Abstract
Evolutionary and ecological situations in a species’ native and invasive ranges can be drastically different. This is the case for Potamopyrgus antipodarum Gray (1843) a morphologically highly variable freshwater snail native to New Zealand, where sexual and asexual individuals coexist and experience selective pressure by sterilizing endoparasites. By contrast, only a few asexual lineages have been established in invaded regions around the globe, where parasite infection is extremely rare. We analyzed the ecomorphology of 996 native P. antipodarum in a geometric morphometric framework, using brood size as proxy for fecundity, and mtDNA and nuclear SNPs to account for relatedness and identify reproductive mode. As expected, we found genetic and morphological diversity to be higher in native than in invasive snails investigated previously, but surprisingly no higher morphological diversity in sexual versus asexual individuals. The relationships between shell morphology, habitat, and fecundity were complex. Shape variation was primarily linked to genetic relatedness but specific environmental factors including flow rate induced similar shell shapes. By contrast, shell size was largely explained by environmental factors. Fecundity was correlated with size but showed trade-offs with shape in increasingly extreme conditions. With increasing flow and toward small springs, the trend of shell shape becoming wider was reversed, i.e., snails with narrower shells were brooding more embryos. We concluded that both genetic and environmental contributions to variation in shell morphology in P. antipodarum likely play an important role in the ability of this species to adapt to a wide spectrum of habitats.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Ecomorphology, the study of the relationship between organismal morphology and ecological characteristics (Williams 1972; Karr and James 1975), has been a central theme in evolutionary biology because it reflects how organisms can adapt to their environment through their morphology. Naturally, morphologically diverse taxa have been especially suited for ecomorphological studies including famous models like, e.g., the East African lake cichlid fishes (e.g., Fryer and Iles 1972) and Anolis lizards (e.g., Losos 2009). Many gastropod species are highly suited as well to study morphological adaptation as contact with the environment is to large parts mediated through the shell in the majority of species. This implies that shell morphology should be shaped at least in part by evolutionary forces. In land snails, shell shape and size have been related to environmental parameters in several ways (Goodfriend 1986). Shape, for example, is strongly correlated to the inclination of a species’ preferred substrate (Okajima and Chiba 2011). In aquatic snails, shell thickness and armature may be influenced by predation risk (e.g., Holomuzki and Biggs 2006; Le Pennec et al. 2017). Furthermore, it appears that shell morphology can evolve rapidly in geological time scales and respond repeatedly but independently in a similar way to similar selection regimes (e.g., Stankowski 2011; Haase et al. 2013), with the potential to generate parallel ecological speciation (Johannesson et al. 2010; Stankowski 2013).
Although most ecomorphological studies have been conducted at the interspecific level, at least in part because evolutionary trends are easier to detect at higher taxonomic levels, studies on the intraspecific level also present several advantages. In particular, unlike their interspecific counterparts, intraspecific ecomorphological studies are not affected by phylogenetic constraints (Losos and Miles 1994). For this reason, intraspecific studies can be especially well suited to identify adaptive pressures and provide insights to the microevolutionary mechanisms leading to the phenotypic differentiation (Rieseberg et al. 2002; Kingsolver and Pfennig 2007). The ovoviviparous New Zealand mudsnail Potamopyrgus antipodarum Gray (1853) is an excellent candidate for an intraspecific ecomorphological study because it features high variability in shell morphology and occurs in a wide diversity of habitat types (Warwick 1952; Winterbourn 1970; Haase 2008; Verhaegen et al. 2018). Native to New Zealand, obligately asexual lineages of this small fresh and brackish water snail have successfully invaded Australia, Europe, the US, Japan, and Chile within the last 180 years (Ponder 1988; Bowler 1991; Shimada and Urabe 2003; Alonso and Castro-Díez 2012; Collado 2014). Several studies have addressed how asexual P. antipodarum could become such successful invaders, suggesting factors such as high physicochemical tolerance, high reproductive rate, release from parasite pressure, and the ability to detect and avoid novel predators (e.g., Alonso and Castro-Díez 2008; Levri et al. 2017). Several authors have also suggested that variation in P. antipodarum shell morphology can be adaptive with respect to invasibility, playing an important role in the snail’s ability to colonize diverse habitats (e.g., Kistner and Dybdahl 2013, 2014; Verhaegen et al. 2018).
The considerable variability of shell morphology of P. antipodarum is linked to both genetic adaptation and phenotypic plasticity (Kistner and Dybdahl 2013; Verhaegen et al. 2018). Variation in shell morphology in P. antipodarum has been associated with numerous abiotic and biotic factors, including flow rate (Haase 2003), water depth (Jokela et al. 1997), predation (Holomuzki and Biggs 2006), and parasitism (Levri et al. 2005; Lagrue et al. 2007). Several recent P. antipodarum studies (Kistner and Dybdahl 2013, 2014; Vergara et al. 2016; Verhaegen et al. 2018) have used a geometric morphometric approach (Bookstein 1991; Zelditch et al. 2012) to disentangle selective pressures on shell shape and size. Kistner and Dybdahl (2014) found that in invasive US lineages, shell shape was related to flow rate, with wider but shorter shells found in habitats with high flow. By contrast, in invasive European lineages, Verhaegen et al. (2018) found that shell size increased with flow rate. Although no difference in shape was detected between lentic versus lotic habitats, narrower but longer shell shapes produced more brooded embryos under conditions with higher flow rate. Verhaegen et al. (2018) also found that water temperature and latitude as well as factors most likely associated with food availability also influenced shell shape and/or size. The only ecomorphological study on native P. antipodarum of which we are aware that has applied geometric morphometrics investigated the association of shell morphology of lake populations with depth, and previous established frequencies of parasite infections, relative proportions of sexual and asexual individuals, and across-lake genetic structure. However, Vergara et al. (2016) only found a correlation between size and depth, hence assuming higher fecundity for populations in deeper waters with less parasite pressure compared to shallow-water populations.
The evolutionary and ecological situations in P. antipodarum’s native and invasive ranges are markedly different. In New Zealand, P. antipodarum populations are characterized by a long evolutionary history and high diversity of mitochondrial haplotypes and genotypes (e.g., Neiman and Lively 2004; Städler et al. 2005; Paczesniak et al. 2013), frequent coexistence between diploid, obligately sexual and polyploid, obligately asexual individuals (Dybdahl and Lively 1995; Neiman et al. 2011), and intense parasite-imposed selection (Winterbourn 1974; Hechinger 2012). By contrast, invasive P. antipodarum populations have established less than 180 years ago, only a few obligately asexual lineages have successfully invaded (Hauser et al. 1992; Hughes 1996; Jacobsen et al. 1996; Gangloff 1998; Städler et al. 2005), and parasite infection is extremely rare (Gérard and Le Lannic 2003; Zbikowski and Zbikowska 2009; Cichy et al. 2017; Gérard et al. 2017). Verhaegen et al. (2018) investigated adaptation of shell morphology of a few closely related clonal lineages in Europe. Here, we extended our ecomorphological analyses of P. antipodarum to native populations collected from two regions of New Zealand and covering spring, stream, and lake habitats. Similar to Verhaegen et al. (2018), we applied a geometric morphometric framework to relate variation in shell morphology to ten environmental parameters. We used genetic markers to account for relatedness and identify reproductive mode, dissected each snail to establish its sex and to control potential effects of parasite infection on shell morphology (Levri et al. 2005; Lagrue et al. 2007), and counted the number of brooded embryos as proxy for fitness in females. As asexual P. antipodarum reproduce without recombination through ameiotic parthenogenesis (Phillips and Lambert 1989), we expected to find higher genotypic diversity and consequently higher morphological diversity in the native range compared to the invasive ranges according to the frozen niche hypothesis. The frozen niche hypothesis predicts that phenotypic variation should be higher in sexual versus asexual lineages because both genetic variation and the range of phenotypically plastic responses should be limited in the latter (Vrijenhoek 1979). For native populations of P. antipodarum, this prediction would hold as well, provided asexual lineages were uncommon. We then asked whether evidence for adaptation of shell morphology to environmental variables in the native range was similar to evidence for shell adaptation detected in Europe (Verhaegen et al. 2018) or North America (Kistner and Dybdahl 2013, 2014). Although our approach did not directly aim to disentangle genetic adaptation and phenotypic plasticity, morphological variation related to genetic lineage rather than habitat would likely have a genetic basis. Controlling for genetic relatedness, we asked whether genetic adaptation to a particular type of habitat occurred once or in parallel. Our overall goal was to generate a deeper understanding of whether variability of shell morphology is an important factor for the success of P. antipodarum for colonizing a wide spectrum of habitats in New Zealand.
Methods
Sampling
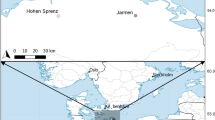
We collected 996 snails at 50 New Zealand freshwater sites during summer (February and March) 2016: 18 sites (337 individuals, 33.17%) in the geographic center of the North Island and 32 sites (659 individuals, 66.83%) in the northwest of the South Island (Supplementary Table 1). We used a dip net to scrape snails off stream and lake bottoms, submerged rocks, and aquatic plants at depths of 0–50 cm and then fixed the snails on site in 70% ethanol. At each site, we recorded water temperature, salinity, and conductivity (Water tester; Milato®, Germany), turbidity (clear, average, or unclear), concentration of nitrates (Test strips from API®, New Zealand), flow rate (estimated by timing three times the travel distance of 1 m by a floating leaf), percent shade, and presence/absence of visually identified predators (fish, crayfish, or crab). Because these water bodies are not continuously monitored for these parameters, we had to assume that our single measurements nevertheless reflect overall differences between sites eventually varying more or less in parallel over the year but certainly not randomly. We are confident that this is indeed the case as we did not detect any unexplainably outlying data, data indicating a punctual, abnormal impact, or contradictory data, for instance when comparing temperature with percent shade, latitude, and altitude. Nitrite concentrations were zero at all sites and therefore discarded from further analyses. Finally, we also categorized each by its status as a lake (7 sites), spring (6 sites, upstream water bodies less than 30 cm wide), type 1 stream (13 sites, width between 30 cm and 1 m), type 2 stream (12 sites, width between 1 and 2 m), and type 3 stream (12 sites, width larger than 2 m).
Geometric morphometrics
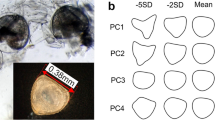
We used geometric morphometrics to analyze shell shape and size independently (Bookstein 1991; Zelditch et al. 2012). We began by individually positioning the shells of up to 20 fully grown snails per site, defining “fully grown” by displaying a continuous apertural lip (Verhaegen et al. 2018), under a Carl Zeiss Discovery V20 microscope on a silicone support, with the aperture facing up and the coiling axis oriented horizontally. We then photographed each snail with an AxioCam MRc camera and a Plan Apo S × 0.63 objective at different magnifications and with a 1-mm scale bar. We also classified shells as smooth, ridged, or spiny (Holomuzki and Biggs 2006). The shell images were transformed into the tps format with the program tpsUtil version 1.64 (Rohlf 2012), 18 Cartesian landmarks (Fig. 1) were placed, and a scale factor was added using the scale bar with tpsDig v.2.22 (Rohlf 2010). Using a Procrustes superimposition, the raw landmarks were centered, scaled, and rotated around a common centroid to minimize the distances between them (Rohlf and Slice 1990). A repeatability test had previously been conducted to ensure the detected variance in shape was unrelated to manipulative error (Verhaegen et al. 2018). For each shell, we used MorphoJ v.1.06 (Klingenberg 2011) to calculate centroid size (CS) as the square root of the summed squared distances of each landmark from the centroid of the landmark configuration (Bookstein 1991). The CS value represents the overall size of the shell and is the only size measurement independent of shape. The heights of the shells with the smallest and the largest CS were also measured with the Zeiss microscope. Because a multiple linear regression of Procrustes coordinates on CS (Monteiro 1999) (R2 = 0.107; mean squared error < 0.001; p < 0.001) revealed allometry, i.e., shape varying with size, we used the regression residuals instead of the coordinates in our further analyses to correct for size (Klingenberg 2016). The variation in shape among individuals was first visualized using a principal component analysis (PCA) and by use of wireframe graphs. Pairwise population comparisons were based on Procrustes distances, a measure of the absolute magnitude of the shape deviation (Klingenberg and Monteiro 2005), tested with 10,000 permutations in MorphoJ.
Landmarks (LM) used in geometric morphometric analyses: apex (LM1); intersection of sutures with the shell outline (LMs 2–7); most external right (LM8) and left (LM9) points of the body whorl; highest (LM10), lowest (LM13), most external left (LM12) and right (LM11) point of the aperture; dotted auxiliary lines indicate how landmarks 14–18 were placed
Dissections
After we photographed each snail, we dissolved the shells in 0.5 M EDTA (pH 7.5) for 3 days. The exposed soft bodies were then dissected under a microscope in order to determine the sex of the snails (presence/absence of a penis), the presence of macroparasites in the body cavity, and to count the number of brooded embryos in females. All snails were collected during the summer months, the presumptive reproductive peak, suggesting that we were likely to capture estimates of fecundity that represent or are close to fecundity maxima for most or all of the snails (Schreiber et al. 1998; McKenzie et al. 2013). Any embryos were removed and the remaining tissue was placed into 96% ethanol for preservation for DNA extraction.
Genetics and population genetic analyses
The dissected snail tissues were sent to LGC Genomics (Berlin, Germany; www.lgcgroup.com) for DNA extraction, mtDNA sequencing, and SNP genotyping. The DNA was extracted with the sbeadex™ lifestock kit in conjunction with an RNase treatment. A 497-bp fragment of the mitochondrial cytochrome b gene (cyt b; primers 5′-TTCTTTATTAGGACTTTGTTTAGG-3′ and 5′-TTTCACCGTCTCTGTTTAGCC-3′; Neiman and Lively 2004) was sequenced on an Illumina MiSeq V3 platform and clustered into haplotypes with CD-HIT-EST v.4.6.1 (Li and Godzik 2006). The sequences were then trimmed to a length of 431 bp to be compared to previously identified haplotypes on the NCBI database using the nucleotide BLAST (Altschul et al. 1990). The haplotype distribution was mapped and a median joining haplotype network built with PopART v.1.7 (Leigh and Bryant 2015).
We genotyped 48 of the 50 SNPs used in Verhaegen et al. (2018), which included 16 SNPs designed by Paczesniak et al. (2013) and 32 by Verhaegen et al. (2018) (Supplementary Table 2), using KASP™ assays. SNP loci that were fixed across all individuals and individuals with missing data at six or more loci were excluded from subsequent analyses. We assigned SNP genotypes using an infinite alleles model distance index and setting the maximum distance threshold (Rogstad et al. 2002) to zero for two genotypes to be considered the same in GenoDive v.2.0 (Meirmans and Van Tienderen 2004). We used the SNP genotypes to distinguish between asexual and sexual individuals: individuals with unique genotypes relative to all other snails were considered sexual while individuals with shared genotypes were considered asexual. It is important to mention that this approach means that we cannot exclude the possibility that asexual individuals with rare genotypes would be mistakenly categorized as sexual. For all subsequent population genetic analyses, we included all sexual individuals and one asexual individual per genotype per sampling site. We evaluated the presence of Hardy–Weinberg equilibrium (HWE) and genotypic linkage disequilibrium (LDE) for each SNP locus in each sampling site in GENEPOP v.4.7.0 (Rousset 2008). In order to assess if sexual and asexual individuals can be distinguished based on the diversity of the SNPs, we calculated the probability that two sexual individuals share the same genotype by chance, the combined non-exclusion probability, with Cervus v.3.0.7 (Kalinowski et al. 2007).
The number of genetic clusters was estimated with a K-means clustering analysis based on the lowest Bayesian information criterion (BIC) (Schwarz 1978). We used a discriminant analysis of principal components (DAPC) (Jombart et al. 2010) within the R package adegenet v.2.0.1 (Jombart 2008) to visualize the relationships among clusters. DAPC is a discriminant analysis with, as first step, a transformation of the data through PCA, which ensures that the variables are uncorrelated and their number less than the number of analyzed individuals (Jombart et al. 2010). The distribution range of the K clusters was mapped by modifying the R script written by F. Jay (Jay et al. 2012) available online (http://membres-timc.imag.fr/Olivier.Francois/pops.html).
To assess the genetic distances between sites, we calculated pairwise Slatkin’s linearized fixation indices based on FST (Slatkin 1995) in Arlequin v.3.5.2.2 (Excoffier and Lischer 2010). We then used these values to build an unrooted neighbor-joining (NJ) tree in PAST, reflecting evolutionary relationships among populations. This NJ tree was then mapped with GenGIS v.2.5.3 (Parks et al. 2013). The presence of genetic structure among geographical regions (Supplementary Table 1) or water body types was assessed with two analyses of molecular variance (AMOVA) (Excoffier et al. 1992), as implemented in GenoDive. The correlation between geographical distances (in meters) and the genetic distances (Slatkin’s linearized FST) among sites was evaluated with a Mantel test in PASSaGE v.2 (Rosenberg and Anderson 2011). The matrix of pairwise geographical distances was created with the R package geosphere v.1.5-7 (Karney 2013) using the coordinates of the sampling sites as the input file.
Statistical analyses
We tested for correlations between shape distances (Procrustes) and genetic distances across sites with a Mantel test as implemented in PASSaGE. A partial Mantel test was then used to assess the relationship of shape and geographical distances, using a matrix of pairwise genetic distances to control for the effect of genetic similarity. In a second series of tests, we replaced shape with CS using pairwise differences of median CSs.
We used a generalized linear model (GLM) (binomial error family) to evaluate whether the ratio of asexual–sexual individuals within sites was affected by water body type. We used another binomial GLM to assess whether the risk of infection with parasites was linked to a particular water body type, island, genetic cluster, mt haplotype, or reproductive mode.
We used a linear mixed model (LMM) to address whether and how shape was affected by the environmental factors, parasite infection, sex, and reproductive mode using PC1 as response variable and setting the mtDNA haplotypes and the SNP-based clusters as random factors to account for genetic relatedness. Salinity and longitude were removed from the environmental variables because each of these two variables was positively correlated with conductivity (Kendall’s tau = 0.964, z = 41.604, p < 0.001) and latitude (tau = 0.448, z = 20.759, p < 0.001), respectively. We used the same set of environmental variables and random genetic factors in all LMMs and generalized linear mixed models (GLMM) following here. We used a similar approach to assess the influence of the environmental variables on CS, applying a LMM on the inverse CS to obtain normally distributed errors. Because we expected that sexual snails would harbor a wider range of shape and size than asexuals, we used Fligner–Killeen median tests to determine whether there were significant differences between sexual versus asexual PC1 and CS variance. To correct for the difference in sample sizes between asexual and sexual individuals, the Fligner–Killeen median tests were repeated again 100 times, both for PC1 and CS, this time by randomly subsampling a matching number of asexuals. The effects of the environmental variables, shell shape (PC1), and CS variables on spininess were estimated with a GLMM (binomial error family). We eliminated the ridged snails from the analysis, leaving only smooth and spiny shell categories as response variables, because we could not definitively determine whether ridged shells might reflect spiny shells that had lost their spines. Finally, we used an LMM with the logarithmically transformed number of embryos as a proxy for fitness for uninfected female snails brooding at least one embryo and the environmental variables, PC1, and CS as fixed factors to assess the influence of each of these three factors on fecundity. We also included the interactions of PC1 and CS with the environmental factors in the model. Because we found CS to have a strong effect on fecundity, we also ran a LMM to test the effects of the environmental factors, PC1, and their interactions on the number of embryos corrected for CS. Finally, subsequent to those (G) LMMs on shell shape, size, armature, and snail fecundity that did reveal a difference between reproductive modes, we implemented additional (G) LMMs for each reproductive mode separately. All (G) LMMs were run in R with the lme4 v.1.1-13 package (Bates et al. 2015) and built by dropping terms based on type-II Wald χ2 tests of the Anova function available from the car package (Fox and Weisberg 2011). Marginal and conditional R2 were calculated for each LMM using the r.squared GLMM function (Nakagawa and Schielzeth 2013; Johnson 2014) of the MuMIn v.1.40.0 package (Bartoń 2017). A marginal R2 (R2(m)) represents the proportion of variance explained by the fixed factors alone, and a conditional R2 (R2(c)) explains the proportion by both the fixed and random factors (Vonesh et al. 1996). The effects v.3.2 package (Fox 2003) was used to visualize the positive and negative effects of the fixed factors. All statistical tests were executed in PAST v.3.14 (Hammer et al. 2001), MorphoJ, or R v.3.3.3 (R Development Core Team 2011). Non-parametric tests were used if normal distributions were rejected by a Shapiro–Wilk test.
Results
Genetics
We successfully sequenced a 497-bp segment of mitochondrial cyt b in 979/996 individuals (98.29%), and we detected seven different mtDNA haplotypes (Figs. 2 and 3). Only two haplotypes were 100% identical to haplotypes already present in GenBank: haplotype 01 (GenBank accession number: AY570182) (588 individuals, 60.06%) and 37 (AY570216) (137 individuals, 13.99%). We named the five new haplotypes cybA (MH092996) (225 individuals, 22.98%), cybB (MH092997) (18 individuals, 1.84%), cybC (MH092998) (9 individuals, 0.92%), cybD (MH092999) (1 individual, 0.10%), and cybE (MH093000) (1 individual, 0.10%). The closest matches we could find for those five haplotypes with previously published haplotypes were haplotypes 22 (AY570201, difference to cybA at one site), 37 (cybB, ten sites), 27 (AY570206, cybC, four sites), 31 (AY570210, cybD, two sites), and 30 (AY570209, cybE, three sites). Haplotypes cybD and cybE were discarded in further analyses because they were only represented by one individual each.
Median joining network for the seven cytochrome b haplotypes found in 979 New Zealand Potamopyrgus antipodarum and sampling sites. Each branch represents a single nucleotide substitution and short transversal lines as well as small black circles unsampled haplotypes. Size of circles is proportional to number of individuals per haplotype
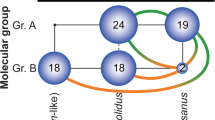
Forty-seven of the 48 SNP loci could be genotyped (failed locus—ss804270590). In addition, 36 of these 47 loci were polymorphic (Supplementary Table 2). In 967 of the 996 (97.09%) individuals, at least 30 of the 36 polymorphic markers could be genotyped; the other 29 individuals were discarded from further statistical analyses involving genetic factors. All SNP loci did not violate statistical expectations for HWE or linkage disequilibrium (p > 0.05 in all cases). We detected 304 different multilocus genotypes. One hundred (32.9%) of these genotypes represented asexual lineages, as we could safely conclude based on the extremely low combined non-exclusion probability of 5.541 × 10−10, which is the probability that two sexual individuals share the same genotype by chance. The number of genotypes among the 20 individuals sampled per site varied between 1 and 20, with up to 9 different asexual genotypes per site (2.76 ± 1.68 − mean ± SD, maximum number of asexual genotypes found at site NZ75). The 304 genotypes were grouped into 16 different clusters (Fig. 4) by K-means clustering. Their distribution is mapped in Fig. 5. We used 34 of the 36 SNP loci to calculate genetic distances (Slatkin’s linearized FST) between sampling sites (Supplementary Fig. 1) and produced a neighbor-joining tree (Supplementary Fig. 2). We excluded two SNPs that exhibited a relatively high fraction of missing data (comp157769_c0_seq1; comp160266_c0_seq4). A Mantel test (Mantel t = 5.433, two-tailed p < 0.001) revealed that genetic distances were structured along a spatial gradient and an AMOVA suggested the same for geographical regions (AMOVA, F = 0.171, SD = 0.017, p = 0.001). We found no evidence for genetic structure among water body types (AMOVA, F = 0.033, SD = 0.009, p = 0.115) (Supplementary Table 3).
Descriptive statistics
Seven hundred sixty-one (76.41%) of the 996 snails were assigned asexual status, with the remaining 209 snails (23.59%) deemed sexual (Supplementary Fig. 3). The asexual–sexual ratio was dependent on the type of water body from which the snails were collected (χ2 = 24.625, df = 4, p < 0.001): the proportion of sexuals was highest in lakes (35.1%), lowest in springs (10.3%), and intermediate in streams (19.7%) (Supplementary Table 4). The majority of snails were female (950, 95.38%) compared to only 46 (4.62%) males, of which 9 were asexual (19.57%). We found parasites in 107 individuals (10.74%), 74 of which were asexuals (9.72% of asexuals) and 33 sexuals (15.79% of sexuals). The risk of infection was influenced by the island (19.64% infections on the North Island compared to 6.24% on the South Island) and type of water body the snails were found in (Supplementary Table 4), their genetic cluster, but not their reproductive mode or haplotype (Supplementary Table 5).
Shape
The three first PCA axes explained a total of 65.80% of the variation in shape across individuals (38.86% by PC1, 14.72% by PC2, and 12.22% by PC3). Snails with negative PC1 values tended to have a shell shape that was narrower but longer than the average shell shape, while snails with positive PC1 values had a shell shape that was shorter but wider than the average shape. Here, we will refer to these two shell shape extremes as “narrow” and “globular,” respectively. Mean shell shape and these two shape extremes are visualized by the wireframe graphs (Fig. 6). Shell shape (Procrustes distances) did not follow a spatial (partial Mantel test t = 1.270, p = 0.204) or a genetic (Mantel test t = 1.8131, p = 0.067) gradient. The variance in shape (PC1) explained in the LMM (Supplementary Table 6) by both the genetic variables as random factors and the environmental variables plus reproductive mode as fixed factors was 61.20% (= R2(c)). The fixed factors alone explained 19.92% (= R2(m)) of the shape variance, i.e., there was a strong genetic effect.
Wireframe representations of the variation in shape (increased ten times) along PC1. The gray wireframes represent the mean shape observed across all sampled individuals; the black wireframes represent the most extreme shapes, with narrow morphs (a) for negative PC1 values and globular morphs (b) for positive PC1 values
The LMM on shape found asexual snails were, on average, narrower in shape than sexual counterparts (PC1asexual = − 0.002 ± 0.033 − mean ± SD; PC1sexual = 0.004 ± 0.0.038). Counter to our predictions, we also found that the shape range for asexuals was wider than for sexuals (Fligner–Kileen median centered χ2 = 6.185, p = 0.013) (Supplementary Fig. 4a); however, this result was only obtained in 48% of our tests with a matching number of sexual and asexual individuals; in the remaining cases, no significant differences were found. Because of the significant differences in shape between asexual and sexual individuals in our total sample, we repeated the LMM on shape within sexual and asexual groups separately. These analyses revealed that both sexual and asexual lake-collected snails were narrower than snails collected from streams (Fig. 7a). Snails were also relatively narrow in high nitrate conditions and relatively globular when flow rate was high. Asexual snails found at higher latitude, altitude, or sunlight exposure were more globular than asexual snails from lower latitudes and altitudes and shadier locations and were narrow relative to other asexuals when conductivity was high, but no effect was found for the sexual snails. We did not find effects of sex, parasitic infection status, island, water temperature, or turbidity on shell shape for either sexual or asexual snails. The R2(c) were 92.80 and 75.01%, and the R2(m) only 5.38 and 8.97%, for the LMMs within asexual and sexual individuals, respectively.
Variation in shell shape (a) and size (b) between water body types. The boxplots show the median (middle line), quartiles (boxes), 1.5 times the interquartile range (IQR) (whiskers), extreme values (dots). The notches extend to ± 1.58 times the IQR divided by the root squared number of observations, overlapping notches being strong evidence that the two medians do not differ (Chambers et al. 1983)
Size
Centroid size varied between 3.42 and 13.94 (6.87 ± 1.90), i.e., the largest snails were more than four times larger than the smallest snails. The smallest snail (found at site NZ24) measured 2.30 mm in height and the largest snail (NZ45) was 9.30 mm high. The population with lowest mean snail size (NZ14, 3.87 ± 0.14) was found on the North Island and the population with the largest mean snail size was from the South Island (NZ52, 11.39 ± 0.77), although the majority of populations with larger snails were found on the North Island (Supplementary Fig. 5). Centroid size followed a spatial (partial Mantel test t = 4.691, p < 0.001) and a genetic gradient (Mantel test t = 2.642, p = 0.008). An effect of genetic relatedness was also detected by the LMM on CS, but most of the variation in size was still explained by the environmental, i.e., fixed variables (R2(m) = 52.73%, R2(c) = 77.35%).
There was no difference is CS (CSasex median = 6.573, CSsex median = 6.747) or in size range (F–K median centered χ2 = 3.308, p = 0.069, CSasex range = 3.420–13.941, CSsex range = 3.523–11.526) (Supplementary Fig. 4b) between asexual and sexual snails, not even when the number of asexuals was subsampled (non-significant in 82% of the replicated tests). Sex, parasites, and altitude did not affect shell size either. All other environmental factors that we evaluated did affect CS: snails were larger on the North Island, at lower latitudes, in the presence of predators, and in warmer water and water with higher conductivity. Snails in current with higher flow rates, nitrate, or sun exposure were smaller in size. The largest snails were found in relatively clear or relatively turbid water, while the smallest snails were found in water of average turbidity. Finally, with respect to water body type, the smallest snails were found in springs, snails of intermediate size in lakes, and the largest snails in streams, with size increasing with stream width (Fig. 7b).
Spininess
The majority of snails had a smooth shell (730 individuals, 73.29%). A total of 162 (16.26%) snails had a spiny shell and 104 snails (10.44%) had ridged shells. The GLMM on spininess (Supplementary Table 6) found a strong effect of the genetic variation on spininess. There was no difference in spininess between asexual and sexual snails. The only environmental variables that we found to affect spininess were temperature (positively correlated), altitude (negatively correlated), and water body type. The largest proportion of spiny-shelled snails was found in lakes (44.53%). In streams, the proportion of spiny shells increased with stream width (7.72% in type 1 streams, 14.36% in type 2, and 26.85% in type 3). We did not find any spiny snails in springs. Finally, the GLMM revealed that spiny snails also had shells that were relatively globular and large compared to smooth-shelled snails.
Fecundity
The number of brooded embryos per female (N = 950) ranged from 0 to 147 (19.75 ± 23.55 − mean ± SD). We did not find any embryos in trematode-infected females, consistent with expectations for these sterilizing parasites. Shell size directly affected fecundity, with larger females brooding more embryos. Fecundity was also affected by the interaction of shell size with the type of water body, the presence of predators, and with latitude. Larger snails brooded more embryos at higher latitudes, in the absence of predators, or in lakes, springs, and type 2 streams (Supplementary Table 6).
When corrected for size, the largest proportion of variation in fecundity was explained by the fixed factors (R2(m) = 33.35%), which included the environmental variables, with or without interaction with shape, while the variance explained by genetic lineage was much lower (R2(c) = 40.87%). Among the environmental variables, latitude and predators had a positive effect and sunlight coverage, altitude, conductivity, and nitrate, a negative effect on fecundity. Water transparency also affected fecundity/CS: snails in turbid water had lower fecundity than their counterparts in clear and, especially, in water of intermediate turbidity. Sexual snails were found to brood more embryos compared to asexual ones. While shell shape alone did not affect fecundity/CS, we did detect interactions with water body type, flow rate, and the presence of predators. In particular, narrow females carried more embryos at high flow sites or in springs than sympatric globular females. When predators were present, globular females brooded significantly more embryos compared to narrow ones (Supplementary Table 6).
Discussion
Our overall goal was to generate a deeper understanding of whether variability of shell morphology is an important factor in P. antipodarum’s successful colonization of a wide spectrum of habitats in New Zealand and in its worldwide invasion. We found, as expected, genetic and morphological diversity to be considerably higher in native relative to invasive populations. Our expectations regarding higher morphological variation in sexual snails were not met, with no apparent differences in the extent of morphological variation between sexual and asexual snails. Finally, we found relationships between shell morphology, habitat, and fecundity to be complex.
Genetic structure
We found seven cyt b haplotypes and 304 SNP genotypes. Thirty-six out of 47 SNP loci were polymorphic, including all 16 polymorphic SNPs found in Europe (Verhaegen et al. 2018). The genetic diversity was, as expected, higher than the genetic diversity observed in Europe, where the same genetic markers revealed only two mitochondrial haplotypes and ten closely related genotypes (Verhaegen et al. 2018). Genetic marker analysis of invasive populations in North America revealed a similar absence of diversity, detecting only three cyt b haplotypes (Dybdahl and Drown 2011). The higher genetic variation of New Zealand P. antipodarum is certainly linked to the presence of sexual reproduction and the longer evolutionary history of the snails in their native range, though we cannot exclude potential roles for sampling effects or post-invasion bottlenecks (Weetman et al. 2002; Städler et al. 2005).
Five of the seven haplotypes were newly detected and two were previously described (Neiman and Lively 2004; Neiman et al. 2011). Our most common haplotype (60.06% of all individuals), 01, was also reported as the most common haplotype by Neiman and Lively (2004) and Neiman et al. (2011) and, like 37, was present on both islands. By contrast, haplotype cybA was mostly confined to the North Island (present in nine North island sites and only a single South island site, NZ64), and cybB was only found in our most northern sampling site in Auckland (NZ90). CybD and cybE were each only found in one individual that were collected from two geographically close sites (NZ 53 and NZ54, respectively) on the northwest coast of the South Island, but they were not closely related to each other (95.82% identity).
SNP-based genetic distances were structured along a spatial gradient indicating isolation by distance. Nevertheless, low FST (< 0.05) values between sites in the northwest of the South Island and the Waikato as well as the mapped NJ tree suggest recent long-distance gene flow, probably mediated by birds (see, e.g., Haase et al. 2010; Zielske et al. 2017).
We found nine polyploid males (19.57% of all males), broadly similar to the 27.02% of all males reported by Neiman et al. (2011). Polyploid males are produced by asexual polyploid females (Neiman et al. 2012); their fertility status remains unclear (Soper et al. 2013, 2016).
More than 75% of our snails had shared multilocus SNP genotypes (100 SNP genotypes) and were thus categorized as asexual, the actual proportion being likely higher as our approach underestimated as expected the number of asexual as shown by the proportion of female individuals (95%). Although the sexual snails represented less than a quarter of our sample, they harbored more than twice as many genotypes (204). The higher proportion of asexual individuals is certainly a consequence of the twofold cost of sexual reproduction (Maynard-Smith Maynard Smith 1971, Maynard-Smith 1978) experimentally demonstrated by Gibson et al. (2017). Lakes tended to have the highest proportion of sexual individuals, with 35.1% of samples from lakes assigned sexual status, followed by streams (19.7%) and springs (10.3%). Our results are generally similar to the frequencies of sexual P. antipodarum reported in earlier studies (Fox et al. 1996, 88% sexuals in lakes; Neiman et al. 2005, 19.6% sexuals in lakes; King et al. 2011, 8.2% sexuals in streams; Paczesniak et al. 2013, 33.7% sexuals in lakes). The higher proportion of sexual individuals likely reflects the higher pressure of parasites present in larger water bodies where fish and water fowl, the final hosts of the parasites, have access. Under the Red Queen hypothesis, the maintenance of sex, hence genotypic variation through recombination, is indeed explained by the presence of co-evolving parasites (Bell 1982), which has been subject of several studies with P. antipodarum as model (e.g., Lively 1987, 1992; Jokela et al. 1997).
Because asexual individuals can only generate genetic variation in offspring through mutation, we expected that sexual individuals would harbor more morphological variation than asexual snails. We could not evaluate whether this prediction was met within sites because we did not collect enough specimens from mixed populations for statistically meaningful comparisons between sexual and asexual individuals. Comparisons between sexual and asexual individuals across our entire sample did not reveal differences in shell size, shape, or spininess between asexual and sexual snails. This result is likely linked at least in part to the marked phenotypic plasticity in shell morphology already observed in P. antipodarum (Verhaegen et al. 2018) and to the fact that asexual snails probably also harbored considerable genetic diversity. It appears that polyploid, asexual individuals originate commonly from diploid, sexual females so that clones and outcrossing snails exhibit similar amounts of variation (Dybdahl and Lively 1995; Neiman and Lively 2004). Finally, we did not detect any difference between sexual and asexual snails in number of brooded embryos, although when corrected for size, fecundity was higher in sexual individuals. These findings are thus largely in line with a previous study showing no sexual/asexual difference in three other life history traits in P. antipodarum (Larkin et al. 2016).
Evidence for adaptive variation in shell morphology
We detected a much higher level of morphological variation in shape, size, and armature in New Zealand snails than for a similar sample collected in Europe. For example, we found that shell height of adult snails varied between 2.30 and 9.30 mm, a much wider range than reported for Europe (3.48–5.00 mm) (Verhaegen et al. 2018). In New Zealand, 16.26% of snails had spiny shells, in Europe none (Verhaegen et al. 2018).
Our mixed-model analyses revealed effects of both genetic and environmental variation on shell morphology and fecundity, though the relative contributions of genetics and environment varied across traits. For example, variation in shape and shell armature had mainly a genetic basis, while environmental factors were much more important as determinants of size and fecundity, similar to the findings in our previous study of European P. antipodarum (Verhaegen et al. 2018).
It is tempting to assume that morphological variation associated with genetic variation or environmental parameters in our statistical models reflects genetic adaptation or phenotypic plasticity, respectively. However, only in cases where genetic relatedness has an effect and the effect of environmental parameters is negligible can we assume that the observed variation is largely due to genetic adaptation. Otherwise, disentangling genetic adaptation and phenotypic plasticity and estimating their relative importance is strictly possible only through common garden experiments. However, this would be prohibitive considering the extent of our analyses and it is questionable if natural conditions can be mimicked in the laboratory accurately enough to yield transferable results.
Mantel tests indicated that size but not shape followed a spatial and genetic gradient. By contrast, our (G) LMMs detected a strong effect of genetic relatedness on shape and revealed a weaker effect of genetic relatedness on centroid size. These different outcomes of the Mantel test and (G) LMMs can be reconciled by considering that the Mantel tests were based on pairwise distances between populations whereas the mixed models instead focused on the genetic relatedness in the form of genetic clusters.
Our AMOVAs showed that genetic variation was structured among geographical regions but not across water body types, which themselves were associated with variation in shell morphology. This suggests that similar shell morphologies might have originated on multiple separate occasions. However, without experimental support, the relative contributions of genetic adaptation and phenotypic plasticity cannot be assessed, hence statements about parallel evolution would be premature.
Effects of environmental factors on morphology and fecundity
Flow and water body type
For many small stream organisms, the main risk associated with exposure to high flow rate is being detached and dragged away, with the potential for physical damage and relocation to unsuitable habitats (e.g., Holomuzki and Biggs 1999, 2006). In P. antipodarum, we observed (see also Haase 2003; Verhaegen et al. 2018) that the snails avoided exposure to stronger currents by staying close to the edge of streams or seeking shelter behind stones or in vegetation. The wide variation of shell morphology in P. antipodarum also appears to be related to habitat and corresponding flow conditions, with generally larger and wider shells found at higher flow rates (Haase 2003; Kistner and Dybdahl 2013, 2014; Verhaegen et al. 2018). Accordingly, we expected that variation in shell morphology would be at least in part related to water body type and potentially reflect adaptation to flow regimes. Largely in accordance with our expectations, we found the largest snails in lakes and wide streams, and the smallest snails in springs. Although shells became more globular as flow rate increased, snails with relatively narrower and smaller shells had higher brood sizes, corrected for shell size, than more globular counterparts under high-flow conditions. We speculate that larger and broader snails also have a relatively larger foot (see Raffaelli 1982 for a review in Littorina) and thereby may compensate for increased drag and lift (Statzner and Holm 1989; Weissenberger et al. 1991; Vogel 1994) by increasing attachment strength to the substrate. Altogether, the similarity of this result from New Zealand snails to those reported for invasive European P. antipodarum by Verhaegen et al. (2018) suggests that this size/shape/fecundity/flow rate relationship is a general feature of P. antipodarum.
Fluctuations in the amount of water probably affect inhabitants of small springs to a greater extent than for larger bodies of water, with the implication that spring-dwelling organisms might experience a substantially higher risk of desiccation. In this situation, small body size might provide advantages with respect to withdrawing to interstitial water remnants during drier periods. This advantage may even increase for relatively narrower snails with their smaller apertures reducing further the desiccation risk, as they were brooding more embryos than more globular spring dwelling P. antipodarum.
Almost twice as many spiny shells were found in lakes than in streams. Spininess might generate both advantages (predator deterrence) and disadvantages (seston buildup; increasing the drag and thus the dislocation risk in lotic habitats) (Holomuzki and Biggs 2006). The existence of such a trade-off might explain the higher fraction of spiny snails in lakes versus streams. Our enthusiasm for this possibility is weakened by the fact that we did not find a direct effect of flow rate on spininess and that we found a higher fraction of spiny snails in larger versus smaller streams. This latter observation could be connected to a likely increase of predation risk in wider streams, which would also help to explain why no spiny snails were found in springs. Similar to Holomuzki and Biggs (2006), we also found a positive association between spines and temperature, implying shell armature is the result of a complex interaction of various environmental factors.
Abiotic factors
In P. antipodarum, temperature and conductivity affect life history traits like growth rate and fecundity (e.g., Dybdahl and Kane 2005; Herbst et al. 2008; Levri et al. 2014). In accordance with previous studies (e.g., McKenzie et al. 2013; Verhaegen et al. 2018), increasing temperature as well as decreasing latitude and sunlight coverage had positive effects on CS and fecundity.
Conductivity can affect shell size and fecundity in various ways. High conductivity likely indicates the presence of calcium necessary for building the shell and producing embryos. Low ion concentrations, in turn, may increase the costs for calcium uptake and lead to osmotic stress (Herbst et al. 2008) affecting growth or fecundity. Consistent with this logic, conductivity had a positive effect on size; however, it had a negative effect on fecundity. We speculate that the latter might be linked to the presence of other compounds causing higher conductivity that negatively affect fecundity.
Nitrogen compounds are naturally present in freshwater ecosystems, but can increase to toxic levels via anthropogenic introduction (Jensen 1995, 2003; Stumm and Morgan 1996). In particular, high concentrations of nitrogen compounds can be toxic for aquatic animals (Jensen 1995, 2003) and negatively affect dissolved oxygen concentration as a consequence of eutrophication (Schindler et al. 1973). While P. antipodarum has relatively high tolerance to short-term nitrate toxicity (Alonso and Camargo 2003), we detected a negative effect of nitrates in New Zealand P. antipodarum, similar to our results from invasive European P. antipodarum (Verhaegen et al. 2018).
Biotic factors
Parasite infection did not have an effect on shell morphology. This result was surprising in light of the fact that infected snails were sterilized, assuming the potential shift of resource allocation in infected snails from fecundity to growth (Negovetic and Jokela 2001; Levri et al. 2005). Our result also differed from that of previous studies (Levri et al. 2005; Lagrue et al. 2007) suggesting that infected P. antipodarum were wider and less spiny than uninfected specimens.
Predators are important drivers of modifications of shell morphology in mollusks (Vermeij 1995). Different types of predation are associated with different types of shell modifications in aquatic gastropods. For example, in Elimia livescens, relatively elongated shells with relatively narrow apertures are more entry-resistant (e.g., against crayfish; Krist 2002). Other studies on Littorina obtusata and Physa sp. have shown that more globular individuals are more crush resistant (Seeley 1986; DeWitt et al. 2000). Here, we found that across our sample as a whole, the presence of predators was associated with snails with relatively larger shells. By contrast, within asexual P. antipodarum, predator presence was associated with narrower shells. Fecundity was positively correlated with shell size in the absence of predators but negatively correlated with shell size when predators were present, indicating a potential trade-off between fecundity and predator avoidance. Because spiny-shelled P. antipodarum also tended to be relatively globular, this could indicate spines to play an additional protective role against crush-type attacks. It is also important to note that any inferences from these data must be viewed with caution in light of the fact that our failure to detect predators does not mean that predators were absent. Future studies that employ more powerful methods (e.g., environmental DNA detection) of predator detection are needed to more definitively establish a connection, if any, between P. antipodarum shell morphology and predator pressure (e.g., Ficetola et al. 2008; Jerde et al. 2011; Goldberg et al. 2013).
Conclusions
We found evidence for complex relationships between shell morphology, habitat, and fitness in P. antipodarum. Genetic relatedness played a primary role in shape variation, though we also discovered evidence for expression of similar shapes in lineages that experienced similar environments, e.g., flow regime. By contrast, size variation was determined primarily by environmental parameters, with genetic variation playing a relatively minor but still significant role. Like other P. antipodarum studies (e.g., McKenzie et al. 2013; Verhaegen et al. 2018), we found a strong and positive relationship between fecundity and size. However, there was a trade-off with shape. In increasingly extreme conditions, i.e., with increasing flow and toward small springs, the trend of shell shape becoming wider was reversed, i.e., snails with narrower shells were brooding more embryos. As principal driver of morphological adaptation, we assumed dislocation risk increasing with current. Further experiments are however needed to actually quantify the differences in drag and lift experienced by the different morphotypes, as well as the differences in attachment strength, and to show if these differences are biologically meaningful.
We did not detect any major differences in the morphological variation of sexual and asexual snails and in the relationship of this variation to different habitat types. This absence of marked sexual/asexual differences is almost certainly at least in part a consequence of the frequent generation of asexual lineages from sexual females, freezing genetic variation across the entire spectrum harbored in the sexuals. We also found evidence for a substantial role of phenotypic plasticity in the expression of shell morphology; the extent to which our evidence for the expression of similar phenotypes in unrelated snails in similar habitats is due to plasticity versus genetic factors will require follow-up studies targeting the role of genetics and environment in the determination of the shell phenotype. The pronounced plasticity of shell morphology, both genetic and phenotypic, is certainly an important factor for the success of P. antipodarum colonizing a wide spectrum of habitats and as an invasive species, in particular as this species combines the benefits of both genetic recombination and the quantitative, reproductive advantage of the enormous multitude of asexual lineages.
References
Alonso, Á., & Camargo, J. A. (2003). Short-term toxicity of ammonia, nitrite, and nitrate to the aquatic snail Potamopyrgus antipodarum (Hydrobiidae, Mollusca). Bulletin of Environmental Contamination and Toxicology, 70(5), 1006–1012. https://doi.org/10.1007/s00128-003-0082-5.
Alonso, Á., & Castro-Díez, P. (2008). What explains the invading success of the aquatic mud snail Potamopyrgus antipodarum (Hydrobiidae, Mollusca)? Hydrobiologia, 614(1), 107–116.
Alonso, Á., & Castro-Díez, P. (2012). The exotic aquatic mud snail Potamopyrgus antipodarum (Hydrobiidae, Mollusca): state of the art of a worldwide invasion. Aquatic Sciences, 74(3), 375–383. https://doi.org/10.1007/s00027-012-0254-7.
Altschul, S. F., Gish, W., Miller, W., Myers, E. W., & Lipman, D. J. (1990). Basic local alignment search tool. Journal of Molecular Biology, 215, 403–410.
Bartoń, K. (2017). MuMIn: Multi-Model Inference. R package version 1.40.0. available at: https://CRAN.R-project.org/package=MuMIn.
Bates, D., Mächler, M., Bolker, B., & Walker, S. (2015). Fitting linear mixed-effects models using lme4. Journal of Statistical Software, 67(1), 1–48. https://doi.org/10.18637/jss.v067.i01.
Bell, G. (1982). The masterpiece of nature: the evolution and genetics of sexuality. CUP Archive.
Bookstein, F. L. (1991). Morphometric tools for landmark data. New York: Cambridge University Press.
Bowler, P. A. (1991). The rapid spread of the freshwater hydrobiid snail Potamopyrgus antipodarum (gray) in the middle Snake River, southern Idaho. Proceedings of the Desert Fishes Council, 21, 173–182.
Chambers, J. M., Cleveland, W. S., Kleiner, B., & Tukey, P. A. (1983) Graphical methods for data analysis. Wadsworth & Brooks/Cole.
Cichy, A., Marszewska, A., Parzonko, J., Żbikowski, J., & Żbikowska, E. (2017). Infection of Potamopyrgus antipodarum (Gray, 1843) (Gastropoda: Tateidae) by trematodes in Poland, including the first record of aspidogastrid acquisition. Journal of Invertebrate Pathology, 150(August), 32–34. https://doi.org/10.1016/j.jip.2017.09.003.
Collado, G. A. (2014). Out of New Zealand: molecular identification of the highly invasive freshwater mollusk Potamopyrgus antipodarum (Gray, 1843) in South America. Zoological Studies, 53(1), 1–9. https://doi.org/10.1186/s40555-014-0070-y.
Development Core Team, R. (2011). R: a language and environment for statistical computing. Vienna: R foundation for Statistical Computing.
DeWitt, T. J., Robinson, B. W., & Wilson, D. S. (2000). Functional diversity among predators of a freshwater snail imposes an adaptive trade-off for shell morphology. Evolutionary Ecology Research, 2(2), 129–148.
Dybdahl, M. F., & Drown, D. M. (2011). The absence of genotypic diversity in a successful parthenogenetic invader. Biological Invasions, 13, 1663–1672.
Dybdahl, M. F., & Kane, S. L. (2005). Adaptation vs. phenotypic plasticity in the success of a clonal invader. Ecological Society of America, 86(6), 1592–1601.
Dybdahl, M. F., & Lively, C. M. (1995). Diverse, endemic and polyphyletic clones in mixed populations of a freshwater snail (Potamopyrgus antipodarum). Journal of Evolutionary Biology, 8(3), 385–398. https://doi.org/10.1046/j.1420-9101.1995.8030385.x.
Excoffier, L., & Lischer, H. E. L. (2010). Arlequin suite ver 3.5: a new series of programs to perform population genetics analyses under Linux and Windows. Molecular Ecology Resources, 10(3), 564–567. https://doi.org/10.1111/j.1755-0998.2010.02847.x.
Excoffier, L., Smouse, P. E., & Quattro, J. M. (1992). Analysis of molecular variance inferred from metric distances among DNA haplotypes: application to human mitochondrial DNA restriction data. Genetics, 131(2), 479–491. https://doi.org/10.1007/s00424-009-0730-7.
Ficetola, G. F., Miaud, C., Pompanon, F., & Taberlet, P. (2008). Species detection using environmental DNA from water samples. Biology Letters, 4(4), 423–425. https://doi.org/10.1098/rsbl.2008.0118.
Fox, J. (2003). Effect displays in R for generalised linear models. Journal of Statistical Software, 8(15), 1–27. https://doi.org/10.2307/271037.
Fox, J., & Weisberg, S. (2011). An R companion to applied regression (2nd ed.). Thousand Oaks: Sage.
Fox, J., Dybdahl, M. F., Jokela, J., & Lively, C. M. (1996). Genetic structure of coexisting sexual and clonal subpopulations in a freshwater snail (Potamopyrgus antipodarum). Evolution, 50(4), 1541–1548. https://doi.org/10.2307/2410890.
Fryer, G., & Iles, T. D. (1972). The cichlid fishes of the Great Lakes of Africa: Their biology and evolution. Scotland: Oliver and Boyd Press.
Gangloff, M. M. (1998). The New Zealand mud snail in western North America. Aquatic Nuisance Species, 2, 25–30.
Gérard, C., & Le Lannic, J. (2003). Establishment of a new host–parasite association between the introduced invasive species Potamopyrgus antipodarum (smith) (Gastropoda) and Sanguinicola sp. Plehn (Trematoda) in Europe. Journal of Zoology, 261, 213–216. https://doi.org/10.1017/S0952836903004084.
Gérard, C., Miura, O., Lorda, J., Cribb, T. H., Nolan, M. J., & Hechinger, R. F. (2017). A native-range source for a persistent trematode parasite of the exotic New Zealand mudsnail (Potamopyrgus antipodarum) in France. Hydrobiologia, 785(1), 115–126. https://doi.org/10.1007/s10750-016-2910-8.
Gibson, A. K., Delph, L. F., & Lively, C. M. (2017). The two-fold cost of sex: experimental evidence from a natural system. Evolution Letters, 1(1), 6–15. https://doi.org/10.1002/evl3.1.
Goldberg, C. S., Sepulveda, A., Ray, A., Baumgardt, J., & Waits, L. P. (2013). Environmental DNA as a new method for early detection of New Zealand mudsnails (Potamopyrgus antipodarum). Freshwater Science, 32(3), 792–800. https://doi.org/10.1899/13-046.1.
Goodfriend, G. a. (1986). Variation in land snail shell form and its causes: a review. Systematic Zoology, 35(2), 204–223.
Haase, M. (2003). Clinal variation in shell morphology of the freshwater gastropod Potamopyrgus antipodarum along two hill-country streams in New Zealand. Journal of the Royal Society of New Zealand, 33(2), 549–560.
Haase, M. (2008). The radiation of hydrobiid gastropods in New Zealand: a revision including the description of new species based on morphology and mtDNA sequence information. Systematics and Biodiversity, 6(1), 99–159. https://doi.org/10.1017/S1477200007002630.
Haase, M., Naser, M. D., & Wilke, T. (2010). Ecrobia grimmi in brackish Lake Sawa, Iraq: indirect evidence for long-distance dispersal of hydrobiid gastropods (Caenogastropoda: Rissooidea) by birds. Journal of Molluscan Studies, 76(1), 101–105. https://doi.org/10.1093/mollus/eyp051.
Haase, M., Esch, S., & Misof, B. (2013). Local adaptation, refugial isolation and secondary contact of alpine populations of the land snail Arianta arbustorum. Journal of Molluscan Studies, 79(3), 241–248. https://doi.org/10.1093/mollus/eyt017.
Hammer, Ø., Harper, D. A. T., & Ryan, P. D. (2001). PAST: Paleontological statistics software package for education and data analysis. Palaeontologia Electronica, 4(1), 9.
Hauser, L., Carvalho, G. R., Hughes, R. N., & Carter, R. E. (1992). Clonal structure of the introduced freshwater snail Potamopyrgus antipodarum (Prosobranchia: Hydrobiidae), as revealed by DNA fingerprinting. Proceedings of the Biological Sciences, 249, 19–25.
Hechinger, R. F. (2012). Faunal survey and identification key for the trematodes (Platyhelminthes: Digenea) infecting Potamopyrgus antipodarum (Gastropoda: Hydrobiidae) as first intermediate host. Zootaxa, 27(3418), 1–27.
Herbst, D. B., Bogan, M. T., & Lusardi, R. A. (2008). Low specific conductivity limits growth and survival of the New Zealand mud snail from the upper Owens River, California. Western North American Naturalist, 68(3), 324–333. https://doi.org/10.3398/1527-0904(2008)68[324:LSCLGA]2.0.CO;2.
Holomuzki, J. R., & Biggs, B. J. F. (1999). Distributional responses to flow disturbance by a stream-dwelling snail. Oikos, 87(1), 36–47.
Holomuzki, J. R., & Biggs, B. J. F. (2006). Habitat-specific variation and performance trade-offs in shell armature of New Zealand mudsnails. Ecological Society of America, 87(4), 1038–1047.
Hughes, R. N. (1996). Evolutionary ecology of parthenogenetic strains of the prosobranch snail, Potamopyrgus antipodarum (Gray) = P. jenkinsi (Smith). Malacological Review, 28, 101–114.
Jacobsen, R., Forbes, V. E., & Skovgaard, O. (1996). Genetic population structure of the prosobranch snail Potamopyrgus antipodarum (gray) in Denmark using PCR-RAPD fingerprints. Proceedings: Biological Sciences, 263, 1065–1070.
Jay, F., Manel, S., Alvarez, N., Durand, E. Y., Thuiller, W., Holderegger, R., Tarbelet, P., & François, O. (2012). Forecasting changes in population genetic structure of alpine plants in response to global warming. Molecular Ecology, 21(10), 2354–2368. https://doi.org/10.1111/j.1365-294X.2012.05541.x.
Jensen, F. B. (1995). Nitrogen metabolism and excretion. Uptake and effects of nitrite and nitrate in animals. Boca Raton: CRC Press.
Jensen, F. B. (2003). Nitrite disrupts multiple physiological functions in aquatic animals. Comparative Biochemistry and Physiology - A Molecular and Integrative. Physiology, 135(1), 9–24. https://doi.org/10.1016/S1095-6433(02)00323-9.
Jerde, C. L., Mahon, A. R., Chadderton, W. L., & Lodge, D. M. (2011). “Sight-unseen” detection of rare aquatic species using environmental DNA. Conservation Letters, 4(2), 150–157. https://doi.org/10.1111/j.1755-263X.2010.00158.x.
Johannesson, K., Panova, M., Kemppainen, P., Andre, C., Rolan-Alvarez, E., & Butlin, R. K. (2010). Repeated evolution of reproductive isolation in a marine snail: unveiling mechanisms of speciation. Philosophical Transactions of the Royal Society, B: Biological Sciences, 365(1547), 1735–1747. https://doi.org/10.1098/rstb.2009.0256.
Johnson, P. C. D. (2014). Extension of Nakagawa & Schielzeth’s R2GLMM to random slopes models. Methods in Ecology and Evolution, 5(9), 944–946. https://doi.org/10.1111/2041-210X.12225.
Jokela, J., Lively, C. M., Dybdahl, M. F., & Fox, J. A. (1997). Evidence for a cost of sex in the freshwater snail Potamopyrgus antipodarum. Ecology, 78(2), 452–460. https://doi.org/10.2307/2266021.
Jombart, T. (2008). Adegenet: A R package for the multivariate analysis of genetic markers. Bioinformatics, 24(11), 1403–1405. https://doi.org/10.1093/bioinformatics/btn129.
Jombart, T., Devillard, & Balloux, S. (2010). Discriminant analysis of principal components: a new method for the analysis of genetically structured populations. BMC Genetics, 11(1), 94. https://doi.org/10.1186/1471-2156-11-94.
Kalinowski, S. T., Taper, M. L., & Marshall, T. C. (2007). Revising how the computer program CERVUS accommodates genotyping error increases success in paternity assignment. Molecular Ecology, 16(5), 1099–1106. https://doi.org/10.1111/j.1365-294X.2007.03089.x.
Karney, C. F. F. (2013). Algorithms for geodesics. Journal of Geodesy, 87, 43–55. https://doi.org/10.1007/s00190-012-0578-z.
Karr, J. R., & James, F. C. (1975). Eco-morphological configurations and convergent evolution in species and communities. Ecology and evolution of communities. Cambridge: Harvard University Press.
King, K. C., Jokela, J., & Lively, C. M. (2011). Parasites, sex, and clonal diversity in natural snail populations. Evolution, 65(5), 1474–1481. https://doi.org/10.1111/j.1558-5646.2010.01215.x.
Kingsolver, J. G., & Pfennig, D. W. (2007). Patterns and power of phenotypic selection in nature. BioScience, 57(7), 561–572. https://doi.org/10.1641/B570706.
Kistner, E. J., & Dybdahl, M. F. (2013). Adaptive responses and invasion: the role of plasticity and evolution in snail shell morphology. Ecology and Evolution, 3(2), 424–436.
Kistner, E. J., & Dybdahl, M. F. (2014). Parallel variation among populations in the shell morphology between sympatric native and invasive aquatic snails. Biological Invasions, 16, 2615–2626. https://doi.org/10.1007/s10530-014-0691-4.
Klingenberg, C. P. (2011). MorphoJ: an integrated software package for geometric morphometrics. Molecular Ecology Resources, 11(2), 353–357. https://doi.org/10.1111/j.1755-0998.2010.02924.x.
Klingenberg, C. P. (2016). Size, shape, and form: concepts of allometry in geometric morphometrics. Development Genes and Evolution, 226(3), 113–137. https://doi.org/10.1007/s00427-016-0539-2.
Klingenberg, C. P., & Monteiro, L. R. (2005). Distances and directions in multidimensional shape spaces: implications for morphometric applications. Systematic Biology, 54(4), 678–688. https://doi.org/10.1080/10635150590947258.
Krist, A. C. (2002). Crayfish induce a defensive shell shape in a freshwater snail. Invertebrate Biology, 121(3), 235–242. https://doi.org/10.1111/j.1744-7410.2002.tb00063.x.
Lagrue, C., Mcewan, J., Poulin, R., & Keeney, D. B. (2007). Co-occurrences of parasite clones and altered host phenotype in a snail–trematode system. Parasitology, 37, 1459–1467. https://doi.org/10.1016/j.ijpara.2007.04.022.
Larkin, K., Tucci, C., & Neiman, M. (2016). Effects of polyploidy and reproductive mode on life history trait expression. Ecology and Evolution, 6(3), 765–778. https://doi.org/10.1002/ece3.1934.
Le Pennec, G., Butlin, R. K., Jonsson, P. R., Larsson, A. I., Lindborg, J., Bergström, E., Westram, A. M., & Johannesson, K. (2017). Adaptation to dislodgement risk on waveswept rocky shores in the snail Littorina saxatilis. PLoS One, 12(10), 1–15. https://doi.org/10.1371/journal.pone.0186901.
Leigh, J. W., & Bryant, D. (2015). PopART: full-feature software for haplotype network construction. Methods in Ecology and Evolution, 6(9), 1110–1116.
Levri, E. P., Dillard, J., & Martin, T. (2005). Trematode infection correlates with shell shape and defence morphology in a freshwater snail. Parasitology, 130(6), 699–708. https://doi.org/10.1017/S0031182005007286.
Levri, E. P., Krist, A. C., Bilka, R., & Dybdahl, M. F. (2014). Phenotypic plasticity of the introduced New Zealand mud snail, Potamopyrgus antipodarum, compared to sympatric native snails. PLoS ONE, 9(4), e93985. https://doi.org/10.1371/journal.pone.0093985.
Levri, E. P., Landis, S., Smith, B., Colledge, E., Metz, E., & Li, X. (2017). Variation in predator-induced behavioral changes in introduced and native populations of the invasive New Zealand mud snail (Potamopyrgus antipodarum Gray 1843). Aquatic Invasions, 12, in press, 499–508.
Li, W., & Godzik, A. (2006). Cd-hit: a fast program for clustering and comparing large sets of protein or nucleotide sequences. Bioinformatics, 22(13), 1658–1659.
Lively, C. M. (1987). Evidence from a New Zealand snail for the maintenance of sex by parasitism. Nature, 329, 855–857.
Lively, C. M. (1992). Parthenogenesis in a fresh-water snail—reproductive assurance versus parasitic release. Evolution, 46(4), 907–913. https://doi.org/10.2307/2409745.
Losos, J. B. (2009). Lizards in an evolutionary tree: Ecology and adaptive radiation of anoles. University of California Press.
Losos, J. B., & Miles, D. B. (1994). Adaptation, constraint, and the comparative method: phylogenetic issues and methods. In Ecological morphology: integrative organismal biology (pp. 60–98). University of Chicago Press.
Maynard Smith, J. (1971). The origin and maintenance of sex. In G. C. Williams (Ed.), Group selection. Chicago.
Maynard-Smith, J. (1978). The evolution of sex. Cambridge: Cambridge University Press.
McKenzie, V. J., Hall, W. E., & Guralnick, R. P. (2013). New Zealand mudsnails (Potamopyrgus antipodarum) in Boulder Creek, Colorado: environmental factors associated with fecundity of a parthenogenic invader. NRC Research Press, 36, 30–36. https://doi.org/10.1139/cjz-2012-0183.
Meirmans, P. G., & Van Tienderen, P. H. (2004). GENOTYPE and GENODIVE: two programs for the analysis of genetic diversity of asexual organisms. Molecular Ecology Notes, 4(4), 792–794. https://doi.org/10.1111/j.1471-8286.2004.00770.x.
Monteiro, L. R. (1999). Multivariate regression models and geometric morphometrics: the search for causal factors in the analysis of shape. Systematic Biology, 48(1), 192–199.
Nakagawa, S., & Schielzeth, H. (2013). A general and simple method for obtaining R2 from generalized linear mixed-effects models. Methods in Ecology and Evolution, 4(2), 133–142. https://doi.org/10.1111/j.2041-210x.2012.00261.x.
Negovetic, S., & Jokela, J. (2001). Life-history variation, phenotypic plasticity and maintenance of subpopulation structure in a freshwater snail. Ecology, 82(10), 2805–2815. https://doi.org/10.1890/0012-9658(2001)082[2805:lhvppa]2.0.co;2.
Neiman, M., & Lively, C. M. (2004). Pleistocene glaciation is implicated in the phylogeographical structure of Potamopyrgus antipodarum, a New Zealand snail. Molecular Ecology, 13, 3085–3098.
Neiman, M., Jokela, J., & Lively, C. M. (2005). Variation in asexual lineage age in Potamopyrgus antipodarum, a New Zealand snail. Evolution, 59(9), 1945–1952.
Neiman, M., Paczesniak, D., Soper, D. M., Baldwin, A. T., & Hehman, G. (2011). Wide variation in ploidy level and genome size in a New Zealand freshwater snail with coexisting sexual and asexual lineages. Evolution, 65(11), 3202–3216.
Neiman, M., Larkin, K., Thompson, A. R., & Wilton, P. (2012). Male offspring production by asexual Potamopyrgus antipodarum, a New Zealand snail. Heredity, 109(1), 57–62. https://doi.org/10.1038/hdy.2012.13.
Okajima, R., & Chiba, S. (2011). How does life adapt to a gravitational environment? The outline of the terrestrial gastropod shell. The American Naturalist, 178(6), 801–809. https://doi.org/10.1086/662674.
Paczesniak, D., Jokela, J., Larkin, K., & Neiman, M. (2013). Discordance between nuclear and mitochondrial genomes in sexual and asexual lineages of the freshwater snail Potamopyrgus antipodarum. Molecular Ecology, 22(18), 4695–4710. https://doi.org/10.1111/mec.12422.
Parks, D. H., Mankowski, T., Zangooei, S., Porter, M. S., Armanini, D. G., Baird, D. J., Langille, M. G. I., & Beiko, R. G. (2013). GenGIS 2: geospatial analysis of traditional and genetic biodiversity, with new gradient algorithms and an extensible plugin framework. PLoS One, 8(7), e69885. https://doi.org/10.1371/journal.pone.0069885.
Phillips, N. R., & Lambert, D. M. (1989). Genetics of Potamopyrgus antipodarum (Gastropoda: Prosobranchia): evidence for reproductive modes. New Zealand Journal of Zoology, 16(3), 435–445.
Ponder, W. F. (1988). Potamopyrgus antipodarum: a molluscan colonizer of Europe and Australia. Journal of Molluscan Studies, 54(3), 271–286.
Raffaelli, D. G. (1982). Recent ecological research on some European species of Littorina. Journal of Molluscan Studies, 48(December), 342–354. https://doi.org/10.1093/oxfordjournals.mollus.a065656.
Rieseberg, L. H., Widmer, A., Arntz, A. M., & Burke, J. M. (2002). Directional selection is the primary cause of phenotypic diversification. Proceedings of the National Academy of Sciences, 99(19), 12242–12245. https://doi.org/10.1073/pnas.192360899.
Rogstad, S. H., Keane, B., & Beresh, J. (2002). Genetic variation across VNTR loci in central North American Taraxacum surveyed at different spatial scales. Plant Ecology, 161(1), 111–121.
Rohlf, F.J. (2010). TpsDig, version 2.16. Department of Ecology and Evolution, State University of New York at Stony Brook, USA, available at: http://life.bio. sunysb.edu/morph/bibr28. Accessed 20/04/2016.
Rohlf, F.J. (2012). TpsUtil, file utility program. Version 1.53. Department of Ecology and Evolution, State University of New York at Stony Brook, USA, available at: http://life.bio.sunysb.edu/morph. Accessed 25/04/2016.
Rohlf, F. J., & Slice, D. (1990). Extensions of the Procrustes method for the optimal superimposition of landmarks. Systematic Biology, 39, 40–59.
Rosenberg, M. S., & Anderson, C. D. (2011). PASSaGE: pattern analysis, spatial statistics and geographic exegesis. Version 2. Methods in Ecology and Evolution, 2(3), 229–232. https://doi.org/10.1111/j.2041-210X.2010.00081.x.
Rousset, F. (2008). GENEPOP’007: a complete re-implementation of the GENEPOP software for Windows and Linux. Molecular Ecology Resources, 8(1), 103–106. https://doi.org/10.1111/j.1471-8286.2007.01931.x.
Schindler, D. W., Kling, H., Schmidt, R. V., Prokopowich, J., Frost, V. E., Reid, R. A., & Capel, M. (1973). Eutrophication of Lake 227 by addition of phosphate and nitrate: the second, third, and fourth years of enrichment, 1970, 1971, and 1972. Journal of the Fisheries Board of Canada, 30(10), 1415–1440.
Schreiber, E. S. G., Glaister, A., Quinn, G. P., & Lake, P. S. (1998). Life history and population dynamics of the exotic snail Potamopyrgus antipodarum (Prosobranchia: Hydrobiidae) in Lake Purrumbete, Victoria, Australia. Marine and Freshwater Research, 49, 73–78.
Schwarz, G. (1978). Estimating the dimension of a model. The Annals of Statistics, 6(2), 461–464. https://doi.org/10.1214/aos/1176344136.
Seeley, R. H. (1986). Intense natural selection caused a rapid morphological transition in a living marine snail. Proceedings of the National Academy of Sciences of the United States of America, 83(18), 6897–6901. https://doi.org/10.1073/pnas.83.18.6897.
Shimada, K., & Urabe, M. (2003). Comparative ecology of the alien freshwater snail Potamopyrgus antipodarum and the indigenous snail Semisulcospira spp. Venus, 62, 39–53.
Slatkin, M. (1995). A measure of population subdivision based on microsatellite allele frequencies. Genetics, 139(1), 457–462.
Soper, D. M., Neiman, M., Savytskyy, O. P., Zolan, M. E., & Lively, C. M. (2013). Spermatozoa production by triploid males in the New Zealand freshwater snail Potamopyrgus antipodarum. Biological Journal of the Linnean Society, 110(1), 1–10. https://doi.org/10.1038/jid.2014.371.
Soper, D. M., Hatcher, K. M., & Neiman, M. (2016). Documentation of copulatory behaviour in triploid male freshwater snails. Ethology Ecology and Evolution, 28(1), 110–116. https://doi.org/10.1080/03949370.2015.1030781.
Städler, T., Frye, M., Neiman, M., & Lively, C. M. (2005). Mitochondrial haplotypes and the New Zealand origin of clonal European Potamopyrgus, an invasive aquatic snail. Molecular Ecology, 14, 2465–2473.
Stankowski, S. (2011). Extreme, continuous variation in an island snail: local diversification and association of shell form with the current environment. Biological Journal of the Linnean Society, 104(4), 756–769. https://doi.org/10.1111/j.1095-8312.2011.01748.x.
Stankowski, S. (2013). Ecological speciation in an island snail: evidence for the parallel evolution of a novel ecotype and maintenance by ecologically dependent postzygotic isolation. Molecular Ecology, 22(10), 2726–2741. https://doi.org/10.1111/mec.12287.
Statzner, B., & Holm, T. F. (1989). Morphological adaptation of shape to flow: microcurrents around lotic macroinvertebrates with known Reynolds numbers at quasi-natural flow conditions. Oecologia, 78(2), 145–157. https://doi.org/10.1007/BF00377150.
Stumm, W., & Morgan, J. J. (1996). Aquatic chemistry (3rd ed.). New York: Wiley.
Vergara, D., Fuentes, J. A., Stoy, K. S., & Lively, C. M. (2016). Evaluating shell variation across different populations of a freshwater snail. Molluscan Research, 5818, 1–13. https://doi.org/10.1080/13235818.2016.1253446.
Verhaegen, G., McElroy, K. E., Bankers, L. A., Neiman, N., & Haase, M. (2018). Adaptive phenotypic plasticity in a clonal invader. Ecology and Evolution, 8, 4465–4483. https://doi.org/10.1002/ece3.4009.
Vermeij, G. J. (1995). A natural history of shells. Princeton University Press.
Vogel, S. (1994). Life in moving fluids: the physical biology of flow. New Jersey: Princeton University Press.
Vonesh, E. F., Chinchilli, V. M., & Pu, K. (1996). Goodness-of-fit in generalized nonlinear mixed-effects models. Biometrics, 52(2), 572–587.
Vrijenhoek, R. C. (1979). Factors affecting clonal diversity and coexistence. Integrative and Comparative Biology, 19(3), 787–797. https://doi.org/10.1093/icb/19.3.787.
Warwick, T. (1952). Strains in the mollusc Potamopyrgus jenkinsi (Smith). Nature, 169(4300), 551–552.
Weetman, D., Hauser, L., & Carvalho, G. R. (2002). Reconstruction of microsatellite mutation history reveals a strong and consistent deletion bias in invasive clonal snails, Potamopyrgus antipodarum. Genetics Society of America, 162, 813–822.
Weissenberger, J., Emanns, H. S. A., & Schwoerbelt, J. (1991). Measurement of lift and drag forces in the mN range experienced by benthic arthropods at flow velocities below 1.2 m s −1. Freshwater Biology, 25, 21–31. https://doi.org/10.1111/j.1365-2427.1991.tb00469.x.
Williams, E. E. (1972). The origin of faunas. Evolution of lizard congeners in a complex island fauna: a trial analysis. In Evolutionary biology (pp. 47–89). New York: Springer.
Winterbourn, M. J. (1970). Population studies on the New Zealand freshwater gastropod Potamopyrgus antipodarum (Gray). Journal of Molluscan Studies, 39(2–3), 139–149.
Winterbourn, M. J. (1974). Larval Trematoda parasitizing the New Zealand species of Potamopyrgus (Gastropoda: Hydrobiidae). Mauri Ora, 2, 17–30.
Zbikowski, J., & Zbikowska, E. (2009). Invaders of an invader—trematodes in Potamopyrgus antipodarum in Poland. Journal of Invertebrate Pathology, 101(1), 67–70. https://doi.org/10.1016/j.jip.2009.02.005.
Zelditch, M. L., Swiderski, D. L., & Sheets, H. D. (2012). Geometric morphometrics for biologists: A primer. Academic Press.
Zielske, S., Ponder, W. F., & Haase, M. (2017). The enigmatic pattern of long-distance dispersal of minute freshwater gastropods (Caenogastropoda, Truncatelloidea, Tateidae) across the South Pacific. Journal of Biogeography, 44(1), 195–206. https://doi.org/10.1111/jbi.12800.
Acknowledgements
We thank K. Collier and I. D. Hogg from The University of Waikato/Hamilton/New Zealand for providing logistic support and help with fieldwork. S. Fregin is acknowledged for her assistance in the lab. We are grateful to S. Puechmaille for population genetic advice. G. Uhl is thanked for providing access to the Zeiss microscope. We also thank two anonymous reviewers for their constructive comments on the previous manuscript. This study was part of the Research Training Group 2010 RESPONSE funded by the Deutsche Forschungsgemeinschaft (DFG).
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Supplementary Table 1
(DOCX 33 kb)
Supplementary Table 2
(DOCX 24.1 kb)
Supplementary Table 3
(DOCX 16.3 kb)
Supplementary Table 4
(DOCX 15.6 kb)
Supplementary Table 5
(DOCX 22 kb)
Supplementary Table 6
(DOCX 45.2 kb)
ESM 1
(PDF 768 kb)
Rights and permissions
About this article
Cite this article
Verhaegen, G., Neiman, M. & Haase, M. Ecomorphology of a generalist freshwater gastropod: complex relations of shell morphology, habitat, and fecundity. Org Divers Evol 18, 425–441 (2018). https://doi.org/10.1007/s13127-018-0377-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13127-018-0377-3