Abstract
Aims
The aim of this study was to investigate the effects of elevated CO2 concentration and nitrogen addition on soil organic carbon fractions in subtropical forests where the ambient N deposition was high.
Methods
Seedlings of typical subtropical forest ecosystems were transplanted in ten open-top chambers and grown under CO2 and nitrogen treatments. The treatments included: 1) elevated CO2 (700 μmol mol-1); 2) N addition of 100 kg NH4NO3 ha-1 yr-1; 3) combined elevated CO2 and N addition; and 4) control. We measured soil total organic carbon (TOC), particulate organic carbon (POC), readily oxidizable organic carbon (ROC), and microbial biomass carbon (MBC).
Results
Results showed that elevated CO2 alone did not significantly affect soil TOC, POC and ROC after 4 years of treatment, but increased soil MBC and soil respiration compared to the control. N addition alone had no significant effect neither on soil TOC, POC and ROC, but decreased MBC and soil respiration over time. However, the elevated CO2 and N addition together significantly increased soil POC and ROC, and had no significant effect on soil MBC.
Conclusions
This study indicated that even in N-rich subtropical forest ecosystems, inputs of N are still needed in order to sustain soil C accumulation under elevated CO2.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Global changes such as elevated atmospheric CO2 concentrations and nitrogen (N) deposition will have dramatic impacts on ecosystem productivity and function in the coming decades (Dijkstra et al. 2005; Bradford et al. 2008). Several studies have shown that the changes in elevated CO2 concentrations and N addition may alter soil microbial activity (Glaser et al. 2006; Carney et al. 2007), detrital production (Reich et al. 2006b), labile C input (Bradford et al. 2008), and soil respiration (Deng et al. 2010). But most previous studies of the interactive effects of elevated CO2 and N deposition on soil carbon (C) dynamics have been restricted to the temperate forest and grassland ecosystems where N is limited (Treseder and Allen 2000; Hagedorn et al. 2001; Shaw et al. 2002; Luo et al. 2004; Dijkstra et al. 2005; Reich et al. 2006b; West et al. 2006). There is limited information regarding soil C responses to interactive effects of elevated CO2 and N deposition in subtropical and tropical forest ecosystems where ambient N deposition is high (Luo et al. 2004, 2006).
Subtropical and tropical forest ecosystems play a significant role in global C cycling and store 46 and 11% of the world’s living terrestrial C and total C, respectively (Brown and Lugo 1982). Atmospheric CO2 concentration and N deposition are two primary factors that are concurrently changing in subtropical forests of China (Deng et al. 2010). The availability of soil N in the subtropical forests is considered to be high (Liu et al. 2010) and the response of subtropical forests to elevated CO2 is still not clear. Several studies using meta-analysis (e.g. De Graaff et al. 2006; Van Groenigen et al. 2006) found that elevated CO2 only increased soil C at N addition larger than 30 kg ha-1 yr-1. However, in the temperate forests with limited N, elevated CO2 enhanced C assimilation more than similar forests with high N fertility (Butnor et al. 2003). Thus, there is a need to investigate how elevated CO2 and N deposition interactively influence C processes and C storage in forests with high N availability.
Several different forms of soil organic carbon (SOC) exist in forest soils (Ågren and Bosatta 2002; Kirschbaum 2004; Eliasson et al. 2005; Knorr et al. 2005; Bradford et al. 2008). Individual SOC fractions have different responses to change in soil N availability (Hagedorn et al. 2003) and elevated CO2 (Luo et al. 2004). The labile soil carbon fractions with short turnover times, such as particulate organic carbon (POC), readily oxidizable organic carbon (ROC) and microbial biomass carbon (MBC) are considered biologically available sources of C and respond faster to environment changes than total SOC (Blair et al. 1995; Yang et al. 2009). Consequently, SOC change may be revealed through soil labile carbon fractions in the short-time experiment.
In this study, we used open-top chambers to study the effects of elevated CO2 and N deposition on soil organic carbon fractions in forest ecosystems in Southern China. We grew 48 seedlings of 6 typical subtropical tree species in the chambers and measured soil organic C and its fractions over 4 years. We hypothesized that: 1) elevated CO2 and nitrogen addition treatments would have no significant effect on soil total organic carbon (TOC), because change in TOC was difficult to detect in the short term; 2) elevated CO2 would stimulate labile carbon fractions (POC and ROC) accumulation rates, due to the high ambient N availability in the forest ecosystems; and 3) combined elevated CO2 and N addition treatment would not enhance labile carbon fractions compared to elevated CO2 alone.
Materials and methods
Study site
The experiment was conducted at South China Botanical Garden, Chinese Academy of Sciences, Guangzhou, China (23°20′ N and 113°30′ E). The area has a typical south subtropical monsoon climate, with annual precipitation ranging from 1600 mm to 1900 mm, of which nearly 80% falls in the hot-humid wet/rainy season (April-September) and 20% in the dry season (October-March) (Liu et al. 2008). The annual total solar radiation in the visible waveband reached 4,367.2-4,597.3 MJ m-2 and mean annual temperature was 21.5°C. The N deposition in Guangzhou was 46–73 kg N ha-1 year-1 in the late 1990s (Ren et al. 2000).
Design of open-top chambers
Ten open-top chambers were built for this experiment. Each chamber was 3 m in diameter, 3 m tall and inserted 0.7 m deep into the soil. The above-ground part was wrapped with impermeable and transparent plastic sheets, leaving the top of the chamber totally open. Light intensity in the chamber was 97% of that in open space. Measured rainfall intensity was identical inside and outside of the chambers and the air temperature was not significantly different. The below-ground part was delimited by concrete wall filling with the brick that prevented any lateral or vertical water and/or element fluxes with the outside surrounding soil. Three holes at the bottom of the wall were connected to a stainless steel water collection box. Holes were capped by a 2 mm net to prevent losses other than those of leachates. In the treatment chambers with elevated CO2, the additional CO2 was distributed from a CO2 tank by a transparent PVC pipe with pinholes. A big fan was connected to the pipe to ensure equal distribution of CO2 in the entire chamber. Air was introduced into chambers via the fan at an exchange rate of about 1.5 chamber volumes per minute. The CO2 flux from the tank was controlled by a flow meter and the CO2 concentrations in the chambers were periodically checked using a Li-Cor 6400 (Li-Cor Inc., Lincoln, Nebraska, USA).
The soils used in the experiment were collected from a nearby evergreen broad-leaved forest after harvesting from 26 February 2005 to 3 March 2005. Simultaneously, the soil was collected at three different layers (0–20, 20–40 and 40–70 cm depth) that were homogenized separately and used to fill the below-ground part of the chambers. The lateritic soil with its chemical properties (before the soil was collected) was shown by Liu et al. (2008). On 10 March 2005, one to two years old seedlings grown in a nursery were transplanted into the chambers without damaging the roots. All the chambers were planted with 48 randomly selected seedlings with 8 seedlings each of the following 6 species: Castanopsis hystrix Hook.f. & Thomson ex A.DC, Syzygium hancei Merr. et Perry, Pinus massoniana Lambert, Schima superba Gardn. and Champ., Acmena acuminatissima (Blume) Merr. et Perry, and Ormosia pinnata (Lour.) Merr. These 6 species were selected because they are all native and all widely distributed in southern China. No significant difference in height and basal diameter of the plant seedling was found among all treatments at the beginning of the experiment (Deng et al. 2010).
Experimental design
The experimental treatments started in April 2005. Three chambers received elevated CO2 and ambient N deposition treatment (HC), two chambers received N addition and ambient CO2 treatment (HN), three chambers received elevated CO2 and N addition treatment (HCHN), and two chambers were used as controls (CK) without elevated CO2 or N addition. The HC and HCHN chambers were parallel arranged, and the distance between the adjacent chambers was 5 m. The HN and CK chambers were 50 m from the HC and HCHN chambers, and the distance between the adjacent chambers was 5 m. The elevated CO2 chambers were controlled at ca. 700 μmol mol-1. The N addition treatments were achieved by spraying liquid fertilizer on seedlings once a week for a total amount of NH4NO3 at 100 kg ha-1 yr-1. No other fertilizer was used. Since the walls of the chambers below-ground blocked lateral and vertical water fluxes, the seedlings were watered with tap water. About 600 mm extra water was applied in each chamber per year. All the chambers received the same amount of water as the control (CK) chambers.
Sample collection and measurements
Soil samples were collected in July of 2009. Twelve cores were collected randomly in each chamber from 0–20 cm soil layers using a standard soil sampling tube (2.5 cm inside diameter). One sample consisted of four cores, and 3 samples were collected in one chamber. Total of 30 samples were collected in this study. The fresh soil samples were passed through a 2 mm sieve to remove rocks and plant roots. Portions of fresh soils were used for microbial biomass carbon measurements. Sub-samples were air-dried and ground 2 mm for TOC and other C fractions measurements.
Particulate organic carbon (POC 53–2000 μm) was measured using the method described by Cambardella and Elliott (1992). Ten grams of the <2 mm air-dried soil was dispersed with 50 mL of 5 g·L-1 sodium hexametaphosphate solution by hand shaking the mixture for 5 min then set on a reciprocal shaker (90 r ·min-1) for 18 h. The dispersed soil sample was passed subsequently through 53 μm stainless steel sieve and rinsed thoroughly with distilled water. The material remaining on the sieve, defined as the POC fraction, was dried at 50°C for 12 h, weighed and finely ground 0.15 mm for carbon measurement.
Readily oxidizable organic carbon (ROC) was determined with 333 mmol L-1 KMnO4 oxidation (Blair et al. 1995, 1997). The air-dried soil, passed through a 2-mm sieve, was taken and passed through a 149 μm sieve. Sub-samples of soil containing 15–30 mg C were weighed into 100 mL plastic centrifuge tubes and 25 mL of 333 mmol KMnO4 were added to each tube. The tubes were tightly sealed and after shaking for 1 h at 250 r ·min-1, were centrifuged for 5 min at 2000 r ·min-1 and the supernatants diluted 1:250 with deionized water. The absorbance of the diluted samples and standards were read on a UVspectrophotometer at 565 nm. The change in the concentration of KMnO4 was used to estimate the amount of carbon oxidized, assuming that 1 mmol MnO4 is consumed in the oxidation of 9 mg carbon (Tirol-Padre and Ladha 2004).
Microbial biomass carbon (MBC) was determined with the chloroform fumigation-extraction method (Joergensen and Brookes 1990). Soil TOC was measured following the Dichromate (0.8 mol·L-1) heating-oxidation (Liu 1996). Soil pH was determined by mixing 10 g soil with 25 ml of deionized CO2-free water for 5 min, leaving it for 30 min for the soil and water to separate and then measuring the pH of supernatant using a glass electrode (Liu 1996).
Soil respiration was measured once a week using an infrared gas analyzer Li-Cor 6400 (Li-Cor Inc, Lincoln, NE, USA) connected with soil chamber from 26 May 2006 to December 2008 (Deng et al. 2010). The measurements were made between 9:00 am and 12:00 pm local time. The soil respiration chamber (with a foam gasket) was put on the soil collars making an air-tight seal. Soil respiration was measured three times for each soil collar. Soil respiration in a treatment chamber was calculated as the mean of four collar measurements. Soil moisture of the top 5 cm soil layer was measured on five random locations within a treatment chamber using a PMKit at the same time when the soil respiration measurements were being taken.
Tree height and basal diameter were measured at the time of planting in early March 2005 and then assessed six times in August 2005, January 2006, May 2006, January 2008, September 2008, and January 2009. Tree height was measured from the soil-stem surface to the tip of the apical bud, and the basal diameter was assessed at the soil surface. To measure tree biomass, one plant of each species in every chamber was harvested in January in 2006, 2007, 2008, and 2009, respectively. The plant was separated into roots, stems, and leaves. Plant samples were oven-dried at 60°C before weighing. A traditional plant growth function was developed for different component biomass estimation (Whittake and Woodwell 1986; Wen et al. 1997): W = a(D 2 H) b, where W is dry biomass of plant components including roots, stems, and leaves; D is plant basal diameter; H is height; and a and b are regression coefficients. The above-ground tree biomass in each chamber was the sum of all tree stem and leave biomass. The below-ground tree biomass was the sum of all tree root biomass.
Statistical analysis
The three soil samples per chamber were averaged before statistical analyses. Two-way analysis of variance (ANOVA) was used to examine CO2, N and their interactive effects on soil organic carbon concentration (TOC), particulate organic carbon (POC), readily oxidizable organic carbon (ROC), microbial biomass carbon (MBC) and pH. One-way ANOVA with Tukey’s HSD test was used to examine different treatments effects on soil TOC, POC, ROC and pH. Repeated measures ANOVA was used to examine the effects of different treatments on soil respiration, moisture and tree biomass at different measurement occasions. All the tests were performed using SPSS software (version 11.5; SPSS Inc).
Results
Soil pH
Results of two-way ANOVA showed that CO2 and N significantly influenced soil pH, but no interaction effect of CO2 and N was detected (Table 1). The elevated CO2 only (HC), N addition only (HN), and combined elevated CO2 and N addition (HCHN) treatments significantly decreased soil pH in the 0–20 cm layers compared to the control (CK) (Fig. 1). Soil pH under the combined elevated CO2 and N addition treatment was significantly lower than that under elevated CO2 only and N addition only treatments. There was no significant difference between HC and HN treatments.
Soil total organic carbon (TOC)
There was no significant difference among the four treatments (Fig. 2a). Averaged TOC for the 0–20 cm depth showed a trend of decline in the treatment order: HN (10.126 ± 1.936 g kg-1) > HCHN (9.340 ± 0.786 g kg-1) > HC (8.942 ± 0.823 g kg-1) > CK (7.733 ± 2.011 g kg-1).
Soil total organic carbon (TOC), particulate organic carbon (POC), readily oxidizable organic carbon (ROC) and microbial biomass carbon (MBC) under different CO2 and N addition treatments in July of 2009. HC: elevated CO2 and ambient N deposition; HN: N addition and ambient CO2; HCHN: elevated CO2 and N addition; CK: control. Different letters across treatments denote significant difference at p < 0.05
Soil particulate organic carbon (POC)
The interaction of elevated CO2 and N addition affected the soil POC significantly (p < 0.05) (Table 1).The elevated CO2 alone (HC) or N addition alone (HN) treatment had no significant effect on soil POC compared to the control. But the POC was significantly higher in the combined elevated CO2 and N addition treatment compared to elevated CO2 only treatment (Fig. 2b). In the top soil layer (0–20 cm), the HCHN treatment had the highest POC, followed by the HN and CK treatments, and the HC treatments had the least POC concentration.
Soil readily oxidizable organic carbon (ROC)
There was significant effect on soil ROC among the four treatments (Fig. 2c). In the 0–20 cm soil layer, the average soil ROC followed the order: HCHN (1.904 ± 0.249 g kg-1) > HN (1.547 ± 0.160 g kg-1) > CK (1.261 ± 0.113 g kg-1) > HC (1.169 ± 0.186 g kg-1). The combined elevated CO2 and N addition (HCHN) treatment had the highest ROC content and was significantly different from the control. The N addition alone (HN) and elevated CO2 alone (HC) had no significant effect on soil ROC, but the HN treatment had higher ROC than HC treatment.
Soil microbial biomass carbon
The N addition alone (HN) treatment significantly decreased soil MBC (Table 1; Fig. 2d), compared to the control (CK). On the contrary, the elevated CO2 alone (HC) treatment significantly increased soil MBC. Both HC and combined elevated CO2 and N addition (HCHN) treatments had higher MBC concentrations than the HN treatment.
Above- and below- ground biomass, soil respiration and moisture
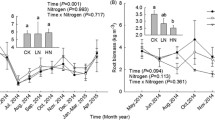
The elevated CO2 alone (HC), N addition alone (HN), and combined elevated CO2 and N addition (HCHN) treatments increased the above- and below-ground biomass (Fig. 3). Both above- and below-ground biomass was lower under the HC treatment than the HN and HCHN treatments.
From 2006 to 2008, the elevated CO2 alone treatment had increased soil respiration and soil moisture (Table 2). The N addition alone (HN) treatment increased soil respiration in 2006, but had no significant effect on soil respiration in 2007 and 2008. The HN treatment decreased soil moisture. The N addition alone (HN) treatment increased soil respiration in three years, and only increased soil moisture in the first two years. In 2008, the HCHN had no significant effect on soil moisture.
Discussion
Effect of elevated CO2 on soil organic carbon fractions
Our results showed that elevated CO2 alone (HC) had no significant effect on soil particulate organic carbon (POC) and readily oxidizable organic carbon (ROC) (Fig. 2b, c). This was a little surprising, as we had hypothesized that POC and ROC may be enhanced by elevated CO2, since they are labile organic carbon fractions and have short turnover times (Yang et al. 2009). Previous studies showed that elevated CO2 may stimulate plant-derived C (Baldock et al. 1992; Tirol-Padre and Ladha 2004; Grandy and Neff 2007). Absence of significant changes in POC and ROC in this study may be due to the balance of carbon inputs and outputs. We found that the above- and below-ground biomass increased under elevated CO2 treatment (Fig. 3). These increases may have promoted an accumulation of particulate organic matter, as shown in a grazed grassland (Allard et al. 2005). However, new carbon inputs can also accelerate the decomposition of SOC and increase soil carbon turnover (Taneva et al. 2006; Fontaine et al. 2004). Indeed, the elevated CO2 treatment in this study significantly enhanced soil respiration and increased soil microbial biomass carbon (Table 2, Fig. 3d). Enhanced soil moisture in the elevated CO2 treatment may also contribute to the higher litter decomposition and nutrient mineralization rates (Liu et al. 2008; Deng et al. 2010). As a result, elevated CO2 reduced the new-C residence times leading to no significant effect on POC and ROC. Similar results were also reported by Lichter et al. (2005) who found that elevated CO2 treatment produced no detectable effect on the C content of any particulate organic matter size fraction.
There was no significant effect of elevated CO2 alone (HC) on TOC (Fig. 2a). This was consistent with our hypothesis. Since no change in ROC or POC was found as discussed above, it was understandable that elevated CO2 alone did not significantly affect soil TOC in the short term. However, in several studies elevated CO2 reduces soil carbon content (Carney et al. 2007; Langley et al. 2009). The controversy may be caused by the difference in N availability. Due to the high ambient N availability in subtropical forests, N supply may meet the need of enhanced plant growth under elevated CO2 in the short term. In the long term, however, the increasing soil respiration and microbial activity under elevated CO2 treatment may alter the rate the total organic carbon decomposition (Fontaine et al. 2004) which could reduce soil carbon stock.
Effect of N addition on soil organic carbon fractions
For the similar reasons as the elevated CO2 alone (HC) treatment, N addition alone (HN) treatment also had no significant effect on soil POC, ROC, and TOC (Fig. 2b, c). We found that the N addition alone treatment enhanced above- and below-ground biomass (Fig. 3) as well as promoted soil respiration in the first 2 years of the experiment (Table 2). This was inconsistent with some other studies that reported increases in soil POC and ROC with N deposition treatments (Diekow et al. 2005; Li et al. 2010). The possible reason could be that N addition treatment experiments in these studies were implemented at least 6 years (Diekow et al. 2005; Li et al. 2010), and our experiment just was carried out for only 4 years.
One thing worth mentioning is that the effect of the N addition alone treatment on soil respiration had been weakened over time (Table 2, Deng et al. 2010). This was consistent with our result that the N addition alone treatment decreased the soil microbial biomass carbon (MBC) (Fig. 2d). The N addition often lead to soil acidification (Fig. 1) especially in tropical forests where the acidic soils often will inhibit soil microbial activity (Waldrop et al. 2004). Meanwhile, soil microbial activity was closely related to soil moisture (Deng et al. 2010). In our study, N addition significantly decreased soil moisture (Table 2), which would suppress the microbial activity. Hence, we speculate that the POC and ROC may be increased under N addition in the long-term.
Effect of combined elevated CO2 and N addition treatment on soil organic carbon fractions
Soil ROC and POC contents under the combined elevated CO2 and N addition treatment were higher than those under other treatments (Fig. 2c). The combined elevated CO2 and N addition treatment enhanced labile carbon derived-plant accumulation compared to the elevated CO2 alone treatment. An increase in plant growth under elevated CO2 caused N to accumulate in plant biomass. Soil N availability was expected to constrain sustainability of plant growth response to elevated CO2 in the long term (Oren et al. 2001; Finzi et al. 2002; Reich et al. 2006a). Although the subtropical soil had a high N availability, the young seedlings used in this experiment grew quickly and required more soil N (Deng et al. 2010). Elevated CO2 together with N addition increased above- and below-ground biomass more than elevated CO2 alone (Fig. 3). This also indicated that more N is needed to meet high N demand under elevated CO2 treatment in the long-term (Luo et al. 2004; Reich et al. 2006a).
Furthermore, the combined elevated CO2 and N addition treatment had no significant effect on soil MBC (Fig. 2d). The combined elevated CO2 and N addition treatment had increased soil moisture in the first 2 years, but had no significant effect in 2008. The likely reason was that the effect of elevated CO2 on soil moisture was reduced by N addition treatment. MBC has been found to be closely related to soil moisture (Kucera and Kirkham 1971; Deng et al. 2010).
The combined elevated CO2 and N addition treatment had no significant effect on TOC in the short-term experiment (Fig. 2a). Although the combined elevated CO2 and N addition treatment increased the new-C (root biomass and labile soil organic carbon) inputs to the soil, the soil respiration was also enhanced. No significant change in TOC was detected in this study over 4 years. Since the combined elevated CO2 and N addition treatment was more favorable for soil C accumulation than elevated CO2 alone treatment, we think that even in the subtropical forest where soil N availability is high, N addition is still needed to meet the enhanced N demand by elevated CO2 treatment.
Conclusions
Both elevated CO2 and N addition alone had no significant effect on soil labile organic carbon (POC and ROC). While the elevated CO2 alone increased the microbial activity (MBC and soil respiration), N addition alone decreased the microbial activity. However, combined elevated CO2 and N addition treatment had stronger effects on accumulation rates of labile organic carbon (POC and ROC) than elevated CO2 or N addition alone, but had no significant influence on MBC. Our results demonstrated that even in the N-rich subtropical forest ecosystems, more exogenous N is needed to maintain higher C inputs to soil under CO2 enrichment.
Abbreviations
- C:
-
carbon
- CK:
-
control
- HC:
-
elevated CO2 and ambient N deposition
- HCHN:
-
elevated CO2 and N addition
- HN:
-
N addition and ambient CO2
- MBC:
-
microbial biomass carbon (mg kg-1)
- N:
-
nitrogen
- POC:
-
particulate organic carbon (g kg-1)
- ROC:
-
readily oxidizable organic carbon (g kg-1)
- SM:
-
soil moisture (%)
- SR:
-
soil respiration (kg CO2 m-2 yr-1)
- TOC:
-
soil total organic carbon (g kg-1)
References
Ågren GI, Bosatta E (2002) Reconciling differences in predictions of temperature response of soil organic matter. Soil Biol Biochem 34:129–132
Allard V, Newton PCD, Lieffering M, Soussana JF, Carran RA, Matthew C (2005) Increased quantity and quality of coarse soil organic matter fraction at elevated CO2 in a grazed grassland are a consequence of enhanced root growth rate and turnover. Plant Soil 276:49–60
Baldock JA, Oades JM, Waters AG, Peng X, Vassallo AM, Wilson MA (1992) Aspects of the chemical structure of soil organic materials as revealed by solid-state 13C NMR spectroscopy. Biogeochem 16:1–42
Blair GJ, Lefroy RDB, Lisle L (1995) Soil carbon fractions based on their degree of oxidation, and the development of a carbon management index for agricultural system. Aust J Agric Res 46:1459–1466
Blair GJ, Lefroy RDB, Singh BP, Till AR (1997) Development and use of a carbon management index to monitor changes in soil C pool size and turnover rate. In: Cadisch G, Giller KE (eds) Driven by nature: plant litter quality and decomposition. CAB International, Wallingford, UK, pp 273–281
Bradford MA, Fierer N, Reynolds JF (2008) Soil carbon stocks in experimental mesocosms are dependent on the rate of labile carbon, nitrogen and phosphorus inputs to soils. Funct Ecol 22:964–974
Brown S, Lugo AE (1982) The storage and production of organic matter in tropical forests and their role in the global carbon cycle. Biotropica 14:161–187
Butnor JR, Johnsen KH, Oren R, Katul AG (2003) Reduction of forest floor respiration by fertilization on both carbon dioxide-enriched and reference 17-year-old loblolly pine stands. Glob Change Biol 9:849–861
Cambardella CA, Elliott ET (1992) Particulate soil organic matter changes across a grassland cultivation sequence. Soil Sci Soc Am J 56:777–783
Carney KM, Hungate BA, Drake BG, Megonigal JP (2007) Altered soil microbial community at elevated CO2 leads to loss of soil carbon. PANS 104:4990–4995
de Graaff MA, van Groenigen KJ, Six J, Hungate B, van Kessel C (2006) Interactions between plant growth and soil nutrient cycling under elevated CO2: a meta-analysis. Glob Change Biol 12:2077–2091
Deng Q, Zhou GY, Liu JX, Duan HL, Zhang DQ (2010) Responses of soil respiration to elevated carbon dioxide and nitrogen addition in young subtropical forest ecosystems in China. Biogeosci 7:315–328
Diekow J, Mielniczuk J, Knicker H, Bayer C, Dick DP, Kögel-Knabner I (2005) Carbon and nitrogen stocks in physical fractions of a subtropical Acrisol as influenced by long-term no-till cropping systems and N fertilization. Plant Soil 268:319–328
Dijkstra FA, Hobbie SE, Reich PB, Knops JMH (2005) Divergent effects of elevated CO2, N fertilization, and plant diversity on soil C and N dynamics in a grassland field experiment. Plant Soil 272:41–42
Eliasson PE, McMurtrie RE, Pepper DA, Strömgren M, Linder S, Ågren GI (2005) The response of heterotrophic CO2 flux to soil warming. Glob Change Biol 11:167–181
Finzi AC, DeLucia EH, Hamilton JG, Richter DD, Schlesinger WH (2002) The nitrogen budget of a pine forest under free CO2 enrichment. Oecologia 132:567–578
Fontaine S, Bardoux G, Abbadie L, Mariotti A (2004) Carbon input to soil may decrease soil carbon content. Ecol Letters 7:314–320
Glaser B, Millar N, Blum H (2006) Sequestration and turnover of bacterial- and fungal-derived carbon in a temperate grassland soil under long-term elevated atmospheric pCO2. Glob Change Biol 12:1521–1531
Grandy AS, Neff JC (2007) Molecular, C dynamics downstream: the biochemical decomposition sequence and its impact on soil organic matter structure and function. Sci Total Environ 404:297–307
Hagedorn F, Maurer S, Egli P, Blaser P, Bucher JB, Siegwolf R (2001) Carbon sequestration in forest soils: effects of soil type, atmospheric CO2 enrichment, and N deposition. Eur J Soil Sci 52:619–628
Hagedorn F, Spinnler D, Bundt M, Blaser P, Siegwolf R (2003) The input and fate of new C in two forest soils under elevated CO2. Glob Change Biol 9:862–872
Joergensen RG, Brookes PC (1990) Ninhydrin-reactive nitrogen measurements of microbial biomass in 0.5 M K2SO4 soil extracts. Soil Boil Biochem 22:1023–1027
Kirschbaum MUF (2004) Soil respiration under prolonged soil warming: are rate reductions caused by acclimation or substrate loss? Glob Change Biol 10:1870–1877
Knorr W, Prentice IC, House JI, Holland EA (2005) Long-term sensitivity of soil carbon turnover to warming. Nat 433:298–301
Kucera CL, Kirkham DR (1971) Soil respiration studies in tall-grass prairie in Missouri. Ecol 52:912–915
Langley JA, McKinley DC, Wolf AA, Hungate BA, Drake BG (2009) Priming depletes soil carbon and releases nitrogen in a scrub-oak ecosystem exposed to elevated CO2. Soil Biol Biochem 41:54–60
Li YF, Jiang PK, Chang SX, Wu JS, Lin L (2010) Organic mulch and fertilization affect soil carbon pools and forms under intensively managed bamboo (Phyllostachys praecox) forests in southeast China. J Soils Sediments 10:739–747
Lichter J, Barron SH, Bevacqua CE, Finzi AD, Irving KF, Stemmier EA, Schlesinger WH (2005) Soil carbon sequestration and turnover in pine forest after six years of atmospheric CO2 enrichment. Ecol 86:1835–1847
Liu GS (1996) Soil physical and chemical analysis, description of soil profiles. Standard Press of China, Beijing
Liu JX, Zhang DQ, Zhou GY, Faivre-Vuillin B, Deng Q, Wang CL (2008) CO2 enrichment increases nutrient leaching from model forest ecosystems in subtropical China. Biogeosci 5:1783–1795
Liu JX, Zhou GY, Zhang DQ, Xu ZH, Duan HL, Deng Q, Zhao L (2010) Carbon dynamics in subtropical forest soil: effects of atmospheric carbon dioxide enrichment and nitrogen addition. J Soils Sediments 10:730–738
Luo YQ, Su B, Currie WS, Dukes JS, Finzi A, Hartwig U, Hungate B, McMurtrie RE, Oren R, Parton WJ, Pataki DE, Shaw MP, Zak DR, Field CB (2004) Progressive nitrogen limitation of ecosystem responses to rising atmospheric carbon dioxide. BioSci 54:731–739
Luo ZB, Calfapietra C, Liberloo M, Scarascia-Mugnozza S, Polle A (2006) Carbon partitioning to mobile and structural fractions in poplar wood under elevated CO2 (EUROFACE) and N fertilization. Glob Change Biol 12:272–283
Oren R, Ellsworth DS, Johnsen KH, Phillips N, Ewers BE, Maier C, Schafer KVR, McCarthy H, Hendrey G, McNulty SG, Katul GG (2001) Soil fertility limits carbon sequestration by forest ecosystems in a CO2-enriched atmosphere. Nat 411:469–472
Reich PB, Hobbie SE, Lee T, Ellsworth DS, West JB, Tilman D, Knops JMH, Naeem S, Trost J (2006a) Nitrogen limitation constrains sustainability of ecosystem response to CO2. Nat 440:922–925
Reich PB, Hungate BA, Luo YQ (2006b) Carbon-nitrogen interactions in terrestrial ecosystems in response to rising atmospheric carbon dioxide. Annu Rev Ecol Evol Syst 37:611–636
Ren R, Mi FJ, Bai NB (2000) A chemo metrics analysis on the data of precipitation chemistry of China. Journal of Beijing Polytechnic University 26:90–95
Shaw MR, Zavaleta ES, Chiarielle NR, Cleland EE, Mooney HA, Field CB (2002) Grassland responses to global environmental changes suppressed by elevated CO2. Science 198:1987–1990
Taneva L, Pippen JS, Schlesinger WH, Gonzalea-Meler MA (2006) The turnover of carbon pools contributing to soil CO2 and soil respiration in a temperate forest exposed to elevated CO2 concentration. Glob Change Biol 12:983–994
Tirol-Padre A, Ladha JK (2004) Assessing the reliability of permanganate-oxidizable carbon as an index of soil labile carbon. Soil Sci Soc Am J 68:969–978
Treseder KK, Allen MF (2000) Mycorrhizal fungi have a potential role in soil carbon storage under elevated CO2 and nitrogen deposition. New Phytol 147:189–200
van Groenigen KJ, Six J, Hungate BA, De Graaff MA, van Breemen N, van Kessel C (2006) Element interactions limit soil carbon storage. PNAS 103:6572–6573
Waldrop MP, Zak DR, Sinsabaugh RL, Gallo M, Lauber C (2004) Nitrogen deposition modifies soil carbon storage through changes in microbial enzymatic activity. Ecol Appl 14:1172–1177
Wen DZ, Wei P, Kong GH, Zhang QM, Huang ZL (1997) Biomass study of the community of Castanopsis chinensis+Cryptocarya concinna+Schima superba in a southern China reserve. Acta Ecologica Sinica 17:497–504
West JB, Hobble SE, Reich PB (2006) Effects of plant species diversity, atmospheric [CO2], and N addition on gross rated of inorganic N release from soil organic matter. Glob Change Biol 12:1400–1408
Whittake RH, Woodwell GM (1986) Dimension and production relations of trees and shrubs in the Brookhaven forest, New York. J Ecology 56:1–25
Yang YS, Guo JF, Chen GS, Yin YF, Gao R, Lin CF (2009) Effects of forest conversion on soil labile organic carbon fractions and aggregate stability in subtropical China. Plant Soil 323:153–162
Acknowledgments
This work was jointly funded by Strategic Priority Research Program-Climate Change: Carbon Budget and Relevant Issues״ of the Chinese Academy of Sciences (XDA05050205), National Basic Research Program of China (2009CB421101), key projects in the national science & technology pillar program (2009BADC6B07), Guangdong Provincial Natural Science Foundation of China (8351065005000001) and Dinghushan Forest Ecosystem Research Station. We thank Prof. Dafeng Hui for helpful comments on an earlier version of the manuscript and Ms. Chloe Davidson for English improvement.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible Editor: Ute Skiba.
Rights and permissions
About this article
Cite this article
Chen, X., Liu, J., Deng, Q. et al. Effects of elevated CO2 and nitrogen addition on soil organic carbon fractions in a subtropical forest. Plant Soil 357, 25–34 (2012). https://doi.org/10.1007/s11104-012-1145-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11104-012-1145-3