Abstract
Purpose
The purpose of this study was to identify the frequency and possible cause of diffuse intrathoracic uptake on post-therapy I-131 scans in thyroid cancer patients.
Methods
We retrospectively reviewed 781 post-therapy scans of 755 thyroid cancer patients who underwent total thyroidectomy and radioactive iodine therapy between January and December 2010. Diffuse intrathoracic uptake on post-therapy scans was examined, and clinical patient characteristics including sex, age, regimen for thyroid-stimulating hormone (TSH) stimulation (thyroid hormone withdrawal or recombinant human TSH injection), TSH, thyroglobulin (Tg) and anti-thyroglobulin antibody (anti-Tg Ab) levels, therapeutic dose of radioactive iodine therapy and prior history of radioactive iodine therapy were recorded.Scan findings were correlated with chest CT, chest radiographs, laboratory tests and/or clinical status. Diffuse intrathoracic uptake without evidence of pathologic condition was categorized as indeterminate. The association between clinical characteristics and intrathoracic uptake were analyzed for negative intrathoracic uptake and indeterminate uptake groups.
Results
Diffuse intrathoracic uptake on post-therapy scans was demonstrated in 39 out of 755 (5.2 %) patients, among which 3 were confirmed as lung metastasis. The 14 patients that showed high Tg or anti-Tg Ab levels were considered to be at risk of having undetected micrometastasis on other imaging modalities. The remaining 22 were indeterminate (2.9 %).Upon comparison of negative intrathoracic uptake and indeterminate uptake groups, TSH stimulation by thyroid hormone withdrawal was shown to be significantly correlated with diffuse intrathoracic uptake (p < 0.05).
Conclusion
The frequency of diffuse intrathoracic uptake on post-therapy scans was 5.2 % and could be seen in thyroid cancer patients with underlying lung metastasis as well as those without definite pathologic condition. In the latter, there was a higher frequency for diffusely increased intrathoracic uptake in those who underwent thyroid hormone withdrawal rather than recombinant human TSH injection.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The radioactive iodine scan plays an important role in the management of patients with differentiated thyroid cancer who have undergone total thyroidectomy by detecting functioning thyroid tissue with expression of sodium-iodide symporters (NIS). However, it is well known that a variety of unexpected radioactive iodine uptakes in non-thyroidal tissue can produce false-positive results [1–5]. The mechanism is not fully understood, and such non-specific radioactive iodine accumulation is a diagnostic pitfall.
The lung is one of the most common sites of distant metastases in thyroid cancer patients. Radioactive iodine scans, along with the thyroblobulin (Tg) level, play an important role in the diagnosis of lung metastasis. Diffuse intrathoracic uptake is also known to be observed in other benign lesions [6], as well as lung metastases, but other possible causes are not clearly established. Particular attention must be paid to diffuse intrathoracic uptake on post-therapy scans without specific pathological foci on other imaging studies. Accurate interpretation of diffuse intrathoracic uptake is clinically significant for the therapeutic management of these patients.
In this study, we retrospectively identified the frequency of diffuse intrathoracic uptake and suggest a possible etiology for diffuse intrathoracic uptake on post-therapy scans in thyroid cancer patients.
Materials and Methods
Patient Population
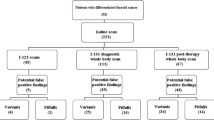
The subjects of this study were patients who had received radioactive iodine therapy with a dose of 3.7 GBq or higher for well-differentiated thyroid cancer between January and December 2010. Those who had insufficient TSH, Tg and anti-Tg data and with less than 2 years of follow-up were excluded from the analysis. Those who showed focal uptake on the scans were also excluded. The remaining scans with negative intrathoracic uptake or diffuse intrathoracic uptake were analyzed (Fig. 1). This study was approved by the institutional review board at our institution. Informed consent was waived because of the retrospective design of this study.
Radioactive Iodine Therapy
Radioactive iodine therapy was performed in thyroid cancer patients who underwent total thyroidectomy. Before radioactive iodine therapy, thyroxine (T4) was discontinued for at least 4 weeks, with tri-iodothyronine (T3) replacement for the first 2 weeks or recombinant human TSH (rh-TSH) injection to raise the serum TSH level above 30 μU/ml.
rh-TSH was used in the patients who were predicted to have insufficient endogenous TSH stimulation due to thyroid hormone withdrawal; those with risk of progressive disease due to extensive distant metastases; those with risk of life-threatening complications of hypothyroidism due to underlying diseases including ischemic heart disease and renal insufficiency; or those who had refused thyroid hormone withdrawal [7, 8].
Patients also followed a low-iodine diet (LID) for at least 2 weeks. After thyroid hormone withdrawal and LID, I-131 diagnostic scans were performed 30 h following radioactive iodine administration with a dose of 74 MBq.
Patients who were prepared with rh-TSH injection were administrated with serial injections prior to admission and underwent F-18 FDG PET/CT instead of an I-131 diagnostic scan. A therapeutic dose of I-131, ranging from 3.7 to 9.25 GBq, was determined by postoperative pathology results, I-131 diagnostic scan or F-18 FDG PET/CT findings, and thyroblobulin and anti-thyroblobulin antibody (anti-Tg Ab) levels.
Tg, Anti-Tg Ab and TSH Measurement
Serum levels of TSH, Tg and anti-Tg Ab were obtained when a dose of 74 MBq radioactive iodine was administrated in patients with thyroid hormone withdrawal or those who had received rh-TSH injection preparations on the day of the second rh-TSH injection (D2) and 3 days later (D5). Tg levels exceeding 2 ng/ml in patients with thyroid hormone withdrawal or above 1 ng/ml in patients with rh-TSH injection and an anti-Tg Ab of 100 IU/ml were taken to be Tg positive and anti-Tg positive, respectively [9, 10].
Post-Therapy I-131 Scans
Post-therapy I-131 scans were performed 5 to 6 days following radioactive iodine therapy. Anterior and posterior images were acquired using an E-CAM dual detector system (Siemens, Erlangen, Germany) with high-energy parallel hole collimators. Each 30-min imaging study was performed using a peak of 364 keV with 15 to 20 % energy windows. Delayed image acquisition or lateral projection was carried out if clinically warranted.
Image Analysis and Interpretation
In the post-therapy scans, chest uptake was considered to be positive when the activity exceeded that of the arm or thigh. Uptakes related to skin inflammation and contamination were excluded by correlation with the patient records or clinical status at the time, which may have affected the post-therapy scans, by correlation with the view of the lateral projection or other imaging studies. Diffuse uptake was defined as that with an indistinguishable focus of prominent activity in the positive uptake. All scans were divided into ‘negative,’ ‘equivocal’ and ‘positive,’ and ‘diffuse uptake’ was determined as when at least two out of the three independent nuclear physicians arrived at a consensus of ‘positive.’ To evaluate interobserver and intraobserver agreement, the images were interpreted twice by each of three experienced nuclear physicians without knowledge of each other’s finding.
In cases that showed diffuse uptake, the underlying pathologies were identified by correlation with chest radiographs taken within 1 week or chest CT scans taken within 2 weeks. Pulmonary metastasis was defined by confirmation of histology, identification of nodules that enlarged during a minimum follow-up period of 6 months on chest radiographs or chest CT scans, or when findings of lung metastases were identified on radioactive iodine scans with elevated serum Tg levels (>2 ng/ml) on follow-up [11–13].
Clinical conditions including age, sex, histology, regimen of TSH stimulation (hormone withdrawal or rh-TSH injection), therapeutic dose of radioactive iodine, prior history of radioactive iodine therapy and laboratory tests such as serum levels of TSH, Tg and anti-Tg Ab were recorded. Uptake in scans of patients without identified underlying pathology was categorized as indeterminate. As the possibility of micrometastases not visualized on chest radiographs or chest CT could not be completely ruled out, Tg or anti-Tg Ab positive patients were excluded from the indeterminate uptake group. Clinical characteristics and laboratory test results were compared between negative intrathoracic uptake and indeterminate uptake groups. The primary endpoint of this study was to analyze the cause of diffuse intrathoracic uptake found after radioactive iodine therapy, to suggest its clinical significance and to establish its relation with rh-TSH. For this, all patients were observed for a minimum follow-up period of 2 years.
Statistical Analysis
Agreement within and between observers with overall interpretation of the scans (negative, equivocal, positive) was calculated. Agreement was expressed as observed agreement (%) and by calculating the kappa statistics using the following grading: poor agreement κ ≤0.40, moderate κ = 0.41−0.60, good κ = 0.61−0.80 and excellent κ > 0.80.
The Student’s t-test was used to compare the laboratory tests such as the serum level of TSH, Tg and anti-Tg Ab between the indeterminate uptake and negative intrathoracic uptake groups, and the chi-square test was used to compare clinical conditions such as sex, histology, regimen of TSH stimulation, therapeutic dose of radioactive iodine and prior history of radioactive iodine therapy between the two groups. A T-test was also done to compare TSH levels between patients with indeterminate uptake and those with negative intrathoracic uptake who had undergone hormone withdrawal as well as between those prepared by hormone withdrawal and rh-TSH within the negative intrathoracic uptake group. A p-value of less than 0.05 was considered statistically significant. Analysis was performed using SPSS, version 13.0 (SPSS, Inc.), for Windows (Microsoft).
Results
Patient Characteristics
A total 781 post-therapy scans of 755 patients were reviewed in this study, among which 47 showing focal uptake on post-therapy scans were excluded from the analysis. Of the remaining 734 scans of 708 patients, 39 showed diffuse intrathoracic uptake, and 669 showed negative intrathoracic uptake on the post-therapy scan (Figs. 2 and 3). Twenty-six patients underwent post-therapy scans twice, but there were no cases where discordant scan findings were present. The mean age of the 708 patients was 48.6 ± 14.1 years (range 13–85 years; 127 males and 581 females). Of these patients, 705 had papillary cancer (99.7 %), two follicular cancer (0.2 %) and one Hurthle cell carcinoma (0.1 %). Five hundred sixty patients underwent hormone withdrawal (79.1 %), and 148 underwent preparation with rh-TSH injections (20.9 %). Six hundred fifty-three patients underwent the first radioactive iodine therapy (92.2 %), and 55 patients had a prior history of radioactive iodine therapy (7.8 %); 465 patients were treated with a therapeutic dose of 3.7 GBq (65.7 %), and 243 patients were treated with a dose ranging from 5.5 to 9.25 GBq (34.3 %) (Table 1).
Post-therapy scan of a 53-year-old female following radioactive iodine therapy with a dose of 3.7 GBq shows diffuse uptake in the chest (arrows). Uptake was more prominent on the posterior view with the patient in the supine position. No underlying pathology was found, and the pattern was categorized into the indeterminate uptake group
Intra- and Interobserver Agreement Between Readers
Intraobserver agreement was good for all readers (κ = 0.61−0.74), while interobserver agreement was moderate to good (κ = 0.51−0.62). Inter- and intraobserver agreements were 90–96 and 93–96 %, respectively, when the results were interpreted as negative, equivocal or positive. (Table 2).
Classification of Diffuse Intrathoracic Uptake Group
Three of 39 patients that showed diffuse intrathoracic uptake on post-therapy scans were confirmed to have pulmonary metastases upon correlation with chest CT, high Tg levels and clinical history. The remaining 36 patients that showed diffuse intrathoracic uptake showed no abnormal results on imaging studies. Of these, ten patients had Tg level greater than 2 ng/ml, and four patients had anti-Tg levels exceeding 100 IU/ml. The remaining 22 patients showed no abnormal results on other imaging studies and laboratory tests, and they were categorized into the indeterminate uptake group (2.9 %) (Table 3). There was no evidence of lung metastasis on imaging studies, including the radioactive iodine scan, and serum Tg levels during the follow-up period of 2 years. In these cases, uptake was more prominent on posterior view images. All were correlated with post-therapy scans and chest radiographs. Five of these were further correlated with chest CT scans. Only two scans showed a minimal volume of pleural effusion on CT scans of patients who did not have any abnormal findings on chest radiographs (Fig. 4).
a Post-therapy scan of a 32-year-old female following radioactive iodine therapy with a dose of 5.5 GBq shows diffuse uptake in the chest (arrows), namely indeterminate uptake. b On the chest radiograph, no abnormal findings were visualized. c On chest CT images within 2 weeks of post-therapy scans, a minimal amount of both pleural effusions (arrows) was visualized. Therefore, the diffuse uptake was thought to be related to bilateral pleural effusion
Indeterminate Uptake Group vs. Negative Intrathoracic Uptake Group
Upon comparison of the 22 indeterminate uptake patients and 669 in the negative intrathoracic uptake group, there was no statistical difference in the age, serum level of TSH, Tg or anti-Tg Ab levels. There was also no statistical difference in the sex distribution of each group, therapeutic dose of radioactive iodine or the presence prior history of radioactive iodine therapy.
There was a tendency to higher TSH levels in patients who showed negative thoracic uptake than in those with indeterminate uptake, although this was not statistically significant (p = 0.081), with TSH levels of 76.9 ± 9.8 and 67.7 ± 14.6, respectively (Fig. 5). The TSH levels in the negative thoracic uptake group of the rh-TSH-using group and negative thoracic uptake group of the hormone-withdrawal group showed a statistically significant difference (p = 0.001), with 171.5 ± 14.1 and 67.7 ± 14.6, respectively (Fig. 6). An interesting finding was that when the frequency of indeterminate uptake for the two groups with different regimens for TSH stimulation was investigated, the frequency was 4.0 % in the hormone withdrawal group, whereas there were no patients with indeterminate uptake in the group that used rh-TSH.
Assessment of the correlation between the regimen for TSH stimulation and the indeterminate uptake group showed that the thyroid hormone withdrawal method had a strong correlation with an indeterminate uptake pattern (p = 0.012) (Table 4).
Discussion
Several imaging modalities are used to diagnose lung metastases in thyroid cancer patients. Of these, radioactive iodine scans play a significant role. Post-therapy scans of thyroid cancer patients often show radioactive iodine uptake in the chest, and it is clinically important to distinguish between true lung metastases and false-positive uptakes in order to avoid unnecessary treatment.
An interesting finding was that that there were diffuse patterns of radioactive iodine uptake even without identified underlying pathology (2.9 %), and when these were compared to the negative intrathoracic uptake group, there was a higher frequency of positive uptake when the thyroid hormone was withdrawn. Diffuse uptake showed a difference from that of bronchiectasis or inactive pulmonary tuberculosis, which demonstrated focal uptake patterns corresponding to pathologic focus [14–16]. One previous study reported that diffuse uptake can be seen in bronchiectasis [6]. However, in our study the fact that abnormal chest radiograph findings were not seen in all patients and that abnormalities such as inactive pulmonary tuberculosis and evidence of bronchiectasis were not seen in the 18 patients who had received chest CT during the entire period of the hospital visit, including the radioactive iodine therapy preparation period, suggest the possibility of other causes.
A diffuse uptake pattern, on the other hand, is associated with pleural effusion. Some of these showed unilateral diffuse uptake, and the fact that the uptake was more prominent on the posterior view and not localized to the mediastinum (as in pericardial effusion) further supports this explanation [2, 17]. It is well known that patients with hypothyroidism often have pleural effusion, pericardial effusion, ascites and peripheral edema [18, 19]. As previously reported, hypothyroidism causes increased vascular permeability, which results in pleural effusion through transudates and/or exudates, and it is believed to be visualized through the uptake of transudates and exudates [20, 21].
It is well known that vascular endothelial growth factor (VEGF) is expressed in a number of normal adult tissues, including the kidney, lung, uterus, ovary, brain, heart, skin, pituitary gland and macrophages [22]. VEGF is produced by thyroid follicular epithelial cells in response to stimulation of the TSH receptor. Secreted VEGF might stimulate VEGF receptors on endothelial cells, leading to increased vascular permeability [23].
It has also been reported that in patients with hypothyroidism, large molecules such as glycosaminoglycans move to the interstitial space, producing an osmotic effect that leads to the movement of fluids from intravascular space to the extravascular space [24]. These mechanisms are believed to be the cause of pericardial effusion and pleural effusion in patients with hypothyrodism.
Pleural effusion in patients with hypothyroidism is minimal, which is why detection is difficult using other imaging modalities. Most are asymptomatic, so these patients generally do not require treatment other than thyroid hormone replacement. Although a significant proportion of these patients has pleural effusion, it is believed that this number is underestimated [25].
Because CT scans were generally taken preoperatively, or when evaluation for lung metastasis was warranted because of high serum Tg levels, there was a lower proportion of patients in the indeterminate uptake group that underwent chest CT who had normal serum Tg levels. Another factor was that patients with hypothyroidism only had minimal volumes of pleural effusion, as stated previously, which makes it difficult to detect on chest radiographs.
Another interesting finding from this study was that the TSH levels of the negative intrathoracic uptake group (96.9 ± 16.1) were higher than those of the indeterminate uptake group (76.9 ± 9.8) (p = 0.061). The mean TSH level of the negative intrathoracic uptake group patients who used rh-TSH (171.5 ± 14.1) was significantly different from that of the negative thoracic uptake group who underwent hormone withdrawal (67.7 ± 14.6) (p = 0.001), suggesting that this was due to the use of rh-TSH. It has been reported that the use of rh-TSH produces higher TSH levels compared to thyroid hormone withdrawal [26]. Actually, the TSH level of the indeterminate uptake group of hormone withdrawal patients was higher (76.9 ± 9.8) than that of the negative intrathoracic uptake group of hormone withdrawal patients (67.7 ± 14.6); this was not statistically significant (p = 0.081). According to previous studies, pleural effusion due to hypothyroidism occurred in hypothyroidism patients with lower TSH levels than those of the hypothyroidism patients in this study [23, 25]. It has been reported that there is no correlation between the clinical manifestation and TSH levels in hypothyroidism patients; we therefore believe that the presence of pleural effusion is related more to the presence of hypothyroidism itself than to TSH levels [27].
The lung is an extrathyroidal tissue that expresses NIS, and other such extrathyroidal tissues include the salivary glands, stomach, mammary glands, colon and ovary. The presence of radioactive iodine uptake in extrathyroidal tissue is thought to be due to NIS expression [21]. Thyroid transcription factor-1 (TTF-1) is a transcription factor that plays an important role in the thyroid-specific expression of NIS. Another interesting study has shown that of the various extrathyroidal tissues, NIS mRNA along with TTF-1 mRNA is expressed only in the lung [28]. The lung showed a significant difference in NIS expression for hypo- and hyperthyroid status, suggesting that it may have an effect on the post-therapy scan of patients with hypothyroidism [28].
There have been reports of lung metastases in patients with well-differentiated thyroid cancer, with radioactive iodine uptake but without detectable Tg levels, which can lead to a doubt in judgment [29]. There is a possibility of lung metastasis in thyroid cancer, which shows radioactive iodine uptake in the indeterminate uptake group, but without Tg expression. According to previous studies, the incidence of distant metastasis in the initial diagnosis of well-differentiated thyroid cancer is 1–9 %, of which 53 % exist solely in the lung [30, 31]. Of these, histologically 2.35 % are from papillary cancer and 10.5 % from follicular cancer [31]. Of the indeterminate uptake group, all were papillary carcinomas. Even after exclusion of those patients with elevated Tg, 2.9 % patients showed indeterminate uptake, suggesting the unlikelihood of all being lung metastases.
The post-therapy scan, along with the serum Tg level, is an important diagnostic tool for the detection of metastases in thyroid cancer patients. However, it should be noted that it is difficult to differentiate between lung metastases and benign lesions using radioactive iodine uptake patterns alone. As shown in this study, diffuse intrathoracic uptakes are visualized on post-therapy scans in both indeterminate uptake and pulmonary metastasis groups.
Some limitations of this study include the fact that not all patients had available tomographic images, which may have resulted in erroneous classification. Accuracy of the classification may be further improved using SPECT/CT. A second limitation is the potential variability in the LID of the patients. As a result, there may be variation of the body iodine pool between the patients, which may affect the uptake of radioactive iodine. Another limitation was that as a retrospective study, clinical relevant factors such as cold history were not considered. Finally, we defined Tg levels of 2 ng/ml in patients with thyroid hormone withdrawal or 1 ng/ml in patients with rh-TSH injection, and anti-Tg Ab levels of 100 IU/ml as being positive, in order to exclude lung micrometastases. However, because this was not basesd upon absolute criteria, it was difficult to clearly differentiate these patients from those with micrometastases.
Future studies of this kind should be done by addressing these limitations.
Conclusion
A total of 39 patients (5.2 %) showed diffuse intrathoracic uptake on post-therapy scans. Diffuse intrathoracic uptake was also found in patients with confirmed pulmonary metastasis or risk of undetected micrometastasis as well as in patients without identified underlying pathology (2.9 %) with a higher frequency of showing positive uptake in patients who underwent thyroid hormone withdrawal. Therefore, an accurate interpretation of radioactive iodine scans with correlation of other imaging studies, clinical history and serum Tg levels has clinical importance for avoiding unnecessary treatment.
References
Gultekin SS, Dilli A, Arikok AT, Bostanci H, Hasdemir AO. The false-positive radioiodine I-131 uptake in the foreign body granuloma located in gluteal adipose tissue. Radiol Oncol. 2012;46(1):28–31. doi:10.2478/v10019-011-0016-5.
Mitchell G, Pratt BE, Vini L, McCready VR, Harmer CL. False positive 131I whole body scans in thyroid cancer. Br J Radiol. 2000;73(870):627–35.
Nair N, Basu S, Pakhale H. Unusual uptake of radioiodine in the chest in a patient with thyroid carcinoma. Br J Radiol. 2004;77(913):63–7.
Yoon S, Hong I. Ovarian teratoma mimicking metastasis on I-131 scan: a case report. Nucl Med Mol Imaging. 2013;47(1):52–4. doi:10.1007/s13139-012-0167-3.
Lee EK, Kim KW, Park SY, Park YJ, Kim YT, Chung JK, et al. Mediastinal uptake misinterpreted as metastasis in papillary thyroid cancer. J Korean Endocr Soc. 2007;22(6):460–4.
Triggiani V, Moschetta M, Giagulli VA, Licchelli B, Guastamacchia E. Diffuse 131I lung uptake in bronchiectasis: a potential pitfall in the follow-up of differentiated thyroid carcinoma. Thyroid Off J Am Thyroid Assoc. 2012;22(12):1287–90. doi:10.1089/thy.2011.0439.
Luster M, Lippi F, Jarzab B, Perros P, Lassmann M, Reiners C, et al. rhTSH-aided radioiodine ablation and treatment of differentiated thyroid carcinoma: a comprehensive review. Endocr-Relat Cancer. 2005;12(1):49–64. doi:10.1677/erc.1.00830.
Mazzaferri EL, Kloos RT. Using recombinant human TSH in the management of well-differentiated thyroid cancer: current strategies and future directions. Thyroid Off J Am Thyroid Assoc. 2000;10(9):767–78.
Cooper DS, Doherty GM, Haugen BR, Kloos RT, Lee SL, Mandel SJ, et al. Revised American Thyroid Association management guidelines for patients with thyroid nodules and differentiated thyroid cancer. Thyroid Off J Am Thyroid Assoc. 2009;19(11):1167–214. doi:10.1089/thy.2009.0110.
Han JM, Kim WB, Yim JH, Kim WG, Kim TY, Ryu JS, et al. Long-term clinical outcome of differentiated thyroid cancer patients with undetectable stimulated thyroglobulin level one year after initial treatment. Thyroid Off J Am Thyroid Assoc. 2012;22(8):784–90. doi:10.1089/thy.2011.0322.
Cho SW, Choi H, Yeom GJ, Lim JA, Moon JH, Park DJ, et al. Long-term prognosis of differentiated thyroid cancer with lung metastasis in Korea and its prognostic factors. Thyroid Off J Am Thyroid Assoc. 2013. doi:10.1089/thy.2012.0654.
Lin JD, Chao TC, Chou SC, Hsueh C. Papillary thyroid carcinomas with lung metastases. Thyroid Off J Am Thyroid Assoc. 2004;14(12):1091–6. doi:10.1089/thy.2004.14.1091.
Ilgan S, Karacalioglu AO, Pabuscu Y, Atac GK, Arslan N, Ozturk E, et al. Iodine-131 treatment and high-resolution CT: results in patients with lung metastases from differentiated thyroid carcinoma. Eur J Nucl Med Mol Imaging. 2004;31(6):825–30. doi:10.1007/s00259-004-1460-x.
Ahn BC, Lee SW, Lee J, Kim C. Pulmonary aspergilloma mimicking metastasis from papillary thyroid cancer. Thyroid Off J Am Thyroid Assoc. 2011;21(5):555–8. doi:10.1089/thy.2010.0448.
Bakheet SM, Hammami MM, Powe J, Bazarbashi M, Al Suhaibani H. Radioiodine uptake in inactive pulmonary tuberculosis. Eur J Nucl Med. 1999;26(6):659–62.
Bakheet SM, Powe J, Hammami MM. Radioiodine uptake in the chest. J Nucl Med Off Publ Soc Nucl Med. 1997;38(6):984–6.
Maslack MM, Wilson CA. Iodine-131 accumulation in a pericardial effusion. J Nucl Med Off Publ Soc Nucl Med. 1987;28(1):133.
Ji JS, Chae HS, Cho YS, Kim HK, Kim SS, Kim CW, et al. Myxedema ascites: case report and literature review. J Korean Med Sci. 2006;21(4):761–4.
Mahajan SK, Machhan PC, Sood BR, Taneja S, Raina R, Thakur S, et al. Pitting oedema in hypothyroidism. J Assoc Physicians India. 2003;51:885.
Nascimento C, Bridji B, Dejax C, Schlumberger M, Leboulleux S. Thoracic 131I uptake after previous pneumonectomy in patients treated for differentiated thyroid cancer. Clin Nucl Med. 2012;37(6):587–90. doi:10.1097/RLU.0b013e3182485146.
Oh JR, Ahn BC. False-positive uptake on radioiodine whole-body scintigraphy: physiologic and pathologic variants unrelated to thyroid cancer. Am J Nucl Med Mol Imaging. 2012;2(3):362–85.
Soh EY, Duh QY, Sobhi SA, Young DM, Epstein HD, Wong MG, et al. Vascular endothelial growth factor expression is higher in differentiated thyroid cancer than in normal or benign thyroid. J Clin Endocrinol Metab. 1997;82(11):3741–7.
Hataya Y, Akamizu T, Kanamoto N, Moriyama K, Shimatsu A, Nakao K. A case of subclinical hypothyroidism developing marked pleural effusions and peripheral edema with elevated vascular endothelial growth factor. Endocr J. 2007;54(4):577–84.
Stathatos N, Wartofsky L. Perioperative management of patients with hypothyroidism. Endocr Metab Clin N Am. 2003;32(2):503–18.
Gottehrer A, Roa J, Stanford GG, Chernow B, Sahn SA. Hypothyroidism and pleural effusions. Chest. 1990;98(5):1130–2.
Carvalho MR, Ferreira TC, Leite V. Evaluation of whole-body retention of iodine-131 ((131)I) after postoperative remnant ablation for differentiated thyroid carcinoma—thyroxine withdrawal versus rhTSH administration: a retrospective comparison. Oncol Lett. 2012;3(3):617–20. doi:10.3892/ol.2011.523.
Zulewski H, Muller B, Exer P, Miserez AR, Staub JJ. Estimation of tissue hypothyroidism by a new clinical score: evaluation of patients with various grades of hypothyroidism and controls. J Clin Endocrinol Metab. 1997;82(3):771–6.
Harun-Or-Rashid M, Asai M, Sun XY, Hayashi Y, Sakamoto J, Murata Y. Effect of thyroid statuses on sodium/iodide symporter (NIS) gene expression in the extrathyroidal tissues in mice. Thyroid Res. 2010;3(1):3. doi:10.1186/1756-6614-3-3.
Singh B, Bollmann R, Ahmadzadehfar H, Biersack HJ, Ezziddin S. Unusual case of well-differentiated papillary thyroid carcinoma lacking thyroglobulin expression while still concentrating radioiodine. Br J Radiol. 2006;79(945):e84–7. doi:10.1259/bjr/62250180.
Lee J, Soh EY. Differentiated thyroid carcinoma presenting with distant metastasis at initial diagnosis clinical outcomes and prognostic factors. Ann Surg. 2010;251(1):114–9. doi:10.1097/SLA.0b013e3181b7faf6.
Shaha AR, Ferlito A, Rinaldo A. Distant metastases from thyroid and parathyroid cancer. ORL J Oto-rhino-laryngol Relat Spec. 2001;63(4):243–9.
Conflict of interest
The authors declare no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Choi, H.S., Kim, S.H., Park, S.Y. et al. Clinical Significance of Diffuse Intrathoracic Uptake on Post-Therapy I-131 Scans in Thyroid Cancer Patients. Nucl Med Mol Imaging 48, 63–71 (2014). https://doi.org/10.1007/s13139-013-0234-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13139-013-0234-4