Abstract
Chemical cues play an important role in short-range communication of butterflies, remarkably in sexual attraction and mate choice. Differentiated scale patches on the wings of male butterflies, the androconia, are involved in the emission of pheromones. Here, we describe the androconial morphology of six sympatric species of Neotropical sulphur butterflies belonging to two genera of the Colias-clade (Pieridae) based on SEM imaging. Gas chromatography-mass spectrometry analyses were used to access the chemical compositions of androconial secretions, which were comparatively investigated to determine species-specific trends and to verify if they yield a phylogenetic signal. The androconial patches from all species are differentiated from the non-androconial male wing surface and exhibit morphological features that may act in both preventing the volatilization of secretions and facilitating the release of semiochemicals, such as high density and length of scales and large perforations in the upper lamellae. A total of 55 compounds were exclusive to the androconia, and unique chemical profiles are present in each butterfly species, verified through multivariate analysis. The majority of androconial compounds were autapomorphic for each species and only four were dominant in more than one species. Cluster analyses placed the two species of Anteos in a single clade, but otherwise evidenced low similarities in the androconial secretion compositions among species, and a moderate correlation between genetic distances and chemical dissimilarities was obtained. Our findings suggest that androconial substances are involved in mating-oriented strategies and might be associated with the evolutionary history of the reproductive isolation of sulphurs.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Visual and chemical cues play complementary roles in the sexual behaviour of butterflies (Constanzo and Monteiro 2007; Smadja and Butlin 2009). While visual cues are involved in both long- and short-range intraspecific interactions, chemical cues are preponderant at close interactions among the Papilionoidea (Siberglied 1984; Vane-Wright and Boppré 1993). The relative importance of each stimulus on butterfly mate recognition and mate choice may vary according to species (Papke et al. 2007; Constanzo and Monteiro 2007), but this multimodal signalling plays a pivotal role in pre-zygotic reproductive isolation among sympatric closely related species (Bacquet et al. 2015). Thus, it is assumed that at least one of these components (e.g. colour pattern, UV reflection, pheromones) is species-specific.
Some Coliadinae butterflies (Lepidoptera: Pieridae) are given the vernacular denomination of ‘sulphurs’, which is associated to the bright white/yellowish colour of their wings from the deposition of pterin pigments on the scales (Watt 1964). In northeastern Brazil, there are six sympatric species of sulphur butterflies belonging to the monophyletic Colias–clade (sensu Wahlberg et al. 2014), among which males are readily distinguished from one another by wing colour pattern. Since visual stimuli play a major role in conspecific recognition of butterflies, including Pieridae—revised on Kemp and Rutowski (2011)—, a first glance assumption can be that the pronounced differences in wing colour patterns would suffice for females to recognise conspecific males. However, males of all six species have differentiated, sex-specific scale patches on their wings—the androconia. These structures are involved in the emission of chemical compounds exclusive to male lepidopterans—especially butterflies and diurnal moths—which function as aphrodisiacs (Pliske and Eisner 1969; Nieberding et al. 2008; Yildizhan et al. 2009) or anti-aphrodisiacs (Estrada et al. 2011). Size, number and location of the androconia vary depending on the species, and their morphology appears to be related to the storage and discharge of pheromones produced by the secretory cells which are usually located at the base of the androconia (Kristensen and Simonsen 2003). For example, during courtship and for aggregation purposes, male Ithomiini butterflies (Nymphalidae, Danainae) display otherwise concealed erectile alar fringes involved in the dispersion of volatile compounds (Schulz et al. 2004). The androconia of male pierid butterflies are typically arranged in dense clusters of scales (Barth 1960) that do not have any protection to prevent evaporation of secretions besides the overlapping of the wings, when at rest. Maybe because of this lack of specialization in concealment, highly volatile androconial compounds would be disadvantageous and, in fact, the androconial chemical bouquets of pierids such as Colias (Coliadinae) may present large amounts of heavier and less volatile compounds (Grula et al. 1980). On the other hand, compounds with higher volatility, such as geranial and neral, occur in high concentrations in Pieris (Pierinae) and are released mainly during flight activity (Andersson et al. 2007).
Results are contrasting regarding a phylogenetic signal of androconial secretions in different groups of butterflies. When the chemical compounds of sympatric, mimetic species were analysed, limited phylogenetic signal was found within both Ithomiini (Schulz et al. 2004) and Heliconiini (Mann et al. 2017) (Nymphalidae: Danainae and Heliconiinae, respectively). A sophisticated convergent aposematism (e.g. bright colour, high-contrast patterns) might cause confusion in intraspecific recognition, as observed in Heliconius (Estrada and Jiggins 2008). Thus, it is reasonable to assume that in order to avoid hybridization, distinct chemical signatures must be adopted between closely related comimetics (Mérot et al. 2015). Nonetheless, androconial secretions of mimetic milkweed butterflies (Nymphalidae: Danainae) exhibit strong phylogenetic chemical signalling in the species level (Schulz et al. 1993). In the same way, a strong correlation was demonstrated to occur between secretions of androconia and genetic distances of partially sympatric species of Pyrgus (Hesperiidae: Pyrginae) (Hernández-Roldán et al. 2014). Species of this genus are morphologically very similar but do not rely on mimicry.
In this study, we present a different scenario, in which six closely related, apparently non-mimetic species belonging to the Colias-clade co-occur. We aimed to (1) describe the morphology of the androconia of the six species and access the biological significance of key features; (2) investigate whether there are chemical compounds exclusive to the androconial patches in relation to the remaining non-androconial wing surface and to those of the wings of conspecific females and (3) compare the composition of androconial secretions among the six species and verify whether there is phylogenetic signal for this trait.
Materials and methods
Studied butterflies
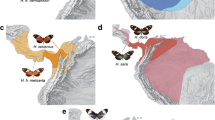
We investigated the sulphurs Anteos clorinde (Godart, [1824]), A. menippe (Hübner, [1818]), Phoebis argante (Fabricius, 1775), P. philea (Linnaeus, 1763), P. marcellina (Cramer, 1777) and Phoebis statira (Cramer, 1777) (Fig. 1). We followed the classification of Murillo-Ramos et al. (2018), who proposed Aphrissa as a synonym of Phoebis, and of Núñez et al. (2020), who raised P. marcellina to the species rank. All these species are typical of sunny, open areas, sympatric and to some extent, synchronic in northeastern Brazil in the same manner to what happens on most of their range (DeVries 1987; Brown Jr. 1992).
Male dorsal wing colour patterns of the Colias-clade butterflies of northeastern Brazil: (a) Anteos clorinde, (b) Phoebis argante, (c) A. menippe (d) P. philea, (e) P. statira, and (f) P. marcellina. Details of the androconia (circles to the right), showing the shapes and colour distinction from the surrounding scales. The white arrow indicates a tuft of hair-like scales below the androconial patch
Scanning electron microscope imaging and description of androconial scales
The androconial patches and surrounding wing areas of dry mounted males belonging to the six studied species were excised from the wings using surgical scissors which were rinsed with hexane and dried between uses. The samples were placed in aluminium stubs with double-sided carbon adhesive tape and coated with 80% gold/20% palladium in a Jeol Datum Ion Sputter JFC-1100. The metalized samples were examined in a Zeiss EVO LS15 scanning electron microscope and images were obtained at an electron high tension of 10 kV. The density of scales was estimated by counting one quadrant of a 1 mm2 area and multiplying the value by four. The length of the scales (both androconial and ordinary) was measured from base to apex for each species (mean ± standard deviation; n = 10 per species).
The overall shape, perforations (windows) and density of the scales surrounding the androconial patches vary noticeably, depending on the area of the wing they cover. Therefore, for comparative purposes, we considered only the ordinary scales laterally adjacent to the androconia. To describe the scales, we followed Downey and Allyn (1975) with adaptations. The nomenclature for the microstructures of the scales follows Ghiradella (1989). For the wing areas and venation nomenclature, we followed DeVries (1987).
Sampling and chemical analyses of androconial secretions
Butterflies were sampled in the municipalities of Recife (8° 03′ 10″ S, 34° 56′ 51″ W) and Buíque (8° 35′ 11″ S, 37° 08′ 52″ W), state of Pernambuco, northeastern Brazil. All individuals were captured in situ visiting flowers of Ixora coccinea L. (Rubiaceae) or Bougainvillea spectabilis Willd. (Nyctaginaceae) from 09:00 to 13:00 h.
The methodology of sampling, injection and chemical analyses of the androconial secretions was adapted from Mann et al. (2017). Immediately following capture, the individuals were sacrificed by thorax compression while inside the insect net. Androconial patches on the wings of each male (n = 5–12/species) were excised and inserted into clear 2-ml silanized glass vials containing hexane (≥ 99.7% purity, Sigma–Aldrich, USA; bidistilled prior to use). In order to latter exclude from our analyses the chemical compounds not exclusive to the androconia, the same procedure was applied to equivalent excised wing areas from conspecific females (n = 2/species) and to non-androconial male wing areas (n = 1/species). In all cases, the butterflies were manipulated by the thorax to avoid contamination of the wings. Solvent negative controls were obtained for each sampling event (n = 6). The crude solvent extracts were filtered through a silanized glass Pasteur pipette plugged with silanized glass wool (Sigma-Aldrich, USA) to remove floating scales and other debris, then concentrated to approximately 25 μl under a laminar N2 flow and kept at − 24 °C refrigeration until further processing procedures.
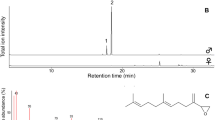
For gas chromatography-mass spectrometry (GC-MS) analyses, we used a gas chromatograph coupled to a mass spectrometer (GC-MS; Agilent 7890A™ gas chromatograph, Agilent 5975C Series MSD™ mass spectrometer) and equipped with a non-polar HP-5ms column (Agilent J&W; 30 m × 0.25 mm d.i., 0.25 μm film thickness). A split/splitless inlet was fitted with an Agilent Thermal Separation Probe (TSP). For each sample, 1 μl of the eluate was injected into quartz microvials which were then inserted in the TSP vial holder with the inlet set to split mode (1:1) and the injector temperature set to 250 °C. GC oven temperature was set at 60 °C for 2 min and then increased at a rate of 10 °C/min-1 to 280 °C, which was held steady for 12 min. The carrier gas flow was maintained at a constant pressure of 7.3 psi. MS Source and quadrupole temperatures were set at 230 °C and 150 °C, respectively. Mass spectra were taken at 70 eV in EI mode with a scanning speed of 1.0 scan-s from m/z 35–450.
After the chromatograms were obtained, their peak areas were integrated by using the software MSD ChemStation E.02.01.1177 (Agilent Technologies, Palo Alto, USA) to obtain the total ion current signal. A homologous series of linear alkanes (C8–C40) was used to determine linear retention indices (RI) of the compounds (Van den Dool and Kratz 1963), which were then tentatively identified by comparing their mass spectra and retention indices with those of reference samples available from personal and commercial mass spectral libraries (FFNSC 2, MassFinder 4, NIST14 and Wiley Registry™ 9th ed.). Mass spectra of target compounds and their calculated retention indices were also cross referenced with data published by Hayashi et al. (1977), Grula et al. (1980), Honda and Kawatoko (1982) and Yildizhan et al. (2009). Additionally, the authors consulted mass spectrometry specialist Prof. Dr. Stefan Schulz (Technische Universität Braunschweig, Germany) for input on identifications. We only considered peaks exclusive to the androconial extracts and that were present in more than two individuals, which were used to determine the relative percentages of each compound per sample. In that way, some compounds such as the cuticular hydrocarbon 13-methylheptacosane—an important male pheromone of the sulphur Colias philodice Godart, 1819 (Grula et al. 1980)—were excluded from our characterization of androconial secretions because they were present in female or non-androconial male wing extracts as well. For comparative analyses of androconial extracts, we established as a threshold that compounds should be present at over 1% of the total individual peak area in at least one of the analysed samples for any species. Compounds with relative concentrations higher than 10% of total peak area were classified as ‘dominant’, whereas ‘minor compounds’ were present at concentrations between 1 and 10%. We considered compounds that eluted latter than pentacosane (RI = 2500) as lowly volatile (Table 2), according to Mann et al. (2017), which also worked on a non-polar column (HP-5ms). Non-polar GC columns are made with poorly selective stationary phases, and thus commonly applied to separate non-polar compounds roughly based on volatility.
Statistical analyses
The presence/absence of componds and their relative percentages were used to generate Jaccard- and Bray Curtis-based similarity matrices respectively, from which non-metric multidimensional scaling analyses were performed in PAST 4.02 (Hammer et al. 2001). To verify if the chemical profile within each category (i.e. species) was significantly different, ANOSIM analyses wereperformed. In order to avoid bias from excessive weight of major compounds, their relative amounts were submitted to a a priori square root transformation.
To verify whether the results reflect the phylogenetic arrangement of the studied species, resulting dendrograms were compared to the most recent phylogeny of the group (Murillo-Ramos et al. 2018) and a Mantel test was performed to correlate the matrices of chemical dissimilarity and genetic distance using the packages Vegan (Oksanen et al. 2013) and Ade4 (Dray and Dufour 2007) in R software (R Core Team 2016). The genetic distance matrix was built by calculating pairwise distances of the mitochondrial cytochrome C oxidase 1 (COI) gene using the Kimura 2-parameter (K2P) model (Kimura 1980), with the data available from the Barcode of Life Data System (BOLD) platform (Ratnasingham and Hebert 2007) (Supplementary table S1) with MEGA-X 10.1 (Kumar et al. 2018). We excluded A. menippe from this analysis because genetic data is not available for this species.
Results
Morphology of androconial scales
The androconia of the forewings are always on the ventral side while those of the hindwings are dorsally positioned (Table 1). Unless specified otherwise in Table 1, the scale sockets are always visible, pedicels are positioned at the basis of the median line and the longitudinal ridges on the lamina are parallel and straight (Fig. 2). The colour of androconial scales is always paler than that of the surrounding ordinary scales (Fig. 1) and contrarily to the latter, the morphology of androconial scales is relatively constant intraspecifically. Androconial scales are 1.5 to 4 times more densely arranged in the wing surface than the ordinary scales (Table 1, Fig. 2) and inserted in the wing membrane at wider angles (not measured). The windows (perforations) and the respective crossribs on the upper lamella are absent or less evident in the periphery of the scales and are usually less evenly arranged than that of the ordinary scales (Table 1, Fig. 2).
SEM photographs of wing scales of the Colias-clade butterflies of northeastern Brazil. The androconial scales are on the upper portion of the images and the ordinary scales, on the lower portion. At × 500 magnification: (a) Anteos clorinde, (b) A. menippe, (c) Phoebis marcellina; at × 200 magnification: (d) P. argante, (e) P. philea, (f) P. statira. Details of the scales (circles to the right), showing the striae, crossribs and windows are at 2.5 k magnification, with exception of (c) and (e), at 5 k magnification. Details on circles to the left: (d) differentiated socket of hair at 5 k magnification and (f) dissimilar shapes of ordinary scales at 1.5 k magnification
Androconial secretions chemistry
A total of 55 compounds were found exclusively in the androconial extracts of the six species, of which 41 were autapomorphic (Table 2, Supplementary table S2). The most complex androconial chemical profile was obtained from Phoebis philea, with the highest number of both overall and species-exclusive compounds (23 and 14, respectively). It also presented the highest number of compounds with high RIs (suggesting lower volatility), and it is in this amplitude that the most dominant constituents appear (Table 2; Supplementary table S2). This also occurred in P. statira, but the profiles of the androconial extracts of the two other congenerics are rather distinct. Only a few compounds identified in P. argante and P. marcellina have high RIs, whereas the most dominant ones are much more volatile: in P. marcellina, benzyl salicylate, an unassigned hexadecenoic acid, and hexadecanoic acid and in P. argante, 6,10,14-trimethyl-pentadecan-2-ol, which comprises ca. 90% of the total blend (Table 2). Androconial solvent extracts of P. argante contained the least number of compounds (6) and most restricted molecular weight amplitude (Supplementary table S2); nonetheless, as only five samples were analysed, it is plausible that this species was under sampled.
As a general observation, the chemical blends of androconial extracts from all species revealed few dominant compounds: two or three compounds corresponded to over 50% of the total blend, while a wider array of compounds occurred in lower concentrations (Table 2; Supplementary table S2).
Although there were slight intraspecific variations in the androconial chemical composition, each species exhibited a characteristic chemical profile (p = 0.001, Global-R = 0.999, Bray Curtis-based; p = 0.001, Global-R = 1, Jaccard-based) and the NMDS ordination segregated the samples (or individuals) in a strongly grouped distribution, relative to species (Fig. 3, Supplementary figure S1). Some compounds were found in androconial extracts of more than one species, but only four were dominant (≥ 10%/species) across species: benzyl salicylate in A. menippe and P. marcellina; hexadecanoic acid in P. statira and P. marcellina; an unidentified compound (unassigned compound 5) in P. statira and P. philea and an unidentified aliphatic ester (unid. aliphatic ester 5) in both species of Anteos (Table 2). These co-occurrences of dominant compounds heavily influenced the clustering pattern, as evidenced by the comparison of presence/absence to the relative abundances data (Supplementary figure S2). The Bray Curtis analysis yielded higher similarities among males of the two species of Anteos; the males of A. clorinde did not even form a cluster of their own, but were rather clustered together with A. menippe (Fig. 4, Supplementary figure S2 - A). Furthermore, higher similarities were obtained between P. marcellina and Anteos spp. than between the former and its congenerics (Fig. 4). Although some dominant compounds co-occurred in two, sometimes three of the investigated species of Phoebis (Table 2, Supplementary table S1), no androconial chemical synapomorphy was identified for the genus as a whole. The four species in our study presented very low similarity values, and the chemical blend of P. argante actually only shared a single minor compound—linoleic acid—with congenerics (Table 2; Supplementary table S2). As result, all samples of P. argante formed an outgroup distant from the remainder sulphurs (Fig. 4, Supplementary figure S1). The Mantel tests revealed moderate positive correlations between genetic distances and chemical dissimilarities of the group (r = 0,593, p = 0.001, Bray Curtis-based; r = 0.509, p = 0.001, Jaccard-based) (Fig. 5, Supplementary figure S3).
Comparison between the (a) phylogenetic tree adapted from Murillo-Ramos et al. (2018) and (b) the dendrogram based on the Bray Curtis similarity index from the androconial chemistry of the Colias-clade butterflies of northeastern Brazil
Discussion
Morphology of androconial scales
The overall morphology of the wing scales of the six investigated species is typical of higher Lepidoptera: hollow structures that contain numerous striae and transversal crossribs that delimit perforations along the upper lamella (Kristensen 1970). The androconial patches, however, are clearly differentiated from the typical scales of the surrounding wing areas by peculiar features such as paler colour, higher density and length of scales and larger windows in the lamella. There is no apparent specialized structure on the wing membrane to protect those patches and reduce volatilization of secretions of the studied butterflies, such as the ‘pocket-like cavities’ in the Danaini (Boppré 1993) and Ithomiini (Schulz et al. 2004). Nevertheless, the androconial patches of all species are invariably placed on the ‘friction areas’ of the wings, be it ventrally (post-basal to submedial region of CuA2) or dorsally (post-basal to medial region of Sc + R1). These areas remain overlapped when the animal is at rest, a mechanism suggested by Rutowski (1980) as a mean of reducing the volatilization of androconial secretions in species of Colias. This indeed seems to be the case of the Colias-clade sulphurs, given that there are no androconial patches located in other areas of the wings.
Compared to ordinary scales, androconial scales of the Colias-clade butterflies were longer (with the exception of P. marcellina and P. argante), and more compactly packed, with densities 1.5 to 4 times higher than that of ordinary scales. Similar ratios (≈ 2 androconial: 1 ordinary) were observed in species of Eurema and Colias (Pieridae: Coliadinae) (Vetter and Rutowski 1979), but not in Pieris (Pieridae: Pierinae), in which scent scales are widely distributed on the ventral surface of the wings, with a much lower density than that of ordinary scales (Yoshida et al. 2000). High density of scales may have a complementary effect to the abovementioned overlapping of the wings, as a preventive apparatus to the volatilization of secretions. In fact, most of the area occupied by androconial scales remain concealed by the upper and adjacent scales, with only the distal portions exposed.
The analysis of the micro sculpture patterns also revealed differences between androconial and ordinary scales. More prominent crossribs and larger windows occur in the upper lamella of the androconial scales, especially in the mid portion. The biological significance of the latter trait is not clear, but it may be related to a rapid release of male pheromones during courtship behaviour. According to Barth (1960), a suitable area in which volatilization of the secretion takes place is a distinguishing feature of androconial scales. Thus, by increasing the contact surface, the enlarged windows in a scale-dense region may contribute to the release of androconial secretions into the air or even facilitate the contact of less volatile compounds with the sensorial surface of conspecific female antennae.
What appeared to be a more specialized structure for releasing pheromonal secretions was found only on the androconia of P. argante males, in the form of a dense patch of long hair-like scales. Males of the remaning species lack those hairs on the androconial patches, but present similar features (‘fringes’) on the anal margin of the forewings (Barth 1960; Bergström and Lundgren 1973), a ‘friction area’ that is close to the ventral androconia and likely to rub into them during flight or courtship behaviour. Friction of the androconial patches by hovering or buffeting the wings near females is a widespread male behaviour among the Coliadinae (Rutowski 1978; Silberglied and Taylor 1978; Vetter and Rutowski 1979; Kan and Hidaka 1997). So, those fringes might have an analogous function to that of the long hair-like scales of P. argante and the potential to act as a facilitator for the release of androconial secretions. This could be considered as a composite system—an area where secretions are produced, associated to a structure that aids their release—as proposed by Barth (1960).
Androconial secretions chemistry
A wide array of compounds exclusive to androconial extracts were found for all investigated species. These chemicals occur precisely in the androconial patches described in this study, which demonstrates that, at least in the wings, their storage and release is restricted to these morphologically specialized areas. Also, we have shown that the composition of androconial secretions is species-specific, suggesting that these compounds take part in intraspecific chemical communication. Behavioural experiments have consistently demonstrated that certain male wing secretions function as sex pheromones in butterflies (Constanzo and Monteiro 2007; Darragh et al. 2017) including Pieridae (Taylor 1973; Silberglied and Taylor 1978; Grula et al. 1980; Rutowski 1980; Kan and Hidaka 1997; Andersson et al. 2007; Yildizhan et al. 2009). Although we did not conduct such experiments, the absence of androconial constituents from the wings of conspecific females offers compelling evidence of their involvement in mate recognition and/or attraction.
Our results revealed distinct chemical profiles for each investigated species of sulphur. Among butterflies, the degree of chemical differentiation of androconial profiles seems to vary case by case, as shown in studies with sympatric Nearctic and Palearctic Pieridae. Colias philodice and C. eurytheme Boisduval, 1852 present significant qualitative compositional differences (Grula et al. 1980), whereas for the closely related Pieris melete Ménétriés, 1857 and P. napi Linaeus, 1758, very similar androconial chemical profiles were evidenced. In this later case, marked dissimilarities were restricted to the concentrations of two stereoisomeric monoterpenes (neral and geranial) and a species-specific presence of another (linalool) (Hayashi et al. 1977). Among the species in our study, large qualitative and quantitative chemical differences were observed, which reinforces the highly species-specific character of the constituents in androconial secretions. This strong differentiation of sex-related traits, if properly recognised by the females, may act alongside the also very distinct species-specific male colour patterns, leading to a robust pre-zygotic isolation mechanism. The complementary use of visual and chemical signs to recognise conspecific males is documented for female butterflies (Silberglied and Taylor 1978; Constanzo and Monteiro 2007). Nevertheless, major differentiation of chemical profiles per se does not necessarily imply a more efficient mechanism of sexual isolation. Female butterflies may rely on different compound concentrations or on the presence of a few or even single constituents within the total androconial chemical blend of conspecific males for mate recognition (Hayashi et al. 1977; Yildizhan et al. 2009). Further investigation is required to elucidate the roles of individual compounds and their blends in the behaviour of the Colias-clade sulphurs.
The most diverse androconial blend among the species in our study belongs to Phoebis philea. It presents a dominance of lowly volatile compounds (those eluting later than pentacosane on a non-polar GC column), which may be a clue to behavioural aspects of courtship of this species. If in fact those compounds are involved as semiochemicals in mate recognition and quality assessment, we could expect substantial androconia-antennae contact between males and females. This behaviour was indeed reported for P. marcellina (Rutowski 1983), in whose androconial secretions we identified a predominance of more volatile compounds than those of P. philea. Presumed lowly volatile pheromones are also found in other species of Pieridae (Rutowski 1980; Grula et al. 1980; Sappington and Taylor 1990) and Danainae (Meinwald et al. 1969; Meinwald et al. 1974; Schulz et al. 1993), in which direct contact occurs during courtship (Pliske and Eisner 1969). On the other hand, high volatile compounds were characteristic of particular species such as limonene in P. marcellina and farnesene in A. menippe and P. marcellina. It is believed that butterflies also use volatiles as mating cues at close-range communication (Boppré 1984). Therefore, such volatiles may not be negligible just because of their low concentrations in relation to the major compounds.
Few of the major compounds found in our study were previously reported in butterflies: 13-methylpentacosane as a contact sex pheromone in the sulphur Colias philodice (Grula et al. 1980), hexadecanoic acid in the male hairpencils of many species of Danaini (Schulz et al. 1988; Schulz et al. 1993) and 2-phenylethyl hexadecanoate as a major androconial compound in the nymphalid genus Bicyclus (Wang et al. 2014). On the other hand, many compounds we identified in sulphurs were previously unknown from Pieridae and Lepidoptera. For minor blend constituents, this may be due to advances achieved in the analytical methodology when compared to the results of previous exploratory works on the androconial chemistry of Pieridae, which date to ca. 40 years ago (Hayashi et al. 1977; Kuwahara 1979; Grula et al. 1980; Honda and Kawatoko 1982). Nonetheless, in the case of major constituents, because we are dealing with species of a poorly investigated Neotropical clade from a chemistry standpoint, a number of novelties were arguably expected to occur. For example, as far as we are aware, benzyl salicylate has not been recorded as an lepidopteran semiochemical to date. It is nonetheless reported as a male sex pheromone component of the Florida woods cockroach (Eurycotis floridana Walker, 1868; Farine et al. 1994) and a constituent in floral scents across different families of angiosperms (Knudsen et al. 2006). It is present in three of the six Colias-clade species in our investigation, being a major constituent in the androconial secretions of A. menippe and dominant in P. marcellina. The compound has fixative properties (Sturm and Peters 2007), which could arguably interfere with the volatility and tenacity of other compounds in the androconial blends.
When compared to recent phylogenies of the group (Wahlberg et al. 2014; Murillo-Ramos et al. 2018; Núñez et al. 2020), the chemical similarity of the androconial blends reflected the phylogenetic proximity between the two investigated species of Anteos. However, neither the phylogenetic relationships among the four species of Phoebis nor between the two genera were supported. When treated as a whole, a moderate correlation between genetic distance and chemical dissimilarity was in fact noticed for the group, but aside the co-occurrence of a dominant unidentified aliphatic ester between the species of Anteos, assumed to be an informative synapomorphy, no such feature occurred among Phoebis. Because we are dealing with elements directly involved in mating and species recognition, a strong differentiation in such features is expected among closely related species in order to ensure reproductive isolation. Therefore, our results did not yield evidence of a conclusive phylogenetic signal on the chemical composition of androconial secretions for the Colias-clade butterflies.
Conclusions and perspectives
The investigation on the scale morphology of males evidenced androconial patches that are clearly differentiated from the non-androconial wing surface and exhibit particular features that could facilitate the release of semiochemicals, such as higher density of scales, higher length and larger windows in the upper lamellae, in comparison to the ordinary scales. Furthermore, the analyses of the androconial extracts of all six studied species revealed a wide array of species- and gender-exclusive compounds—some of which novel for Lepidoptera. The species-specific nature of the blends of the sympatric and ecologically similar species point towards the value of such diversification in mating-oriented strategies of butterflies. Nevertheless, a number of questions arose from our results: are all the androconial chemical components involved in sexual interactions or are some rather cues used in male-male communication? Are they directed exclusively to mate recognition or are there compounds that indicate male fitness/quality? Do they act as a backup for visual stimuli or do they provide distinct information? And, on which sensory cues, visual or chemical, do female butterflies rely more strongly? Some of these questions were assessed for Neartic pierids, and conclusions varied among species. Very diverse and species-specific androconial chemical blends occur in the Colias-clade sulphurs of northeastern Brazil; we expect that a wide array of different strategies are involved in their sex-oriented chemical communication as well.
Data availability
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.
References
Andersson, J., Borg-Karlson, A. K., Vongvanich, N., & Wiklund, C. (2007). Male sex pheromone release and female mate choice in a butterfly. Journal of Experimental Biology, 210, 964–970.
Bacquet, P. M. B., Brattström, O., Wang, H. L., Allen, C. E., Löfstedt, C., Brakefield, P. M., et al. (2015). Selection on male sex pheromone composition contributes to butterfly reproductive isolation. Proceedings of the Royal Society B: Biological Sciences, 282.
Barth, R. (1960) Órgãos odoríferos dos Lepidópteros. Boletim do Parque Nacional do Itatiaia 7, Ministério da Agricultura, Brazil.
Bergström, G. and Lundgren, L. (1973) Androconial secretion of three species of butterflies of the genus Pieris (Lep., Pieridae). Zoon a Journal of Zoology (Supplement 1), 67–75.
Boppré, M (1984) Chemically mediated interactions between butterflies. In R. I. Vane-Wright and P. R. Ackery (Eds.), The Biology of Butterflies (pp. 259-275). Simposia of the Royal Entomological Society of London 11, London and New York: Academic Press.
Boppré, M. (1993) The American monarch: courtship and chemical communication of a peculiar Danaine butterfly. In S. B. Malcolm. and M. P. Zalucki (Eds.), Biology and Conservation of the Monarch Butterfly (Vol. 38, pp. 29–41). Science Series. Natural History Museum of Los Angeles County, Los Angeles.
Brown Jr., K. S. (1992). Borboletas da Serra do Japi: diversidade, hábitats, recursos alimentares e variação temporal. In L. P. C. Morellato (Ed.), História Natural da Serra do Japi - Ecologia e preservação de uma área florestal no Sudeste do Brasil (pp. 142–187). Campinas: Editora da Unicamp.
Constanzo, K., & Monteiro, A. (2007). The use of chemical and visual cues in female choice in the butterfly Bicyclus anynana. Proceedings of the Royal Society B: Biological Sciences, 274, 845–851.
Core Team, R. (2016). R: a language and environment for statistical computing. R Software Version, 3(3), 1 http://www.r-project.org. Accessed 02 Feb 2020.
Darragh, K., Vanjari, S., Mann, F., Gonzalez-Rojas, M. F., Morrison, C. R., Salazar, C., et al. (2017). Male sex pheromone components in Heliconius butterflies released by the androconia affect female choice. PeerJ, 5, 3953.
DeVries, P. J. (1987). The butterflies of Costa Rica and their natural history. Volume I: Papilionidae, Pieridae and Nymphalidae. New Jersey: Princeton University Press.
Downey, J. C. and Allyn, A. C. (1975) Wing scale morphology and nomenclature. Bulletin of the Allyn Museum 31 Florida Museum of Natural History, Gainesville, Florida.
Dray, S., & Dufour, A. (2007). The ade4 package: implementing the duality diagram for ecologists. Journal of Statistical Software, 22(4), 1–20.
Estrada, C., & Jiggins, C. D. (2008). Interspecific sexual attraction because of convergence in warning coloration: is there a conflict between natural and sexual selection in mimetic species? Journal of Evolutionary Biology, 21(3), 749–760.
Estrada, C., Schulz, S., Yildizhan, S., & Gilbert, L. E. (2011). Sexual selection drives the evolution of antiaphrodisiac pheromones. Evolution, 6510, 2843–2854.
Farine, J.-P., Le Quere, J. L., Duffy, J., Everaerts, C., & Brossut, R. (1994). Male sex pheromone of cockroach Eurycotis floridana (Walker) (Blattidae, Polyzosteriinae): role and composition of tergites 2 and 8 secretions. Journal of Chemical Ecology, 20, 2291–2306.
Ghiradella, H. (1989). Structure and development of iridescent butterfly scales: lattices and laminae. Journal of Morphology, 202, 69–88.
Grula, J. W., McChesney, J. D., & Taylor, O. R. (1980). Aphrodisiac pheromones of the sulfur butterflies Colias eurytheme and C. philodice (Lepidoptera: Pieridae). Journal of Chemical Ecology, 6, 241–256.
Hammer, Ø., Harper, D. A. T., & Ryan, P. D. (2001). PAST: Paleontological statistics software package for education and data analysis. Palaeontologia Electronica, 4(1), 9.
Hayashi, N., Kuwahara, Y., & Komae, H. (1977). The scent scale substances of male Pieris butterflies Pieris melete and Pieris napi. Experientia, 34, 684.
Hernández-Roldán, J. L., Bofill, R., & Dapporto, L. (2014). Morphological and chemical analysis of male scent organs in the butterfly genus Pyrgus (Lepidoptera: Hesperiidae). Organisms Diversity and Evolution, 14, 269–278.
Honda, K., & Kawatoko, M. (1982). Exocrine substances of the white cabbage butterfly, Pieris rapae crucivora (Lepidoptera: Pieridae). Applied Entomology and Zoology, 17, 325–331.
Kan, E., & Hidaka, T. (1997). Role of male scent in the mating behavior of Pieris melete Ménétriès (Lepidoptera: Pieridae). Journal of Ethology, 15, 87–93.
Kemp, D. J., & Rutowski, R. L. (2011). The role of coloration in mate choice and sexual interactions in butterflies. Advances in the Study of Behavior, 43, 55–92.
Kimura, M. (1980). A Simple method for estimating evolutionary rates of base substitutions through comparative studies of nucleotide sequences. Journal of Molecular Evolution, 16, 111–120.
Knudsen, J. T., Eriksson, R., Gershenzon, J., & Ståhl, B. (2006). Diversity and distribution of floral scent. The Botanical Review, 72, 1–120.
Kristensen, N. P. (1970). Morphological observations on the wing scales in some primitive Lepidoptera (Insecta). Journal of Ultrastructure Rresearch, 30, 402–410.
Kristensen, N. P., & Simonsen, T. J. (2003). Hairs’ and cells. In N. P. Kristensen & T. J. Simonsen (Eds.), Lepidoptera, moths and butterflies vol. 2: morphology, physiology, and development, Handbuch der Zoologie/ Handbook of Zoology IV/36 (pp. 9–22). Berlin and New York: Walter de Gruyter.
Kumar, S., Stecher, G., Li, M., Knyaz, C., & Tamura, K. (2018). MEGA X: molecular evolutionary genetics analysis across computing platforms. Molecular Biology and Evolution, 35, 1547–1549.
Kuwahara, Y. (1979). Scent scale substances of male Pieris melete Ménétriès (Pieridae: Lepidoptera). Applied Entomology and Zoology, 14, 350–355.
Mann, F., Vanjari, S., Rosser, N., Mann, S., Dasmahapatra, K. K., Corbin, C., Linares, M., Pardo-Diaz, C., Salazar, C., Jiggins, C., & Schulz, S. (2017). The scent chemistry of Heliconius wing androconia. Journal of Chemical Ecology, 43, 843–857.
Meinwald, J., Meinwald, Y. C., & Mazzocchi, P. H. (1969). Sex pheromone of the queen butterfly: chemistry. Science, 164, 1174–1175.
Meinwald, J., Boriack, C. J., Schneider, D., Boppré, M., Wood, W. F., & Eisner, T. (1974). Volatile ketones in the hairpencil secretion of danaid butterflies (Amauris and Danaus). Experientia, 30(7), 721–723.
Mérot, C., Frérot, B., Leppik, E., & Joron, M. (2015). Beyond magic traits: multimodal mating cues in Heliconius butterflies. Evolution, 69, 2891–2904.
Murillo-Ramos, L., Torres, R. H., Águila, R. N., & Ayazo, R. (2018). New insights on the taxonomy and phylogenetic relationships of the Neotropical genus Phoebis (Pieridae: Coliadinae) revealed by molecular and morphological data. Zootaxa, 44571, 179–188.
Nieberding, C. M., De Vos, H., Schneider, M. V., Lassance, J. M., Estramil, N., Andersson, J., et al. (2008). The male sex pheromone of the butterfly Bicyclus anynana: towards an evolutionary analysis. Plos One, 3, e2751.
Núñez, R., Genaro, J. A., Pérez-Asso, A., Murillo-Ramos, L., Janzen, D. H., Hallwachs, W., Wahlberg, N., & Hausmann, A. (2020). Species delimitation and evolutionary relationships among Phoebis New World sulphur butterflies (Lepidoptera, Pieridae, Coliadinae). Systematic Entomology, 45(2), 481–492.
Oksanen, F. J., Oksanen, A., Blanchet, F. G., Kindt, R., Legendre, P. (2013). Vegan: community ecology package. R package Version 2.4-3. https://CRAN.R-project.org/package=vegan. Accessed 19 Feb 2020.
Papke, R. S., Kemp, D. J., & Rutowski, R. L. (2007). Multimodal signalling: structural ultraviolet reflectance predicts male mating success better than pheromones in the butterfly Colias eurytheme L (Pieridae). Animal Behavior, 73, 47–54.
Pliske, T. E., & Eisner, T. (1969). Sex pheromone of the queen butterfly: biology. Science, 164, 1170–1172.
Ratnasingham, S. and Hebert, P. D. N. (2007) Bold: the barcode of life data system. http://www.barcodinglife.org. Accessed 19 Feb 2020.
Rutowski, R. L. (1978). The courtship behaviour of the small sulphur butterfly, Eurema lisa (Lepidoptera: Pieridae). Animal Behavior, 26, 892–903.
Rutowski, R. L. (1980). Male scent-producing structures in Colias butterflies: function, localization, and adaptive features. Journal of Chemical Ecology, 6, 13–26.
Rutowski, R. L. (1983). Courtship leading to copulation in the cloudless sulphur, Phoebis marcellina (Pieridae). Journal of Research on the Lepidoptera, 22, 249–253.
Sappington, T. W., & Taylor, O. R. (1990). Genetic sources of pheromone variation in Colias eurytheme butterflies. Journal of Chemical Ecology, 16, 2755–2770.
Schulz, S., Francke, W., & Boppré, M. (1988). Carboxylic acids from hairpencils of male Amauris butterflies (Lep.: Danainae). Biological Chemistry, 369(2), 633–638.
Schulz, S., Boppré, M., & Vane-Wright, R. I. (1993). Specific mixtures of secretions from male scent organs of African milkweed butterflies (Danainae). Philosophical Transactions of the Royal Society B: Biological Sciences, 342, 161–181.
Schulz, S., Beccaloni, G., Brown, K. S., Boppré, M., Freitas, A. V. L., Ockenfelsd, P., et al. (2004). Semiochemicals derived from pyrrolizidine alkaloids in male ithomiine butterflies (Lepidoptera: Nymphalidae: Ithomiinae). Biochemical Systematics and Ecology, 32, 699–713.
Siberglied, R. E. (1984). Visual communication and sexual selection among butteflies. In R. I. Vane-Wright & P. R. Ackery (Eds.), The biology of butterflies (pp. 207–233). London: Academic Press.
Silberglied, R. E., & Taylor, O. R. (1978). Ultraviolet reflection and its behavioral role in the courtship of the sulfur butterflies, Colias eurytheme and C. philodice (Lepidoptera, Pieridae). Behavioral Ecology and Sociobiology, 3, 203–243.
Smadja, C., & Butlin, R. K. (2009). On the scent of speciation: the chemosensory system and its role in premating isolation. Heredity, 102, 77–97.
Sturm, W. and Peters, K., (2007) Perfumes. Ullmann’s Encyclopedia of Industrial Chemistry, 6th edn, Wiley, VCH.
Taylor, O. R. (1973). Reproductive isolation in Colias eurytheme and C. philodice (Lepidoptera: Pieridae). Use of olfaction in mate selection. Annals of the Entomological Society of America, 66, 621–626.
Van den Dool, H., & Kratz, P. D. (1963). A generalization of the retention index system including linear temperature programmed gas-liquid partition chromatography. Journal of Chromatography A, 110, 463–471.
Vane-Wright, R. I., & Boppré, M. (1993). Visual and chemical signalling in butterflies: functional and phylogenetic perspectives. Philosophical Transactions of the Royal Society B, 340, 197–205.
Vetter, R. S., & Rutowski, R. L. (1979). External sex brand morphology of three sulphur butterflies (Lepidoptera: Pieridae). Pyche a Journal of Entomology, 85(4), 383–393.
Wahlberg, N., Rota, J., Braby, M. F., Pierce, N. E., & Wheat, C. W. (2014). Revised systematics and higher classification of pierid butterflies (Lepidoptera: Pieridae) based on molecular data. Zoologica Scripta, 43, 641–650.
Wang, H. L., Brattström, O., Brakefield, P. M., Francke, W., & Löfstedt, C. (2014). Identification and biosynthesis of novel male specific esters in the wings of the tropical butterfly, Bicyclus martius sanaos. Journal of Chemical Ecology, 40, 549–559.
Watt, W. (1964). Pteridine components of wing pigmentation in the butterfly Colias eurytheme. Nature, 201, 1326–1327.
Yildizhan, S., van Loon, J., Sramkova, A., Ayasse, M., Arsene, C., Broeke, C., et al. (2009). Aphrodisiac pheromones from the wings of the small cabbage white and large cabbage white butterflies, Pieris rapae and Pieris brassicae. ChemBioChem, 10, 1666–1777.
Yoshida, A., Noda, A., Yamana, A., & Numata, H. (2000). Arrangement of scent scales in the male wings of the small white cabbage butterfly (Lepidoptera: Pieridae). Entomological Science, 3(2), 345–349.
Acknowledgements
We thank Renato Portela Salomão for his help with statistical analysis and Bruna Bezerra, Simão Vasconcelos, Robert Robbins and an anonymous reviewer for important insights to the manuscript. We are in great debt to Prof. Stephan Schulz, who kindly aided us in the challenging task of identifying many of the compounds found in the androconial extracts.
Funding
This study was supported by the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior – CAPES (Finance Code 001), which also provided a PhD scholarship to CEB Nobre, a Msc scholarship to LAS Lucas and a post-doctoral scholarship to ACD Maia (PNPD/CAPES 88882.306329/2018-01). ACD Maia is also supported by Fundação de Amparo à Ciência e tecnologia do Estado de Pernambuco – FACEPE (#BCT-0057-2.04/17).
Author information
Authors and Affiliations
Contributions
Carlos Eduardo B. Nobre conceived, designed and performed the research, collected and analysed the data, contributed with reagents and materials, prepared figures and tables, authored the drafts of the paper, approved the final draft.
Layse A. S. Lucas contributed with analysis tools, analysed the data, reviewed the drafts of the paper and approved the final draft.
Rafael J. R. Padilha contributed with materials and analysis, prepared figures and approved the final draft.
Daniela M. A. F. Navarro contributed with reagents, materials and analysis tools, and approved the final draft.
Luiz C. Alves contributed with reagents, materials and equipment, and approved the final draft.
Artur C. D. Maia conceived and designed the research, analysed the data, contributed with reagents and materials, edited figures, reviewed the drafts of the paper and approved the final draft.
Corresponding author
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Nobre, C.E.B., Lucas, L.A.d., Padilha, R.J.R. et al. Specialized androconial scales conceal species-specific semiochemicals of sympatric sulphur butterflies (Lepidoptera: Pieridae: Coliadinae). Org Divers Evol 21, 93–105 (2021). https://doi.org/10.1007/s13127-020-00475-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13127-020-00475-8