil enzymes via changes in soil properties (Iyyemperumal and Shi 2008). Previous studies found that N addition altered the activities of soil β-glucosidase, invertase, and urease which are involved in C and N cycling in grassland and forest ecosystems (Ajwa et al. 1999; Saiya-Cork et al. 2002; Iyyemperumal and Shi 2008; Dalmonech et al. 2010). Furthermore, such changes in soil enzyme activities have been found to be correlated with the degradation of SOM and plant litters in response to N addition.
The content of inorganic N in wetland soil is typically low, and N is commonly the most limited nutrient which directly affects the productivity of wetland ecosystem (Mitsch and Gosselink 2000). In recent decades, reclamation of natural wetlands has been one of the major land use changes in Northeast China. Sanjiang Plain, the largest freshwater marshland in China, has experienced intensive and extensive cultivation over the past 50 years (Zhao 1999). More and more marshes have been drained for conversion into agricultural lands, and the natural marshes have received N input from the adjacent agricultural lands due to the fertilization. Nitrogen addition might affect the ecosystem C balance, and alter the C storage as well as C cycling rate in wetland soils through biological and chemical changes (Min et al. 2011). Studies have shown that exogenous N significantly increased the CH4 emission rate and N2O fluxes (Zhang et al. 2007a, b) and increased the litter decomposition rate (Song et al. 2011) in the freshwater marshes in the Sanjiang Plain, and thus may cause substantial losses of soil C in these wetlands. However, the mechanism of the impact of N on the stability and turnover of soil C is not well understood. More studies are needed to better understand the effect of N addition on microbial enzyme activity and thus nutrient dynamics in wetland ecosystem.
The primary objective of this study was to assess the short-term responses of aboveground biomass and enzyme activities of C. angustifolia litter in the freshwater marsh in the Sanjiang Plain of Northeast China. For a better understanding of the effect of N addition on marshland C cycling in this ecosystem, we also examined the soil chemical and microbial properties and their complex interactions with N addition. The changes of microbial enzyme (i.e., β-glucosidase, invertase, and urease) activity both in soils and litter following one growing season of N addition were investigated. Soil microbial biomass carbon (MBC), dissolved organic carbon (DOC) as well as soil N (i.e., NH4 +-N, NO3 −-N, and total N) were also examined. The hypothesis was that N addition to N limited marshland ecosystems would promote marshland vegetation growth and change the litter C/N ratio due to the elevated soil N availability. We also expected stimulation of soil microbial activities (enzyme activities and MBC) under N addition.
Materials and Methods
Site Description
The freshwater marsh is located in the Sanjiang Marsh Wetland Experimental Station, Chinese Academy of Sciences (47°35’N, 133°31’E), in Sanjiang Plain, Northeast China. The study area features an annual average temperature of 2.5 °C with a growing season of 125 days. The annual precipitation is 550–600 mm, more than 60 % of which concentrates in the period from June to August. The typical type of seasonal waterlogged C. angustifolia (90 % area coverage; few other plants included) marsh was selected as the field experiment. The soil in the study site is primarily meadow marsh soil.
Experimental Design
The experimental setup was designed with two levels of N fertilization and one control (i.e. no N addition). Each experimental treatment had triplicate plots, giving a total of 9 experimental plots. Board walks giving access to the whole experimental area were installed to minimize further impacts to the plots. Plastic frames (PVC 1.5 × 1.5 m and 0.8 m deep) were installed to prevent horizontal movement and lateral loss of the added N, and each plot was separated by a 1-m buffer zone. In order to imitate the exogenous N addition found in nature, ammonium nitrate (NH4NO3) was applied every month (from May to September) during the whole growing season of 2011, with a rate of 0 (N0), 12 (N1), and 24 g N m−2 year−1 (N2), respectively. At the same time, the unfertilized plots were watered with the same amounts (1 L) of surface marsh water. The freshwater marsh may receive some inputs of N leached from agricultural activities (approximately 5.8 g N m−2 year−1; Zhang et al. 2007a) and from atmospheric deposition (0.8 g N m−2 year−1; Sun and Liu 2007), but the N addition levels used in this study were greater than the current total exogenous N input to this ecosystem.
Sample Collection and Analysis
Soil samples were collected from 0–15 cm and 15–30 cm layer by taking four soil cores from each plot on 3 October 2011 (about 30 days after the last N addition in September). After removal of plant and organic debris, soil samples were sieved through a 2-mm sieve, mixed thoroughly, and then divided into two subsamples. One subsample was stored at 4 °C until determination of MBC, DOC, NH4 +-N and NO3 −-N contents, and the second subsample was freeze-dried and used to determine activities of soil enzymes (β-glucosidase, invertase, and urease). Freeze drying of samples is a recommended procedure prior to the biochemical analysis of flooded soil (Liu et al. 2009) and the freeze-drying procedure maintains stable enzyme activity (Sundari et al. 2000). All the biological and biochemical analyses were conducted within 2 weeks of soil collection.
Soil MBC was measured using the fumigation–extraction method (Wu et al. 1990). Fumigated and non-fumigated soils were extracted with 0.5 mol L−1 K2SO4 solution by shaking for 30 min. Organic C in the extracts was analyzed using high-temperature combustion method (Multi N/C 2100 TOC analyzer, Analytik Jena, Germany). The MBC was calculated using the following equation: MBC = E C /0.45, where E C was the difference in organic C between fumigated and non-fumigated samples.
Soil DOC was assayed following the procedures presented by Ghani et al. (2003). Soil samples were extracted with 30 mL of distilled water by shaking for 30 min. Next, the samples were centrifuged for 20 min at 3500 rpm. All supernatants were filtered through a 0.45 μm filter into separate vials for C analysis. Total dissolved C and inorganic C in the water were measured using a Multi N/C 2100 analyzer (Analytik Jena, Germany). Soil DOC was calculated by determining the difference between total dissolved C and dissolved inorganic C.
Soil mineral N (NH4 +-N + NO3 −-N) was extracted with 2 mol L−1 KCl solution. After extraction, NH4 +-N was analyzed using the indophenol blue spectrophotometric method, and NO3 −-N was analyzed by UV spectrophotometry at 220 nm and 275 nm (Lu 2000). The measurement at two wavelengths allows correction for the interference due to dissolved organic matter, by calculating the difference between both absorbance readings. Soil pH was determined in a soil: water slurry (1:2.5, w/w; PHS-3C, Rex Shanghai, China).
Soil invertase and urease activities were assayed following the methods of Guan (1986). Urease activity assay was based on the determination of the NH4 +-N released when 1 g soil was incubated with 10 mL of 10 % aqueous urea, and 20 mL of citric acid buffer (pH 6.7) at 37 °C for 24 h. The NH4 +-N released was determined by the indophenol blue method. Results were expressed as mg NH4 +-N g−1 24 h−1. Invertase activity was determined by incubating 1 g soil with 15 mL of 8 % sucrose solution and 5 mL of phosphate buffer (pH 5.5) at 37 °C for 24 h, and measuring reducing sugars as glucose by the colorimetry at 578 nm with 3,5-dinitrosalicylic acid. The results were expressed as mg glucose g−1 24 h−1. Soil β-glucosidase activity was assayed by the method of Tabatabai (1994). One gram soil was weighed into a 50 mL flask, mixed with 0.25 mL of toluene, 4 mL of modified universal buffer (MUB) (pH 6.0), and 1 mL of 0.5 mol L−l p-nitrophenyl-β-D-glucoside (pNG) solution, and incubated for 1 h at 37 °C. The reaction was terminated by adding 1 mL of 0.5 mol L−l CaC12 and 4 mL of 0.1 mol L−1 pH 12 tris(hydroxymethyl)aminomethane (THAM) buffer. The soil suspension was allowed to develop a yellow color, and the color intensity was determined using a spectrophotometer at 410 nm. Soil β-glucosidase activity was expressed as μg pNG g−1 h−1. Each analysis was done in triplicate. All the enzyme activities were expressed on a per-gram dry soil basis.
The above-ground biomass in the sampling site was sampled by using a 0.25 m2 quadrat at the end of the growing season. Plant material was clipped above the soil surface and divided into two subsamples. One subsample was oven-dried at 80 °C until constant weight. Above-ground biomass was calculated using the following equation: above-ground biomass = B/0.25, where B is the biomass obtained from the 50 cm × 50 cm area, the unit of 0.25 is m2. The second subsample was freeze-dried and used for determination of enzyme activities. β-glucosidase, invertase, and urease activities in C. angustifolia litter were determined according to the above methods described for soil samples. The total C amount was determined by the dry combustion method using a Multi N/C 2100 analyzer (Analytik Jena, Germany). The total N amount was measured by the Kjeldahl method using the Kjeltec Auto Analyzer (Behr Labor Technik, Germany).
Statistical Analyses
Statistical analyses were conducted with SPSS 11.5 package. Means (n = 3) and standard errors (SE) were calculated. One-way analysis of variance (ANOVA) was used to determine the differences between different N treatments. All data were normally distributed and met the assumptions of the ANOVA (data not shown). Significance for all statistical analysis was accepted at the α = 0.05 level.
Results
Above-ground Biomass, C, N Concentrations, and Enzyme Activities of C. angustifolia Litter
Added N affected the growth of C. angustifolia, which was reflected in the changes of biomass. The average increase for the two N treatments was 40 % (N1) and 32 % (N2), respectively, in comparison to the control (Table 1). The biomass was not significantly different between the N1 and N2 treatments (P < 0.05). Litter C and N concentrations both increased in response to N addition. The higher content of N in the litter for the N addition treatment led to significant decrease in C/N ratios, from 152.9 (N0) to 102.3 (N1) and 73.7 (N2). The N addition resulted in a significant increase of litter β-glucosidase (Fig. 1a) and invertase (Fig. 1b) activities in comparison to the control. The activities of β-glucosidase in the litter of N addition treatments were all markedly higher than that of control treatment, but there were no significant differences between N1 and N2 treatments (P > 0.05). Litter invertase activities increased with increasing N addition level, and reached the maximum value for N2 treatment (1390 mg glucose g−1 24 h−1), 2.42 times of control treatment (575 mg glucose g−1 24 h−1). However, there was no effect of N addition on litter urease activities (Fig. 1c).
Effects of N addition on a β-glucosidase, b invertase, and c urease activities of C. angustifolia litter after one growing season of N addition. N0, N1, N2 represent applications of 0, 12, and 24 g N m−2 year−1, respectively, applied over the entire growing season. The different lowercase letters on the bars indicate significant differences at P < 0.05 level between the different treatments
Soil MBC and DOC Concentrations
The MBC concentrations ranged from 861.8 to 1977.8 μg g−1 in the topsoil (0–15 cm) and from 330.3 to 895.6 μg g−1 in the subsoil (15–30 cm) (Fig. 2a). The N1 addition resulted in an increase in soil MBC, 2.3 and 2.7 times of control in the topsoil and subsoil, respectively. However, no difference was observed in MBC between N2 addition plot and control plot. Soil DOC concentrations ranged from 131.4 to 166.4 μg g−1 in the topsoil and from 78.6 to 131.4 μg g−1 in the subsoil. One growing season of N addition did not significantly change DOC concentrations compared with the control treatment in both soil layers (Fig. 2b).
Changes in a soil microbial biomass C (MBC) and b dissolved organic C (DOC) in the 0–15 cm and 15–30 cm layers after one growing season of N addition. N0, N1, N2 represent applications of 0, 12, and 24 g N m−2 year−1, respectively, applied over the entire growing season. The different capital letters and lowercase letters on the bars indicate significant differences at P < 0.05 level between the different treatments in topsoil and subsoil, respectively
Soil NH4 +-N, NO3 −-N, and Total N Concentrations
After one growing season of N addition, NH4 +-N concentrations in the topsoil decreased for N1 treatment but did not significantly change for N2 treatment. The N2 addition significantly increased NH4 +-N concentrations in the subsoil, 2.4 times of the control treatment (Fig. 3a). NO3 −-N concentrations increased with increasing N addition level in the topsoil, but did not alter in the subsoil (Fig. 3b). Topsoil total N did not change after N addition; however, significant increase of total N in subsoil for N addition was observed (Fig. 3c).
Changes in soil a NH4 +-N, b NO3 −-N, and c TN concentrations in the 0–15 cm and 15–30 cm layers after one growing season of N addition. N0, N1, N2 represent applications of 0, 12, and 24 g N m−2 year−1, respectively, applied over the entire growing season. The different capital letters and lowercase letters on the bars indicate significant differences at P < 0.05 level between the different treatments in topsoil and subsoil, respectively
Soil Enzymatic Activities
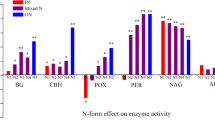
Our results showed that responses of soil enzyme activities to N addition varied with different N addition levels and soil depth (Fig. 4). Compared with the control, soil β-glucosidase, invertase, and urease activities significantly increased for N2 treatment in the topsoil (Fig. 4a, b, c). However in the subsoil, there were no significant changes in β-glucosidase activities for N addition treatment; invertase activities reached the highest value for N1 treatment (16.3 mg glucose g−1 24 h−1) but decreased significantly as N addition level increased (Fig. 4b); urease activities increased with increasing N addition level and reached the maximum value at N2 treatment (0.7 mg NH4 +-N g−1 24 h−1), 3.5 times of control treatment (0.2 mg NH4 +-N g−1 24 h−1) (Fig. 4c).
Changes in soil a β-glucosidase, b invertase, and c urease activities in the 0–15 cm and 15–30 cm layers after one growing season of N addition. N0, N1, N2 represent applications of 0, 12, and 24 g N m−2 year−1, respectively, applied over the entire growing season. The different capital letters and lowercase letters on the bars indicate significant differences at P < 0.05 level between the different treatments in topsoil and subsoil, respectively
Soil pH
The soil pH ranged from 5.57 to 5.68 in the topsoil and ranged from 5.71 to 6.02 in the subsoil. Nitrogen addition had a significant effect on soil pH in the subsoil, following the decreasing order of: N0 (6.02) > N1 (5.85) > N2 (5.71). However, it only reached the significant level for N2 treatment (5.57) compared with control treatment (5.68) in the topsoil.
Discussion
In the C. angustifolia freshwater marsh, N addition over one growing season led to increased vegetation productivity, as reflected by significant increases in above-ground biomass. This was consistent with our initial hypothesis and other studies in which the amount of standing dead material and litter was higher in N addition treatments than in the control plots (Berendse et al. 2001; Zhang et al. 2007a). Increase of the aboveground biomass indicated that the added N had been readily available for plant growth. The C. angustifolia litter in the N addition plots contained significantly higher concentrations of C and N, agreeing with the results of an earlier study (Zhang et al. 2007a). The significant increase in litter N concentration and decrease in C/N ratio were likely the result of increased N availability in the fertilized plots, allowing greater N uptake and accumulation by the vegetation biomass (Manning et al. 2008).
Nutrient mineralization from plant litter occurs via the enzymatic activities of the microbial communities that become established on the litter surfaces (Kourtev et al. 2002). In this study, we found that N addition over one growing season increased C. angustifolia litter invertase and β-glucosidase activities. Both enzymes are responsible for the breakdown of plant litter and involved in the C cycling. Invertase catalyzes the hydrolysis of sucrose (one of the most abundant soluble sugars in plants) to glucose and fructose (Frankenberger and Johanson 1983). β-glucosidase is responsible for the degradation of cellobiose (a disaccharide produced during the enzymatic hydrolysis of cellulose) to glucose (Eriksson and Wood 1985). Addition of N has also been observed to increase invertase activities in aspen litter (Chigineva et al. 2011) and β-glucosidase activities in Acer saccharum (Saiya-Cork et al. 2002), dogwood and maple litters (Carreiro et al. 2000). The stimulation of litter invertase and β-glucosidase activities may result in positive effects of N addition on litter decomposition rate in marshland systems. Similarly, Magill and Aber (1998) and Fang et al. (2007) found that high concentrations of N stimulate litter degradation rates initially; however, chronic N addition generally has either no effect or negative effect on litter decomposition. Therefore, future study is needed for the long-term effects of N addition on litter decomposition.
High litter quality and quantity would improve soil physical and chemical properties, and exert an important influence on soil biological activity and nutrient cycling (Cross and Schlesinger 1999; Li et al. 2004; Carrera et al. 2005). MBC is an important index of biological status in soil since it is both a source of labile nutrients and an agent of transformation and cycling of organic matter and plant nutrients in soil (Sicardi et al. 2004; Xue et al. 2006). The MBC concentrations were significantly higher in N1 addition plot than in the control plot in both soil layers. The results of Zhang and Zak (1998) and Wang et al. (2010) also indicated that N addition typically increased microbial biomass shortly following initial addition, and soil microbial biomass has been shown to be increased by optimum and balanced fertilization (Shen 2010). Therefore, increased MBC could be caused by the N enrichment and litter accumulation. This may be because N addition could provide N that is essential to microbial cell synthesis (Geisseler et al. 2010). Meanwhile, recently added C (via root exudation or root death) was a readily available source of substrate for microbial activity (Salome et al. 2010). In contrast to our results, Chen et al. (2012) reported that N addition over 4 years significantly decreased soil moisture and led to soil acidification, which would suppress microbial activity and decrease the soil MBC.
Dissolved organic matter comprises only a small part of soil organic matter. The concentration of DOC can decrease as a result of sorption, precipitation or mineralization by soil microorganisms (Mavi et al. 2012). Furthermore, sorption of DOC to mineral surfaces is the primary sink for DOC in mineral soil (Currie et al. 1996; Cory et al. 2004). The Harvard Forest experiment showed that after 4 years of sampling, there were small changes in the DOC concentrations (McDowell et al. 1998). In our study, N addition showed no significant impact on soil DOC concentrations except for N1 treatment in subsoil. The lowest DOC concentration was accompanied by the highest MBC and NO3 −-N concentrations in this soil. In addition, NO3 −-N could compete directly with DOC for adsorption sites, thus NO3 −-N accumulation decreases sequestration of DOC into soil organic matter (Pregitzer et al. 2004; Smemo et al. 2006).
Our results showed that soil total N concentrations in topsoil did not significantly change after one growing season of N addition, confirming the observations of Moscatelli et al. (2008) in a poplar plantation. This was because that the external N may leach into the subsoil. Pregitzer et al. (2004) found that 65–70 % of experimentally added N annually leached out in a chronic N deposition treatment. Subsoil total N concentrations significantly increased in N1 and N2 plots, which may have been caused by the retention of the added N in this soil layer. Soil NH4 +-N concentrations decreased for N1 treatment in topsoil. One explanation for this decrease is that along with the external N, greater NH4 +-N is absorbed and immobilized by plants and soil microbes or adsorbed to cation exchange surfaces in soils (Gundersen and Rasmussen 1995; Moldan et al. 1995). However, the higher concentration of added NH4 +-N in the N2 plots alone could meet the plant and microbial requirements, thus not leading to a decrease in soil NH4 +-N concentrations in these plots. Added N increased NO3 −-N concentrations in topsoil probably because the N application might have stimulated microbial nitrification (Carroll et al. 2003; Xue et al. 2006) and consequent accumulation of NO3 − -N in this soil layer. However, soil NO3 −-N concentrations in the subsoil did not significantly change after one growing season of N addition.
Soil enzyme activity was measured as an indicator of microbial activity in our study. Activities of β-glucosidase, invertase, and urease were significantly greater in the topsoil than in the subsoil. Increases in soil enzyme activities were evident for the higher level of N addition, and several studies have reported that N addition increased the activities of β-glucosidase (Manning et al. 2008; Liu et al. 2011b), invertase (Wang et al. 2008; Shen et al. 2010), and urease (Saiya-Cork et al. 2002; Xue et al. 2006; Wang et al. 2010). The increase in soil enzyme activities was potentially a microbial response to increased litter (fresh organic) C, the higher soil N availability could also have played a role (Manning et al. 2008; Salome et al. 2010). Nitrogen addition may cause a shift in soil microbial community composition and subsequently alter microbial production of enzymes (Liu et al. 2011b). Allison et al. (2008) found that adding N to the soil stimulates the synthesis of β-glucosidase by saprotrophic microbes to meet the microbial demand for C. Invertase plays a critical role in releasing low molecular weight sugars that are important as energy sources for microorganisms (Zhou et al. 2012), and it was one of the degradative enzymes found to be primarily responsible for litter decomposition in a coniferous forest fertilized with N (Wang et al. 2010). Microbial production of urease in soils may be repressed through end product inhibition or by-products formed from the microbial assimilation of excessive N (McCarty et al. 1992). However, the large quantity of protein-rich litter entering the system was probably more important than mineral N availability in determining urease production (Allison et al. 2006; Wang et al. 2008). Our study suggests that N addition may stimulate the decomposition of organic matter and reduce the stabilization of organic C in soil by increasing enzyme activities.
Conclusion
Our study shows that in C. angustifolia freshwater marshes, N addition could promote vegetation growth and increase C and N concentrations in above-ground litter. The decreased C/N ratios and increased β-glucosidase and invertase activities in litter caused by N addition generally stimulated litter decomposition. Soil MBC significantly increased because of low level of N addition. Soil β-glucosidase, invertase, and urease activities significantly increased in response to high level of N addition. As the enhanced enzyme activity in the context of N enrichment apparently accelerates decomposition of SOM, this might result in long-term reductions in the capability of these marshlands to store carbon.
References
Acosta-Martínez V, Cruz L, Sotomayor-Ramírez D, Pérez-Alegría L (2007) Enzyme activities as affected by soil properties and land use in a tropical watershed. Appl Soil Ecol 35:35–45
Ajwa HA, Dell CJ, Rice CW (1999) Changes in enzyme activities and microbial biomass of tallgrass prairie soil as related to burning and nitrogen fertilization. Soil Biol Biochem 47:769–777
Allison SD, Nielsen C, Hughes RF (2006) Elevated enzyme activities in soils under the invasive nitrogen-fixing tree Falcataria moluccana. Soil Biol Biochem 38:1537–1544
Allison SD, Czimczik CI, Treseder KK (2008) Microbial activity and soil respiration under nitrogen addition in Alaskan boreal forest. Glob Chang Biol 14:1156–1168
Berendse F, van Bremen N, Rydín H, Buttler A, Heijmans M, Hoosbeek M, Lee J, Mitchell E, Saarinen T, Vasander H, Wallén B (2001) Raised atmospheric CO2 levels and increased N deposition cause shifts in plant species composition and production in Sphagnum bogs. Glob Chang Biol 7:591–598
Carreiro MM, Sinsabaugh RL, Repert DA, Parkhurst DF (2000) Microbial enzyme shifts explain litter decay responses to simulated nitrogen deposition. Ecology 81:2359–2365
Carrera AL, Vargas DN, Campanella MV, Bertiller MB, Sain CL, Mazzarino MJ (2005) Soil nitrogen in relation to quality and decomposability of plant litter in the Patagonian Monte, Argentina. Plant Ecol 181:139–151
Carroll JA, Caporn SJM, Johnson D, Morecroft MD, Lee JA (2003) The interactions between plant growth, vegetation structure and soil processes in semi-natural acidic and calcareous grasslands receiving long-term inputs of simulated pollutant nitrogen deposition. Environ Pollut 121:363–376
Chen Y, Hogberg P (2006) Gross nitrogen mineralization rates still high 14 years after suspension of N input to a N-saturated forest. Soil Biol Biochem 38:2001–2003
Chen XM, Liu JX, Deng Q, Yan JH, Zhang DQ (2012) Effects of elevated CO2 and nitrogen addition on soil organic carbon fractions in a subtropical forest. Plant Soil 357:25–34
Chigineva NI, Aleksandrova AV, Marhan S, Kandeler E, Tiunov AV (2011) The importance of mycelial connection at the soil–litter interface for nutrient translocation, enzyme activity and litter decomposition. Appl Soil Ecol 51:35–41
Cory RM, Green SA, Pregitzer KS (2004) Dissolved organic matter concentration and composition in the forests and streams of Olympic National Park, WA. Biogeochemistry 67:269–288
Cross AF, Schlesinger WH (1999) Plant regulation of soil nutrient distribution in the Northern Chihuahuan desert. Plant Ecol 145:11–25
Currie WS, Aber JD, McDowell WH, Boone RD, Magill AH (1996) Vertical transport of dissolved organic C and N under long-term N amendments in pine and hardwood forests. Biogeochemistry 35:471–505
Dalmonech D, Lagomarsino A, Moscatelli MC, Chiti T, Valentini R (2010) Microbial performance under increasing nitrogen availability in a Mediterranean forest soil. Soil Biol Biochem 42:1596–1606
Eriksson KE, Wood TM (1985) Biodegradation of cellulose. In: Takayoshi T (ed) Biosynthesis and Biodegradation of Wood Components. Academic, Orlando, pp 469–503
Fang H, Mo JM, Peng SL, Li ZA, Wang H (2007) Cumulative effects of nitrogen additions on litter decomposition in three tropical forests in southern China. Plant Soil 297:233–242
Fontaine S, Barot S, Barrà P, Bdioui N, Mary B, Rumpel C (2007) Stability of organic carbon in deep soil layers controlled by fresh carbon supply. Nature 450:277–280
Frankenberger WT Jr, Johanson JB (1983) Factors affecting invertase activity in soils. Plant Soil 74:313–323
Galloway JN, Zhao DW, Thomson VE, Chang LH (1996) Nitrogen mobilization in the United States of America and the People’s Republic of China. Atmos Environ 30:1551–1561
Galloway JN, Cowling EB, Seitzinger SP, Socolow RH (2002) Reactive nitrogen: too much of a good thing? Ambio 31:60–63
Geisseler D, Horwath WR, Joergensen RG, Ludwig B (2010) Pathways of nitrogen utilization by soil microorganisms, A review. Soil Biol Biochem 42:2058–2067
Ghani A, Dexter M, Perrott KW (2003) Hot-water extractable carbon in soils: a sensitive measurement for determining impacts of fertilization, grazing and cultivation. Soil Biol Biochem 35:1231–1243
Guan SY (1986) Soil enzymology and research method. Agricultural Press, Beijing (in Chinese)
Gundersen P, Rasmussen L (1995) Nitrogen mobility in a nitrogen limited forest at Klosterhede, Denmark, examined by NH4NO3 addition. For Ecol Manag 71:75–88
Guo P, Wang CY, Jia Y, Wang Q, Han GM, Tian XJ (2011) Responses of soil microbial biomass and enzymatic activities to fertilizations of mixed inorganic and organic nitrogen at a subtropical forest in East China. Plant Soil 338:355–366
Huang ZQ, Clinton PW, Baisden WT, Davis MR (2011) Long-term nitrogen additions increased surface soil carbon concentration in a forest plantation despite elevated decomposition. Soil Biol Biochem 43:302–307
Iyyemperumal K, Shi W (2008) Soil enzyme activities in two forage systems following application of different rates of swine lagoon effluent or ammonium nitrate. Appl Soil Ecol 38:128–136
Kim JG, Rejmánková E (2004) Decomposition of macrophytes and dynamics of enzyme activities in subalpine marshes in Lake Tahoe basin, U.S.A. Plant Soil 266:303–313
Kourtev PS, Ehrenfeld JG, Huang WZ (2002) Enzyme activities during litter decomposition of two exotic and two native plant species in hardwood forests of New Jersey. Soil Biol Biochem 34:1207–1218
Li YQ, Xu M, Sun OJ, Cui WC (2004) Effects of root and litter exclusion on soil CO2 efflux and microbial biomass in wet tropical forests. Soil Biol Biochem 36:2111–2114
Liu YY, Yao HY, Huang CY (2009) Assessing the effect of air-drying and storage on microbial biomass and community structure in paddy soils. Plant Soil 317:213–221
Liu XJ, Duan L, Mo JM, Du EZ, Shen JL, Lu XK, Zhang Y, Zhou XB, He CN, Zhang FS (2011a) Nitrogen deposition and its ecological impact in China: an overview. Environ Pollut 159:2251–2264
Liu YY, Dell E, Yao HY, Rufty T, Shi W (2011b) Microbial and soil properties in bentgrass putting greens: impacts of nitrogen fertilization rates. Geoderma 162:215–221
Lu RK (2000) Soil agrochemistry analysis method. Chinese Agrotechnology Press, Beijing (in Chinese)
Magill AH, Aber JD (1998) Long-term effects of experimental nitrogen additions on foliar litter decay and humus formation in forest ecosystems. Plant Soil 203:301–311
Manning P, Saunders M, Bardgett RD, Bonkowski M, Bradford MA, Ellis RJ, Kandeler E, Marhan S, Tscherko D (2008) Direct and indirect effects of nitrogen deposition on litter decomposition. Soil Biol Biochem 40:688–698
Mavi MS, Marschner P, Chittleborough DJ, Cox JW, Sanderman J (2012) Salinity and sodicity affect soil respiration and dissolved organic matter dynamics differentially in soils varying in texture. Soil Biol Biochem 45:8–13
McCarty GW, Shogren DR, Bremner JB (1992) Regulation of urease production in soil by microbial assimilation of nitrogen. Biol Fertil Soils 12:261–264
McDowell WH, Currie WS, Aber JD, Yano Y (1998) Effects of chronic nitrogen amendments on production of dissolved organic carbon and nitrogen in forest soils. Water Air Soil Pollut 105:175–182
Min K, Kang H, Lee D (2011) Effects of ammonium and nitrate additions on carbon mineralization in wetland soils. Soil Biol Biochem 43:2461–2469
Mitsch WJ, Gosselink JG (2000) Wetlands [M]. Van Nostrand Reinhold Company Inc., New York, pp 89–125
Moldan F, Hultberg H, Nystrom U, Wright RF (1995) Nitrogen saturation at Gardsjon, southwest Sweden, induced by experimental addition of ammonium nitrate. For Ecol Manag 71:89–97
Moscatelli MC, Lagomarsino A, De Angelis P, Grego S (2008) Short- and medium-term contrasting effects of nitrogen fertilization on C and N cycling in a poplar plantation soil. Forest Ecol Manag 255:447–454
Pregitzer KS, Zak DR, Burton AJ, Ashby JA, MacDonald NW (2004) Chronic nitrate additions dramatically increase the export of carbon and nitrogen from northern hardwood ecosystems. Biogeochemistry 68:179–197
Saiya-Cork KR, Sinsabaugh RL, Zak DR (2002) The effects of long term nitrogen deposition on extracellular enzyme activity in an Acer saccharum forest soil. Soil Biol Biochem 34:1309–1315
Salinas-Garcia JR, Hons FM, Matocha JE, Zuberer DA (1997) Soil carbon and nitrogen dynamics as affected by long-term tillage and nitrogen fertilization. Biol Fertil Soils 25:182–188
Salome C, Nunan N, Pouteau V, Lerch TZ, Chenu C (2010) Carbon dynamics in topsoil and in subsoil may be controlled by different regulatory mechanisms. Glob Chang Biol 16:416–426
Shen WS, Lin XG, Shi WM, Min J, Gao N, Zhang HY, Yin R, He XH (2010) Higher rates of nitrogen fertilization decrease soil enzyme activities, microbial functional diversity and nitrification capacity in a Chinese polytunnel greenhouse vegetable land. Plant Soil 337:137–150
Sicardi M, Garcia-Prechac F, Frioni L (2004) Soil microbial indicators sensitive to land use conversion from pastures to commercial Eucalyptus grandis (Hill ex Maiden) plantations in Uruguay. Appl Soil Ecol 27:125–133
Smaill SJ, Clinton PW, Greenfield LG (2008) Nitrogen fertiliser effects on litter fall, FH layer and mineral soil characteristics in New Zealand Pinus radiata plantations. For Ecol Manag 256:564–569
Smemo KA, Zak DR, Pregitzer KS (2006) Chronic experimental NO3 − deposition reduces the retention of leaf litter DOC in a northern hardwood forest soil. Soil Biol Biochem 38:1340–1347
Song CC, Liu DY, Yang GS, Song YY, Mao R (2011) Effect of nitrogen addition on decomposition of Calamagrostis angustifolia litters from freshwater marshes of Northeast China. Ecol Eng 37:1578–1582
Sun ZG, Liu JS (2007) Nitrogen cycling of atmosphere-plant-soil system in the typical Calamagrostis angustifolia wetland in the Sanjiang Plain, Northeast China. J Environ Sci (China) 19:986–995
Sundari SK, Adholeya A (2000) Retention of enzyme activity following freeze-drying the mycelium of ectomycorrhizal isolates: part II. Enzymes acting upon carbon compounds. World J Microbiol Biotechnol 16:865–868
Tabatabai MA (1994) Methods of soil analysis: microbiological and biochemical properties. in: Madison (Eds.) Soil Science Society of America Book Series pp. 236–268
Tripathi S, Chakraborty A, Chakrabarti K, Bandyopadhyay BK (2007) Enzyme activities and microbial biomass in coastal soils of India. Soil Biol Biochem 39:2840–2848
Vestgarden LS, Selle LT, Stuanes AO (2003) In situ soil nitrogen mineralization in a Scots pine (Pinus sylvestris L.) stand: effects of increased nitrogen input. For Ecol Manag 176:205–216
Vitousek PM, Aber JD, Howarth RW, Likens GE, Matson PA, Schindler DW, Schlesinger WH, Tilman DG (1997) Human alteration of the global nitrogen cycle: sources and consequences. Ecol Appl 7:737–750
Wang QK, Wang SL, Liu YX (2008) Responses to N and P fertilization in a young Eucalyptus dunnii plantation: Microbial properties, enzyme activities and dissolved organic matter. Appl Soil Ecol 40:484–490
Wang CT, Long RJ, Wang QL, Liu W, Jing ZC, Zhang L (2010) Fertilization and litter effects on the functional group biomass, species diversity of plants, microbial biomass, and enzyme activity of two alpine meadow communities. Plant Soil 331:377–389
Wu J, Joergensen RG, Pommerening B, Chaussod R, Brookes PC (1990) Measurement of soil microbial biomass C by fumigation-extraction-an automated procedure. Soil Biol Biochem 22:1167–1169
Xue D, Yao HY, Huang CY (2006) Microbial biomass, N mineralization and nitrification, enzyme activities, and microbial community diversity in Tea Orchard soils. Plant Soil 288:319–331
Zhang WD, Wang SL (2012) Effects of NH4 + and NO3 − on litter and soil organic carbon decomposition in a Chinese fir plantation forest in South China. Soil Biol Biochem 47:116–122
Zhang QH, Zak JC (1998) Effects of water and nitrogen amendment on soil microbial biomass and fine root production in a semi-arid environment in west Texas. Soil Biol Biochem 30:39–45
Zhang LH, Song CC, Wang DX, Wang YY (2007a) Effects of exogenous nitrogen on freshwater marsh plant growth and N2O fluxes in Sanjiang Plain, Northeast China. Atmos Environ 41:1080–1090
Zhang LH, Song CC, Wang DX, Wang YY, Xu XF (2007b) The variation of methane emission from freshwater marshes and response to the exogenous N in Sanjiang Plain Northeast China. Atmos Environ 41:4063–4072
Zhao KY (1999) Mires in China. Science Press, Beijing (in Chinese)
Zhou XB, Zhang YM, Downing A (2012) Non-linear response of microbial activity across a gradient of nitrogen addition to a soil from the Gurbantunggut Desert, northwestern China. Soil Biol Biochem 47:67–77
Acknowledgments
We would like to thank the reviewers for their helpful and constructive review of this paper. This work was funded by the National Natural Science Foundation of China (No. 41125001, 41103038, 40930527), the Key Project of Chinese Academy of Sciences (KZCX2-YW-JC301), and the “Strategic Priority Research Program-Climate Change: Carbon Budget and Related Issues” of the Chinese Academy of Sciences (XDA05050508). We thank Dr Aixin Hou for her helpful remarks on an earlier version of this manuscript.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Song, Y., Song, C., Li, Y. et al. Short-term Effect of Nitrogen Addition on Litter and Soil Properties in Calamagrostis angustifolia Freshwater Marshes of Northeast China. Wetlands 33, 505–513 (2013). https://doi.org/10.1007/s13157-013-0407-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13157-013-0407-z