Abstract
A combined approach was used to study the diversity, distribution and variability of the ameirid genus Nitokra in the uppermost reaches of the Carey palaeochannel, as very little is known about habitat invasions of stygofauna in general and inland dispersal of this predominantly marine genus in particular. A 70-km-long stretch of several disconnected calcrete subterranean habitats, known as Yeelirrie, has previously shown to harbour up to ten sympatric and parapatric congeners of the miraciid genus Schizopera and six allopatric congeners of the parastenocaridid genus Kinnecaris, in addition to 11 other species of copepods. The diversity of the genus Nitokra is much smaller, with only two allopatric species in the entire area. Nitokra esbe sp. nov. is a short-range endemic, recorded in a single bore in the most downstream part of Yeelirrie. In contrast, both molecular and morphological data indicate that Nitokra yeelirrie sp. nov. is widespread here, showing one of the largest distribution ranges of any subterranean copepod in Yeelirrie. Phylogenetic analysis of Nitokra populations based on the COI gene shows N. esbe as a sister clade to other Nitokra sequences, which does not exclude the possibility of an ‘active upstream’ dispersal model, proposed for other copepods of marine origin here. High levels of COI sequence divergence (∼10 %) among specimens of N. yeelirrie collected 8 km apart suggest the potential for considerable population differentiation or restricted gene flow within an apparently single large calcrete body. A table of the most important morphological characters for all 79 valid world species of Nitokra is presented, and replacement names are provided for four junior homonyms. An overview of the conservation status of the entire Yeelirrie stygofauna was also provided.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The Yeelirrie area of Western Australia has received due attention recently because of an unprecedented diversity of copepods (Karanovic and Cooper 2011a, b, 2012), representing more than 70 % of the previously recorded α-diversity in the whole Yilgarn region of Western Australia (Karanovic 2004), although it comprises less than 3 % of the region’s surface. This 70-km-long stretch of several disconnected calcrete subterranean habitats in the uppermost reaches of the Carey palaeochannel has been previously shown to harbour up to ten sympatric and parapatric congeners of the miraciid genus Schizopera Sars, 1905 (Karanovic and Cooper 2012) and six allopatric congeners of the parastenocaridid genus Kinnecaris Jakobi, 1972 (Karanovic and Cooper 2011a), in addition to about 11 other species of copepods (T. Karanovic, unpublished data). Their reconstructed molecular phylogenies revealed that both explosive radiation and multiple colonisations are responsible for this richness and that copepods of marine origin most probably colonised this palaeochannel using active dispersal upstream, while those of freshwater origin dispersed mostly downstream (Karanovic and Cooper 2011a, 2012). In this paper, we study the ameirid genus Nitokra Boeck, 1865, which is also very abundant in Yeelirrie; we use this term to describe indiscriminantly the existing pastoral station, one of tributaries of the Carey palaeochannel (as Yeelirie palaochanel), and the system of calcrete deposits in this area; for more information about calcrete geology, geomorphology and distribution in Western Australia, see Humphreys (2001, 2006).
The majority of ameirids are free-living benthic or interstitial marine animals, but a few species are also found in association with flatworms (Liddell 1912), medusae (Humes 1953) and malacostracan crustaceans (Chappuis 1926; Bowman 1988). Although primarily marine, they have successfully radiated into freshwater and can be found today from marine abyssal depths (Corgosinho and Martínez Arbizu 2010) to freshwater caves (Karanovic 2000), with an especially rich and diverse fauna discovered recently in the calcrete aquifers of Western Australia (Karanovic 2004, 2006, 2010; Karanovic and Hancock 2009) and in calcrete-free fractured Archaean greenstone (Karanovic et al. 2013). With more than 300 valid species worldwide (Boxshall and Halsey 2004), the Ameiridae Monard, 1927 is the third largest harpacticoid family, just after the Canthocamptidae Sars, 1906 and Miraciidae Dana, 1846.
The ameirids are currently classified into 46 valid genera (Walter and Boxshall 2013) and two subfamilies: Ameirinae Monard, 1927 and Stenocopiinae Lang, 1944. A sexually dimorphic basal spine on the first swimming leg is the most important synapomorphy that unites all ameirids, and this character state has (probably) been secondarily lost in only a few species (Lee and Huys 2002; Karanovic 2006; Karanovic and Hancock 2009). Nitokra is the largest ameirid genus, containing nearly 80 valid species (Karanovic and Pesce 2002), with a notoriously difficult and problematic taxonomy (Gómez et al. 2012). In addition to numerous synonyms (Lang 1948, 1965a) and the erroneous spelling of the genus name (as Nitocra) for more than 100 years (Bowman 1988), there are several pairs of still unresolved homonyms (Karanovic and Pesce 2002).
The first mention of any Nitokra in Australia was that of the cosmopolitan and polymorphic Nitokra lacustris (Shmankevich, 1875) by Dussart and Defaye (1990) in their list of world continental copepods. However, it is not clear what this record was based upon, if not on an erroneous interpretation of a casual mention of this species by Bayly (1972, p. 237) in his overview of osmotic behaviour of animals in saline continental waters (also confirmed by personal communication from Dr. Ian Bayly on 30 November 2013). Adams and Stauber (2008) reported on a personal communication with two colleagues about ecotoxicology tests done on the cosmopolitan Nitokra spinipes Boeck, 1865, implying that the original material has been collected somewhere in Australia, but without any explicit data. This record should be considered uncertain until it can be verified by a specialist. Only two species of Nitokra have been confirmed so far from Australia: the endemic Nitokra humphreysi Karanovic and Pesce 2002, described from anchialine groundwaters of the Cape Range karst area in northwestern Western Australia (Karanovic and Pesce 2002), and the relatively widely distributed Australo-Pacific N. lacustris pacifica Yeatman 1983, reported so far from crab holes in Western Samoa, Tonga, and Fiji (Yeatman 1983), temporary brackish water pools in Papua New Guinea (Fiers 1986), and bore holes in calcrete aquifers in arid Western Australia (Karanovic 2004).
Arid Western Australia is famous for numerous isolated calcrete aquifers that lie along palaeodrainage channels and range in diameter from tens of kilometres to hundreds of metres (Humphreys 2001, 2006). The highly porous and carbonate-rich sediments here represent an ideal habitat for various groups of stygofauna (aquatic subterranean fauna), including dytiscid beetles (Watts and Humphreys 2006, 2009 and references therein), amphipods (Finston et al. 2007; King et al. 2012), isopods (Wilson 2008), bathynellids (Cho et al. 2006a, b), ostracods (Karanovic 2007) and copepods (Karanovic 2004, 2006). Previous genetic and morphological studies suggested that individual calcretes are equivalent to closed island habitats, which have been isolated for millions of years (Cooper et al. 2008). The majority of stygobitic species evolved within individual calcretes, possibly following an independent colonisation by epigean ancestors (Cooper et al. 2002, 2007, 2008; Guzik et al. 2008; Leys et al. 2003; Leys and Watts 2008). Phylogeographic studies of dytiscid beetles (Cooper et al. 2002; Leys et al. 2003), amphipods (Cooper et al. 2007; Bradford et al. 2010), isopods (Cooper et al. 2008) and bathynellids (Guzik et al. 2008) have confirmed the presence of monophyletic groups restricted to single calcretes. The diversity of the stygofauna is mostly dependent on the size of the calcrete and typically includes one to three species from each major group, most of them endemic to that site (Karanovic 2004, 2006, 2007; Finston et al. 2007; Leys and Watts 2008; Allford et al. 2008; Bradford et al. 2010). However, very little is known about habitat invasions of stygofauna in general and inland dispersal of predominantly marine groups in particular. Here, we use a combined approach, based on morphological and molecular analyses, to study the diversity, distribution and variability of the ameirid genus Nitokra in subterranean calcrete aquifers in Yeelirrie, in an attempt to shed some additional light on the more general questions of their habitat invasion and subsequent dispersal.
Material and methods
Most samples studied here were collected in the Yeelirrie area (Karanovic and Cooper 2011a, 2012), as a part the Yeelirrie Subterranean Fauna Survey 2009–2011, commissioned by BHP Billiton Yeelirrie Development Company Pty Ltd. Two outgroups for our molecular phylogenies came from the family Canthocamptidae Brady, 1880, and their COI sequence data are available from GenBank: Australocamptus hamondi Karanovic 2004 (also collected in the Yeelirrie calcrete) and Elaphoidella humphreysi Karanovic 2006 (collected from the Pilbara region of Western Australia). Locality data and numbers of specimens are listed separately for each Nitokra species, and all type material is deposited in the Western Australian Museum (WAM), Perth. Some additional material is kept as voucher specimens by Subterranean Ecology but will ultimately also be deposited in the WAM (not listed herein).
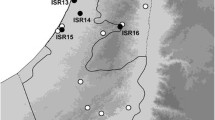
Samples were collected with haul nets (mesh size 50 or 150 μm) from groundwater bores. Bores are holes mainly made by mining companies or agricultural enterprises for the purpose of water monitoring and abstraction or mineral exploration. They are usually from 5 to 20 cm in diameter and may be lined entirely, or in part, by PVC tubing (the casing). This tubing may be open only at the bottom, or it may be pierced at one or more levels by holes of various sizes (‘slots’). The top may be securely capped or entirely open to the elements. Some bores record the water pressure at a given level in the aquifer (piezometers), while others, equipped with windmills or solar pumps, provide water for pastoral use. Haul nets are simple plankton nets of different sizes suitable for the bores; their collars can range from 20 to 150 mm in diameter and are made of stainless steel. For each sample, a weighed net (using simple fishing leads) was lowered down into the bore with a bottle screwed onto its distal part and then hauled through the water column, usually six times. Samples were preserved in the field in cold 100 % ethanol, kept on ice or in a refrigerator and sorted in a laboratory. Each sample was given a unique four-digit lab code, which was used throughout the investigation; these codes are also presented in this paper for all material examined (prefix seLN). The same number is also used for our COI sequences. Bores established for hydrogeological work, mineral exploration and water monitoring have prefixes or suffixes of relevance only to that drilling program. These codes are cited in the material examined for each species to aid in specifying the location, although precise coordinates are also provided. More than 500 samples were taken from close to 200 bores in 2009 and 2010, most of them distributed along 21 bore lines (see Fig. 15).
Specimens for morphological observation were dissected and mounted on microscope slides in Faure’s medium, which was prepared following the procedure described by Stock and von Vaupel Klein (1996), and dissected appendages were then covered by a coverslip. For the urosome or the entire animal, two human hairs were mounted between the slide and coverslip, so the parts would not be compressed. By manipulating the coverslip carefully by hand, the whole animal or a particular appendage could be positioned in different aspects, making possible the observation of morphological details. During the examination, the water slowly evaporated and the appendages eventually remained in a completely dry Faure’s medium, ready for long-term storage. All line drawings were prepared using a drawing tube attached to a Leica MB2500 phase-interference compound microscope, with N-PLAN (×5, ×10, ×20, ×40 and ×63 dry) or PL FLUOTAR (×100 oil) objectives. Specimens that were not drawn were examined in propylene glycol and, after examination, were again preserved in 100 % ethanol. Photographs of whole specimens were taken in propylene glycol with a Leica DFC420 micro-camera attached to a Leica M205C dissecting microscope. The software package Leica Application Suite (LAS), version 3.5.0, was used to create a multifocal montage image. Specimens for scanning electron micrography (SEM) were dehydrated in progressive ethanol concentrations, transferred into pure isoamyl acetate, critical-point dried, mounted on stubs, coated in gold and observed under a Hitachi S-4700 microscope on the in-lens detector, with an accelerating voltage of 10 kV and working distances between 12.9 and 13.2 mm; micrographs were taken with a digital camera. Digital photographs were processed and combined into plates using Adobe Photoshop CS4.
Morphological terminology follows Huys and Boxshall (1991), except for caudal ramus setal numbering (not used) and small differences in the spelling of some appendages (antennula, mandibula, maxillula instead of antennule, mandible, maxillule), as an attempt to standardise the terminology for homologous appendages in different crustacean groups. Descriptions were shortened by making them comparative, and only the first species is described in full. Biospeleological terminology follows Humphreys (2000). Morphological characters for comparative analysis of all valid species of Nitokra are prepared in a tabular form, with the different armature formulae recorded as follows: antenna—number of setae on first/second exopodal segment (most species have a one-segmented exopod); mandibula—number of setae on basis/endopod lateral, endopod apical; maxilla—number of setae on proximal/distal coxal endites; first swimming leg—length to width index of first endopodal segment; intercoxal sclerite of second swimming leg—presence (+) or absence (−) of spinules; second to fourth swimming legs exopods and endopods (a widely used formula in harpacticoid copepods; see Lang 1948, 1965)—number of elements on segments first inner/second inner/third inner, apical, outer; fifth leg—number of armature elements on exopod/endopod; female fifth leg—length to with index of exopod; sixth leg—number of setae; anal operculum—number of spinules.
Specimens for molecular analysis were examined without dissection under a compound microscope (objective ×63 dry) in propylene glycol (CH3CH(OH)CH2OH). After examination, they were returned to 100 % ethanol. DNA was extracted using the GENTRA Puregene extraction method (Qiagen; www.qiagen.com) according to the manufacturer’s protocol for fresh tissues. PCR amplifications of a 623-bp fragment from the mitochondrial COI gene were carried out with the ‘universal’ primers LCOI490 and HCO2198 (Folmer et al. 1994). The use of these primers, however, proved problematic in many cases and hence additional ‘nested’ primers (M1321-1323) were designed by Ms. Kathleen Saint (South Australian Museum) from preliminary copepod COI sequence data and used in combination with the primers of Folmer et al. (1994) to improve the PCR amplification efficiency (see Karanovic and Cooper 2011a, 2012). An initial PCR amplification used the combination LCOI490/HCO2198; then, 1 μL of product was used to seed-nested PCRs in the following combinations: M1323/HCO2198 or M1321/M1322. PCR amplifications were carried out in 25-μL volumes containing 4 mM MgCl2, 0.20 mm dNTPs, 1× PCR buffer (Applied Biosystems), 6 pmol of each primer and 0.5 U of AmpliTaq Gold (Applied Biosystems). PCR amplification was performed under the following conditions: 94 °C for 9 min, then 34 cycles of 94 °C for 45 s, annealing 48 °C for 45 s, 72 °C for 60 s, with a final elongation step at 72 °C for 6 min. PCR products were purified using a vacuum plate method, and sequencing was undertaken using the ABI prism Big Dye Terminator Cycle sequencing kit (PE Applied Biosystems, Foster City, CA). Sequencing was carried out on an ABI 3700 DNA analyser, and sequences were edited and manually aligned in SeqEd version 1.0.3 (Applied Biosystems). For this study, DNA was extracted and the COI fragment successfully PCR-amplified from 11 copepod specimens (Table 1).
An initial BLAST analysis of GenBank was carried out to confirm the copepod origin of the sequence data, and the sequences were also translated into protein using MEGA version 5 (Tamura et al. 2011) to confirm the presence of an open reading frame. Phylogenetic analyses of the COI sequence data were conducted using a maximum likelihood (ML) approach. These analyses were conducted both in PAUP* (using the Geneious (version 6.05; Biomatters Ltd, www.geneious.com) interface; Swofford 2002) and using the program RAxML and the WEB-based RAxML ‘black box’ version 7.7.1 (http://phylobench.vital-it.ch/raxml-bb/; Stamatakis et al. 2008) provided by the Vital-IT Unit of the Swiss Institute of Bioinformatics. The ML analyses were conducted by applying a single general time-reversible (GTR) model (Rodríguez et al. 1990) to the COI data and with unequal variation at sites modelled using a gamma (G) distribution (Yang 1996). Support for branches was estimated employing the bootstrap option in RAxML, using 100 bootstrap pseudoreplicates. Average DNA sequence divergences among sequences and taxa were estimated using the program Geneious (version 6.05) using both a patristic distance based on PAUP* ML analyses with a GTR + G model and p-distances.
Taxonomic section
Subphylum Crustacea Brünich, 1772
Class Maxillopoda Dahl, 1956
Suclass Copepoda H. Milne Edwards, 1840
Order Harpacticoida Dana, 1846
Family Ameiridae Monard, 1927
Subfamily Ameirinae Monard, 1927
Genus Nitokra Boeck, 1865
Type locality
Australia, Western Australia, Yilgarn region, Yeelirrie station, bore line F, bore YU1, 27.142601° S, 119.853144° E.
Specimens examined
Holotype female dissected on one slide (WAM C47251), allotype male dissected on one slide (WAM C47250), three paratype males and four paratype females together on one SEM stub (WAM C47249), one paratype female (WAM C47252) dissected on one slide, six paratype males and 11 paratype females together in alcohol (WAM C47253), one female destroyed for DNA sequence (amplification successful), all collected from type locality, 15 March 2010, leg. T. Karanovic and G. Perina (seLN8492).
Six paratypes (three males and three females) together on one SEM stub (WAM C47248a), three paratype males and 10 paratype females and 11 paratype copepodids together in alcohol (WAM C47248b), all collected from Yeelirrie station, bore line 1.5, bore YYAC33, 27.169565° S, 119.871815° E, 16 March 2010, leg. S. Callan and N. Krawczyk (seLN8360).
Nitokra yeelirrie sp. nov., SEM photographs, a–c paratype female 1 from bore YYAC33, d–g paratype female 2 from bore YYAC33, h paratype female 3 from bore YYAC33: a habitus, ventro-lateral; b fifth pedigerous somite and genital double-somite, ventro-lateral; c swimming legs, ventro-lateral; d habitus, lateral; e fifth pedigerous somite and genital double-somite, lateral; f fourth and fifth urosomites, lateral; g anal somite and caudal rami, lateral; h anal somite and caudal rami, dorso-posterior
Nitokra yeelirrie sp. nov., SEM photographs, a–d paratype male 1 from bore YYAC33, e–h paratype male 2 from bore YYAC33: a, habitus, lateral; b, cephalic shield, lateral; c, tergites of free prosomites, lateral; d, first three urosomites, lateral; e, habitus, ventral; f, mouth appendages, ventral; g, first and second swimming legs, ventral; H, fifth and sixth legs, ventral
Two paratype females on one SEM stub (WAM C47254) from Yeelirrie station, bore line 3.5, bore YYAC284, 27.173127° S, 119.906857° E, 11 January 2010, leg. P. Bell and G. Perina (seLN7647).
One female destroyed for DNA sequence (amplification successful), from Yeelirrie station, bore line 3.5, bore YYAC284, 27.173127° S, 119.906857° E, 17 March 2010, leg. S. Callan and N. Krawczyk (seLN8387).
One paratype male on one SEM stub (WAM C47255) from Yeelirrie station, bore line K, bore YYHC085B, 27.247824° S, 120.054676° E, 20 March 2010, leg. T. Karanovic and S. Callan (seLN8418).
One female destroyed for DNA sequences (amplification unsuccessful), from Yeelirrie station, bore line K, bore YYHC085B, 27.247824° S, 120.054676° E, 18 March 2010, leg. T. Karanovic and S. Callan (seLN7131).
One paratype male and one paratype female together on one SEM stub (WAM C47256), three paratype females and one paratype copepodid together in alcohol (WAM C47257), all collected from Yeelirrie station, bore line N, bore YYHC0067B, 27.306433° S, 120.224542° E, 23 September 2010, leg. S. Callan and G. Perina (seLN10:0394).
One paratype female in alcohol (WAM C47258) from Yeelirrie station, bore line 5, bore YYAC0014A, high flow pump, 27.185507° S, 119.929233° E, 30 August 2009, leg. P. Bell and S. Callan (seLN6591).
Four paratype males and 18 paratype females (eight ovigerous) together in alcohol (WAM C47259), one female destroyed for DNA sequences (amplification unsuccessful), all from Yeelirrie station, bore line 1, bore YYD22, 27.167304° S, 119.870456° E, 15 March 2010, leg. S. Callan and N. Krawczyk (seLN8496).
Two paratype males and seven paratype females (one ovigerous) and four paratype copepodids together in alcohol (WAM C47260) from Yeelirrie station, bore line 1, bore YYD22, 27.167304° S, 119.870456° E, 1 September 2009, leg. P. Bell and G. Perina (seLN6610).
Four paratype males and three paratype females together in alcohol (WAM C47261) from Yeelirrie station, bore line 1, bore YYAC0015A, 27.170329° S, 119.868869° E, 16 March 2010, leg. S. Callan and N. Krawczyk (seLN8349).
Two paratype males and three paratype females (two ovigerous) together in alcohol (WAM C47262) from Yeelirrie station, bore line 1, bore YYD22, 27.167304° S, 119.870456° E, 20 March 2010, leg. T. Karanovic and S. Callan (seLN8411).
Two paratype males and two paratype females (one ovigerous) together in alcohol (WAM C47263) from Yeelirrie station, bore line 1, bore YYD26, 27.164033° S, 119.873196° E, 20 March 2010, leg. T. Karanovic and S. Callan (seLN8297).
Three paratype females and six paratype copepodids together in alcohol (WAM C47264), one females destroyed for DNA sequence (amplification unsuccessful), all from Yeelirrie station, bore line 1, bore YYD26, 27.164033° S, 119.873196° E, 15 March 2010, leg. S. Callan and N. Krawczyk (seLN8479).
One ovigerous paratype female in alcohol (WAM C47265) from Yeelirrie station, bore line 1, bore YYAC0015A, 27.170329° S, 119.868869° E, 11 January 2010, leg. P. Bell and G. Perina (seLN7688).
Two paratype males and one ovigerous paratype female together in alcohol (WAM C47266) from Yeelirrie station, bore line 1, bore YYAC0016A, 27.170328° S, 119.868867° E, 20 March 2010, leg. T. Karanovic and S. Callan (seLN8417).
One paratype female in alcohol (WAM C47267) from Yeelirrie station, bore line 1, bore YYAC0018C, 27.161503° S, 119.874715° E, 16 March 2010, leg. S. Callan and N. Krawczyk (seLN8355).
One paratype copepodid in alcohol (WAM C47268) from Yeelirrie station, bore line 1, bore YYD26, 27.164033° S, 119.873196° E, 1 September 2009, leg. P. Bell and S. Callan (seLN6605).
Four paratype males and three paratype females (two ovigerous) in alcohol (WAM C47269) from Yeelirrie station, bore line 1.5, bore YYAC35, 27.166173° S, 119.873977° E, 16 March 2010, leg. S. Callan and N. Krawczyk (seLN8366).
Two paratype males, five paratype females and one paratype copepodid together in alcohol (WAM C47270) from Yeelirrie station, bore line 1.5, bore YYAC35, 27.166173° S, 119.873977° E, 11 January 2010, leg. P. Bell and G. Perina (seLN7645).
Four paratype males and two paratype females together in alcohol (WAM C47271) from Yeelirrie station, bore line 1.5, bore YYAC35, 27.166173° S, 119.873977° E, 21 March 2010, leg. T. Karanovic and S. Callan (seLN7126).
Three paratype females and one paratype copepodid together in alcohol (WAM C47272) from Yeelirrie station, line 2, bore YYAC1007A, 27.165236° S, 119.883142° E, 21 March 2010, leg. T. Karanovic and S. Callan (seLN8546).
Two paratype females together in alcohol (WAM C47273) from Yeelirrie station, bore line 2, bore YYAC1004D, 27.174664° S, 119.877343° E, 16 March 2010, leg. S. Callan and N. Krawczyk (seLN8342).
One paratype female in alcohol (WAM C47274) from Yeelirrie station, bore line 2, bore YYAC1004C, 27.174665° S, 119.877345° E, 16 March 2010, leg. S. Callan and N. Krawczyk (seLN8526).
One paratype female in alcohol (WAM C47275) from Yeelirrie station, bore line 2, bore YYAC1007A, 27.165236° S, 119.883142° E, 16 March 2010, leg. S. Callan and N. Krawczyk (seLN8524).
One paratype male in alcohol (WAM C47276) from Yeelirrie station, bore line 2, bore YYAC1004D, 27.174664° S, 119.877343° E, 11 January 2010, leg. P. Bell and G. Perina (seLN7690).
Five paratype females and one paratype copepodid in alcohol (WAM C47277), one female destroyed for DNA sequence (amplification unsuccessful), all from Yeelirrie station, bore line 2, bore YYAC1004C, 27.174665° S, 119.877345° E, 21 March 2010, leg. T. Karanovic and S. Callan (seLN8555).
One paratype female in alcohol (WAM C47278) from Yeelirrie station, bore line 3, bore YYAC118, 27.174573° S, 119.889727° E, 12 November 2009, leg. P. Bell and G. Perina (seLN7389).
One female destroyed for DNA sequence (amplification unsuccessful) from Yeelirrie station, bore line 3, bore YYAC118, 27.174573° S, 119.889727° E, 21 March 2010, leg. T. Karanovic and S. Callan (seLN8538).
Two paratype females (one ovigerous) together in alcohol (WAM C47279) from Yeelirrie station, bore line 3.5, bore YYAC328, 27.175601° S, 119.907658° E, 12 November 2009, leg. P. Bell and G. Perina (seLN7433).
One paratype ovigerous female and one paratype copepodid in alcohol (WAM C47280), one female destroyed for DNA sequence (amplification successful), all from Yeelirrie station, bore line 3.5, bore YYAC328, 27.175601° S, 119.907658° E, 17 March 2010, leg. T. Karanovic and S. Callan (seLN8393).
One paratype female in alcohol (WAM C47281) from Yeelirrie station, bore line 4, bore YYAC0005, 27.172292° S, 119.916822° E, 30 August 2009, leg. S. Eberhard and S. Callan (seLN6505).
Three paratype males, nine paratype females and two paratype copepodids together in alcohol (WAM C47282), from Yeelirrie station, bore line F, bore YU1, 27.142601° S, 119.853144° E, 18 March 2010, leg. T. Karanovic and S. Callan (seLN8565).
One paratype male and two paratype females together in alcohol (WAM C47283), from Yeelirrie station, bore line F, bore YU1, 27.142601° S, 119.853144° E, 1 September 2009, leg. P. Bell and S. Callan (seLN6613).
Nitokra yeelirrie sp. nov., SEM photographs, a–f paratype male 1 from bore YU1, g, h paratype male 2 from bore YU1: a habitus, dorsal; b cephalic shield, dorsal; c rostrum, dorsal; d tergites of free prosomites and first urosomite, dorsal; e second to fifth urosomites, dorsal; f anal somite and caudal rami, dorsal; g habitus, lateral; h first three urosomites with fifth and sixth legs, lateral
One male destroyed for DNA sequence (amplification unsuccessful) from Yeelirrie station, bore line F, bore YYHC0139, 27.138090° S, 119.853130° E, 17 March 2010, leg. T. Karanovic and G. Perina (seLN8427).
One male destroyed for DNA sequence (amplification unsuccessful) from Yeelirrie station, bore line F, bore YU2, 27.137264° S, 119.853169° E, 17 March 2010, leg. T. Karanovic and G. Perina (seLN8427).
Etymology
The species is named after its type locality, Yeelirrie station. The name is a noun in apposition.
Description
Female (based on holotype and 11 paratypes). Total body length, measured from tip of rostrum to posterior margin of caudal rami (excluding appendages and caudal setae), from 396 to 508 μm (456 μm in holotype). Preserved specimens yellowish. Nauplius eye not visible. Prosome comprising cephalothorax with completely fused first pedigerous somite and three free pedigerous somites; urosome six-segmented, comprising fifth pedigerous somite, genital double-somite (fused genital and first abdominal somites) and three free abdominal somites. Short sclerotised joint between prosome and urosome wider on ventral side. Habitus (Figs. 1a, d and 4a) cylindrical and slender, gently tapering towards posterior end, podoplean boundary between prosome and urosome inconspicuous; prosome/urosome index about 1.1 and greatest width in dorsal view at posterior end of cephalothorax. Body length/width index about 4; cephalothorax 1.2 times as wide as genital double-somite. Free pedigerous somites without pronounced lateral or dorsal expansions, pleural plates only partly covering coxae of swimming legs in lateral view. Integument relatively strongly chitinised and without cuticular windows or pits anywhere. Surface ornamentation of somites consisting of 83 pairs and two unpaired pores and sensilla, several rows of minute spinules on all somites except cephalothorax, and posterior row of large spinules on all urosomites. Distal frill of all somites except anal one serrated, serrations resembling small brushes and becoming larger towards posterior end of body.
Nitokra yeelirrie sp. nov., SEM photographs, a–c paratype female 1 from bore YU1, d–h paratype female 2 from bore YU1: a habitus, ventral; b first swimming leg, ventral; c anal somite and caudal rami, ventral; d habitus, lateral; e rostral region and first antennular segments, lateral; f fifth pedigerous somite and genital double-somite, lateral; g fourth and fifth urosomites, lateral; h anal somite and caudal rami, lateral
Rostrum (Fig. 4a) small, membranous, linguiform with relatively sharp tip, reaching just beyond midlength of first antennular segment, about twice as long as wide and demarcated at base; ornamented with two dorsal sensilla.
Cephalothorax (Figs. 1a, d and 4d, e) gradually tapering towards anterior end in dorsal view, almost 1.1 times as long as wide, representing about 23 % of total body length. Surface of cephalic shield ornamented with two pairs of cuticular pores, one unpaired dorsal sensillum, and 31 pairs of long sensilla; seven pairs of sensilla along posterior magin.
Second pedigerous (first free) somite (Figs. 1a, d and 4d) ornamented with ten pairs of long sensilla; dorsalmost pair obviously serially homologous to dorsalmost posterior pair on cephalothorax, but other serial homologies not so obvious. Pair of moon-shaped anterior depressions possibly large cuticular pores, but this is not clear with light microscopy.
Third pedigerous somite (Figs. 1a, d and 4d) ornamented similarly to the second one, only the difference being absence of one pair of lateral sensilla (nine in total) and absence of anterior moon-shaped depressions; all sensilla obviously serially homologous to those on the second pedigerous somite.
Fourth pedigerous somite (Figs. 1a, d and 4d) ornamented with unpaired antero-dorsal pore and seven pairs of long sensilla along posterior margin; recognising serially homologous pairs not as easy as with two previous somites.
Fifth pedigerous (first urosomal) somite (Figs. 1e, 4f and 5a) ornamented with four pairs of sensilla along posterior margin and one pair of lateral pores; posterior row of large spinules interrupted dorsally between two dorsalmost pairs of pores; two short and arched rows of smaller spinules in anterior part of lateral surface; ventral surface smooth.
Genital double-somite (Figs. 1b, e, 4f and 5a) 1.1 times as long as wide (ventral view); internal suture (remnant of segmental fusion) strongly sclerotised and accompanied by serrated frill on outer surface, situated dorsolaterally at 2/5 of double-somite, furnished with four pairs of sensilla, lateral pair of cuticular pores, and uninterrupted posterior row of large spinules; posterior part of genital double-somite ornamented with uninterrupted posterior row of large spinules and four pairs of posterior sensilla, in addition to numerous short, arched rows of minute spinules on dorsal and lateral surfaces; ventral surface of genital double-somite without minute spinules. Female genital complex (Figs. 1b and 5a) with single large copulatory pore, well sclerotised and almost straight copulatory duct, and two ovoid seminal receptacles. Single median genital aperture with small central bulb, covered by fused reduced sixth legs, latter representing 53 % of somite’s width.
Third urosomite (first free abdominal somite) (Figs. 1f, 4g and 5a) ornamented with uninterrupted posterior row of spinules and four pairs of posterior sensilla, in addition to several short, arched rows of minute spinules all around (sparser on ventral surface); all sensilla obviously serially homologous to those on the posterior part of genital double-somite.
Fourth (preanal) urosomite (Figs. 1f, 4g and 5a) without any sensilla or pores, ornamented with uninterrupted posterior row of large spinules and many short, arched rows of minute spinules all around.
Anal somite (Figs. 1g, h, 4c, h and 5a) cleft medially in posterior half, ornamented with pair of large dorsal sensilla, three pairs of lateral cuticular pores, posterior row of large spinules (those on dorsal surface very large), and several short rows of minute spinules; small internal sclerotised ridge visible on ventral side; anal operculum convex, narrow and short, not reaching posterior margin of anal somite, representing 41 % of somite’s width, ornamented with seven large spinules along posterior margin and row of slender spinules on inner surface; anal sinus ornamented with two parallel diagonal rows of hair-like spinules on each side, widely open, with weakly sclerotised walls, and without any chitinous projections, but longest hair-like spinules forming two posterior whips.
Caudal rami (Figs. 1g, h, 4c, h and 5a) small and widely spaced, about half as long as anal somite, 1.4 times as long as wide in ventral view, nearly parallel and cylindrical (but with dorsal diagonal ridge), with space between them about 1.5 times ramus width; each ramus armed with seven armature elements (three lateral, one dorsal and three apical); ornamentation consisting of several large spinules at base of lateral setae, six or seven slender spinules along posterior margin ventrally (at base of inner apical setae), several slender spinules along posterior half of inner margin, several slender spinules at base of dorsal seta, row of minute posterior spinules laterally, and two ventral pores. Dorsal seta relatively short and slender, smooth, inserted close to postero-median corner, about twice as long as caudal ramus, triarticulate at base (i.e. inserted on two pseudojoints). Lateral setae all smooth and slender, inserted close to each other at about three-fourth length of ramus; central lateral seta minute, hardly half as long as ramus, while two others about twice as long as ramus. Inner apical seta smooth and slender, slightly shorter than ramus. Middle apical seta strongest, with breaking plane, sparsely bipinnate, and nearly as long as entire body. Outer apical seta also with breaking plane, strong, and sparsely bipinnate, half as long as middle apical seta.
Antennula (Figs. 1d, 4a, d, e and 6a) eight-segmented, joined to cephalothorax with small triangular pseudosegment laterally, approximately as long as cephalothorax, ornamented with long tubular pore on dorsal surface of first segment (Fig. 4e), arched row of slender spinules on ventral surface of first segment, and two large spinules on posterior surface of fourth segment. Long, slender aesthetasc on fourth segment fused basally with adjacent large seta and reaching beyond tip of appendage by length of last three segments combined; slender apical aesthetasc on eighth segment fused basally with two apical setae, forming apical acrothek. Setal formula: 1.9.7.4.2.3.4.7. Only seta on first segment unipinnate, all other setae smooth. Two lateral setae on seventh segment and four lateral setae on eighth segment biarticulate at base (i.e. inserted on small pseudojoint), all other setae uniarticulate. Subapical seta on the fifth segment with breaking planes. Length ratio of antennular segments from proximal end and along caudal margin 1:1.3:0.7:1.2:0.7:1.1:0.85:1.3.
Antenna (Fig. 6b) relatively short, composed of coxa, allobasis, one-segmented endopod and one-segmented exopod, although ancestral segmentation between basis and first endopodal segment visible near base of exopod. Coxa very short, unarmed and unornamented. Allobasis long and strong, cylindrical, more than five times as long as coxa and about 2.1 times as long as wide, ornamented with several large spinules along inner margin proximally, unarmed. Endopod longest segment in limb, narrower proximally than distally, about 2.3 times as long as its greatest width and 1.1 times as long as basis, ornamented with two surface frills subdistally and longitudinal row of strong spinules along inner margin, armed laterally with two spines flanking thin seta; apical armature consisting of five geniculate setae, longest one fused basally to additional smaller seta which not geniculate and bearing proximal tuft of fine setules; longest seta bipinnate, others finelly unipinnate. Exopod slightly shorter than width of allobasis, with narrow basal part and somewhat wider distal part, about 2.3 times as long as its greatest width, unornamented, armed with three curved, strong and bipinnate apical setae, all slightly longer than exopod.
Labrum (Fig. 6c) large compared with cephalothorax, trapezoidal, rigidly sclerotised, with relatively short and slightly convex cutting edge, ornamented subapically with two rows of seven or eight strong spinules, apically with numerous minute spinules, two parallel longitudinal rows of hair-like spinules on posterior surface, and two circular fields of gustatory papillae on posterior surface.
Paragnaths (Fig. 6d) ellipsoidal, about 1.8 times as long as wide, with several parallel rows of spinules of different length apically, few spinules laterally in proximal part, as well as two parallel rows of spinules along inner margin of each lobe; lobes fused basally with median linguiform plate, this being ornamented apically with row of hair-like spinules (not shown in Fig. 6d).
Mandibula (Fig. 6e) with wide cutting edge on elongate coxa, armed with two bicuspidate strong teeth in ventral part, two rows of unicuspidate slender teeth (or strong spinules) in middle, two bicuspidate fine teeth (or strong spinules) in dorsal part, and single dorsal unipinnate seta. Palp uniramous, comprising basis and one-segmented endopod. Basis about 2.4 times as long as wide, armed with single large but soft brush seta, ornamented with arched row of small spinules near base of endopod. Endopod small and unornamented, about half as long as basis and 1.3 times as long as wide; armed with four slender and smooth apical setae.
Maxillula (Fig. 6f) with large praecoxa; arthrite rectangular, unornamented, armed with two anterior surface setae, three lateral and four apical elements (probably three spines and one seta); three apical spines with apical combs resting on distal tip of labrum. Coxal endite shorter than praecoxal arthrite, armed apically (on inner margin) with one curved and stout bipinnate seta and two smooth, slender setae. Basis significantly shorter than coxal endite, armed with five smooth setae apically and subapically. Endopod represented by minute but distinct segment, unornamented, armed with two slender and smooth setae apically.
Maxilla (Fig. 6g) large, with broad base, unornamented. Proximal endite of syncoxa well developed although not strongly sclerotised, not highly mobile, somewhat bulbous, armed with single large but soft apical brush seta. Distal endite of syncoxa cylindrical, well sclerotised and highly mobile, armed apically with one strong unipinnate seta (fused basally to endite), and two smooth and slender setae; smooth setae of subequal length, about 1.4 times as long as unipinnate seta, and 2.3 times as long as endite. Basis drawn out into long claw, with shorter spiniform and curved seta at base, ornamented with minute spinules along convex margin. Endopod represented by minute segment basally fused to basis, armed with two long and smooth apical setae of subequal length; endopodal setae about 1.3 times as long as basal seta, all reaching to three-fourth length of basal claw.
Maxilliped (Fig. 6h) with short and stout syncoxa, 1.2 times as long as wide, ornamented with short proximal row of spinules on posterior surface, armed with single bipinnate seta subapically. Basis about 1.8 times as long as wide and 1.4 times as long as syncoxa, unarmed, ornamented with longitudinal row of long spinules along inner margin distally and one short row of spinules on outer-distal corner. Endopod represented by long, curved claw, slightly longer than basis, smooth, accompanied at base by minute smooth seta.
All swimming legs (Figs. 1c, 4b and 7) composed of small triangular praecoxa, rectangular coxa, short basis, and elongated three-segmented exopod and endopod, with coxae on opposite legs connected by small intercoxal sclerite. Praecoxa, coxa, and intercoxal sclerite without armature; intercoxal sclerite also without ornamentation. All exopods and endopods ornamented with large spinules along outer margin, and first and second exopodal and endopodal segments with serrated distal-inner frill.
Nitokra yeelirrie sp. nov., line drawings, a–f holotype female, g, h allotype male: a first swimming leg, anterior; b endopod of second swimming leg, anterior; c third exopodal segment of second swimming leg, anterior; d intercoxal sclerite of second swimming leg, anterior; e endopod of third swimming leg, anterior; f fourth swimming leg, anterior; g basis of first swimming leg, anterior; h endopod of third swimming leg, anterior
First swimming leg (Figs. 1c, 4b and 7a) shorter than other legs, and with outwardly directed, prehensile endopod. Praecoxa larger than in other legs, with long transverse row of large spinules on anterior surface. Coxa about as long as outer margin of praecoxa, 2.4 times as wide as long, with several transverse rows of spinules of various sizes on anterior surface, and one longitudinal row of slender, long spinules on posterior surface, close to outer margin. Intercoxal sclerite small, basically pentagonal in shape but with deeply concave distal margin. Basis shorter and narrower than coxa, 2.3 times as wide as greatest length, almost pentagonal in shape, with inner margin twice as long as outer margin; armed with short bipinnate spine on outer marigin and even shorter bipinnate spine on anterior surface close to inner-distal corner, latter slightly shorter than inner margin of basis; ornamentation consisting of posterior row of large spinules on anterior surface and another row of large spinules at base of inner distal spine. Exopod about 1.2 times as long as endopod; all three segments of similar width; first segment only 0.8 times as long as second or third, armed with single long outer spine 1.2 times as long as segment; second segment with equally long outer spine and slightly shorter inner seta, additionally ornamented with cuticular pore on anterior surface and row of hair-like spinules along inner margin; third segment with three outer strong spines and two distal prehensile setae, inner distal seta 1.3 times as long as entire exopod, 1.6 times as long as outer distal seta, 2.1 times as long as distal outer spine, 3.4 times as long as central outer spine, and 4.3 times as long as proximal outer spine. First endopodal segment 1.75 times as wide and 1.6 times as long as second endopodal segment, armed with single strong inner seta about 1.7 times as long as segment and reaching distal tip of endopod; second endopodal segment slightly wider and shorter than third endopodal segment, armed with single strong inner seta 1.5 times as long as segment and reaching distal tip of endopod; third endopodal segment with three distal armature elements; outer spine strong and 1.35 times as long as segment, central seta geniculate and 2.5 times as long as segment, inner seta slender and 1.5 times as long as segment.
Second swimming leg (Figs. 1c, 7b–d) with fewer spinules on coxa than in first leg, and with larger intercoxal sclerite, without inner distal spine on basis, none of its rami or setae prehensile, and with additional cuticular pore on first exopodal segment. First exopodal segment only half as long as second and 0.4 times as long as third exopodal segment; first and second exopodal segments armed as in first leg; third exopodal segment with three outer spines, two distal setae (outer one spiniform) and two inner setae; length ratio of armature elements on third exopodal segment (starting from proximal outer spine) 1:1.35:2.2:4.5:4.8:5.3:2.1. Endopod only as long as first two exopodal segments combined; first two endopodal segments both about 1.4 times as wide as third endopodal segment but third endopodal segment twice as long as first and 1.5 times as long as second; first endopodal segment unarmed; second endopodal segment armed with spiniform inner seta twice as long as segment; third endopodal segment armed with three elements (outer spine, distal seta, and inner seta), their length ratio (starting from outer spine) 1:2.3:1.6; outer spine on third endopodal segment about as long as its segment
Third swimming leg (Figs. 1c and 7e) very similar to second swimming leg, except outer basal armature element slender and long seta, and third endopodal segment with one additional inner seta; length ratio of armature elements on third endopodal segment (starting from outer spine) 1:2.1:2.4:1.6; outer spine on third endopodal segment slightly shorter than segment.
Fourth swimming leg (Fig. 7f) very similar to third swimming leg, except distal inner seta on third exopodal segment longer and much more spiniform.
Fifth leg (Figs. 1b, e, 4f and 5a) biramous, composed of baseoendopod and one-segmented exopod. Opposite baseoendopods connected by minute intercoxal sclerite. Long spinules on both inner and outer margins on exopod and endopodal lobe. Basal armature consisting of single slender outer seta inserted on long setophore. Endopodal lobe nearly trapezoidal, short, armed with four elements, outermost endopodal element more slender and 1.8 times as long as either other endopodal armature or endopodal lobe. Exopod almost ovoid, about twice as long as wide, armed with three smooth and three bipinnate slender setae, length ratio of exopodal armature (starting from proximal outer seta) 1:1.3:2.6:1.7:3.1:2.2.
Sixth leg (Figs. 1b, e and 5a) minute cuticular plate, with small inner notch (probably basally fused inner spine) and single short outer seta.
Male (based on allotype and seven paratypes). Genital somite and first abdominal somite not fused. Habitus (Figs. 2a, e, 3a, g), rostrum (Fig. 3c), ornamentation of cephalic shield (Figs. 2b and 3b), ornamentation of tergites of free pedigerous somites (Figs. 2c and 3d), ornamentation of urosomites (Figs. 2d, 3e, f, h and 5b), anal operculum (Fig. 3f), caudal rami (Figs. 3f and 5b), antenna, mouth appendages (Fig. 2f), exopod and endopod of first swimming leg (Fig. 2g), second swimming leg (Fig. 2g), most of third swimming leg, and fourth swimming leg as in female.
Antennula (Fig. 6i) prehensile, nine-segmented, with geniculation between sixth and seventh segments. Ornamentation consisting of ventral row of spinules and dorsal tubular pore on first segment, several cuticular sutures on third segment, two cuticular keels with pore on tip on sixth segment, and three cuticular keels on seventh segment, each with pore on tip. Armature of first and last two segments as in female. Second segment similar to that of female, but with one additional seta. Third segment much wider and more complex than in female, armed with 10 setae, two of them on separate dorsal lobe. Fourth segment with slightly longer subapical aesthetasc than in female, additionally armed with bunch of 12 long and very slender setae on ventral and anterior surfaces, one short spiniform seta, and two short elements, each with two rows of scale-like spinules and pore on tip. Fifth and sixth segment each with single short element with two rows of scale-like spinules and pore on tip, very similar to those on fourth segment. Sixth segment with single long subapical seta.
First swimming leg (Figs. 2g and 7g) with inner distal spine of basis smooth and modified, its distal part inflated and split, beak-like.
Third swimming leg (Fig. 7h) with distal armature element on third endopodal segment smooth and much shorter than in female.
Fifth leg (Figs. 2h, 3h and 5b) with baseoendopods fused medially, each endopodal lobe armed with only two elements of about same length (outer one smooth and slender, inner strong and bipinnate), with endopodal lobe unornamented or with one or two large spinules on outer margin. Exopod similar to that of female in shape and armature, except for common asymmetry in size of innermost armature element, smooth inner margin, and all setae except innermost strong seta being smooth.
Sixth leg (Figs. 2h, 3h and 5b) simple cuticular plate, slightly asymmetrical (usually right leg larger and better demarcated at base), armed with three slender setae; outermost seta unipinnate, two others smooth; central seta 2.5 times as long as outer seta and three times as long as inner seta, reaching beyond posterior margin of third urosomite.
Variability
In addition to hundreds of specimens examined using light microscopy, we studied seven males and nine females from five different bore lines using a scanning electron microscope (see “Specimens examined” section). Remarkably, minute details of somite and appendage ornamentation show almost no variability (see Figs. 1, 2, 3 and 4; representing only a small selection among hundreds of SEM photos taken), with only tiny differences in relative sizes of some spinules in homologous rows (compare Figs. 1e, f, 2d, 3h, 4f, g). The armature formula of all appendages is also very constant, except for the common asymmetry in size of the innermost armature element on the male fifth leg exopod (Fig. 5b).
Type locality
Australia, Western Australia, Yilgarn region, Yeelirrie station, bore SB14-1, 27.344283° S, 120.307708° E.
Specimens examined
Holotype female dissected on one slide (WAM C47286), allotype male dissected on one slide (WAM C47285), and five paratypes (three males and two females) together on one SEM stub (WAM C47284), all collected from type locality, 18 March 2010, leg. T. Karanovic and S. Callan (seLN8182).
Three females destroyed for DNA sequence (one amplification successful) from type locality, 16 March 2010, leg. T. Karanovic and G. Perina (seLN8517).
Etymology
The species is named after its type locality, bore SB14-1, i.e. with first two letters of the bore code spelled out. The name is a noun in apposition.
Description
Female (based on holotype and three paratypes). Habitus (Fig. 8a), colour of preserved specimens, and serration of hyaline fills of somites very similar to those of N. yeelirrie. Most ornamentation of somites easy to homologise and same as in N. yeelirrie, although generally with fewer rows of minute spinules on most somites.
Nitokra esbe sp. nov., SEM photographs, a–e, paratype female 1, f, g paratype female 2, h allotype male: a habitus, ventro-lateral; b fifth pedigerous somite and genital double-somite, ventro-lateral; c genital field, ventro-lateral; d left caudal ramus, ventro-lateral; e second swimming leg, ventro-lateral; f urosome, ventral; g antenna, posterior; h central part of right antennula, dorsal. Arrows point at most prominent diagnostic features
Cephalic shield with additional unpaired dorsal pore just behind rostrum; all other pores and sensilla as in N. yeelirrie.
Tergite of second pedigerous somite without anterior row of minute spinules and with one less pair of dorsal sensilla than in N. yeelirrie (fourth pair from dorsal side missing).
Tergite of third pedigerous somite also without fourth pair of sensilla as viewed from dorsal side.
Tergite of fourth pedigerous somite without dorsal unpaired pore and also with slightly different relative positions of two dorsal pairs of sensilla than in N. yeelirrie (dorsalmost pair inserted more anteriorly than next pair in N. esbe).
Fifth pedigerous somite (Fig. 8a, b) without lateral arched rows of large spinules in anterior half (arrowed in Fig. 8a), but other ornamentation as in N. yeelirrie, including same number and position of sensilla and pores, as well as dorsally interrupted posterior row of large spinules.
Genital double-somite (Figs. 8b, c, f and 11a) ornamentation and length/width ratio very similar to those in N. yeelirrie, but genital field very different. Genital operculum formed by fused sixth legs much simpler, without central bulb, and with reduced armature (arrowed in Fig. 8b). Copulatory pore much smaller and rounder, and additional ridge present between copulatory pore and genital operculum (both arrowed in Fig. 8c). Copulatory duct narrow, not well sclerotised, and syphoned (Fig. 11a). Seminal receptacles smaller and rounder that in N. yeelirrie.
Third and fourth urosomites (Figs. 8f and 11a) as in N. yeelirrie.
Anal somite (Figs. 8d and 11a) generally as in N. yeelirrie, but ventral internal ridge better developed and positioned more posteriorly, additional ventral pair of pores present, and dorsolateral pair of pores much larger and positioned much closed to posterior margin. Anal operculum with six strong posterior spinules.
Caudal rami (Figs. 8d and 11a) much shorter than in N. yeelirrie, especially in ventral view (arrowed in Fig. 8d), only about half as long as wide, but ornamentation and armature same as in N. yeelirrie.
Antennula (Fig. 11b) with simple pore on first segment (not tubular), proportionately larger second segment, and proportionately shorter fourth and sixth segments; other ornamentation and all armature as in N. yeelirrie.
Antenna (Fig. 8g) with almost completely divided (arrowed in Fig. 8g) and much more elongate basis and first endopodal segment; other segmentation, armature, and ornamentation as in N. yeelirrie.
Labrum and paragnaths as in N. yeelirrie.
Mandibula (Fig. 8c) with slightly shorter basis, but all segmentation, armature, and ornamentation as in N. yeelirrie.
Maxillula and maxilla as in N. yeelirrie.
Maxilliped (Fig. 11d) with spinules along concave margin of endopodal claw, limb generally more slender than in N. yeelirrie, and with smaller spinules on basis.
First swimming leg (Fig. 11e) with segmentation, armature, and ornamentation as in N. yeelirrie, but first endopodal segment 2.7 times as long as wide and slightly longer than first two exopodal segments combined.
Second swimming leg (Figs. 8e, 11f–h) with segmentation, all endopodal armature and ornamentation, and most exopodal armature as in N. yeelirrie; intercoxal sclerite with two arched rows of large spinules; third exopodal segment with only one (posterior) inner seta and with two spinules on inner margin.
Third swimming with leg segmentation, armature, and ornamentation as in N. yeelirrie, except third exopodal segment with only one inner seta, as in second leg.
Fourth swimming leg (Fig. 11i) as in N. yeelirrie, except proximal inner seta proportionally much shorter.
Fifth leg (Figs. 8f, 11a and j) with segmentation and general armature as in N. yeelirrie, but exopod proportionately much shorter (arrowed in Fig. 8f), and outermost endopodal element only about as long as other endopodal elements (arrowed in Fig. 8f).
Sixth leg (Figs. 8c and 11a) armed with single minute smooth seta (arrowed in Fig. 8c).
Male (based on allotype and two paratypes). Genital somite and first abdominal somite not fused. Habitus (Figs. 9a and 10a), rostrum (Fig. 9c), ornamentation of cephalic shield (Figs. 9b, c and 10b), ornamentation of tergites of free pedigerous somites (Figs. 9d and 10c), ornamentation of urosomites (Figs. 9e–h, 10d–f), anal operculum (Fig. 9g), caudal rami (Figs. 9g and 10f), antenna (Fig. 10b), mouth appendages (Fig. 10b), exopod and endopod of first swimming leg (Fig. 10c), second swimming leg (Fig. 10c), most of third swimming leg, and most of fourth swimming leg as in female.
Nitokra esbe sp. nov., SEM photographs, paratype male 1: a habitus, dorsal; b cephalic shield, dorsal; c rostrum, dorsal; d tergites of free prosomites, dorsal; e first three urosomites, dorsal; f last three urosomites and caudal rami, dorsal; g anal somite and caudal rami, dorsal; h posterior-dorsal corner of anal somite, dorsal. Arrows point at most prominent diagnostic features
Nitokra esbe sp. nov., SEM photographs, paratype male 2: a habitus, lateral; b cephalon, lateral; c free prosomites, lateral; d first two urosomites with fifth and sixth legs, lateral; e third, fourth and fifth urosomites, lateral; f anal somite and caudal rami, lateral; g central part of left antennula, anterior surface; h distal part of antennulae, lateral. Arrows point at most prominent diagnostic features
Antennula (Figs. 8h, 9c, 10g, h and 12a) with segmentation, geniculation, and most armature and ornamentation as in N. yeelirrie, except first segment with simple dorsal pore (arrowed in Fig. 9c), third segment with one additional seta on anterior surface, and fourth segment with only three smooth, slender setae in proximal half (normal condition in family), i.e. without additional tuft of slender setae (or very elongated and slender spinules) found in N. yeelirrie.
First swimming leg (Fig. 12b) with inner distal spine on basis smooth and modified, its distal part inflated and split, forming a beak-like structure, slightly less inflated distally than in N. yeelirrie.
Third swimming leg (Fig. 12c) with distal armature element on third endopodal segment smooth and shorter than in female, as in N. yeelirrie, but with proportionally much shorter outer spine.
Fourth swimming leg (Fig. 12d) without proximal inner seta on third exopodal segment; other armature and all ornamentation as in female.
Fifth leg (Figs. 10d and 12e) segmentation and armature as in N. yeelirrie, but exopod much shorter (arrowed in Fig. 10d); basis with additional pore on anterior surface but without any spinules.
Sixth leg (Figs. 10d and 12e) a simple cuticular plate, slightly asymmetrical (right leg larger and better demarcated at base), armed with single long seta on outer margin.
Variability
The availability of specimens to check for the examination of morphological variability was much more modest than for the previous species (four males and three females; see “Specimens examined” section); also, it was not possible to observe all morphological features on all specimens because of different mounting positions on the SEM stub. Thus, it remains to be checked whether the difference in the armature formula of the third exopodal segment of the fourth leg between one paratype male and one paratype female (Figs. 11i and 12d) represents just individual variability or a form of sexual dimorphism. The former possibility seems more plausible, as the proximal inner seta on this segment is already reduced in size (when compared to other setae) in the female, and completely absent in the male. The fine ornamentation of the somites and appendages seems to be remarkably conservative.
Nitokra esbe sp. nov., line drawings, holotype female: a urosome, ventral; b antennula without armature, dorsal; c mandibular palp, anterior; d maxilliped, posterior; e endopod of first swimming leg, anterior; f endopod of second swimming leg, anterior; g intercoxal sclerite of second swimming leg, anterior; h third exopodal segment of second swimming leg, anterior; i third exopodal segment of fourth swimming leg, anterior; j exopod of fifth leg, anterior
Molecular results
DNA was extracted, and the COI fragment successfully PCR-amplified from 11 copepod specimens (Table 1) using a nested combination of primers. Obtained sequences translated into protein using MEGA were shown to have no evidence of stop codons, ambiguities or insertions-deletions indicative of non-functional copies of COI. BLAST analyses of GenBank revealed that the obtained sequences are copepod in origin and not contaminants. One unverified COI-like sequence attributed to ‘Nitocra sp.’ on GenBank was included in our analysis (accession number KF673354.1). This sequence and those obtained from our study of Yeelirie specimens are not just the only COI sequence data available so far for the genus Nitokra, but for the entire family Ameiridae.
The ingroup taxa formed a monophyletic group in all analyses, confirming therefore that the unverified sequence from GenBank most probably does belong to the genus Nitokra. The topology of the resulting cladograms did not differ depending on the parameters used (Fig. 13). All clades were supported with very high bootstrap values (90 % or higher), except the clade that suggests a sister relationship between Nitokra sp. and N. yeelirrie, which was moderately supported (bootstrap value of 69 %). All morpho-species were also well defined in our molecular analyses, and average pairwise divergence values between morpho-species were found to be very high, with the lowest p-distance (19.9 %) being between the two canthocamptid species, A. hamondi and E. humphreysi (Table 2). This is not surprising, since they belong to two distinct and well-defined genera (see Karanovic and Cooper 2011b). The average p-distances between specimens of N. yeelirrie and N. esbe were in excess of 21 %, which is the most surprising result of our analyses. Expectedly, the p-distances between canthocamptid and ameirid taxa were very high, ranging from 20.4 to 25.8 %. None of our analyses suggested a sister relationship between the two new species from Yeelirrie, with the unidentified but clearly distinct species (with p-distances from other congeners in excess of 21 %) Nitokra sp. nested in between.
Maximum likelihood cladogram based on COI sequence data from 11 copepod specimens from Yeelirrie (Australocamptus hamondi Karanovic, 2004; Nitokra esbe sp. nov.; Nitokra yeelirrie sp. nov.) and the Pilbara region (Elaphoidella humphreysi Karanovic, 2006) (see Table 1) of Western Australia. Nitokra sp. is an unverified COI-like sequence from GenBank, its accession number prefixed. The scale bar represents genetic distance, specimen codes correspond to those in Table 1, and the numbers above branches represent bootstrap values calculated from 100 pesudoreplicates
Divergence values within morpho-species were lowest between four specimens of A. hamondi (0 %) that were all collected from the same bore and are possibly kin. Those between specimens of E. humphreysi were much higher (up to 6.4 %), even though they were collected from a relatively small calcrete in the Pilbara region (but from different bores). The highest divergence values (from 2.3 to 9.8 %) were recorded between specimens of N. yeelirrie, all of which collected from the biggest calcrete in Yeelirrie. Specimens 8387 and 8393 were collected from two different bores on the same bore line (bore line 3.5), while specimen 8492 was collected from bore line F, some 8 km away (see Table 1 and Fig. 15).
We also calculated patristic distances between our taxa (distances measured along the ML tree, based on a GTR + G model of sequence evolution) (Table 2, above diagonal), even though they are not commonly used in publications related to barcoding (but see Lefébure et al. 2006). Their main advantage is that they do not show the saturation that p-distances show and are therefore more reliable for comparison of species that are not very closely related (Korber et al. 2000; Fourment and Gibbs 2006). As with p-divergences, patristic distances were also smallest between A. hamondi and E. humphreysi (46.7 %). Largest patristic distances were between E. humphreysi and Nitokra sp. (90.3 %), while those between E. humphreysi and N. yeelirrie were the second largest (84.2 %). Patristic distances between the two species of Nitokra from Yeelirrie were also very high, in excess of 50 %.
Discussion
Taxonomic and nomenclatural problems in the genus Nitokra
As was noted by Karanovic and Pesce (2002), there are several homonyms in this genus. Because they have not been resolved in the meantime, we feel obliged to rename junior taxa here. None of the junior homonyms already has an available junior synonym, and they are all currently regarded as valid species. Although it is customary to dedicate new names to people who created junior homonyms, we feel this course of action to be somewhat unjust and decided to dedicate the new names to researchers who proposed the senior homonyms. In that fashion we propose Nitokra lacustis richardi nom. nov. as a replacement name for N. lacustris incerta Chappuis 1933, a junior primary homonym of N. incerta (Richard, 1893); Nitokra langi nom. nov. as a replacement name for Nitokra baltica Arlt 1983, a junior primary homonym of Nitokra fallaciosa baltica Lang 1965a, b; Nitokra mediterranea jakubisiaki nom. nov. as a replacement name for N. mediterranea pontica Apostolov 1980, a junior primary homonym of Nitokra pontica Jakubisiak, 1938; and Nitokra pori nom. nov. as a replacement name for Nitokra stygia (Apostolov 1976), a junior secondary homonym of Nitokra affinis stygia Por 1968 (see also Table 3).
Bowman (1988) pointed out that the correct original spelling of the genus name is Nitokra, correcting the erroneous spelling Nitocra which had then been in use for more than 100 years. Subsequent researchers have used both spellings, those opting for Nitocra arguing for its preservation in accordance with usage (Mielke 1993, 1997; Huys 2009; Gómez et al. 2012). Although Bowman’s (1988) argumentation was based on the correct interpretation of the then valid Zoological Code, the current Code (International Commission on Zoological Nomenclature ICZN 1999) indeed provides for the preservation of an incorrect subsequent spelling (Article 33.3.1) when it is in prevailing usage. However, prevailing usage is not clearly defined. This leaves room for various interpretations and perhaps introduces more instability than strict adherence to the priority rule, as was already noted by Dubois (2010). Prevailing usage is defined in the Glossary of the Code as follows: ‘Of a name: that usage of the name which is adopted by at least a substantial majority of the most recent authors concerned with the relevant taxon, irrespective of how long ago their work was published’. As noted by Dubois (2010), this raises a few points. How big is a ‘substantial’ majority? What is the cut-off for ‘most recent’? How ‘concerned’ does an author have to be with the taxon to be taken into account? In this paper, we use the correct original spelling, expecting that the new version of the Code will undoubtedly go back to the strict priority rule in most cases. Any other action that would involve counting authors (how do we score papers with multiple co-authors?) or papers using those two spellings would be premature, unnecessarily difficult, and time-consuming, and any given count is bound to change in a few years.
As with many other large and old marine copepod genera, Nitokra is rich in subjective synonyms at the species-group level (Lang 1948, 1965). Most of them were conceived as a consequence of a widely held belief that most marine species of copepods have cosmopolitan distributions, a view that has been repeatedly challenged in recent times for most copepod groups (Reid 1998; Lee 2000; Grishanin et al. 2005; Hamrova et al. 2012; Karanovic and Cho 2012; Karanovic and Krajicek 2012), as well as for other small aquatic animals (Rowe et al. 2007; Petrusek et al. 2009; Xu et al. 2009, 2011; Xiang et al. 2011). Undoubtedly, some of these ‘widely distributed’ species represent species complexes, but older descriptions are generally not detailed enough to allow reinstatement of previously synonymised taxa. One solution to this problem would be a reexamination of various type materials, if still available and in good condition, in combination with detailed combined morphological/molecular studies of newly collected specimens from type localities. Unfortunately, with the current rate of habitat destruction and anthropogenic translocation (Karanovic and Krajicek 2012), it is quite possible that many marine harpacticoids described 50 years ago do not live in their type locality any more, their type habitat no longer exists (see Karanovic et al. 2013), or they have become (and are) distributed around the world in ship ballast waters (Reid and Pinto-Coelho 1994; Lee 1999; Horvath et al. 2001).
In Table 3, we list all currently valid species of Nitokra, along with some of their most prominent morphological features. Those were compiled from publications, not from original observations (except for the two new species described here) and in most cases, take no account of intraspecific variability (we report the character state that was considered normal or most common). Explanations for the various armature formulae used in Table 3 are given in the “Material and methods” section. Many original species descriptions were based on only a few morphological characters, and additional data are compiled from subsequent publications attributed to the same species. Besides renaming junior homonyms, no other taxonomic or nomenclatural action was taken in compiling this list (i.e. we honoured almost all widely accepted synonyms). However, this may be a good place to draw attention to several problematic species.
It may be clear even from Table 3 that Nitokra delaruei Soyer, 1975 probably does not belong to this genus, as it differs markedly from all other species in the segmentation of the antennal exopod, shape and ornamentation of the anal operculum, and size and shape of the transformed inner spine on the male first leg basis (see Soyer 1975). However, without a robust phylogeny of ameirids (see Karanovic and Hancock 2009; Karanovic et al. 2013), it may be more practical to keep it in this genus than to place it incertae sedis, also because its males are still unknown.
Sexual dimorphism reported by Bruno et al. (2002) for Nitokra evergladensis may be indicative of two separate species, as it involves most of the swimming legs and even details of urosomite ornamentation. Their identification was not based on specimens in copula or even from the same locality, and differences reported are highly unusual for this genus. In our N. esbe, one can observe the difference in the armature formula of the fourth leg third exopodal segment, but the missing inner proximal seta (male condition) is already reduced in size in the female compared to congeners.
As was noted by Karanovic and Pesce (2002), Nitokra balli may be a junior subjective synonym of Nitokra uenoi. This view was mostly based on a newly discovered population from Christmas Island, Australia (T. Karanovic, unpublished data). The senior author has been unsuccessfully trying ever since to locate the type material of the latter species. Some of the species in our list were originally described as subspecies, and later on elevated to specific rank. Undoubtedly, some others will have a similar destiny in the future, as several ‘subspecies’ have widely overlapping distributional ranges. In order not to confuse the reader any further, we have refrained from proposing these sorts of rearrangements. Recently Gómez et al. (2012) proposed elevating Nitokra reducta fluviatilis and Nitokra sewelli husmanni to full specific rank, based on a comparison of published original drawings, ‘until the variability of these two species is properly assessed’. Reexamination and redescription of their types (some of which are cited as still available) would be worthwhile to test this hypothesis.
Morphological affinities of the Yeelirrie species
Both new species have a unique armature formula of the swimming legs in the entire genus (see Table 3), which could be interpreted as their set of synapomorphies. They also share armature formulae of endopods of third and fourth legs that can be found additionally only in N. lacustris richardi nom. nov. (see Chappuis 1933), while Nitokra reunionensis Bozic, 1969 has the same armature of the fourth leg endopod but a different configuration on the third leg endopod (see Bozic 1969). N. yeelirrie sp. nov. and N. esbe sp. nov. additionally share the armature formula of the female fifth leg, with six setae on the exopod and four on the endopodal lobe (Figs. 5a and 11a, j), which is relatively rare in Nitokra, being only found in five other species: Nitokra blochi Soyer, 1975 from the marine interstitial of Kerguelen, southern Indian Ocean; Nitokra cari Petkovski, 1954 from the marine interstitial of the Adriatic Sea, eastern Mediterranean; N. lacustris richardi from the marine interstitial in the vicinity of a freshwater spring on Curaçao, southern Carribean Sea; N. pontica Jakubisiak, 1938 from the western coast of the Black Sea; and Nitokra pseudospinipes Yeatman 1983 from crab holes in Tonga and Fiji, South Pacific (see Chappuis 1933; Lang 1948; Petkovski 1954; Soyer 1975; Yeatman 1983; Apostolov and Marinov 1988). These widely different and disjunct localities and the many morphological differences in other respects between all these species strongly suggest that the present female fifth leg armature formula originated convergently a number of times, although perhaps not separately in the two new species, which additionally share the armature formula of the male sixth leg (see Table 3). However, based on the available morphological evidence alone, it would be difficult to speculate that the two new species had an immediate common ancestor, as several important synapomorphies are simple reductions and may have originated independently a number of times in subterranean habitats (Gibert et al. 1994; Monchenko and von Vaupel Klein 1999; Culver and Pipan 2009).
As was mentioned in the “Introduction” section, only two other Nitokra are known from Australia: N. humphreysi and N. lacustris pacifica. Both differ significantly from our two new species. N. humphreysi has only five (and sometimes only four) setae on the female fifth leg exopod, in addition to different armature formulae of the mandibular palp, maxillar endites and swimming legs (with the first endopodal segment of the second to fourth legs bearing an inner seta, and the third segment with one additional seta; see Table 3), as well as details of the ornamentation of most somites. Additionally, males of N. humphreysi have three armature elements on the fifth leg endopodal lobe. Although both N. humphreysi and the two new species from Yeelirrie have four elements on the female fifth leg endopodal lobe, their nature is different. In the former, the innermost spine (or spiniform seta) has been lost from the ancestral five-element formula (see Karanovic and Pesce 2002), while in the latter, it is the outermost seta that has been lost (Figs. 5a and 11a). Thus, our new species are only remotely related to N. humphreysi. On the other hand, N. lacustris pacifica shares a number of morphological characters with our new species, including serrated hyaline fringes of the urosomites, armature of the mandibular basis, mandibular endopod with no lateral seta, armature of the maxilla, and most of the swimming legs armature formula (although N. lacustris pacifica has one additional seta on the third endopodal segment of the third and fourth legs). A major difference is in the number of elements on the female fifth leg endopodal lobe, with N. lacustris pacifica having the plesiomorphic five-element formula. In fact, almost all differences between N. lacustris pacifica and the two new species are plesiomorphies in the former, i.e. its morphology is very close to what we would imagine an ancestor of our two new species looked like (although probably not the immediate ancestor, as the two new species share a number of synapomorphies mentioned above).
However, the two new species are not very closely related, as indicated both by high divergence rates in the COI gene and a number of morphological differences (many of them indicated by arrows in Figs. 8, 9 and 10). Some of the major differences include the segmentation of the antenna (basis and first endopodal segment distinct in N. esbe; compare Figs. 6b and 8g), armature formula of the swimming legs (third exopodal segment of the second and third leg without a proximal inner seta in N. esbe; compare Figs. 7c and 11h), relative size of the first leg first endopodal segment (much longer in N. esbe; compare Figs. 7a and 11e), length of the fifth leg exopod (shorter in N. esbe; compare Figs. 1b and 8f), length of the outermost element on the female fifth leg endopodal lobe (shorter in N. esbe; compare Figs. 5a and 11a), and the length of the caudal rami (shorter in N. esbe; compare Figs. 4c and 8d). Other minor differences include details of ornamentation of some somites and appendages. For example, the intercoxal sclerite of the second swimming leg has two groups of large spinules in N. esbe (Fig. 11g), but it is completely smooth in N. yeelirrie (Fig. 7d). The first segment of the antennula has a prominent tubular pore in N. yeelirrie (Fig. 4e), but only a simple pore in N. esbe (Fig. 9c). The ornamentation of somites differs in the presence/absence of several pores and sensilla, most notably that of an unpaired dorsal pore at the base of the rostrum (compare Figs. 3c and 9c), as well as a pair of dorsal sensilla on each free pedigerous prosomite (compare Figs. 3d and 9d). Also, the size and relative positions of some pores differ in these two species, especially as concerns the dorso-lateral pair of pores on the anal somite (compare Figs. 1g and 9h). All these are indicative of interspecific variability, when compared with other copepod groups, for which a combined approach was used to delineate between closely related congeners (Karanovic and Cooper 2011a, 2012; Hamrova et al. 2012; Karanovic and Krajicek 2012).
Phylogeography and implications for habitat colonisation
Our phylogenetic analysis of Nitokra populations based on the COI gene shows N. esbe as forming a basal clade relative to the other two taxa, which could be interpreted as being in congruence with an ‘active upstream’ dispersal model, as this species was recorded from bore SB14-1 (southeastern corner in Fig. 15), the most downstream part of the Yeelirrie palaeochannel. This model has been demonstrated for several other copepods belonging to genera of marine origin here (see Karanovic and Cooper 2012), including Schizopera leptafurca Karanovic and Cooper, 2012, between bore lines K and 1, and Schizopera akation Karanovic and Cooper, 2012, between bore SB14-1 and bore line 1.
Under this model, the ancestor of Nitokra would have colonised the upper reaches of the Carey palaochannel by active dispersal upstream, possibly from the marine interstitial, where this genus is quite common. However, as no sister relationship between N. yeelirrie and N. esbe was recovered in our molecular phylogenies (see Fig. 13), it is very unlikely that they represent a single colonisation event here, despite the fact that they share a number of morphological synapomorphies (see above). If they ever shared an immediate common ancestor, it was probably in the very distant past, as suggested by the very high p-divergence values (in excess of 21 %, see Table 2). It is therefore reasonable to assume that their allopatric distribution is not a consequence of recent speciation, as is the case with six allopatric species from the parastenocaridid genus Kinnecaris in Yeelirrie (see Karanovic and Cooper 2011a). Considering the very wide distribution of N. yeelirrie, however, it is likely that some dispersal to the lowest parts of this palaeochannel (now occupied by N. esbe) occurred during its long independent evolutionary history, as is suggested both by the very high divergence rates in our molecular analyses and by the nested position of Nitoka sp.
Competition for the same resources may be one of the reasons that the Yeelirrie Nitokra species maintain allopatric ranges, as they differ very little in size and habitus shape. As demonstrated by Karanovic and Cooper (2012) for the miraciid genus Schizopera, sympatric and parapatric congeners in Yeelirrie either differ very significantly in size or some other prominent morphological character (i.e. caudal rami shape), as these are very competitive environments, with up to ten different species of copepods (and up to four congeners) in a single sampling bore (see Fig. 14). It must be noted that N. yeelirrie is the largest harpacticoid in Yeelirrie (compare body sizes in Fig. 14), but that does not restrict its dispersal abilities outside the main calcrete, as it was also recorded on bore lines K and N (see Fig. 15).
Montage image from light dissecting microscope showing seven sympatric species from a single sampling event in a typical Yeelirrie bore (bore YYD26 in this case): a Halicyclops sp., adult female; b Schizopera uranusi Karanovic and Cooper, 2012, adult female; c S. leptafurca Karanovic and Cooper, 2012, adult female; d S. akation Karanovic and Cooper, 2012, ovigerous female; e Nitokra yeelirrie sp. nov., adult female; f Pseudectinosoma sp., adult female; g Kinnecaris uranusi Karanovic and Cooper, 2011, adult female
Map of the Yeelirrie area, showing some of the sampling localities (bores and wells). All other sampling bores lie on the following 21 bore lines (from northwest to southeast): P, Q, O, E, A, G, H, F, 1, 1.5, 2, 3, 3.5, 4, 5, 6, C, K, D, L and N. The general water flow in the palaeochannel is also in this direction. The inset shows the location of the area in Australia. Nitoka esbe sp. nov. was recorded from a single bore (SB14-1) in a small calcrete furthest downstream (near lower right corner of the map), while N. yeelirrie sp. nov. was recorded from the following bore lines: F, 1, 1.5, 2, 3, 3.5, 4, 5, K and N. Map from Karanovic and Cooper (2012)
The phylogeography of N. yeelirrie (Fig. 13) provides some evidence for population substructuring or reduced gene flow within the biggest calcrete body in Yeelirrie, with the presence of divergent haplotypes (p-distance ∼10 %: specimens 8387 and 8393 from bore line 3.5 vs specimen 8492 from bore line F, some 8 km upstream; Fig. 15). However, we sampled but a fraction of its entire population for sequencing, and more molecular data are needed to assess the phylogeography and patterns of dispersal of this species. Our amplification success rates were very low with the fresh material (see “Specimens examined” section), less than 30 % in 2010. Additional attempts were made in 2013 (this material is not listed above) but were all unsuccessful, probably due to DNA degradation. With such a limited sample size, we cannot exclude the possibility that standing variation in the original coloniser, and then chance loss of haplotypes and mutations of new ones would produce a similar cladogram, and these all need to be taken into account when testing hypotheses about history.
Significance of the Yeelirrie palaeochannel
The Yeelirrie area, which contains one of the largest calcretes in the uppermost reaches of the Carey palaeochannel (Timms 1992), is only about 70 km long and less than 10 km wide, but the surface area of suitable habitats (calcretes) is probably less than 100 km2 (see Fig. 15). Although this is significantly less than 3 % of the whole surface area of the Yilgarn region, it harbours an unprecedented copepod diversity. As mentioned in the “Introduction” section, the diversity of stygofauna is mostly dependent on the size of the calcrete and typically includes one to three species from each major group. An example of a typical Yilgarn calcrete is that at Sturt Meadows, where multiple molecular studies from a very dense grid of bores (115 bore holes in an area of 3.5 km2) suggested only three species of copepods, one cyclopoid and two harpacticoids (Allford et al. 2008; Bradford et al. 2010; S. Cooper, unpublished data). In a preliminary survey of the entire Yilgarn region, based on a study of 164 different localities, Karanovic (2004) recorded 31 species of copepods classified into 16 genera, five families and two orders. In contrast, so far, we have been able to distinguish 27 different species and subspecies of copepods in Yeelirrie alone, belonging to six families and two orders, including an endemic monotypic parastenocaridid genus Dussartstenocaris Karanovic and Cooper, 2011 (see Karanovic and Cooper 2011b), six allopatric species of the parastenocaridid genus Kinnecaris (see Karanovic and Cooper 2011a), up to ten different species of the miraciid genus Schizopera (see Karanovic and Cooper 2012), one species of the canthocamptid genus Australocamptus Karanovic, 2004 (also used as one of the outgroups in the molecular analyses reported here), two species of the ameirid genus Nitokra (described in this paper), and at least seven other species awaiting formal description (T. Karanovic, unpublished data). This suggests that Yeelirrie harbours more than 45 % of the entire regional copepod diversity, but it does not take into account between 10 and 20 other species discovered in other areas of Yilgarn since 2004 (T. Karanovic, unpublished data), so a more accurate figure would be between 35 and 40 %. This is still in an incredible diversity, when compared to the surface area. Other recent surveys of copepod stygofauna in calcrete areas of similar size near Lake Way and Lake Maitland (T. Karanovic, mostly unpublished data), both quite close to Yeelirrie and also done with a similarly intensive sampling effort, revealed much less diversity (15 and 10 species, respectively). For example, at Lake Way, only one species of Kinnecaris was identified (see Karanovic and Cooper 2011a).
It is difficult to say why Yeelirrie is so rich, especially because there is no hint of any significant difference from other areas of the Yilgarn, either on its surface (see Fig. 16) or in its aquifer geochemistry. We doubt that any external factors are responsible for this unprecedented groundwater copepod diversity, especially because ongoing morphological and molecular studies on other major groups in this calcrete show them to be much less diverse, containing the usual number of one to three species. For example, amphipods are represented by a single new species of Chiltoniidae (T. Finston personal communication), ostracods by a single new species of Candonopsis Vavra, 1891 (family Candonidae) (I. Karanovic personal communication), syncarids by two new species of Atopobathynella Schminke, 1973 (family Parabathynellidae) and one of Bathynellidae (T. Finston personal communication), and diving beetles by two new species of Limbodessus Guignot, 1939 and one of Paroster Sharp, 1882 (family Dytiscidae) (C. Watts, personal communication). This may suggest different ages and colonisation histories for different groups, and the overall impression is (see also Karanovic and Cooper 2011a, b, 2012) that repeated colonisations into different fragmented bodies of the Yeelirrie calcrete, along with explosive radiation in some groups, are responsible for this copepod species richness. As different groups of copepods invaded this palaeochannel from inland freshwater and downstream saline systems, they created opportunities for each other, probably facilitating further speciation. The majority of copepods here are short-range endemics (sensu Eberhard et al. 2009), with the relatively wide distribution of N. yeelirrie being a notable exception. The high uranium content (Needham 2009) in the largest calcrete may also be a factor contributing towards higher mutation rates. Yeelirrie is Australia’s second biggest unmined uranium deposit, originally discovered in 1972 by the Western Mining Corporation (WMC) and experimentally mined between 1980 and 1983. From 1983, it was used as a pastoral station again, but the site rehabilitation was not finished until 2003. From 2005, WMC was acquired by the mining giant BHP Billiton, and a renewed interest in uranium mining in Western Australia led to the reactivation of the project in 2008, with series of environmental impact assessment studies done until 2011. All copepod material in this paper was collected as part of that survey. The mine was acquired by Cameco Corp. in 2012 and was scheduled for production in 2014 but is now ear-marked to supply new production when it is needed. Uranium mining in Yeelirrie may involve almost complete removal of the largest calcrete (which actually contains the ore) and dewatering of a very large area, thus severely impacting the core stygofauna habitat for and possibly causing extinction of the exceptionally diverse copepod and other locally endemic stygofauna. We hope that this and previous papers will contribute to recognition and adequate protection of the unique and highly significant stygofauna assemblage here.
Photographs of different habitats and sampling localities in the Yeelirrie area: a top of the largest calcrete with mature eucalypt trees, between bore lines 2 and 3; b sampling at Wirraway bore, on bore line A; c spinifex grasses and small bushes along bore line Q; d exposed calcrete sediments on top of a small calcrete, near bore line O; e claypan near bore line G, one of the major recharge points for the largest Yeelirrie calcrete; f the Yeelirrie area functioning as an extensive pastoral station
References
Adams, M. S., & Stauber, J. L. (2008). Marine whole sediment toxicity tests for use in temperate and tropical Australian enviroments: current status. Australasian Journal of Ecotoxicology, 14, 155–167.
Allford, A., Cooper, S. J. B., Humphreys, W. F., & Austin, A. D. (2008). Diversity and distribution of groundwater fauna in a limestone aquifer: does sampling alter the story? Invertebrate Systematics, 22, 127–138.
Apostolov, A. (1973). Sur diverses Harpacticoides (Copépodes) de la Mer Noire. Zoologischer Anzeiger, 190, 88–110.
Apostolov, A. (1976). New species of Harpacticoida (Copepoda) from Bulgaria. Zoologicheskiĭ Zhurnal, 55, 453–458 [in Russian with English abstract].
Apostolov, A. (1980). Deux forms nouvelles du genre Nitocra Boeck (Copépoda, Harpacticoida) de la mer Noire (côte bulgare). Acta Zoologica Bulgarica, 15, 36–42.
Apostolov, A., & Marinov, T. M. (1988). Copepoda Harpacticoida (morski kharpaktikoidi), S’stav i razprostranenie na mezopsamalnite vidove ot podrazred Harpacticoida (Copepoda, Crustacea) v akvatoriyata na B’lgarskoto Chernomorsko krabrezhie. Fauna Bolgarii Izdanie B’lgarski Akadmii Aedibus Academiae Scientificae Bulgaricae Sofia, 18, 1–384 [in Bulgarian].
Arlt, G. (1983). Taxonomy and ecology of some harpacticoids (Crustacea, Copepoda) in the Baltic Sea and Kattegat. Zoologische Jahrbücher, Abteilung für Systematik, 110, 45–85.
Bayly, I. A. E. (1972). Salinity tolerance and osmotic behaviour of animals in athalassic saline and marine hypersaline waters. Annual Review of Ecology and Systematics, 3, 233–268.
Behning, A. (1936). Miscellanea aralo-caspica. Internationale Revue der Gesamten Hydrobiologie und Hydrographie, 33, 141–149.
Bodin, P. (1970). Copépodes Harpacticoides marins des environs de la Rochelle, 1. Espéces de la vase intertidale de Chatelaillon Tethys, 2, 385–436.
Bowman, T. E. (1988). Nitokra sphaeromata, a new harpacticoid copepod crustacean associated with the wood-boring isopod, Sphaeroma peruvianum, in Costa Rica. Proceedings of the Biological Society of Washington, 101, 171–175.
Boxshall, G. A., & Halsey, S. H. (2004). An introduction to copepod diversity. London: The Ray Society.
Bozic, B. (1964). Copépodes Harpacticoïdes et Cyclopoïdes de la Réunion, II. Plage St. Pierre. Bulletin du Muséum National D’Histoire Naturelle Zoologie 2e série, 36, 481–499.
Bozic, B. (1965). Copépodes de quelques petits estuaires Méditerranéens. Bulletin du Muséum National D’Histoire Naturelle Zoologie, 2e série, 37, 351–356.
Bozic, B. (1969). Copépodes harpacticoïdes de la Réunion. Bulletin du Muséum National D’Histoire Naturelle Zoologie, 2e série, 41, 867–882.
Bradford, T., Adams, M., Humphreys, W. F., Austin, A. D., & Cooper, S. J. B. (2010). DNA barcoding of stygofauna uncovers cryptic amphipod diversity in a calcrete aquifer in Western Australia’s arid zone. Molecular Ecology Resources, 10, 41–50.
Brian, A. (1928). I copepodi bentonici marini. Archivio Zoologico Italiano, 12, 293–343.
Brian, A. (1929). Richerche faunistiche delle isole italiane dell’Egeo. Archivo Zoologico Italiano, 13, 269–281.
Bruno, M. C., Reid, J. W., & Perry, S. A. (2002). New records of harpacticoid copepods from Everglades National Park (Florida, U.S.A.): description of Nitokra evergladensis, new species (Ameiridae), supplementary description of Attheyella americana, and redescription of Bryocamptus newyorkensis (Canthocamptidae). Journal of Crustacean Biology, 22, 834–854.
Chang, C. Y. (2007). Two harpacticoid species of genera Nitokra and Ameira (Harpacticoida: Ameiridae) from brackish waters in Korea. Integrative Biosciences, 11, 247–253.
Chang, C. Y. (2010). Continental harpacticoida. Invertebrate fauna of Korea, 21(4). Seoul: National Institute of Biological Resources, Ministry of Environment.
Chappuis, P. A. (1923). Description de deux harpacticides nouveaux de Transylvanie. Buletinul Societatii de Stiinte din Cluj 2e serie, 21, 23–26.
Chappuis, P. A. (1926). Harpacticiden aus der Kiemenhöhle des Flusskrebses. Archiv für Hydrobiologie, 17, 515–520.
Chappuis, P. A. (1930). Copepoda Harpacticoida von der insel Luzon, Philippinen. Philippine Journal of Science, 41, 143–147.
Chappuis, P. A. (1933). Süss- und Brackwasser-Copepoden von Bonaire, Curaçao und Aruba. In: Zoologische Ergebnisse einer Reise nach Bonaire, Curaçao und Aruba im Jahre 1930. Zoologische Jahrbücher, Abteilung für Systematik Ökologie und Geographie der Tiere, 64, 391–404.
Chappuis, P. A. (1934). Süsswasser Harpacticiden aus dem hawaiischen Inselgebiet. Buletinul Societatii de Stiinte din Cluj, 7, 631–635.
Cho, J.-L., Humphreys, W. F., & Lee, S.-D. (2006a). Phylogenetic relationships within the genus Atopobathynella Schminke, 1973 (Bathynellacea, Parabathynellidae): with the description of six new species from Western Australia. Invertebrate Systematics, 20, 9–41.
Cho, J.-L., Park, J.-G., & Ranga Reddy, Y. (2006b). Brevisomabathynella gen. nov. with two new species from Western Australia (Bathynellacea, Syncarida): the first definitive evidence of predation in Parabathynellidae. Zootaxa, 1247, 25–42.
Chullasorn, S., Kangtia, P., & Klangsin, P. (2014). A new species of Nitokra Boeck, 1865 (Copepoda: Harpacticoida: Ameiridae) from a brown alga in Thailand. Proceedings of the Biological Society of Washington, 127, 122–137.
Cooper, S. J. B., Hinze, S., Leys, R., Watts, C. H. S., & Humphreys, W. F. (2002). Islands under the desert: molecular systematics and evolutionary origin of stygobitic water beetles (Coleoptera: Dytiscidae) from central Western Australia. Invertebrate Systematics, 16, 589–598.
Cooper, S. J. B., Bradbury, J. H., Saint, K. M., Leys, R., Austin, A. D., & Humphreys, W. F. (2007). Subterranean archipelago in the Australian arid zone: mitochondrial DNA phylogeography of amphipods from central Western Australia. Molecular Ecology, 16, 1533–1544.
Cooper, S. J. B., Saint, K. M., Taiti, S., Austin, A. D., & Humphreys, W. F. (2008). Subterranean archipelago: mitochondrial DNA phylogeography of stygobitic isopods (Oniscidea: Halniscus) from the Yilgarn region of Western Australia. Invertebrate Systematics, 22, 195–203.
Corgosinho, P. H. C., & Martínez Arbizu, P. (2010). Ameiridae Boeck and Argestidae Por revisited, with establishment of Parameiropsidae, a new family of Harpacticoida (Crustacea, Copepoda) from deep-sea sediments. Helgoland Marine Research, 64, 223–255.
Culver, D., & Pipan, T. (2009). The biology of caves and other subterranean habitats. Oxford: Oxford University Press.
Dubois, A. (2010). Zoological nomenclature in the century of extinctions: priority vs. ‘usage’. Organisms, Diversity and Evolution, 10, 259–274.
Dussart, B. H. (1967). Les Copépodes des eaux continentales d’Europe occidentale (Vol. 1). Paris: Calanoïdes et Harpacticoïdes. N. Boubée et Cie.
Dussart, B., & Defaye, D. (1990). Répertoire mondial des crustacés copépodes des eaux intérieures, III. Harpacticoïdes. Crustaceana Supplement, 16, 1–384.
Eberhard, S. M., Halse, S. A., Williams, M. R., Scanlon, M. D., Cocking, J., & Barron, H. J. (2009). Exploring the relationship between sampling efficiency and short-range endemism for groundwater fauna in the Pilbara region, Western Australia. Freshwater Biology, 54, 885–901.
Fiers, F. (1986). New and interesting copepods (Crustacea, Copepoda) from brackish waters of Laing Island (Northern Papua New Guinea); Léopold III biological station, Laing Island, contribution no. 96. Bulletin de l’Institut Royal des Sciences Naturelles de Belgique Biologie, 56, 99–120.
Finston, T. L., Johnson, M. S., Humphreys, W. F., Eberhard, S., & Halse, S. (2007). Cryptic speciation in two widespread subterranean amphipod genera reflects historical drainage patterns in an ancient landscape. Molecular Ecology, 16, 355–365.
Folmer, O., Black, M., Hoeh, W., Lutz, R., & Vrijenoek, R. (1994). DNA primers for amplification of mitochondrial cytochrome c oxidase subunit 1 from diverse metazoan invertebrates. Molecular Marine Biology and Biotechnology, 3, 294–299.
Fourment, M., & Gibbs, M. J. (2006). PATRISTIC: a program for calculating patristic distances and graphically comparing the components of genetic change. BMC Evolutionary Biology, 6, 1.
Galhano, M. H. (1968). Two new interstitial Ameiridae (Copepoda Harpacticoidea) from Portugal. Publicações do Instituto de Zoologia ’Dr. Augusto Nobre, 104, 9–21.
Gibert, J., Danielopol, D. L., & Stanford, J. A. (1994). Groundwater ecology. London: Academic.
Gómez, S., Carrasco, N. K., & Morales-Serna, F. N. (2012). A new species of Nitocra Boeck, 1865 (Harpacticoida, Ameiridae, Ameirinae) from South Africa, with notes on its ecology and remarks on the status of Nitocra sewelli husmanni Kunz, 1976. ZooKeys, 244, 33–58.
Grishanin, A. K., Rasch, E. M., Dodson, S. L., & Wyngaard, G. A. (2005). Variability in genetic architecture of the cryptic species complex of Acanthocyclops vernalis (Copepoda). I. Evidence from karyotypes, genome size, and ribosomal DNA sequences. Journal of Crustacean Biology, 25, 375–383.
Guzik, M. T., Abrams, K. M., Cooper, S. J. B., Humphreys, W. F., Cho, J.-L., & Austin, A. D. (2008). Phylogeography of the ancient Parabathynellidae (Crustacea: Bathynellacea) from the Yilgarn region of Western Australia. Invertebrate Systematics, 22, 205–216.
Hamrova, E., Krajicek, M., Karanovic, T., Cerny, M., & Petrusek, A. (2012). Congruent patterns of lineage diversity in two species complexes of planktonic crustaceans, Daphnia longispina (Cladocera) and Eucyclops serrulatus (Copepoda), in East European mountain lakes. Zoological Journal of the Linnean Society, 166, 754–767.
Horvath, T. G., Whitman, R. L., & Last, L. L. (2001). Establishment of two invasive crustaceans (Copepoda: Harpacticoida) in the nearshore sands of Lake Michigan. Canadian Journal of Fisheries and Aquaculture Science, 58, 1261–1264.
Humes, A. G. (1953). Two new semiparasitic harpacticoid copepods from the coast of New Hampshire. Journal of the Washington Academy of Sciences, 43, 360–373.
Humphreys, W. F. (2000). Background and glossary. In H. Wilkens, D. C. Culver, & W. F. Humphreys (Eds.), Ecosystems of the world, 30: subterranean ecosystems (pp. 3–14). Amsterdam: Elsevier.
Humphreys, W. F. (2001). Groundwater calcrete aquifers in the Australian arid zone: the context to an unfolding plethora of stygal biodiversity. Records of the Western Australian Museum Supplement, 64, 63–83.
Humphreys, W. F. (2006). Aquifers: the ultimate groundwater-dependent ecosystems. Australian Journal of Botany, 54, 115–132.
Huys, R. (2009). Unresolved cases of type fixation, synonymy and homonymy in harpacticoid copepod nomenclature (Crustacea: Copepoda). Zootaxa, 2183, 1–99.
Huys, R., & Boxshall, G. A. (1991). Copepod evolution. London: The Ray Society.
International Commission on Zoological Nomenclature (ICZN). (1999). International code of zoological nomenclature (4th ed.). London: The International Trust for Zoological Nomenclature.
Jakobi, H. (1956). Novas espécies de Harpacticoidea (Copepoda-Crustacea) provenientes de regioes de água salobra da costa São Paulo-Paraná. (Neue Harpacticoiden-Arten (Copepoda-Crustacea) aus den Brackwassergebieten der Küste Sao Paulo-Paraná). Dusenia Curitiba, 7, 159–171.
Karanovic, T. (2000). Nitocrella longa n. sp. (Crustacea, Copepoda, Harpacticoida) from subterranean waters of Montenegro (SE Europe). Mitteilungen aus dem Museum für Naturkunde in Berlin. Zoologische Reihe, 76, 75–83.
Karanovic, T. (2004). Subterranean copepoda from Arid Western Australia. Crustaceana Monographs, 3, 1–366.
Karanovic, T. (2006). Subterranean copepods (Crustacea, Copepoda) from the Pilbara region in Western Australia. Records of the Western Australian Museum Supplement, 70, 1–239.
Karanovic, I. (2007). Candoninae ostracods from the Pilbara region in Western Australia. Crustaceana Monographs, 7, 1–432.
Karanovic, T. (2010). First record of the harpacticoid genus Nitocrellopsis (Copepoda, Ameiridae) in Australia, with descriptions of three new species. International Journal of Limnology, 46, 249–280.
Karanovic, T., & Cho, J.-L. (2012). Three new ameirid harpacticoids from Korea and first record of Proameira simplex (Crustacea: Copepoda: Ameiridae). Zootaxa, 3368, 91–127.
Karanovic, T., & Cooper, S. J. B. (2011a). Molecular and morphological evidence for short-range endemism in the Kinnecaris solitaria complex (Copepoda: Parastenocarididae), with descriptions of seven new species. Zootaxa, 3026, 1–64.
Karanovic, T., & Cooper, S. J. B. (2011b). Third genus of paratenocaridid copepods from Australia supported by molecular evidence (Copepoda, Harpacticoida). In D. Defaye, E. Suárez-Morales, & J. C. von Vaupel Klein (Eds.), Crustaceana monographs, studies on freshwater copepoda: a volume in honour of Bernard Dussart (pp. 293–337). Leiden: Brill.
Karanovic, T., & Cooper, S. J. B. (2012). Explosive radiation of the genus Schizopera on a small subterranean island in Western Australia (Copepoda : Harpacticoida): unravelling the cases of cryptic speciation, size differentiation and multiple invasions. Invertebrate Systematics, 26, 115–192.
Karanovic, T., & Hancock, P. (2009). On the diagnostic characters of the genus Stygonitocrella (Copepoda, Harpacticoida), with descriptions of seven new species from Australian subterranean waters. Zootaxa, 2324, 1–85.
Karanovic, T., & Krajicek, M. (2012). When anthropogenic translocation meets cryptic speciation globalised bouillon originates; molecular variability of the cosmopolitan freshwater cyclopoid Macrocyclops albidus (Crustacea: Copepoda). International Journal of Limnology, 48, 63–80.
Karanovic, T., & Pesce, G. L. (2002). Copepods from ground waters of Western Australia, VII. Nitokra humphreysi sp. nov. (Crustacea: Copepoda: Harpacticoida). Hydrobiologia, 470, 5–12.
Karanovic, T., Eberhard, S. M., Perina, G., & Callan, S. (2013). Two new subterranean ameirids (Crustacea : Copepoda : Harpacticoida) expose weaknesses in the conservation of short-range endemics threatened by mining developments in Western Australia. Invertebrate Systematics, 27, 540–566.
Kiefer, F. (1949). Freilebende Ruderfusskrebse (Crustacea Copepoda). In: The Amstrong College Zoological Expedition to Siwa Oasis (Libyan Desert) 1935. Proceedings of the Egyptian Academy of Sciences, 4, 62–112.
Kiefer, F. (1955). Freilebende Ruderfusskrebse (Crustacea Copepoda) aus türkischen Binnengewässern, II. Cyclopoida und Harpacticoida. Istanbul Üniversitesi Fen Fakültesi Hidrobiologi seri B, 2, 108–132.
Kiefer, F. (1960). Psammobionte Ruderfusskrebse (Crust. Cop.) aus dem Gebiet der Unterweser und von der Insel Helgoland. Zoologischer Anzeiger, 165, 29–37.
King, R. A., Bradford, T., Austin, A. D., Humphreys, W. F., & Cooper, S. J. B. (2012). Divergent molecular lineages and not-so-cryptic species: the first descriptions of stygobitic chiltoniid amphipods (Talitroidea: Chiltoniidae) from Western Australia. Journal of Crustacean Biology, 32, 465–488.
Korber, B., Muldoon, M., Theiler, J., Gao, F., Gupta, R., Lapedes, A., Hahn, B. H., Wolinsky, S., & Bhattacharya, T. (2000). Timing the ancestor of the HIV-1 pandemic strains. Science, 288, 1789–1796.
Kornev, P. N., & Chertoprud, E. C. (2008). Copepod crustaceans of the order Harpacticoida of the White Sea: morphology, systematics, ecology. Moscow: Biology Faculty, Moscow State University, Tovarishchestvo Nauchnikh Izdanii KMK [In Russian].
Kunz, H. (1975). Copepoda Harpacticoidea aus dem Litoral des südlichen Africa. Kieler Meeresforschunhen, 31, 179–212.
Kunz, H. (1976). Eine neue Unterart der Gattung Nitocra (Copepoda, Harpacticoidea) aus Norddeutschland. Gewässer und Abwässer, 60(61), 27–34.
Kunz, H. (1983). Harpacticoiden (Crustacea: Copepoda) aus dem Litoral der Azoren. Arquipelago, Serie Ciencias Naturales, 4, 117–208.
Lang, K. (1948). Monographie der Harpacticiden (Vol. 1, 2). Lund: Hakan Ohlsson’s Boktryckeri.
Lang, K. (1965a). Copepoda Harpacticoidea from the Californian Pacific coast. Kungliga Svenska Vetensk-Akademiens Handlingar, Fjarde Serien, Stockholm, 10, 1–560.
Lang, K. (1965b). Copepoda Harpacticoidea aus dem Küstengrundwasser dicht bei idem Askölaboratorium. Arkiv för Zoologi, 18, 73–83.
Lee, C. E. (1999). Rapid and repeated invasions of fresh water by the copepod Eurytemora affinis. Evolution, 53, 1423–1434.
Lee, C. E. (2000). Global phylogeography of a cryptic species complex and reproductive isolation between genetically proximate ‘populations’. Evolution, 54, 2014–2027.
Lee, W., & Huys, R. (2002). A new genus of groundwater Ameiridae (Copepoda, Harpacticoida) from boreholes in Western Australia and the artificial status of Stygonitocrella Petkovski, 1976. Bulletin of the Natural History Museum London (Zoology), 68, 39–50.
Lefébure, T., Douady, C. J., Gouy, M., & Gibert, J. (2006). Relationship between morphological taxonomy and molecular divergence within Crustacea: proposal of a molecular threshold to help species delimitation. Molecular Phylogenetics and Evolution, 40, 435–447.
Leys, R., & Watts, C. H. (2008). Systematics and evolution of the Australian subterranean hydroporine diving beetles (Dytiscidae), with notes on Carabhydrus. Invertebrate Systematics, 22, 217–225.
Leys, R., Watts, C. H. S., Cooper, S. J. B., & Humphreys, W. F. (2003). Evolution of subterranean diving beetles (Coleoptera: Dytiscidae: Hydroporini, Bidessini) in the arid zone of Australia. Evolution, 57, 2819–2834.
Liddell, J. A. (1912). Nitocrameira bdellurae, nov. gen. et sp., a copepod of the family Canthocamptidae, parasitic in the egg-cases of Bdellura. Journal of the Linnean Society Zoology, 32, 87–94.
Marcus, A. (1968). Copepoda from the midlittoral zone of the Black Sea, Rumanian shore, I. Nitocra elongate n. sp. Travaux du Museum National d’Histoire Naturelle “Grigore Antipa”, 9, 15–24.
Marcus, A., & Por, F. D. (1961). Die Copepoden der polyhalinen Lagunen Sinoe (Schwarzes Meer – Rumänische Küsten). Acta Museui Macedonici Scientiarum Naturalium, 7, 105–126.
Mielke, W. (1993). Species of the taxa Orthopsyllus and Nitocra (Copepoda) from Costa Rica. Microfauna Marina, 8, 247–266.
Mielke, W. (1997). Interstitial fauna of Galapagos, XXXIX. Copepoda, part 7. Microfauna Marina, 11, 115–152.
Miura, Y. (1962). Subterranean harpacticoid copepods of the Amami Group of the Ryukyu Islands. Annotationes Zoologicae Japonenses, 35, 95–105.
Monard, A. (1935). Étude sur la faune des harpacticoïdes marins de Roscoff. Travaux de la Station Biologique de Roscoff, 13, 5–88.
Monchenko, V. I., & von Vaupel Klein, J. C. (1999). Oligomerization in Copepoda Cyclopoida as a kind of orthogenetic evolution in the animal kingdom. Crustaceana, 72, 241–264.
Needham, S. (2009). Yeelirrie uranium deposit in Western Australia. Canberra: Parliament of Australia, Department of Parliamentary Service, Background Note.
Noodt, W. (1952). Subterrane Copepoden aus Norddeutschland. Zoologischer Anzeiger, 148, 331–343.
Noodt, W. (1953). Bemerkenswerte Copepoda Harpacticoidea aus dem Eulitoral der deutschen Meeresküste. Zoologischer Anzeiger, 151, 5–20.
Noodt, W. (1954). Copepoda Harpacticoidea aus dem limnishcen Mesopsammal der Türkei. Istanbul Üniversitesi Fen Fakültesi Hidrobiologi seri B, 2, 27–40.
Noodt, W. (1957). Zur Kenntnis von Nitocra reducta Schäfer (Copepoda Harpacticoida). Zoologischer Anzeiger, 159, 179–184.
Pesce, G. L. (1983). Contributo alla conoscenza degli Arpacticoidi delle acque soterranee della regione Pugliese (Crustacea: Copepoda). Thalassia Salentina, 13, 62–81.
Petkovski, T. K. (1954). Harpacticiden des Grundwassers unserer Meeresküste. Acta Musei Macedonici Scientiarum Naturalium, 2, 93–123.
Petkovski, T. K. (1956). Über zwei Harpacticoide Copepoden, Pseudameira kunzi n. sp. und Bryocamptus pygmeus (Sars) f. balcanica n. f. aus Jugoslavien. Folia Balcanica, 1, 9–14.
Petkovski, T. (1976). Nitocra lacustris sinoi Marcus et Por (Copepoda, Harpacticoida) vom Strande des Karibischen Meeres. Musei Macedonici Scientiarum Naturalium, 7, 89–95.
Petrusek, A., Tollrian, R., Schwenk, K., Haas, A., & Laforsch, C. (2009). A “crown of thorns” is an inducible defense that protects Daphnia against an ancient predator. Proceedings of the National Academy of Sciences of the United States of America, 106, 2248–2252.
Por, F. D. (1964). The genus Nitocra Boeck (Copepoda Harpacticoida) in the Jordan Rift Valley. Israel Journal of Zoology, 13, 78–88.
Por, F. D. (1968). Copepods of some land-locked basins on the islands of Entedebir and Nocra (Dahlak Archipelago, Red Sea); Israel South Red Sea expedition, 1962, reports No. 31. Bulletin of the Sea Fisheries Research Station Israel, 49, 32–50.
Reid, J. W. (1988). Cyclopoid and harpacticoid copepods (Crustacea) from Mexico, Guatemala, and Colombia. Transaction of the American Microscopical Society, 107, 190–202.
Reid, J. W. (1998). How “cosmopolitan” are the continental cyclopoid copepods? Comparison of the North American and Eurasian faunas, with description of Acanthocyclops parasensitivus sp. n. (Copepoda: Cyclopoida) from the U.S.A. Zoologischer Anzeiger, 236, 109–118.
Reid, J. W., & Pinto-Coelho, R. M. (1994). An Afro-Asian continental copepod, Mesocyclops ogunnus, found in Brazil; with a new key to the species of Mesocyclops in South America and a review of intercontinental introduction of copepods. Limnologica, 24, 359–368.
Rodríguez, F., Oliver, J. F., Marín, A., & Medina, J. R. (1990). The general stochastic model of nucleotide substitutions. Journal of Theoretical Biology, 142, 485–501.
Roe, K. M. (1958). The littoral harpacticids of the Dalkey (Co. Dublin) area with descriptions of six new species. Proceedings of the Royal Irish Academy section B, 59, 221–255.
Rouch, R. (1972). Deux harpacticides nouveaux de l’ile de Long-Island (Territoire de Papouasie et de Nouvelle-Guinée). Archives de Zoologie Expérimentale et Générale, 113, 147–164.
Rowe, C. L., Adamowicz, S. J., & Hebert, P. D. N. (2007). Three new cryptic species of the freshwater zooplankton genus Holopedium (Crustacea: Branchiopoda: Ctenopoda), revealed by genetic methods. Zootaxa, 1656, 1–49.
Shen, C.-J., Tai, A.-Y., Zhang, C.-Z., Li, Z.-Y., Song, D.-X., Song, Y.-Z., & Chen, G.-X. (1979). Fauna sinica, crustacea, freshwater copepoda. Peking: Science Press [in Chinese].
Soyer, J. (1975). Harpacticoïdes (Crusacés Copépodes) de l’archipel de Kerguelen, 1. quelques forms mésopsammiques. Bulletin du Muséum National D’Histoire Naturelle, Zoologie 3e série, 168, 1169–1223.
Stamatakis, A., Hoover, P., & Rougemont, J. (2008). A rapid bootstrap algorithm for the RAxML web-servers. Systematic Biology, 75, 758–771.
Stock, J. K., & von Vaupel Klein, J. C. (1996). Mounting media revisited: the suitability of Reyne’s fluid for small crustaceans. Crustaceana, 69, 749–798.
Swofford, D. L. (2002). PAUP*, phylogenetic analysis using parsimony (* and other methods), version 4.0b10. Sunderland: Sinauer.
Tamura, K., Peterson, D., Peterson, N., Stecher, G., Nei, M., & Kumar, S. (2011). MEGA5: molecular evolutionary genetics analysis using maximum likelihood, evolutionary distance, and maximum parsimony methods. Molecular Biology and Evolution, 28, 2731–2739.
Timms, B. V. (1992). Lake geomorphology. Glen Osmond: Gleneegles.
Tran, D. L., & Chang, C. Y. (2012). Two new species of harpacticoid copepods from anchialine caves in karst area of North Vietnam. Animal Cells and Systems, 16, 57–68.
Vervoort, W. (1964). Free-living Copepoda from Ifaluk Atoll in the Caroline Islands with notes on related species. Bulletin of the United States National Museum, 236, 1–431.
Walter, T. C., & Boxshall, G. (2013). World of Copepods database. http://www.marinespecies.org/copepoda. Accessed 15 Nov 2013.
Watts, C. H. S., & Humphreys, W. F. (2006). Twenty-six new Dytiscidae (Coleoptera) of the genera Limbodessus Guignot and Nirripirti Watts and Humphreys from underground waters in Australia. Transaction of the Royal Society of South Australia, 130, 123–185.
Watts, C. H. S., & Humphreys, W. F. (2009). Fourteen new Dytiscidae (Coleoptera) of the genera Limbodessus Guignot, Paroster Sharp and Exocelina Broun, from underground waters in Australia. Transactions of the Royal Society of South Australia, 133, 62–107.
Wells, J. B. J. (1967). The littoral Copepoda (Crustacea) of Inhaca Island, Mozambique. Transactions of the Royal Society of Edinburgh, 67, 189–358.
Wells, J. B. J., & Chandrasekhara Rao, G. (1987). Littoral Harpacticoida (Crustacea: Copepoda) from Andaman and Nicobar Islands. Memoirs of the Zoological Survey of India, 16, 1–385.
Willey, A. (1930). Harpacticoid Copepoda from Bermuda, Part I. Annals and Magazine of Natural History, 10, 81–114.
Wilson, C. B. (1932). The copepods of the Woods Hole region, Massachusetts. Bulletin of the United States National Museum, 158, 1–635.
Wilson, G. D. F. (2008). Gondwanan groundwater: subterranean connections of Australian phreatoicidean isopods to India and New Zealand. Invertebrate Systematics, 22, 301–310.
Xiang, X., Xi, Y., Wen, X., Zhang, G., Wang, J., & Hu, K. (2011). Genetic differentiation and phylogeographical structure of the Brachionus calyciflorus complex in eastern China. Molecular Ecology, 20, 3027–3044.
Xu, S., Hebert, P. D. N., Kotov, A. A., & Cristescu, M. E. (2009). The noncosmopolitanism paradigm of freshwater zooplankton: insights from the global phylogeography of the predatory cladoceran Polyphemus pediculus (Linnaeus, 1761) (Crustacea, Onychopoda). Molecular Ecology, 18, 5161–5179.
Xu, L., Han, B. P., Van Damme, K., Vierstraete, A., Vanfleteren, J. R., & Dumont, H. J. (2011). Biogeography and evolution of the Holarctic zooplankton genus Leptodora (Crustacea: Branchiopoda: Haplopoda). Journal of Biogeography, 38, 359–370.
Yang, Z. (1996). Among-site rate variation and its impact on phylogenetic analyses. Trends in Ecology and Evolution, 11, 367–372.
Yeatman, H. C. (1983). Copepods from microhabitats in Fiji, Western Samoa, and Tonga. Micronesica, 19, 57–90.
Acknowledgments
We thank Prof. Supawadee Chullasorn, Ramkhamhaeng University, Bangkok, for sharing with us her still unpublished paper on a new Nitokra from Thailand. The following staff of Subterranean Ecology are acknowledged for their help in the field, as well as for sorting the samples: Giulia Perina, Natalie Krawczyk, Peter Bell and Shae Callan. We are also very grateful to Kathleen Saint (South Australian Museum) for carrying out the PCR and sequencing analyses and for developing the nested primers that were used in combination with universal primers for COI gene amplification. The species described herein were collected as part of the Yeelirrie Subterranean Fauna Survey 2009–2011 commissioned by BHP Billiton Yeelirrie Development Company Pty Ltd.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Karanovic, T., Eberhard, S., Cooper, S.J.B. et al. Morphological and molecular study of the genus Nitokra (Crustacea, Copepoda, Harpacticoida) in a small palaeochannel in Western Australia. Org Divers Evol 15, 65–99 (2015). https://doi.org/10.1007/s13127-014-0193-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13127-014-0193-3