Abstract
Chemical communication in the family Hesperiidae (Lepidoptera) is practically unstudied, even though this group includes approximately 4,000 species and represents a fifth of the world’s butterfly fauna. We present the first comparative morphological and chemical analysis of scent organs for nine species in the genus Pyrgus, the most species-rich hesperiid genus in the Palearctic region. Our results show that the morphology of the two main male scent organs—the costal fold and the tibial tufts—does not differ between species. The chemical analyses detected a total of 125 different compounds exclusively present in these organs. We document great interspecific differences and much narrower intraspecific variability in the chemical profiles. The dynamics of chemical versus genetic distances indicate two different phases: a faster (but more variable) initial chemical divergence at lower genetic divergences (probably related to speciation) and a slower but more constant differentiation (drift). As a result most species can be identified based on their chemical profiles, except for a closely related species pair (P. malvae/P. malvoides) for which hybridisation is common in the contact zone. Our results suggest that the Hesperiidae is a group with great potential for the study of chemical communication that deserves further attention.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Premating assessment of specific traits is the most common cause of reproductive isolation in animals and usually constitutes a capital step in the process of speciation (Templeton 1981; Coyne and Orr 1989; Singh and Kulathinal 2000; Coyne and Orr 2004; Groot et al. 2006; Ritchie 2007). Sexual recognition is thus considered as a major force driving evolution and, alongside ecological selection, contributes to several cases of both sympatric and allopatric speciation (Ritchie 2007; Berlocher and Feder 2002; Kirkpatrik and Ravigné 2002). Insects rely on almost all possible sensory channels to identify suitable sexual partners. Among these, chemical communication plays a fundamental role. Actually, insects have developed one of the most complete systems of interspecific and intraspecific communication by olfactory cues (Grichanov 1998; Wyatt 2003).
In Lepidoptera, two main courtship strategies involving chemical communication exist: either males or females release pheromones to attract mates. Male pheromones are produced in specialised organs that contain scent scales called androconia (Boppré 1989). Female pheromones are typically, but not exclusively, produced by moths, in which the male finds the female at night. These have been extensively studied because synthetic pheromones can be used as a way to control agricultural pests. Indeed, female pheromones from more than 500 moth species have been characterised (Ando et al. 2004; Witzgall et al. 2004; El-Sayed 2009). Lepidoptera female pheromones are classified into type I (75 % of known pheromones), type II (15 %), and miscellaneous (10 %), according to their chemical structure. Primary alcohols and their derivatives, mainly acetates and aldehydes with a long straight chain (C10-C18), comprise the predominant group type I. Polyunsaturated hydrocarbons with a longer straight chain (C17-C23) and their epoxy derivatives comprise the type II pheromones (Ando et al. 2004). Finally, cuticular lipids have been also suggested to play a role in species and gender recognition as contact pheromones (Dapporto 2007).
Male pheromones are typically produced by butterflies, but also by several moths. In this case, males usually find potential mates by visual cues and male pheromones allow the female to test conspecificity and male condition during the courtship (Andersson et al. 2007). Because they act at close distances, these components are usually produced in low quantities. They have been studied in some families of moths (e.g. Ando et al. 2004; Birch and Hefetz 1987) and butterflies (e.g. Nishida et al. 1996; Schulz et al. 1988; Schulz et al. 2004). Several types of male scent organs have been identified in the Lepidoptera: in the form of protruding bifurcate formations (coremata) on genital segments in Arctiidae; androconial scales located on tarsi in Noctuidae, and on the thorax, abdomen or wings in Sphingidae and butterflies; hair-pencils at the base of the abdomen, on the genital area, or on the forewings in moths and in Danainae and Ithomiini butterflies, and on legs in some Pyrginae hesperiids (Ando et al. 2004; Birch and Hefetz 1987; Guillaumin 1963; Birch 1970a; Birch 1970b; Birch 1975; Baker et al. 1981; De Jong 1982; Fitzpatrick and McNeil 1988; Birch et al. 1990; Heath et al. 1992; Boppré 1993). The hair-pencil secretions produced by several species of Lepidoptera have been described (Nishida et al. 1996; Schulz et al. 1988; Schulz et al. 2004; Heath et al. 1992; Phelan et al. 1986; Teal and Tumlinson 1989; Thibout et al. 1994; Huang et al. 1996) and most often these scents have been deemed important in courtship behaviour.
Hesperiidae represents approximately a fifth of the world’s butterfly fauna. Estimations of global diversity within this group of butterflies range from 3,000 to 4,000 species (Robbins 1982; Bridges 1994) grouped in 567 genera (Warren 2006; Warren et al. 2008). In this family, the role and characteristics of male scent organs have been studied in some taxa. In the subfamily Hesperiinae, the pheromone-dispersing apparatus is formed of patches (stigmata) of androconia on the forewings (Pivnick et al. 1992; Wuest 1998). The androconia are tubular scales containing pheromones within the hollow medulla. These scales can break into pieces named osmophores, which constitute a pheromone-dispersing mechanism (Wuest 1998). In laboratory experiments, female antennae of Thymelicus lineola responded strongly to odours from conspecific male forewings but not from other parts of either males or females, but the chemical compounds involved have not been assessed (Pivnick et al. 1992). In Pyrginae, some tribes display androconia in the forewing costal fold (Warren et al. 2009). Scent-producing organs involving parts of the thorax and the abdomen have been described in Pyrgus malvae and in the genus Celaenorrhinus (Guillaumin 1963; Andersson et al. 2007). Moreover, the tibial tufts are a group of specialised setae on the tibia of the hind leg of males presumably used to help in directing the chemical compounds towards the female during courtship. At rest, tibial tufts are hosted between the abdomen scent scales of the ventral plate and the coxal appendix in Pyrgus and Heliopetes species (LSPN 1999; Dyar 1905). Despite the existence of such male scent organ variability, the chemical composition of volatile compounds in Hesperiidae is virtually unknown. Only recently, the chemical composition of a hesperiid (Erynnis montanus) has been studied, and p-cresol and benzothiazole were identified as potential male pheromones produced in the costal part of the wing, and possibly in other unknown scent organs (Ômura and Honda 2011).
In this study we document the morphology and chemistry of the male scent organs of several species in the genus Pyrgus (Lepidoptera: Hesperiidae) (Table S1). This genus was selected as the subject of our study because of several particularities. First of all, it includes about 27 species in the Palaearctic region (De Jong 1972) and it is the most diverse genus of the family in this area. Species are morphologically very similar, and thus it is probable that they rely primarily on chemical cues during courtship rather than on visual ones. We aim to test the hypothesis of taxonomic specificity of chemical profiles in male Pyrgus (Roelofs and Brown 1982; Roelofs and Rooney 2003; Smadja and Butlin 2009) and to investigate the rate of chemical evolution with respect to genetic distance.
Materials and methods
Samples studied and collecting method
Specimens of male Pyrgus were collected in Spain, France, and Romania with a net and immediately placed into clean glassine envelopes. No later than 4 h after they were killed by pressing the thorax they were processed as follows with the help of clean forceps and scissors: the forewings were cut out leaving the base, which could be contaminated by chemicals from the thorax. The area with the costal fold was cut and stored in a 10-ml screw cap headspace glass vial. The rest of the wing was also kept in the same way as blank. The posterior legs, including the tibial tufts, were extracted by pulling them out with forceps and kept in another vial. The rest of the legs were used as blanks. The vials were kept at 5 °C until analysed. The scent organs and blanks from between five and nine males of the same species were pooled in a single vial in order to obtain the required signal intensity and to reduce bias due to potential individual variability. All the males pooled were collected at the same locality and date. The bodies were kept in vials with absolute ethanol for genetic analysis. The specimens were identified by the study of the genitalia when this was necessary. A total of 15 different populations representing nine species were studied (Table S1).
Scanning electron microscope (SEM) photographs
The morphology of the scent organs on the wings, legs, abdomen, and thorax was studied by placing dry samples of each organ and species on aluminium stubs using a double-sided adhesive tape. The samples were then coated with gold using a Sputter Coater SC502. Morphological analysis was performed with a Hitachi S-3,000 N Scanning Electron Microscope at Universidad Autónoma de Madrid, Spain. Photographs were taken at an accelerating voltage of 20 kV and with magnifications ranging from 30× to 5,000×. Measurements of spacing between longitudinal ribs of scales were taken directly from the photographs using the image analysis programme ImageJ 1.43 (Ferreira and Rasband 2010).
Gas chromatography–mass spectrometry (GC-MS) analyses
A solid-phase microextraction (SPME) method using polydimethylsiloxane-divinylbenzene fibers (PDMS/DVB, 65 μm × 2 cm) was coupled to a gas chromatograph-mass spectrometer (GC-MS) in order to sample the volatile components from the scent organs, following a procedure very similar to that described by Andersson et al. (2003). Vials containing the samples and the PDMS/DVB fibres were equilibrated at 60 °C for 30 min in order to adsorb the volatile components from the samples to the fibres and afterwards the volatile components were desorbed by heating the vials at 250 °C for 5 min in the GC injector with a 1 ml/min He gas flow. The emerging gas from the head space was then injected in splitless mode by a CP8200 autosampler. Analyses were conducted on a Varian 3800 GC equipped with a Varian Factor IV column (30 m × 0.25 mm × 0.25 μm) and coupled to a Varian Saturn 2000 ion trap MS. The column was treated with the following temperature programme: (1) 1 min equilibration at 40 °C, (2) heating from 40 °C to 110 °C at a 5 °C/min rate, (3) further heating from 110 °C to 300 °C at 10 °C/min, and (4) 10 min equilibration at 300 °C. The chemicals that were progressively released from the column were analysed by the MS, which was set at 70 eV and programmed to work in a 35–350 Da m/z modus scan during 30 min. Total ion chromatograms of the costal fold and the tibial tufts for each sample were compared to those of the blanks corresponding to the rest of the wing and other legs, respectively.
Analyses of chemical similarity
The results of GC-MS for each population studied were coded as discrete characters. The peak intensities of all the compounds found in each sample were normalised with respect to the area of the most intense peak present in the same sample, which was given an arbitrary value of 3. Accordingly, peaks were coded 1, 2, or 3 depending on whether their intensity was up to 1/3, 2/3, or above 2/3 of the reference peak, respectively, and undetected compounds were coded as zero.
Dissimilarity among the chemical profiles of Pyrgus populations was visualised in a two-dimensional plot obtained by non-metric multidimensional scaling. The dissimilarity matrix was calculated on the three category data by using the Bray-Curtis index using chemical compositions of the two male scent organs for each of the populations studied. The analysis was run in R using the "nmds" function of the package “ecodist” (dissimilarity-based functions for ecological analysis), version 1.2.7.
Specialisation among species in producing chemicals by tibial tufts and costal folds was also tested. For the mean chemical composition of each species we summed the abundance categories of compounds found in tufts and costal folds separately. The resulting values reflect the overall abundance of chemicals produced by each organ in each species. Finally, a Pearson correlation between overall compound abundance of the two organs was computed. In case of specialisation a negative correlation is expected to exist.
Genetic analyses
Total genomic DNA was extracted using Chelex 100 resin, 100–200 mesh, sodium form (Biorad), under the following protocol: one leg was removed and introduced into 100 μl of Chelex 10 %, and 5 μl of Proteinase K (20 mg/ml) was added. The samples were incubated overnight at 55 ºC and were subsequently incubated at 100 ºC for 15 min. Samples were then centrifuged for 10 s at 3,000 rpm. A 658-bp fragment at the 5´ end of the mitochondrial gene COI was amplified by polymerase chain reaction using the primers LepF1 (5´-ATTCAACCAATCATAAAGATATTGG-3´) and LepR1 (5´-TAAACTTCTGGATGTCCAAAAAATCA-3´) (Hebert et al. 2004). Double-stranded DNA was amplified in 25 μl volume reactions containing 14.4 μl autoclaved Milli-Q water, 5 μl 5× buffer, 2 μl 25 mM MgCl2, 0.5 μl 10 mM dNTPs, 0.5 μl of each primer (10 μM), 0.1 μl Taq DNA Polymerase (Promega, 5U/μl), and 2 μl of extracted DNA. The typical thermal cycling profile was as follows: first denaturation at 92 ºC for 60 s, followed by five cycles of 92 ºC for 15 s, 48 ºC for 45 s, and 62 ºC for 150 s, and then by 35 cycles of 92 ºC for 15 s, 52 ºC for 45 s, and 62 ºC for 150 s, and a final extension at 62 ºC for 420 s. PCR products were purified and sequenced by Macrogen Inc. Sequences were edited and aligned using GENEIOUS PRO v6.0.3 created by Biomatters (http://www.geneious.com/). This programme was also used to obtain a neighbour-joining phylogenetic tree. Bootstrap values were obtained using 100 replicates. Sequences can be found in the Barcode of Life Datasystems (http://www.boldsystems.org/) and in the GenBank database under Accession Nos. KJ490920-KJ490929. Mean uncorrected pairwise genetic distances for all the populations sequenced were calculated with MEGA 5 (Tamura et al. 2011).
Combining genetic and chemical data
First of all, the correlation between chemical dissimilarity and genetic distance among the studied populations was tested. We thus performed a Mantel test by using the function provided in the ecodist package. Moreover, to verify if the relationship was linear or curvilinear we fitted a quadratic regression. Furthermore, to verify correspondence between phenotypic traits and genetic relationships, the phylogenetic tree was superimposed to the mean composition of species using the following method. We inferred virtual compositions of nodes in the phylogenetic tree as means of the chemical composition of their two descendants (species or nodes). We then computed a Bray-Curtis dissimilarity matrix among species, inferred nodes and performed NMMDS on the dissimilarity matrices. Then, phylogeny was drawn on the obtained map by connecting nodes as in the COI phylogenetic tree. Long and highly crossing branches indicate weak correlations between genetics and chemical compositions.
Results
Morphological analysis of male scent organs
We have studied the male scent organs in Pyrgus (Fig. 1) by means of scanning electron microscope photographs. Two types exist: androconia on the forewing costal fold (Fig. 2), and a complex of tibial tufts on the hind legs (Fig. 3) plus scent scales on thorax and abdomen (Fig. 4). All nine studied species displayed both organs exclusively in males and their structure was similar across species (Figs. S1, S2). The costal fold on the dorsal side of the male forewing forms a pocket behind the costal vein (Fig. 2a, b). Inside the pocket there are scent scales that are rounded or bifurcated at the distal border (Fig. 2c, d)—as opposed to toothed borders in the rest of scales (Fig. 2e, f)—and with longitudinal and transversal ribs within the scale forming a denser net in comparison to normal scales (Figs. 2f, S 1). Longitudinal ribs in Pyrgus are spaced by 1.36 to 1.69 μm in normal scales, similarly to those described in other species of Lepidoptera (García-Barros and Meneguz 2012), while the spacing is 0.65 to 0.86 μm in scent scales (Table S2). Tibial tufts are a dense group of extremely long modified setae that are located in the tibiae of the posterior legs (Figs. 3, S2). The structure of these setae is tubular—with a hollow medulla—and their external surface is finely striated (Figs. 3c, d, S2). Androconia are also found on scent organs on the thorax and the abdomen (Fig. 4). These organs are on the ventral part of the first abdominal segments (ventral plate, Fig. 4b) and nearby on the thorax. Scales from these areas are similar to those in the costal fold but with a much denser microstructure of ribs (Fig. 4d). When the butterfly is resting, tibial tufts are hosted between the coxal appendix (Fig. 4a2) and the ventral plate (Fig. 4a1), probably absorbing any volatile compounds produced by these organs.
SEM photographs of the male scent organs on the wings of Pyrgus onopordi. a Costal fold from the dorsal side of the forewings. Scale bar 1 mm. b Detail of costal fold. Scale bar 300 μm. c Scent scales (androconia) from the inside of the costal fold. Scale bar 50 μm. d Detail of a scent scale surface. Scale bar 20 μm. e Normal scales outside of the costal fold. Scale bar 100 μm. f Detail of a normal scale surface. Scale bar 20 μm
SEM photographs of the scent organs on the legs of Pyrgus males. a General view of Pyrgus malvoides hind leg showing tibial tufts. Scale bar 1 mm. b Detail of P. malvoides tibial tuft setae and their insertion in the tibia. Scale bar 20 μm. c Detail of the finely striated external surface of P. malvoides tibial tuft setae. Scale bar 3 μm. d Detail showing the hollow structure of tibial tuft setae in Pyrgus sidae. Scale bar 10 μm
SEM photographs of the male scent organs on the abdomen and thorax of Pyrgus onopordi. a General view of the contact area between thorax and abdomen. 1. Area with scent scales on two first abdominal segments (ventral plate). 2. Coxal appendix. 3. Area with scent scales on the thorax. At rest, tibial tufts (Fig. 3a) are hosted between ventral plate and coxal appendix. Scale bar 1 mm. b Scent scales on the ventral plate (above) and scales and setae on the coxal appendix (below). Scale bar 300 μm. c Scent scales (androconia) from the ventral plate. Scale bar 100 μm. d Detail showing the structure of a scent scale. Scale bar 10 μm
Chemical analysis of male scent organs
Total ion chromatograms from scent organs generally displayed more peaks than those of the corresponding blanks, and no peaks were detected exclusively in the blanks (Fig. 5). The chemical compounds in the male scent organs of 15 populations representing nine Pyrgus species are reported in Table S3. A total of 125 compounds were found to be exclusive of the scent organs (31 in the costal fold and 101 in the tibial tufts). The total number of compounds detected in a sample ranged from 6 for Pyrgus bellieri to 31 for P. sidae. The substances found in the two types of scent organs (costal fold and tibial tufts) were usually different, with very few exceptions. The total abundance of compounds occurring in tufts and costal folds was negatively correlated among species (Spearman correlation rho = −0.686, P = 0.041, Fig. 6).
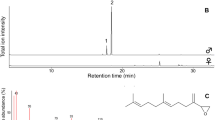
Example of total ion chromatogram (TIC). TIC of the tibial tufts (top) and the blank corresponding to the other legs (bottom) of P. malvoides from Solsonès (Lleida, Spain). The peaks of the sample that do not appear in the corresponding blank have been marked with an arrow and numbered 1–9. The relative intensity of all peaks is 1 except for compound 7, which shows maximum intensity (relative intensity of 3)
Similarity of chemical profiles
In most cases, the chemical profiles of different populations of the same species were identical or similar. Conversely, strong differences between species could be readily appreciated (Fig. 7, Table S3). The mean interspecific Bray-Curtis dissimilarity was 0.921, while the mean intraspecific dissimilarity was 0.291. Accordingly, a Mantel test between genetic distance and Bray-Curtis dissimilarity revealed a strong correlation (see Fig. 8). The relationship between genetic distance and chemical distance was revealed to be curvilinear with a stronger slope at low genetic distance, reaching a plateau around 2.5 % (Fig. 8). Actually both linear (t = 8.110, P < 0.001) and quadratic (t = −5.727, P < 0.001) coefficients showed a highly significant effect. Non-metric multidimensional scaling produced a rather high stress value (0.265), but the dissimilarity pattern was revealed to be very clear: conspecific populations showed small or no differences in the presence/absence of chemicals, and closely related species pairs P. malvae/P. malvoides and P. alveus/P. bellieri were placed near to each other (Fig. 7). When conspecific populations were grouped in single mean cases and a phylogenetic tree (Fig. S3) is superimposed to chemical dissimilarity, the phylogenetic tree is maintained in the representation without particularly long or crossing branches (Fig. 9). The longest branch is represented by P. onopordi, which is relatively distant to the genetically closest species P. cirsii.
Non-metric multidimensional scaling of chemical profiles. Two-dimensional plot representing the dissimilarity pattern in chemical profiles of the Pyrgus male scent organs. Populations representing species or closely related species pairs are encircled. A picture of a male of each species is shown (upper row: upperside; lower row: underside)
Discussion
The morphological study of the Pyrgus scent organs showed that they host different groups of scales, setae and structures. The tibial tufts, together with the ventral plate, coxal appendix, and scent scales from the thorax and first abdominal segments, form a complex organ. In the family Hesperiidae, costal folds are present in many genera of Eudaminae and in the following tribes of Pyrginae: Tagiadini, Erynnini, Pyrgini, and Carcharodini (Warren et al. 2009). Interestingly, in many of these taxa there are some species without a costal fold, which seems to suggest cases of secondary losses. Pyrgus-type tibial tufts are present in Erynnini, Achlyodidini, and Pyrgini, plus Celaenorrhinini (genus Celaenorrhinus), two genera of Coeliadinae (Badamia and Choaspes), and one genus of Eudaminae (Entheus), though these might not all be homologous. It is often presumed that the tibial tufts go hand in hand with the abdominal-thoracic pouch, but at least in the New World species Pyrgus communis the pouch is present (but vestigial), while the tufts are absent (Warren et al. 2009).
The ultrastructure of the long setae, with a hollow medulla and a ridged exterior surface, seems adequate to host and release volatile compounds. The walls of the setae seem to be formed by a spongy material, which might be related to their function (Fig. 3d). The tibial tufts are usually kept hidden within the pocket formed by the coxal appendix and ventral plate (LSPN 1999). Presumably, during courtship males take out the tufts from the pocket and these would then release the volatile compounds. Unfortunately, it is extremely difficult to observe the courtship in the inconspicuous and fast flying Pyrgus at close distance. The lack of marked interspecific differences suggests that the morphology of these organs does not represent a valuable diagnostic character for species identification (Figs. S1, S2). On the other hand, the complexity of these organs and the wide variety of species-specific chemical compounds documented strongly suggest that these butterflies rely on chemical communication during courtship. The existence of a chemically based precopulatory barrier rather than a visual one could explain why Pyrgus species are so similar in their external morphology and why they are so inconspicuously coloured (see Fig. 7).
The high number of compounds found exclusively in the scent organs and not in the corresponding blanks suggests that a remarkable variety of pheromones could exist in Pyrgus. The number of substances detected in the costal fold (between 0 and 12) was generally lower than those in the tibial tufts (Table S3). Indeed, in four species (P. carthami, P. sidae, P. onopordi, and P. serratulae) no compound was detected in the costal fold that was not present in the rest of the wing. However, the number of compounds in the tibial tufts varied strongly across species, with a minimum of two in some populations of P. alveus and P. bellieri, and a maximum of 31 in both populations of P. sidae (Table S3). A first important clue for the function of these chemicals in intraspecific communication relies on the strict negative correlation among species in the abundance of compounds occurring in the costal fold and in tibial tufts (Fig. 6). This finding suggests that two main types of strategies occur in Pyrgus, each mostly relying on the secretion of one of the scent organs. We showed that chemical composition is strongly correlated with genetic distance, but this relationship is curvilinear, with a strong slope at low genetic distance and rapidly reaching a plateau (Fig. 8). In practice, divergence among some allopatric populations is only slightly lower than divergence among species. This indicates that divergence in chemical signalling can arise among allopatric populations in a relatively short time. Then, speciation processes in the form of selection and/or genetic drift can reinforce diversification to the high values observed among species. The strongly ordered pattern obtained in the superimposition of the phylogenetic tree and chemical distances (Fig. 9) actually confirms a gradual hypothesis of divergence with an initial fast step followed by slow differentiation. Pyrgus species are highly differentiated based on the chemical profiles (the mean interspecific Bray-Curtis dissimilarity is 0.921, very close to the theoretical maximum of 1). One case represents an exception, the closely related species pair P. malvae/P. malvoides (Fig. 7). The taxon malvoides was described as a subspecies of P. malvae based on differences in the male genitalia, and it is still debated whether it represents a different species or not. Both species display a parapatric distribution and are known to hybridise regularly in the contact zone (Aistleitner 1996). Thus, a precopulatory barrier does not apparently exist between P. malvae and P. malvoides, which agrees with our observation that their chemical profiles are most similar. The two species P. alveus and P. bellieri have also recently diverged, and their chemical differentiation is intermediate (Fig. 7). These two species seem to have different phenologies and altitudinal preferences (Hernández-Roldán 2005). As a consequence, receptive females are unlikely to meet males of the other species, and the selective pressure to develop a precopulatory barrier must be rather low. As for the rest of the studied species, they widely co-occur (several of them are polivoltine generalists), are very similar in their morphology, behaviour and life history, and yet interspecific mating or hybrids have not been documented, which suggests the existence of a premating barrier among species. Hence, we can affirm that the results of the comparative chemical analysis of the male scent organs in the genus Pyrgus fully agree with the data available on their biology.
We conclude that species-specific chemical profiles are the rule in these butterflies and that they could potentially serve as a premating barrier to hybridisation. Exceptions involve two closely related pairs of taxa for which biological data suggest a lack of prezygotic interspecific barrier. The number of species within the family Hesperiidae that is still not studied from the chemical point of view is enormous. Many of the species display unique scent organs that have not yet been morphologically described in detail. Given the unexpected array of compounds found in Pyrgus, we can affirm that the Hesperiidae is a group with great potential for the study of chemical communication that deserves further attention.
References
Aistleitner, E. (1996). Die Arealgrenzen der beiden Dickkopffalter-Arten Pyrgus malvae L. und Pyrgus malvoides Elw. & Edw. (Lepidoptera Hesperiidae) in Vorarlberg (Österreich) und Liechtenstein. Vorarlb Natursch, 1, 335–344.
Andersson, J., Borg-Karlson, A.-K., & Wiklund, C. (2003). Antiaphrodisiacs in pierid butterflies: a theme with variation! Journal of Chemical Ecology, 29, 1489–1499.
Andersson, J., Borg-Karlson, A.-K., Vongvanich, N., & Wiklundf, C. (2007). Male sex pheromone release and female mate choice in a butterfly. The Journal of Experimental Biology, 210, 964–970.
Ando, T., Inomata, S., & Yamamoto, M. (2004). Lepidopteran sex pheromones. In S. Schulz (Ed.), The chemistry of pheromones and other semiochemicals. Topics in Current Chemistry 239 (pp. 51–96). Berlin: Springer.
Baker, T. C., Nishida, R., & Roelofs, W. L. (1981). Close-range attraction of female oriental fruit moths to herbal scent of male hairpencils. Science, 214, 1359–1361.
Berlocher, S. H., & Feder, J. L. (2002). Sympatric speciation in phytophagous insects: moving beyond controversy? Annual Review of Entomology, 47, 773–815.
Birch, M. C. (1970a). Structure and function of the pheromone-producing brush-organs in males of Phlogophora meticulosa (L.) (Lepidoptera: Noctuidae). Transactions of the Royal Entomological Society of London, 122, 277–292.
Birch, M. C. (1970b). Pre-courtship use of abdominal brushes by the nocturnal moth, Phlogophora meticulosa (L.) (Lepidoptera: Noctuidae). Animal Behaviour, 18, 310–316.
Birch, M. C. (1975). Aphrodisiac pheromones in insects. In M. C. Birch (Ed.), Pheromones (pp. 115–134). New York: Elsevier.
Birch, M. C., & Hefetz, A. (1987). Extrusible organs in male moths and their role in courtship behavior. Bulletin of the Entomological Society of America, 33, 222–229.
Birch, M. C., Poppy, G. M., & Baker, T. C. (1990). Scents and eversible scent structures of male moths. Annual Review of Entomology, 35, 25–58.
Boppré, B. (1989). Chemically mediated interactions between butterflies. In R. I. Vane-Wright & P. R. Ackery (Eds.), The biology of butterflies (pp. 259–275). Princeton: Princeton University Press.
Boppré, B. (1993). The American monarch: courtship and chemical communication of a peculiar danaine butterfly. In S. B. Malcolm & M. P. Zalucki (Eds.), Biology and conservation of the monarch butterfly (pp. 29–41). Los Angeles: Natural History Museum of Los Angeles County.
Bridges, C. A. (1994). Catalogue of the Family-group, Genus-group and Species-group Names of the Hesperiidae (Lepidoptera) of the World. Illinois: Urbana III.
Coyne, J. A., & Orr, H. A. (1989). Two rules of speciation. In D. Otte & J. Ender (Eds.), Speciation and its consequences (pp. 180–207). Sunderland: Sinauer Associates Inc.
Coyne, J. A., & Orr, H. A. (2004). Speciation. Sunderland: Sinauer Associates Inc.
Dapporto, L. (2007). Cuticular lipid diversification in Lasiommata megera and L. paramegera: the influence of species, sex, and population (Lepidoptera, Nymphalidae). Biological Journal of the Linnean Society, 91, 703–710.
De Jong, R. (1972). Systematics and geographic history of the genus Pyrgus in the palaearctic region. Tijdschrift voor Entomologie, 115, 1–121.
De Jong, R. (1982). Secondary sexual characters in Celaenorrhinus and the delimitation of the genus (Lepidoptera, Hesperiidae). Journal of Natural History, 16, 695–705.
Dyar, H. G. (1905). A review of the Hesperiidae of the United States. Journal of the New York Entomological Society, 13, 111–141.
El-Sayed, A. M. (2009). The Pherobase: Database of Insect Pheromones and Semiochemicals. Available: http://www.pherobase.com. Accessed 2012 Jan 24.
Ferreira, T. A. & Rasband, W. (2010). The ImageJ User Guide, Version 1.43. Avalaible: http://rsbweb.nih.gov/ij/docs/user-guide.pdf/. Accessed 2012 Apr 25.
Fitzpatrick, S. M., & McNeil, J. N. (1988). Male scent in lepidopteran communication: the role of male pheromone in mating behaviour of Pseudaletia unipuncta (Haw.) (Lepidoptera: Noctuidae). Memoirs of the Entomological Society of Canada, 146, 131–151.
García-Barros, E., & Meneguz, M. (2012). Estructura de las escamas ventrales de las alas de Coenonympha Hübner, [1819], con especial referencia a C. pamphilus (L., 1758) y su morfotipo lyllus (Esper, 1805) (Lepidoptera: Nymphalidae). SHILAP Revista de lepidopterología, 40(159), 279–293.
Grichanov, I. Y. (1998). Lepidoptera male pheromones in relation to the origin of the pheromone system in insects. Russian Entomological Journal, 7, 77–82.
Groot, A. T., Horovitz, J. L., Hamilton, J., Santangelo, R. G., Schal, C., & Gould, F. (2006). Experimental evidence for interspecific directional selection on moth pheromone communication. Proceedings of the National Academy of Sciences of the United States of America, 103, 5858–5863.
Guillaumin, M. (1963). Le complexe odoriférant thoraco-abdominal de Pyrgus malvae L. (Lep. Hesperiidae). Bulletin de la Societe Entomologique de France, 68, 128–136.
Heath, R. R., Landolt, P. J., Dueben, B. D., Murphy, R. E., & Schneider, R. E. (1992). Identification of male cabbage looper sex pheromone attractive to females. Journal of Chemical Ecology, 18, 441–453.
Hebert, P. D. N., Penton, E. H., Burns, J. M., Janzen, D. H., & Hallwachs, W. (2004). Ten species in one: DNA barcoding reveals cryptic species in the neotropical skipper butterfly Astraptes fulgerator. Proceedings of the National Academy of Sciences of the United States of America, 101, 14812–14817.
Hernández-Roldán, J. L. (2005). Pyrgus bellieri (Oberthür, 1910) en España. Estudio morfométrico comparativo con Pyrgus alveus (Hübner, [1803]) (Lepidoptera: Hesperiidae). Minor thesis dissertation, Universidad Autónoma de Madrid.
Huang, Y., Xu, S., Tang, X., Zhao, Z., & Du, J. (1996). Male orientation inhibitor of cotton bollworm: identification of compounds produced by male hairpencil glands. Entomologia Sinica, 3, 172–182.
Kirkpatrik, M., & Ravigné, V. (2002). Speciation by natural and sexual selection: models and experiments. American Naturalist, 159, 22–35.
LSPN. (1999). Les papillons et leurs biotopes. Egg: Fotorotar AG.
Nishida, R., Schulz, S., Kim, C. S., Fukami, H., Kuwahara, Y., Honda, K., & Hayashi, N. (1996). Male sex pheromone of a giant danaine butterfly, Idea leuconoe. Journal of Chemical Ecology, 22, 949–972.
Ômura, H., & Honda, H. (2011). Pungent odor of the adult skipper butterfly Erynnis montanus (Lepidoptera: Hesperiidae). Applied Entomology and Zoology, 46, 281–286.
Phelan, P. L., Silk, P. J., Northcott, C. J., Tan, S. H., & Baker, T. C. (1986). Chemical identification and behavioral characterization of male wing pheromone of Ephesia elutella (Pyralidae). Journal of Chemical Ecology, 12, 135–146.
Pivnick, K. A., Lavoie-Dornik, J., & McNeil, J. N. (1992). The role of the androconia in the mating behaviour of the European skipper, Thymelicus lineola, and evidence for a male sex pheromone. Physiological Entomology, 17, 260–268.
Ritchie, M. G. (2007). Sexual selection and speciation. Annual Review of Ecology, Evolution, and Systematics, 38, 79–102.
Robbins, R. K. (1982). How many butterfly species? The News of the Lepidopterists´ Society, 41, 214–216.
Roelofs, W. L., & Brown, R. L. (1982). Pheromones and evolutionary relationships of Tortricidae. Annual Review of Ecology and Systematics, 13, 395–422.
Roelofs, W. L., & Rooney, A. P. (2003). Molecular genetics and evolution of pheromone biosynthesis in Lepidoptera. Proceedings of the National Academy of Sciences of the United States of America, 100, 9179–9184.
Schulz, S., Franche, W., & Boppré, M. (1988). Carboxylic acids from hairpencils of male Amauris butterflies (Lep.: Danainae). Biological Chemistry Hoppe Seyler, 369, 633–638.
Schulz, S., Beccaloni, G., Brown, K. S., Boppré, M., Freitas, A. V. L., Ockenfels, P., & Trigo, J. R. (2004). Semiochemicals derived from pyrrolizidine alkaloids in male ithomiine butterflies (Lepidoptera: Nymphalidae: Ithomiinae). Biochemical Systematics and Ecology, 32, 699–713.
Singh, R. S., & Kulathinal, R. J. (2000). Sex gene pool evolution and speciation: A new paradigm. Genes & Genetic Systems, 75, 119–130.
Smadja, C., & Butlin, R. K. (2009). On the scent of speciation: the chemosensory system and its role in premating isolation. Heredity, 102, 77–97.
Tamura, K., Peterson, D., Peterson, N., Stecher, G., Nei, M., & Kumar, S. (2011). MEGA5: molecular evolutionary genetics analysis using maximum likelihood, evolutionary distance, and maximum parsimony methods. Molecular Biology and Evolution, 28, 2731–2739.
Teal, P. E. A., & Tumlinson, J. H. (1989). Isolation, identification and biosynthesis of compounds produced by male hairpencil glands of Heliothis virescens (F.) (Lepidoptera: Lepidoptera). Journal of Chemical Ecology, 15, 413–427.
Templeton, A. R. (1981). Mechanisms of speciation - A population genetic approach. Annual Review of Ecology, Evolution, and Systematics, 12, 23–48.
Thibout, E., Ferary, S., & Auger, J. (1994). Nature and role of the sexual pheromones emitted by males of Acrolepiopsis assectella. Journal of Chemical Ecology, 20, 1571–1581.
Warren, A. D. (2006). The higher classification of Hesperiidae (Lepidoptera: Hesperioidea). PhD dissertation, Oregon State University. Available: http://gradworks.umi.com/32/19/3219773.html. Accessed 2012 Jan 26.
Warren, A. D., Ogawa, J. R., & Brower, A. V. Z. (2008). Phylogenetic relationships of subfamilies and circumscription of tribes in the family Hesperiidae (Lepidoptera: Hesperioidea). Cladistics, 24, 642–676.
Warren, A. D., Ogawa, J. R., & Brower, A. V. Z. (2009). Revised classification of the Hesperiidae (Lepidoptera: Hesperioidea) based on combined molecular and morphological data. Systematic Entomology, 34, 467–523.
Witzgall, P., Lindblom, T., Bengtsson, & Tóth, M. (2004). The Pherolist. Available: http://www-pherolist.slu.se. Accessed 2012 Jan 24.
Wuest, J. (1998). The pheromone dispersing apparatus in some Hesperiinae (Lepidoptera: Hesperiidae). Swiss Journal of Zoology, 105, 813–822.
Wyatt, T. D. (2003). Pheromones and Animal Behaviour. Communication by Smell and Taste. Cambridge: Cambridge University Press.
Acknowledgments
We thank Vlad Dincă (Stockholm University, Stockholm, Sweden, and Institut de Biologia Evolutiva CSIC-UPF, Barcelona, Spain) for his help in obtaining samples and genetic analyses. We are also grateful to Rosa Sedano (Laboratory GC-LC, SIDI from Universidad Autónoma de Madrid, Spain) for performing the chromatography analyses and to Esperanza Salvador, Enrique Rodríguez, and Isidoro Poveda (Laboratory SEM-EDX, SIDI from Universidad Autónoma de Madrid, Spain) for help with scanning electron microscope images. Andrew Warren (McGuire Center for Lepidoptera and Biodiversity, University of Florida, USA) very kindly shared his knowledge on Hesperiidae with us. Funding for this research was provided by Universitat Autònoma de Barcelona (project EME2007-33 to J.L.H.-R., R.B. and R.V.) and by the Spanish Ministerio de Ciencia e Innovación (project CGL2010-21226/BOS to J.L.H.-R. and R.V. and project CGL2004-04680-c10-08/BOS to M.L.M.).
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(PDF 5.30 mb)
Rights and permissions
About this article
Cite this article
Hernández-Roldán, J.L., Bofill, R., Dapporto, L. et al. Morphological and chemical analysis of male scent organs in the butterfly genus Pyrgus (Lepidoptera: Hesperiidae). Org Divers Evol 14, 269–278 (2014). https://doi.org/10.1007/s13127-014-0170-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13127-014-0170-x