Abstract
In this paper, we investigate a collection of Northwest Pacific nudibranch molluscs by means of integrative taxonomy, including morphological analyses, molecular data from the cytochrome c oxidase subunit I, 16S rRNA, histone H3, 28S rRNA, 18S rRNA markers, and ecological data. Two new species, Bathydoris antoni sp. nov. and Dendronotus kurilensis sp. nov., are described, and their phylogenetic relationships reconstructed. We also document two potentially new species of the genera Cadlina (Cadlinidae) and Cuthona (Fionidae s.l.). For the first time, we report molecular data for the Northwest Pacific specimens of Colga pacifica (Polyceridae), Dendronotus patricki (Dendronotidae), Ziminella vrijenhoeki (Paracoryphellidae), and Zeusia herculea (Aeolidiidae). Our molecular data supports the existence of biogeographic connections between the shallow water nudibranch fauna and their continental slope counterparts and communities on both sides of the North Pacific with possible ongoing gene exchange between fauna of both regions. We found two general types of deep shelf and bathyal communities inhabited by nudibranchs in the Northwest Pacific, each characterized by a certain type of fauna and a connectivity with different bathymetric and geographic areas.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Among marine ecosystems, deep-water fauna is one of the most difficult to study using reasonable sample sizes. A number of dedicated expeditions aiming to collect bathyal and abyssal organisms have obtained an order of magnitude smaller sample sizes than those conducted in shallow waters (McClain, 2007). Sampling from great depths is complex, and recovered material is often impacted due to pressure and temperature differences, as well as physical damage inflicted by collecting gear. However, the ocean floor is far from being the desert it was originally believed to be, as demonstrated by the multitude of newly discovered, highly productive communities (Sibuet & Olu, 1998; Van Dover, 2000); preliminary biodiversity estimates predict between 0.5 and 10 million species of deep sea macrofauna exists (Brandt & Malyutina, 2015; Brandt et al., 2007a, b).
From an evolutionary perspective, deep water ecosystems are extremely important to study, as they contain diverse faunas composed, at least in part, by ancient groups predating the Pliocene turnover (Briggs, 2003; Menzies & Imbrie, 1958; Wilson, 1999; Zenkevitch & Birstein, 1953). Conditions on the ocean floor are far from uniform, and have never remained stable for very long; for example temperature, salinity, and oxidation levels have changed multiple times over the Earth’s history (Horne, 1999; Jacobs & Lindberg, 1998; Jeppsson, 1990; Rogers, 2000; Waelbroeck et al., 2001). Groups of organisms living in the deep sea for a long time have evolved to meet challenges of environmental change (Wilson, 1999). At the same time, there are younger groups that colonized deeper waters quite recently and provide a model to study the different stages of adaptation to the novel conditions they encountered (Jablonski & Bottjer, 1991; Jablonski et al., 1983; Linder et al., 2008; Menzies & Imbrie, 1958; Smith & Stockley, 2005). Many unique communities assembled in bathyal and abyssal zones, and their detailed study enhances our understanding of biogeography (Jumars et al., 1990; McClain et al., 2009; Pradillon & Gaill, 2007). For example, deep-sea faunal connections between different biogeographic provinces and regions are of a great interest and link deep water organisms to their littoral and sublittoral counterparts (Baco et al., 1999; Linder et al., 2008; Raupach et al., 2009). It turns out that conflicting and controversial hypotheses on the age and origin of the deep fauna all appear to be partially true (McClain & Hardy, 2010). Paradoxically, while deep sea communities appear to be extremely fragmented in isolated sea mounts or other suitable habitats separated by long distances and the presence of land masses and strong currents, they are much more connected than initially estimated (McClain & Hardy, 2010). Barriers separating deep sea communities appear to be semi-permeable, and evidently different groups of organisms overcame some of them at certain points (Vinogradova, 1997; Zardus et al., 2006). There is also evidence that for many bathyal and abyssal animals, dispersal potential at the larval stage has been greatly underestimated (Pearse & Lockhart, 2004).
The North Pacific region is one of three major marine biodiversity origin hotspots, with high numbers of endemic taxa (Briggs, 2003). There is a significant interest in deep-water research in this region, which is an active area of ongoing biogeographic studies of pelagic and benthic organisms. However, research coverage for different taxa is uneven. For instance, hypotheses on migration, fauna exchange, and connectivity are rather well tested for fish and nektonic organisms (Afonso et al., 2014; Gordeeva, 2014; Milligan et al., 2020), including those inhabiting North Pacific deep waters (Orlov et al., 2020; Orlova et al., 2019). At the same time, many groups of deep-sea benthic invertebrates in this region are poorly known. To mitigate this knowledge gap in the Northwest Pacific, ten expeditions of the R/V Vityaz (USSR) were conducted between the years 1949–1979 and 1983. More recently, the international Russian-German collaboration programs SoJaBio and KuramBio had an enormous impact on the deep-sea fauna biodiversity knowledge (Brandt & Malyutina, 2015; Brandt et al., 2015, 2019; Malyutina & Brandt, 2012, 2013; Saeedi et al., 2020), resulting in a number of publications on the systematics and biogeography of various taxa (Brandt & Malyutina, 2015; Malyutina et al., 2018; etc.). However, not every taxonomic group was covered in these studies. In particular, data on nudibranch molluscs of the Northwest Pacific deep waters remains scarce, with just a handful of species descriptions scattered across several taxonomically focused papers (i.e., Volodchenko, 1941; Martynov & Roginskaya, 2005; Martynov, 2013; Korshunova et al., 2017a, b, 2020a; Martynov et al., 2020).
In this study, we report on the species diversity of nudibranch molluscs of the Northwest Pacific based on specimens collected in two expeditions, covering the lower shelf and bathyal areas off Sakhalin island, the Kuril Islands, and Kamchatka, and discuss their ties to both the shallow water and the Northeast Pacific bathyal faunas. The goal of this paper is to contribute additional data to the known nudibranch biodiversity of the region and provide baseline data for future studies on the evolution and biogeography of this group.
Material and methods
Collection data and community descriptions
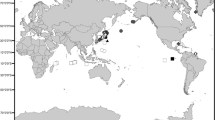
Samples were collected in several locations during the following expeditions: (1) R/V “Akademic Lavrentyev” (Russia) to the Bering Sea, June–July 2018, and (2) R/V “Akademic Oparin” (Russia) to the Sea of Okhotsk, July–August 2019, at depths of 200–900 m (Fig. 1; Table S1). In the first survey, specimens were photographed underwater by a deep-water remotely operated vehicle (ROV), taken by ROV manipulators, and placed into specialized plastic boxes for transport to the surface. In the field laboratory, specimens were photographed again from their dorsal and ventral sides. Specimens found in the second survey were collected by an Agassiz trawl (AGT) and then photographed in the lab. All specimens were fixed in 96% EtOH and stored at −20 °C to prevent DNA degradation. Data on biological communities in sampling locations was collected using ROV photos (1), or by visual inspection of dominant species collected by the AGT (2). Voucher specimens are deposited in the collections of the National Scientific Center of Marine Biology (voucher numbers will be provided after the first revision according to rules of the collection). Detailed sampling localities and voucher numbers for each specimen are given in Table S1.
Morphological studies
All collected specimens were used for the examination of their external morphology under a stereomicroscope. The internal morphology of 13 specimens was also examined, including the digestive and reproductive systems. The buccal mass of each specimen was extracted and soaked in proteinase K solution for 2 h at 60 °C to dissolve connective and muscle tissues. The radula and the jaws were rinsed in distilled water, air-dried, mounted on an aluminium stub, and sputter-coated with gold for visualization under a JEOL JSM 6380 scanning electron microscope (SEM). Features of the jaws were examined by optical stereomicroscopy and SEM on Tescan Vega TS5130MM (Tescan, Czech Republic) and CamScan S2 (Cambridge). For the study of the reproductive system, specimens were dissected from the dorsal side along the midline and examined under a stereomicroscope.
DNA extraction, amplification, and sequencing.
Total genomic DNA was extracted from tissue samples preserved in 96% EtOH (Table S1) following the invertebrate protocol of the Canadian Center for DNA Barcoding (Ivanova et al., 2006). Extracted DNA was used as a template for amplification of partial mitochondrial cytochrome c oxidase subunit I and 16S rRNA and nuclear histone H3 and 28S rRNA; additionally for one species of the genus Dendronotus, we obtained partial 18S rRNA. Reaction conditions and primers are available in Table 1. Polymerase chain reactions were performed with an “HS Taq” kit (Eurogen Lab, Russia), following the manufacturer’s protocol.
For sequencing, 2 μL of amplicons was purified by EtOH + ammonium acetate precipitation (Osterburg et al., 1975) and used as a template for the sequencing reactions with a BigDye Terminator v3.1 sequencing kit by Applied Biosystems. Sequencing reactions were analyzed using an ABI 3500 Genetic Analyser (Applied Biosystems). All novel sequences were submitted to NCBI GenBank (Table S2).
Data processing and phylogenetic reconstruction
All raw reads for each gene were assembled and checked for ambiguities and low-quality data in Geneious R10 (Kearse et al., 2012). Edited sequences were verified for contamination using the BLAST-n algorithm run over the GenBank nr/nt database (Altschul et al., 1990). For phylogenetic reconstruction, datasets obtained in previous studies on the following representative groups were incorporated in the analyses: Bathydoris (Mahguib & Valdés, 2015), Cadlina (Do et al., 2020; Korshunova et al., 2020b), Tritonia (Korshunova & Martynov, 2020; Moles et al., 2021; Valdés et al., 2018), Dendronotus (Ekimova et al., 2019; Korshunova et al., 2017c, 2020a), Ziminella (Korshunova et al., 2017a; Valdés et al., 2018), Cuthona (Cella et al., 2016; Chichvarkhin et al., 2016; Korshunova et al., 2018), and Zeusia (Korshunova et al., 2017b; Valdés et al., 2018). For species of Colga, only a BLAST-n search was done, since molecular data for this genus is very limited. Original data and publicly available sequences were aligned with the MUSCLE (Edgar, 2004) algorithm in MEGA 7 (Kumar et al., 2016). Additionally, all protein-coding sequences were translated into amino acids to verify reading frames and check for stop codons. Saturation was checked by plotting for all specimens including outgroup the total number of pairwise differences (transitions and transversions) against uncorrected p-distances. For the COI and H3 fragments, saturation was further examined separately for the first, second, and third codon positions. Indel-rich regions of the 16S alignment were identified and removed in Gblocks 0.91b (Talavera & Castresana, 2007) with the least stringent settings. Sequences were concatenated by a simple biopython script following Chaban et al. (2019a). Phylogenetic reconstructions were conducted for the concatenated multi-gene partitioned datasets. The best-fit nucleotide evolution model for MrBayes phylogeny reconstruction method was selected in ModelTest-NG v0.1.7 (Darriba et al., 2020; Flouri et al., 2014). Multi-gene analyses were done by applying evolutionary models separately to partitions representing single markers. The Bayesian phylogenetic analyses and estimation of posterior probabilities were performed in MrBayes 3.2 (Ronquist & Huelsenbeck, 2003). Markov chains were sampled at intervals of 500 generations. The analysis was initiated with a random starting tree and ran for 107 generations. Best-fit models for each gene dataset and fragment length (bp) are shown in Table S3. Maximum likelihood phylogeny inference was performed in the HPC-PTHREADS-AVX option of RaxML HPC-PTHREADS 8.2.12 (Stamatakis, 2014) with 1000 pseudoreplicates under the GTRCAT model of nucleotide evolution. Bootstrap values were placed on the best tree found with SumTrees 3.3.1 from DendroPy Phylogenetic Computing Library 3.12.0 (Sukumaran & Holder, 2010). Final phylogenetic tree images were rendered in FigTree 1.4.0 and further modified in Adobe illustrator CS 2015. Uncorrected p-distances were calculated based on COI alignments in MEGA 7 (Kumar et al., 2016).
Haplotype networks
Haplotype networks based on the COI dataset were constructed using PopART 1.7 (http://popart.otago.ac.nz) (Leigh & Bryant, 2015) with the TCS network algorithm (Clement et al., 2002) and a connection limit of 5%. Resulting networks were edited in Adobe illustrator CS 2015 to highlight certain features. For Dendronotus patricki, the 18S dataset was also examined.
Nomenclatural acts
The electronic edition of this article conforms to the requirements of the amended International Code of Zoological Nomenclature (ICZN), and hence, the new names contained herein are available under that Code from the electronic edition of this article. This published work and the nomenclatural acts it contains have been registered in ZooBank, the online registration system for the ICZN. The ZooBank LSIDs (Life Science Identifiers) can be resolved and the associated information viewed through any standard web browser by appending the LSID to the prefix “http://zoobank.org/”. The LSID for this publication is: urn:lsid:zoobank.org:pub:6A397FB3-2799-4A1C-A0A2-11B2190DCE08.
Results
Our molecular and morphological results (see below) revealed 12 nudibranch species belonging to genera Bathydoris, Cadlina, Colga, Tritonia, Dendronotus, Cuthona, Ziminella, and Zeusia. Of those, two species are new to science and are described herein. Additionally, Cadlina sp. and Cuthona sp. likely represent two new species but limited morphological and/or molecular data does not allow us to provide formal species descriptions, so they will remain unnamed until new material becomes available for study. For described species which identity was confirmed by morphological and molecular data, we provide only brief descriptions; the new species are described in full.
-
Systematics
-
Order Nudibranchia Blainville, 1814
-
Suborder Doridina Odhner, 1934
-
Superfamily Bathydoridoidea Bergh, 1891
-
Family Bathydorididae Bergh, 1891
-
Genus Bathydoris Bergh, 1884
-
Bathydoris antoni sp. nov.
-
urn:lsid:zoobank.org:act:F79336D3-0ADE-428C-AE0A-DD0052AAD739
Bathydoris antoni sp. nov, holotype MIMB 42229, external morphology and SEM micrographs of internal morphology. A, dorsal view. B, ventral view. C, lateral view from left. D, lateral view from right. E, jaw plate. F, radula. G, anterior radular portion. H, details of denticulation in rachidian and innermost lateral teeth. I, inner and middle lateral teeth. Scale bars: A–D, 5 mm; E, F, 500 µm; G, I, 100 µm; H, 50 µm. Living photos by Anastassya Maiorova
Reproductive system. A, Bathydoris antoni sp. nov., general view. B, Bathydoris antoni sp. nov., male part and female gland mass partly removed. C, Dendronotus patricki, MIMB 42240. D, Dendronotus kurilensis sp. nov., paratype MIMB 42238. Abbreviations: amp, ampulla; bc, bursa copulatrix; bcd, bursa copulatrix duct; dvd, distal vas deferens; fgm, female gland mass; hd, hermaphroditic duct; ov, oviduct; p, penis; pr, prostate; psh, penile sheath; pvd, proximal vas deferens; rs, receptaculum seminis; va, vagina; vd, vas deferens
Type material: Holotype MIMB 42229, Sea of Okhotsk, Raikoke Is., 48°19′7″ N, 153°09′9″ E, depth 241–400 m, August 18, 2019, R/V Akademik Oparin.
Type locality: Sea of Okhotsk, Raikoke Is., 48°19′7″ N, 153°09′9″ E, depth 241–400 m.
Etymology: This species is named in memoriam of our friend and colleague Dr. Anton Chichvarkhin, Russian marine biologist and researcher of invertebrate systematics and phylogenetics. His professional contributions greatly improved the knowledge on the Far East marine molluscan fauna. His passion for science and sea slug systematics was an inspiration for many of us, and he will be greatly missed.
External morphology (Fig. 2A–D): Living specimen 23 mm in length, body elongate-oval, elevated. Body texture soft, lacking spicules. Numerous swollen papillae with pointed tips of different sizes on lateral margins of notum, most autotomized. Central dorsal surface lacks papillae. Anterior end of notum with 18 conical papillae, about twice as long as the notal papillae. Rhinophores near anterior edge of notum, with wide base, conical, perfoliated with 24–26 lamellae. Four bipinnate branchial leaves near posterior dorsal end. Anal papilla very prominent, situated behind branchial leaves. Buccal area projects from anterior end and bear two conical oral tentacles. Foot irregularly folded, extending posteriorly and laterally beyond notal edge.
Color (Fig. 2A–D): Background color from creamy-white to pale pink with light-orange body surface on anterior and posterior parts of notum and upper parts of foot. Foot light orange. Rhinophoral bases and branchial leaves intense orange. Rhinophores ochre in color. Papillae semitransparent white with subapical orange pigment rings.
Anatomy: Buccal mass strongly muscular, oval. Two thin elongated salivary glands occupy first 1/3 part of body, insert into buccal bulb near esophagus opening. Posteriorly esophagus connects with muscular gizzard possessing internal digitiform folds. Jaws large, strong, triangular chitinous plates lacking denticles and rodlets (Fig. 2E). Radular formula 30 × 33.1.33 (Fig. 2F). Rachidian teeth elongate-triangular, with strong base, cusp small, compressed with 3–6 denticles on each side (Fig. 2G, H). Number of denticles often asymmetric. Innermost lateral teeth smallest, triangular, with strong base and wide conical cusp, denticulation asymmetric, 2–3 denticles on inner side and 5–7 denticles on outer side. Morphology of lateral teeth slightly changes from inner to outer teeth, second inner tooth has wide conical cusp, 3–4 denticles on outer side, but lacks denticles on inner side; next, tooth has triangular cusp and 5–6 denticles at base (Fig. 2I). Outer teeth with very small denticles (2–3) near tooth base and blade-like triangle cusps.
Studied specimen was not fully mature. Ampulla long, convoluted, branched into very short bent oviduct and vas deferens (Fig. 3A, B). Prostatic vas deferens very long and convoluted, widens into small bulbous ejaculatory portion, possibly undeveloped in specimen examined. Bulbous transparent bursa copulatrix with wide convoluted duct connects to vagina. Female gland mass complex including a large gland with many internal loops and another glandular portion below.
Molecular data: Among Bathydorididae only sequences of two species are present in GenBank: Bathydoris clavigera Thiele, 1912 and B. aioca Er. Marcus & Ev. Marcus, 1962; therefore, a comprehensive molecular phylogenetic study was not possible. Nevertheless, our analysis based on four markers (COI, 16S, H3, 28S; see Fig. 4A) places our new species in a highly supported clade (PP = 1), as sister species to B. clavigera and B. aioca (PP = 0.92).
Molecular phylogenetic reconstructions of studied groups. A, genus Bathydoris, Bayesian inference, concatenated dataset of four markers (COI + 16S + H3 + 28S). B, genus Cadlina, maximum likelihood, concatenated dataset of four markers (COI + 16S + H3 + 28S), species-level clades and outgroups (Dendrodoris and Aldisa) are collapsed to a single branch. C, genus Tritonia, maximum likelihood, concatenated dataset of four markers (COI + 16S + H3 + 28S), species-level clades and outgroups are collapsed to a single branch, except target species. Numbers above branches indicate posterior probabilities from Bayesian inference, numbers below branches, bootstrap support from maximum likelihood
Biology: The ecological community was represented by a substrate composed of remains of dead barnacles, with fragmentary hydrozoans and Octocorallia, and less common sponges and ophiuroids.
Remarks: Bathydoris antoni sp. nov. is here placed in the genus Bathydoris based on the presence of shared traits between this species and other members of the genus described in Valdés (2002), specifically the possession of autotomizable papillae on the dorsum, large perfoliate rhinophores, non-retractable gill and a protruding buccal area with two large conical oral tentacles. No other dorid nudibranch group possesses this combination of traits. Moreover, the molecular phylogenetic analysis conforms that all species of Bathydoris sequenced to date, including B. antoni sp. nov., form a monophyletic group. At the same time, Bathydoris antoni sp. nov. is clearly distinguishable from other species of Bathydoris described to date. Four other species of Bathydoris are known from the Pacific Ocean proper, including the northwestern Pacific species B. japonensis Hamatani & Kubodera, 2010, known only from northern Japan, the Northeastern Pacific species B. aioca, which ranges from Baja California to Oregon, and the tropical Indo-Pacific species B. abyssorum Bergh, 1884, and B. spiralis Valdés, 2002. Both B. japonensis and B. aioca are clearly distinguishable from Bathydoris antoni sp. nov. in several regards. Bathydoris japonensis and B. aioca lack denticles on the rachidian and lateral radular teeth (Hamatani & Kubodera, 2010; Valdés & Bertsch, 2000), which are present in B. antoni sp. nov. Moreover, the reproductive anatomy of these three species is clearly distinct, with B. japonensis and B. aioca having a much longer ampulla, as well as a larger, conical penis (Hamatani & Kubodera, 2010; Valdés & Bertsch, 2000), all these organs being much smaller in B. antoni sp. nov. Because the specimen of B. antoni sp. nov. here examined was not fully mature, organ sizes could be unreliable for comparison, but the shape of the penis and the radular morphology should be taxonomically useful traits. Finally, genetically, there is an 11.94% sequence divergence in 16S between B. antoni sp. nov. and B. aioca, but there is no data for B. japonensis.
Of the tropical species, B. spiralis is very different from B. antoni sp. nov. as it has an extremely long deferent duct and very short, triangular rachidian teeth (Valdés, 2002), lacking the characteristic denticles of B. antoni sp. nov., and B. abyssorum has smooth lateral teeth, lacking denticles and a rachidian tooth with only one denticle (Valdés, 2002), again very different from B. antoni sp. nov.
Bathydoris antoni sp. nov. is the second species of Bathydoris described from the northwestern Pacific Ocean and only the eighth valid species described in this genus. Two additional species of Bathydoris are endemic to Antarctic and subantarctic waters (Bathydoris hodgsoni Eliot, 1907 and B. clavigera) and one more to the North Atlantic (Bathydoris ingolfiana Bergh, 1899). Bathydoris is probably paraphyletic (Valdés, 2002), and some authors have placed B. clavigera in the genus Prodoris Baranetz & Minichev, 1995 (Hallas et al., 2017). Based on the results of the molecular phylogeny presented here (Fig. 4A), if B. antoni sp. nov. is included in Bathydoris, there is no support for Prodoris as distinct from Bathydoris. Therefore, we regard Prodoris as a synonym of Bathydoris. But this question, as well as the monophyly of Bathydoris, requires further work and the inclusion of additional species in the molecular analysis, particularly B. spiralis as it is morphologically more divergent.
-
Superfamily Chromodoridoidea Bergh, 1891
-
Family Cadlinidae Bergh, 1891
-
Genus Cadlina Bergh, 1879
-
Cadlina sp.
-
(Fig. 5)
Cadlina sp. MIMB 42230, external morphology and SEM micrographs of internal morphology. A, dorsal view of living specimen, ca. 15 mm in length. B, labial cuticle. C, details of labial cuticle. D, radula. E, rachidian and innermost lateral teeth. F, inner lateral teeth. G, outer lateral teeth. Scale bars: A, 5 mm; B, D, 300 µm; C, 10 µm; E–G, 30 µm. Living photo by Anastassya Maiorova
Material studied: MIMB 42230, Sea of Okhotsk, Iturup Is., 45°44′6″ N, 148°33.7′ E, depth 263–273 m, August 19, 2019, R/V Akademik Oparin, 1 specimen.
External morphology (Fig. 5A): Living specimen 24 mm in length. Notum large, oval, rounded at its anterior and posterior ends. Rhinophores tubular, perfoliated with 14 lamellae, rhinophoral sheath rim with small tubercles. Notum bears numerous small tubercles, larger tubercles also common among small ones. Subepidermal small yellow glands on lateral sides of notum. Eight multi-pinnate branchial leaves surround anal opening, retract in gill cavity. Foot broad, not extending to notal edge.
Color (Fig. 5A): Background color milky-white, semitransparent. Pink portions of digestive system and darker parts visible through body wall. Rhinophores and branchial leaves yellowish beige. Small subapical glands light yellow.
Anatomy: Large buccal complex, salivary glands thin and narrow. Jaws formed by oval labial disk bearing rod-shaped labial cuticular elements, tips bifurcated (Fig. 5B, C). Radular formula 79 × 34.1.34 (Fig. 5D). Rachidian tooth with triangular base, rounded at top, bear 4–6 denticles (Fig. 5E). Innermost lateral teeth with wide base and strong conical cusp, bearing 2–3 inner and 4–6 outer denticles (Fig. 5E). Morphology of lateral teeth slightly changes from inner teeth to outer, second inner lateral teeth lack denticles on inner side, number of outer denticles increases toward outer teeth (Fig. 5F). Last outer lateral teeth elongated, hook-shaped, with 17–20 denticles (Fig. 5G).
Reproductive system triaulic. Ampulla wide, folded, vas deferens moderately long with distinct widened prostatic part. Both bursa copulatrix and receptaculum seminis oval in shape, vagina narrow. Penis with hooks.
Molecular data: On the phylogenetic tree of the genus Cadlina (Fig. 4C) based on three markers (COI, 16S, 28S) the studied specimen forms a singleton that is sister to C. paninae Korshunova, Fletcher, Picton, Lundin, Kashio, N. Sanamyan, K. Sanamyan, Padula, Schrödl & Martynov, 2020 (PP = 0.9, ML = 89). The p-distance between Cadlina sp. and C. paninae comprises 5.1%, between Cadlina sp. and C. kamchatica Korshunova, Picton, Sanamyan & Martynov, 2015 or C. laevis (Linnaeus, 1767) — 5.4%.
Distribution: This species is only known from Iturup Is.
Biology: This species was found in species-rich community of gravel sand, including numerous sponges, hydrozoans, annelids, echinoderms, and crustaceans.
Remarks: Several recent studies on Cadlina diversity revealed a high number of new species described from the North Pacific: Cadlina kamchatica, C. umiushi Korshunova, Picton, Sanamyan & Martynov, 2015, C. paninae, C. japonica Baba, 1937 (Korshunova et al., 2020b; Martynov et al., 2015), C. koreana T. D. Do, D.-W. Jung, H.-J. Kil & C.-B. Kim, 2020 (Do et al., 2020) and C. olgae Chichvarkhin 2016. The latter species was further regarded as a junior synonym of C. umiushi (Chichvarkhin, 2016; Korshunova et al., 2020b). All these species inhabit shallow water except C. japonica, which has wide bathymetric range from 5 to 350 m in depth (Korshunova et al., 2020b). The here specimen of Cadlina sp. described herein represents a separate lineage on the Cadlina phylogenetic tree, being sister to C. paninae, another species recently described from the Kuril Islands. From this species, Cadlina sp. differs by the morphology of the rachidian tooth, which is much slender in C. paninae and has asymmetrical denticles on each side. On the other hand, Cadlina sp. is similar in radular characters to another phylogenetically close species Cadlina laevis (inhabits the North Atlantic and the Arctic), but the latter species has more bright yellow glands on the notum. The reproductive system of Cadlina sp. is similar two both C. laevis and C. paninae. Taking into account the high level of variation in radular characters recently shown for C. laevis (Korshunova et al., 2020b) and limited material studied on both morphological and molecular levels, we suggest that further studies are needed to confirm the species status of the deep-sea Cadlina specimen.
-
Superfamily Polyceroidea Alder & Hancock, 1845
-
Family Polyceridae Alder & Hancock, 1845
-
Genus Colga Bergh, 1880
-
Colga pacifica (Bergh, 1894)
-
(Fig. 6)
Material studied: MIMB 42231, Sea of Okhotsk, Shiashkotan Is., 48°48′3″ N, 154°17′7″ E, depth 285–304 m, August 12, 2019, R/V Akademik Oparin, 2 specimens.
External morphology (Fig. 6A, B): Length of studied specimens up to 22 mm. Body elongated. Dorsal side of notum with three distinct rows of papillae, and additional row of papillae on each notal lateral edge. Sides of hyponotum with small papillae. Oral veil with ~ 20 papillae. Rhinophores bear up to 28 lamellae. Five multipennate branchial leaves surrounding anal opening.
Color (Fig. 6A, B): Notum, rhinophores and branchial leaves orange, foot yellowish white.
Anatomy: Radular formula 16 × 5–6.1.1.1.1.1.5–6 (Fig. 6C). Rachidian tooth rectangular, plate-like. Innermost lateral tooth narrow with beak-shaped curved top. Second innermost lateral tooth broad with rectangular base, beak-shaped top and additional lobe-shaped process at middle part. Outer lateral teeth rectangular plates lacking processes or denticles. Reproductive system triaulic. Ampulla wide, folded. Lobe-shaped, large bursa copulatrix, receptaculum seminis small oval muscular sac. Vas deferens long and convoluted, its prostatic part rather indistinct. Penis with hooks.
Molecular data: A BLAST-n search of COI sequence (GenBank accession number is MZ782097) resulted 93% identical to three sequences of Colga villosa (Odhner, 1907) (KU695600-02), thus confirming distinctness of C. pacifica.
Distribution: North Pacific (Unimak Is., Alaska) and Northwest Pacific from Commander Islands to Southern Kuril Islands, 14–1020 m in depth (Bergh, 1894; Martynov, 1993; Martynov & Baranets, 2002; Volodchenko, 1941).
Biology: This species was found in species-rich community of gravel sand and rocks, with echinoderms, sponges, and large ascidians as common constituents.
Remarks: This species is a common component of the Kuril Islands communities (Martynov & Baranets, 2002; Martynov et al., 2016). Another species in the Northwest Pacific, Colga minichevi Martynov & Baranets, 2002, inhabits shallow waters above 140 m depth (Martynov & Baranets, 2002; Martynov et al., 2016). This species differs from C. pacifica by its external morphology (6–8 rows of papillae on notum, smooth foot edge), coloration (pale white with ochre rhinophores and opaque white pigment on papillae), and in internal features (the rachidian tooth is absent, elongated lateral teeth).
-
Suborder Cladobranchia William & Morton, 1984
-
Superfamily Tritonioidea Lamarck, 1809
-
Family Tritoniidae Lamarck, 1809
-
Genus Tritonia Cuvier, 1798
-
Tritonia tetraquetra (Pallas, 1788)
-
(Fig. 7)
Genus Tritonia, external morphology and SEM micrographs of internal morphology. A, Tritonia tetraquetra MIMB 42248. B, Tritonia tetraquetra MIMB 42249. C, Tritonia psoloides MIMB 42245 in natural environment collected by ROV. D, Tritonia tetraquetra MIMB 42246 in natural environment; a specimen is marked by a white arrow. E, Tritonia tetraquetra MIMB 42246, rachidian and inner lateral teeth. F, Tritonia tetraquetra MIMB 42246, middle lateral teeth. G, Tritonia tetraquetra MIMB 42246, outer lateral teeth. Scale bars: A, B, 5 mm. E, F, 100 µm; G, 200 µm. Living photos by Anastassya Mayorova and team of ROV “Komanch”
Material studied: MIMB 42246, Bering Sea, 55°22′14.9″ N, 167°16′1.2″ E, depth 895 m, June 17, 2018, R/V Akademik Lavrentyev, 1 specimen. MIMB 42247, Sea of Okhotsk, Simushir Is., 47°15′4″ N, 152°10′0″ E, depth 205–222 m, July 03, 2019, R/V Akademik Oparin, 1 specimen. MIMB 42248, Sea of Okhotsk, Urup Is., 46°15′9″ N, 150°15′4″ E, depth 450–460 m, July 05, 2019, R/V Akademik Oparin, 1 specimen. MIMB 42249, Sea of Okhotsk, Iturup Is., 45°44′6″ N, 148°34′0″ E, depth 264–270 m, July 07, 2019, R/V Akademik Oparin, 1 specimen. MIMB 42250, Sea of Okhotsk, Shiashkotan Is., 48°45′4″ N, 154°11′6″ E, depth 498–516 m, August 15, 2019, R/V Akademik Oparin, 1 specimen. MIMB 42251, Sea of Okhotsk, Raikoke Is., 48°19′7″ N, 153°09′9″ E, depth 245–247 m, August 16, 2019, R/V Akademik Oparin, 1 specimen. MIMB 42252, Sea of Okhotsk, Urup Is., 46°16′7″ N 150°16′5″ E, depth 227–210 m, August 18, 2019, 1 specimen. MIMB 42253, Sea of Okhotsk, Iturup Is., 45°44′6″ N, 148°33′7″ E, depth 263–273 m, August 19, 2019, R/V Akademik Oparin, 1 specimen.
External morphology (Fig. 7A, B, D): Length of studied specimens up to 200 mm. Body elongate, relatively narrow, tapering towards posterior end. Up to 17 tripinnate branched dorsolateral appendages. Dorsal surface smooth to slightly rugose. Up to 16 conical appendages on oral veil. Rhinophores elongate and narrow, surrounded by 5–6 tripinnate papillae. Rhinophoral sheaths low, simple. Anal opening on right side, mid-length, reproductive opening on right side, at anterior 1/4 of body length.
Color (Fig. 7A, B, D): Background color white, accompanied by intense orange or yellow-orange pigmentation. Rhinophores from white to yellow. Dorsolateral appendages white, sometimes with opaque white pigment on bifurcated tips.
Anatomy: Large muscular buccal complex, jaws elongated plates, two large salivary glands. Radular formula 52 × 58.1.58. Rachidian tooth broad, central cusp triangular and broad, with 2 denticles on each side (Fig. 7E). Lateral teeth hook-shaped, lacking denticles (Fig. 7F, G). Ampulla large, convoluted. Prostatic vas deferens narrow, expanding to massive copulative organ. Seminal receptacle small, oval.
Molecular data: On the phylogenetic reconstruction based on three markers (COI, 16S, H3) studied, specimens are genetically identical to Tritonia tetraquetra from the Northeast Pacific and Kamchatka (ML = 99; PP = 1) with minor intraspecific variation (Fig. 4C). This lineage is sister to the Tritonia psoloides Aurivillius, 1887 clade (ML = 87; PP = 1).
Biology: This species was found in many localities with different species composition. In the Bering Sea, it was on rocky gravel sand in the vicinity of methane seeps. Dominant benthic species included demosponges, anemones Cribrinopsis sp. and Amphianthus sp., gorgonians Swiftia pacifica (Nutting, 1912) and Isididae, alcyonarians Clavularia sp. Many shrimps, ophiuroids, crabs Munidae, anthozoans Paragorgia sp., and ascidians Styelidae were found on large rocks. In the Sea of Okhotsk, all stations were composed of different bottom sediments (muddy sand, gravel sand, rocks) and included a large number of sponges, hydrozoans, gorgonians, and alcyonarians.
-
Tritonia psoloides Aurivillius, 1887
-
(Fig. 7C)
Material studied: MIMB 42245, Bering Sea, 61°10′40.1″ N, 174°52′14.9″ E, depth 418 m, June 28, 2018, R/V Akademik Lavrentyev, 1 specimen.
External morphology: Body length up to 280 mm. Body elongate, broad, tapering towards posterior end. Up to 18 bi- or tripinnate branched dorsolateral appendages. Dorsal side smooth to slightly rugose. Seventeen conical appendages on oral veil. Rhinophores elongate and narrow, surrounded by 6 tripinnate papillae. Rhinophoral sheaths low, simple. Anal opening on right side mid-length, reproductive opening on right side at anterior 1/4 of body length.
Color: Uniform milky-white with opaque-white tips of appendages.
Internal morphology: The single specimen was not available for study of internal morphology since this large formalin-fixed specimen is a part of permanent exposition in the Museum of National Scientific Center of Marine Biology.
Molecular data: On the phylogenetic reconstruction based on three markers (COI, 16S, H3), the specimen here examined is genetically identical to Tritonia cf. psoloides (CASIZ181055) from the eastern part of the Bering Sea (ML = 70; PP = 1) (Fig. 4C). This lineage is sister to the Tritonia tetraquetra clade (ML = 87; PP = 1). The p-distance in COI marker between these two species ranges between 8.1 and 9.1%.
Distribution: So far only known from the Bering Sea, 140–418 m depth (Korshunova & Martynov, 2020; present study).
Biology: Community of sea urchins Brisaster latifrons (A. Agassiz, 1898). Local seep activity, marked by bacterial tufts and dark bioturbated sediments. Common components of this community include anemones of the genus Monactis and the families Actinostolidae and Sagartiidae, as well as hermit crabs and gastropods. Sea pens are absent from active seep sites; however, they are very abundant components of their distant vicinity.
Remarks: The morphological data of the type specimen of T. psoloides were given in a recent comprehensive review of the family Tritoniidae (Korshunova & Martynov, 2020). Since this species was described from the Bering Sea (61°45′N 180°W), and a specimen genetically distinct from T. tetraquetra was found in the same sea, it was suggested to retain the species name T. psoloides as valid for that specimen. Although we were not able to study the internal morphology, our specimen matches T. psoloides type specimen in external morphology and coloration (Korshunova & Martynov, 2020) and was collected close to the type locality of this species (appr. 250 km). Also, our specimen is similar genetically to GB specimen identified as Tritonia cf. psoloides from the Bering Sea. Therefore, we conclude that our data agrees with Korshunova and Martynov (2020) view and T. psoloides is regarded a valid species.
-
Superfamily Dendronotoidea Allman, 1845
-
Family Dendronotidae Allman, 1845
-
Genus Dendronotus Alder & Hancock, 1845
-
Dendronotus dalli Bergh, 1879
-
(Fig. 8)
Dendronotus dalli Bergh, 1879, external morphology and SEM micrographs of internal morphology. A, MIMB 42234a. B, MIMB 42234c. C, MIMB 42233. D, MIMB 42234c, right jaw plate. E, MIMB 42234c, denticulation of masticatory border. F, MIMB 42234c, radula. G—MIMB 42234c, rachidian tooth, posterior radular portion. H, MIMB 422342c, lateral teeth, posterior radular portion. Scale bars: A–C, 10 mm; D, F, 500 µm; E, G, H, 50 µm. Living photos by Anastassya Maiorova
Material examined: MIMB 42233, Sea of Okhotsk, Urup Is., 46°16′7″ N 150°16′5″ E, depth 227–210 m, August 18, 2019, 1 specimen, 29 mm length. MIMB 42234, Sea of Okhotsk, Sakhalin Is., 52°12′4″ N 144°26′8″ E, depth 218–192 m, July 30, 2019, 8 specimens, from 12 to 38 mm length.
External morphology (Fig. 8A–C): Body up to 53 mm long, laterally compressed, elongate. From 6 to 10 oral appendages, 5 rhinophoral sheath appendages, lateral papilla present, 4–6 pairs of dorsolateral appendages. All appendages with 2–3 main stalks and extensive secondary branching, tertiary branches rare if present. Anal opening on right side of body about midway between first and second pairs of dorsolateral processes. Reproductive openings placed laterally near first pair of dorsolateral processes on right side.
Color (Fig. 8A–C): Milky-white to pink, with opaque white pigment on tips of dorsolateral, rhinophore sheaths and oral appendages.
Anatomy: Jaws elongated plates with strong dorsal process and slightly curved masticatory border (Fig. 8D), bearing a single row of denticles (Fig. 8E). Radular formula in studied specimens 33–36 × 11–12.1.11–12 (Fig. 8F). Rachidian tooth triangular, smooth in adults (Fig. 8G). Lateral teeth elongated narrow plates, slightly curved, with large sharp cusp and 3–4 small denticles at its base (Fig. 8H). Cusp length decreases toward outer teeth. Innermost teeth thin, curved. Outermost teeth plate-like, 2–3 in number. Reproductive system triaulic. Large, folded ampulla with broadening mid-length. Prostate with small number of alveolar glands. Distal part of vas deferens short, expanding into a slightly curved penis. Vagina convoluted, gradually narrows into rounded bursa copulatrix. Small receptaculum seminis near distal part of vagina.
Molecular data: Our phylogenetic reconstruction based on the 4 markers (COI, 16S, H3 and 28S) indicates all Dendronotus dalli specimens are clustered together into a single highly supported group (PP = 1; ML = 93) (Fig. 9A). This confirms identity of studied specimens from the Sea of Okhotsk. On the phylogenetic tree, D. dalli specimens display intraspecific structure with three internal clades, which correspond haplogroups on the haplotype network (Fig. 9B). All studied specimens from the Sea of Okhotsk represent a single haplogroup B, which differs by 9 substitutions from members of the haplogroup A (specimens from the shallow Chukchi Sea) and by 7 substitutions from the haplogroup C (specimens from shallow waters near Kamchatka and British Columbia).
Results of molecular analysis of genus Dendronotus. A, molecular phylogenetic hypothesis, maximum likelihood, concatenated dataset of four markers (COI + 16S + H3 + 28S), species-level clades and outgroups are collapsed to a single branch, except target species. Numbers above branches indicate posterior probabilities from Bayesian inference, numbers below branches, bootstrap support from maximum likelihood. B, Dendronotus dalli, COI haplotype network produced with TCS method in PopART. Colors of circles refer to the geographic origin of each haplotype. The relative size of circles is proportional to the number of sequences of that same haplotype. C, Dendronotus patricki, COI haplotype network (above-left) and haplotype network of predicted 18S haplotypes (below-right), produced with TCS method in PopART. Colors of circles refer to the geographic origin of each haplotype. The relative size of circles is proportional to the number of sequences of that same haplotype
Biology: This species was collected from two localities on the upper continental slope near Sakhalin Is. (1) and near Urup Is., a part of the Kuril Islands (2). In both cases bottom sediments were composed of hard sand gravel. The ecological community in the locality near Sakhalin Is. is represented by echinoderms, with Strongylocentrotus droebachiensis (O.F. Müller, 1776), Crossaster papposus (Linnaeus, 1767), Henricia sp., and large unidentified Crinoidea as dominant species. Urup Is. gravel community includes numerous demosponges, hydrozoans and gorgonians, and a species-rich benthic epifauna.
Distribution: This species displays a wide bathymetric and geographical range, including shallow and upper bathyal areas in the Sea of Japan, the Sea of Okhotsk, the Bering Sea, the Northwest Pacific, from Alaska to British Columbia (Ekimova et al., 2015; Korshunova et al., 2020a), and was also recently found in the Arctic region in the Chukchi Sea (Ekimova et al., 2019).
Remarks: Molecular data indicates divergence into three mitochondrial haplogroups corresponding to inhabitants of different bathymetric zones and biogeographical areas (Fig. 9A, B). Same groups form distinct clades on the phylogenetic reconstructions. However, these specimens represent uniform morphological traits and are not so diverged genetically to be considered a cryptic species. This genetic population structure is most likely a result of temporary isolation into subpopulations that occurred in recent past.
-
Dendronotus patricki Stout, N. G. Wilson & Valdés, 2011
-
= Dendronotus bathyvela Martynov, Fujiwara, Tsuchida, R. Nakano, N. Sanamyan, K. Sanamyan, Fletcher & Korshunova, 2020 syn. nov.
Dendronotus patricki Stout et al. 2011, external morphology and SEM micrographs of internal morphology. A, MIMB 42239. B, MIMB 42240. C, MIMB 42241, dorsal side. D, MIMB 42241, ventral side. E, MIMB 42241, left jaw plate. F, MIMB 42241, denticulation of masticatory border. F, MIMB 42241, radula. G, MIMB 42241, rachidian and lateral teeth, posterior radular portion. H, MIMB 42241, rachidian tooth, posterior radular portion. J, MIMB 42241, lateral teeth, posterior radular portion. Scale bars: A–D, 5 mm; E, G, H, 200 µm; F, I, J, 100 µm. Living photos by Anastassya Maiorova
Material examined: MIMB 42239, Sea of Okhotsk, Sakhalin Is., 49°10′4″ N 145°03′1″ E, depth 262–266 m, July 29, 2019, 1 specimen. MIMB 42240, Sea of Okhotsk, Sakhalin Is., 49°10′4″ N 145°03′1″ E, depth 262–266 m, July 29, 2019, 1 specimen. MIMB 42241, Sea of Okhotsk, Sakhalin Is., 52°45′0″ N 144°24′3″ E, depth 261–282 m, July 30, 2019, 1 specimen.
External morphology (Fig. 10A–D): Body broad, up to 41 mm in length. Large oral veil with up to 9 poorly branched oral appendages, 5 rhinophoral sheath appendages, lateral papilla absent, 5 to 6 pairs of dorsolateral appendages. Appendages stout with large primary stalk and extensive secondary and tertiary branching. Anal opening on right side of body about midway between first and second pairs of dorsolateral processes. Reproductive openings placed laterally near first pair of dorsolateral processes on right side.
Color (Fig. 10A–D): Light pink to brownish. Opaque white large dots covering notum, dorsolateral and oral appendages. Digestive gland visible through skin, variable in color in different specimens from light pink to black.
Anatomy: Jaws oval-shaped plates with strong dorsal process and slightly curved masticatory border, bearing at least three rows of small conical denticles (Fig. 10E, F). Radular formula in studied specimens 30 × 12.1.12 (Fig. 10G). Rachidian tooth triangular, with strong conical cusp and 9–14 small denticles on each side (Fig. 10H, I). Lateral teeth triangular, slightly curved, lacking denticles in most cases (Fig. 10J). Innermost lateral teeth subulate, some possess 1–2 reduced denticles. Reproductive system triaulic (Fig. 3C). Ampulla large, muscular, convoluted. Relatively long narrow proximal part of vas deferens lead to prostate composed of 22 small alveolar glands. Distal part of vas deferens muscular and very convoluted, expanding to slightly curved, large penile sheath. Penis elongated. Vagina folded in midline, gradually narrowing into rounded muscular bursa copulatrix. Small receptaculum seminis near distal part of vagina.
Molecular data: Our phylogenetic reconstruction based on the 4 markers (COI, 16S, H3 and 28S) indicates all Dendronotus patricki specimens are clustered together into a single highly supported group (PP = 1; ML = 90) (Fig. 9A). This confirms identity of studied specimens from the Sea of Okhotsk. The COI haplotype network (Fig. 9C) showed that the type specimen D. patricki from the NE Pacific has 12 substitutions from the Northwest Pacific and the Arctic specimens (p-distance 1.7%). The rest specimens are differed by 1–3 substitutions, with no geographical structure. Analysis of nuclear 18S showed its similarity among all studied specimens (from all localities), with several cases of heterozygous sites: in the positions 654 (C/T in WS9105, MIMB 42239, MIMB 42240; C in others), 679–680 (AA/GG in WS9104, WS9105, MIMB 42240; AA/AG in WS9106; AA in others), 685 (C/T in WS9104, WS9105, MIMB 42240; T in others), and 712 (C/T in WS9104-WS9106, MIMB 42240; T in others). This suggests geneflow across population of distant geographical areas.
Biology: This species was collected in two localities, both composed of fine muddy sand. Other benthic species found in the localities are typical members of the muddy sand community, including Nuculana bivalves, Crossaster sea stars and other echinoderms, numerous shrimps and hermit crabs, sedentary annelids, and buccinid gastropods.
Distribution: According to our data, this species has a wide bathymetric and geographic range, encompassing bathyal and abyssal areas of the Arctic (Ekimova et al., 2019), the NE Pacific (Stout et al., 2011), and the Northwest Pacific (Martynov et al., 2020; present study).
Remarks: Dendronotus patricki was originally described from 1819 m in depth, Monterey Canyon, NE Pacific (Stout et al., 2011). It was also reported from the bathyal zone of Arctic waters (Ekimova et al., 2019). The present study shows that its distribution now includes the Northwest Pacific. Recently, the new species Dendronotus bathyvela Martynov, Fujiwara, Tsuchida, R. Nakano, N. Sanamyan, K. Sanamyan, Fletcher & Korshunova, 2020 was described based on morphological data only (Martynov et al., 2020) from the same geographic area (Japan, off the Pacific coast of Northern Honshu, 303–307 m in depth). This species was suggested to have notable differences from Dendronotus patricki original species description (Stout et al., 2011), i.e., in the coloration and the radular characters. The coloration pattern of the type specimen of D. patricki is uniform pinkish body color, while in D. bathyvela it is brown with opaque white dots. Our specimens of D. patricki have pinkish or brown coloration and numerous white dots on the dorsum (Fig. 10A–D). Regarding radular characters, D. bathyvela has up to 14 lateral teeth on both sides and up to 30 denticles on the rachidian tooth. In our specimens of D. patricki, there are up to 28 denticles on each side of the rachidian tooth. It should be also mentioned that the D. patricki paratype has 11 lateral teeth on each side (Stout et al., 2011: Fig. 4A) and up to 30 denticles on the rachidian tooth (Stout et al., 2011: Fig. 4B). Therefore, our data demonstrates D. patricki has a considerable range of morphological variability encompassing the diagnosis of D. bathyvela. Unfortunately, molecular data for D. bathyvela are not available. However, taking into consideration the morphological similarities of our specimens and the type specimens of D. bathyvela, the proximity of collection the localities of D. patricki in the Northwest Pacific to the type locality of D. bathyvela, and the molecular similarities of the studied specimens, we suggest to consider D. bathyvela as a synonym of D. patricki.
-
Dendronotus zakuro Martynov, Fujiwara, Tsuchida, Nakano, K. Sanamyan, N. Sanamyan, Fletcher & Korshunova, 2020
-
(Fig. 11)
Dendronotus zakuro Martynov et al. 2020, external morphology and SEM micrographs of internal morphology. A, MIMB 42244. B, MIMB 42243. C, MIMB 42244, left jaw plate. D, MIMB 42244, denticulation of masticatory border. E, MIMB 42244, posterior radular portion. F, MIMB 42244, middle radular portion. G, MIMB 42244, anterior radular portion. Scale bars: A, B, 5 mm; C, 500 µm; D, 20 µm; F–J, 100 µm. Living photos by Anastassya Maiorova
Material studied: MIMB 42242, Sea of Okhotsk, Shiashkotan Is., 48°48′3″ N 154°17′7″ E, depth 285–304 m, August 12, 2019, 1 specimen. MIMB 42243, locality, depth and date same as MIMB 42242, 1 specimen. MIMB 42244, locality, depth and date same as MIMB 42242, 1 specimen.
External morphology (Fig. 11A, B): Body up to 52 mm long, laterally compressed, elongate. From five to six oral appendages, five rhinophoral sheath appendages, lateral papilla present, 5–6 pairs of dorsolateral appendages. All appendages with 2–3 main stalks and extensive secondary branching, tertiary branches rare if present. Anal opening on right side of body about midway between first and second pairs of dorsolateral processes. Reproductive opening located laterally near first pair of dorsolateral processes on right side.
Color (Fig. 11A, B): Background color varies from translucent milky-white with an orange tinge to variegated orange-brown. In pale specimens, body pigmentation formed by narrow brown lines on dorsolateral sides between appendages of each row and few white opaque dots. In variegated specimens these lines and dots well visible. Oral veil, rhinophoral and dorsolateral appendage tips orange to brown sometimes with opaque white pigment inside branches.
Anatomy: Jaws elongated plates with strong dorsal process and slightly curved masticatory border (Fig. 11C), bearing a single row of denticles (Fig. 11D). Radular formula in studied specimens 29 × 8–10.1.8–10 (Fig. 11E, F). Rachidian tooth smooth. Lateral teeth elongate, narrow, slightly curved with up to 12 denticles. Inner- and outermost lateral teeth subulate. Reproductive system triaulic. Ampulla relatively small, bent mid-length. Prostate with up to 25 alveoli. Distal vas deferens long and winding, narrowing slightly into curved ejaculatory portion. Wide vagina gradually narrows into muscular bursa copulatrix, receptaculum seminis small, indistinct.
Molecular data: Our phylogenetic reconstruction based on 4 markers (COI, 16S, H3, and 28S) clusters all Dendronotus zakuro specimens together into a single highly supported group (PP = 1; ML = 96) (Fig. 9A). This confirms the identity of studied specimens from deep waters. Intraspecific p-distance of COI marker is 0.9%.
Biology: This species was found in a species-rich community of gravel sand, composed of numerous echinoderms, annelids, sponges, and hydrozoans.
Distribution: Northwest Pacific: Japan, Kamchatka, up to 20 m in depth (Martynov et al., 2020); the Kuril islands, 285–300 m in depth (present study).
Remarks: The species Dendronotus zakuro was recently described from the subtidal zone in two distant geographic localities (Japan and Kamchatka). Our data provide a link between these localities and expand the known bathymetric range of this species to the deep-sea shelf and upper bathyal areas. Our specimens show a different coloration pattern from the type specimens: the latter have bright red to reddish-brown coloration, while the Kuril island specimens are much paler (Fig. 11A, B). This may cause identification difficulties as the same variety in variegated coloration is characteristic of D. kamchaticus Ekimova, Korshunova, Schepetov, Neretina, Sanamyan & Martynov, 2015 (Ekimova et al., 2016a; Korshunova et al., 2016), which also has a similar radular morphology.
-
Dendronotus kurilensis sp. nov.
-
urn:lsid:zoobank.org:act:F8048B84-91A5-4717-9A56-74D9BF185BCF
Dendronotus kurilensis sp. nov., external morphology and SEM micrographs of internal morphology. A, holotype MIMB 42237, dorsal view. B, MIMB 42237, lateral view from left. C, holotype MIMB 42237, lateral view from right. D, paratypes MIMB 42235. E, paratype MIMB 42238. C, MIMB 42235b, right jaw plate. D, MIMB 42235b, denticulation of masticatory border. E, MIMB 42235d, denticulation of masticatory border. F, MIMB 42235b, posterior radular portion. G, MIMB 42235b, rachidian tooth. G, MIMB 42235b, lateral teeth. Scale bars: A–E, 5 mm; F, 200 µm; G, H, 25 µm; I, 50 µm; K, L, 20 µm. Living photos by Anastassya Maiorova
Type material: Holotype: MIMB 42237, Sea of Okhotsk, Urup Is., 46°15′9″ N, 150°15′4″ E, depth 450–460 m, July 05, 2019, R/V Akademik Oparin. Paratypes: MIMB 42236, Sea of Okhotsk, Urup Is., 46°16′7″ N 150°16′5″ E, depth 227–210 m, August 18, 2019. MIMB 42235, Sea of Okhotsk, Urup Is., 46°16′7″ N 150°16′5″ E, depth 227–210 m, August 18, 2019, 4 specimens. MIMB 42238, Sea of Okhotsk, Iturup Is., 45°44′6″ N, 148°34′0″ E, depth 264–270 m, July 07, 2019, R/V Akademik Oparin, 1 specimen.
Etymology: After “Kuril Islands”, the type locality of this species.
Type locality: Sea of Okhotsk, Urup Is., 46°15′9″ N, 150°15′4″ E, depth 450–460 m.
External morphology (Fig. 12A–E): Body up to 30 mm long, laterally compressed, elongate. Oral veil large, with up to six simple conical appendages. Four branched rhinophoral sheath appendages of same size, lateral papilla absent, rhinophores perfoliated with up to 12 lamellae. Up to seven pairs of stout dorsolateral appendages with simple secondary branching. Anal opening on right side of body about midway between first and second pairs of dorsolateral processes. Reproductive openings located laterally near first pair of dorsolateral processes on right side.
Color (Fig. 12A–E): Uniform milky pink to peach. Anterior part of body, dorsolateral appendages and surface between them with bright orange pigmentation.
Anatomy: Jaws elongated plates with strong dorsal process and slightly curved masticatory border (Fig. 12F), bearing row of denticles and two rows of smaller denticles underneath (Fig. 12G, E). Radular formula in studied specimens 29 × 8–10.1.8–10. Rachidian tooth with up to 10 large denticles on each side of reduced cusp, with deep furrows (Fig. 12I, K). Lateral teeth slightly curved with sharp cusp and up to 5 denticles (Fig. 12L). Inner- and outermost laterals subulate. Reproductive system triaulic (Fig. 3D). Ampulla relatively small, convoluted. Prostate with up to 10 alveoli. Distal vas deferens winding, moderate in length, slightly narrowing into curved ejaculatory portion. Wide vagina gradually narrowing into muscular bursa copulatrix, receptaculum seminis small, indistinct.
Molecular data: On our phylogenetic reconstruction based on 4 markers (COI, 16S, H3 and 28S), Dendronotus kurilensis sp. nov. specimens are clustered together into a single highly supported group (PP = 1; ML = 96) (Fig. 9A). This clade is sister to NE Pacific Dendronotus claguei Valdés, Lundsten & N. G. Wilson, 2018 (LACM3554) (PP = 1; ML = 87). Although only H3 data are available for the latter species, it differs from D. kurilensis sp. nov. by four substitutions. Since H3 is a slowly evolving molecular marker, which is commonly identical in sister Dendronotus species (Ekimova et al., 2015, 2016a, b, 2019), the four substitutions seem to be enough molecular divergence to support D. kurilensis sp. nov. distinctness at molecular level. The clade including D. kurilensis sp. nov. and D. claguei is sister to the Dendronotus robustus-Dendronotus patricki clade in the phylogenetic tree.
Biology: This species was found in two localities represented by similar gravel sand communities, with numerous sponges and hydrozoans, annelids, and echinoderms.
Distribution: Known only from the southern Kuril Islands.
Remarks: This species is a representative of a recently discovered clade of deep-sea dendronotids with narrow body and the Dendronotus frondosus radular type (Ekimova et al., 2019; Valdés et al., 2018). It shares many morphological similarities with another member of this group, Dendronotus claguei including body shape, absence of lateral rhinophoral sheath papillae, radular configuration, and details of the reproductive system. However, the two species differ substantially in external appearance: D. claguei possess up to six pairs of very simple conical appendages with almost no branching, asymmetric rhinophoral sheath appendages, longer oral veil appendages, while only four pairs of secondary branched appendages were found in D. kurilensis sp. nov., and rhinophoral sheath appendages are equal in this species. Finally, D. claguei coloration is transparent white instead of the milky-pink to peach coloration of D. kurilensis sp. nov. The two species are also distinct genetically, with 4 substitutions in H3 marker. Other species of the family Dendronotidae do not possess similar combination of external and internal characters. The sister clade of this group is the “robustus” species group, represented by wide-bodied species with unique radular morphology (Ekimova et al., 2019; Korshunova et al., 2020b).
-
Superfamily Fionoidea Gray, 1857
-
Family Fionidae Gray, 1857 s.l.
-
Genus Cuthona Alder & Hancock, 1855
-
Cuthona sp.
-
(Fig. 13)
Material studied: MIMB 42232, Sea of Okhotsk, Shiashkotan Is., 48°48′3″ N 154°17′7″ E, depth 285–304 m, August 12, 2019, 1 specimen.
External morphology (Fig. 13A): Body 6 mm in length, elongate, relatively wide, narrowing posteriorly. Numerous elongate, dorsolateral cerata in dense indistinct rows. Oral tentacles absent, probably due to traumatic removal during sampling. Rhinophores stout, smooth. Reproductive opening placed laterally on anterior body part. Anal opening lateral, posterior to pericardium.
Color (Fig. 13A): Homogenous milky-white. Anterior digestive organs visible through skin as pink mass. Cnidosac areas marked with opaque white dots.
Anatomy: Buccal mass large. Jaws triangular elongate plates, masticatory border with single row of denticles. Radula uniseriate (Fig. 13B, C), 18 rows. Rachidian tooth broad, with non-compressed large conical cusp and 5–6 denticles on each side.
Animal was not fully mature, and its poor preservation state did not allow to study the reproductive system in details.
Molecular data (Fig. 14A, B): According to the molecular phylogenetic analysis of three markers (COI, 16S, H3) our specimen form a singleton, which is sister to Cuthona nana (Alder & Hancock, 1842), C. hermitophila Martynov, Sanamyan & Korshunova, 2015 and C. divae (Er. Marcus, 1961) clades and Bohuslania matsmichaeli Korshunova, Lundin, Malmberg, Picton & Martynov, 2018 (PP = 1; ML = 100) (Fig. 14B).
Results of molecular analysis of different aeolid groups. A, genus Cuthona, COI haplotype network produced with TCS method in PopART. Colors of circles refer to the geographic origin of each haplotype. The relative size of circles is proportional to the number of sequences of that same haplotype. B, molecular phylogenetic hypothesis of genus Cuthona, maximum likelihood, concatenated dataset of three markers (COI + 16S + H3), species-level clades and outgroups are collapsed to a single branch, except Cuthona and Bohuslania species. Numbers above branches indicate posterior probabilities from Bayesian inference, numbers below branches, bootstrap support from maximum likelihood. C, molecular phylogenetic hypothesis of genus Ziminella, Bayesian inference, concatenated dataset of four markers (COI + 16S + H3 + 28S), species-level clades and outgroups are collapsed to a single branch, except target species. Numbers above branches indicate posterior probabilities from Bayesian inference, numbers below branches, bootstrap support from maximum likelihood. C, molecular phylogenetic hypothesis of genus Zeusia, maximum likelihood, concatenated dataset of three markers (COI + 16S + H3), species-level clades and outgroups are collapsed to a single branch, except target species. Numbers above branches indicate posterior probabilities from Bayesian inference, numbers below branches, bootstrap support from maximum likelihood
Biology: This species was found in a species-rich community of gravel sand, including numerous echinoderms, annelids, sponges, and hydrozoans.
Distribution: Known only from the Northern Kuril Islands, deep-water area near Shiashkotan Is.
Remarks: This specimen was severely damaged during collection, which does not allow us to provide a full description of its external and internal characters. This also complicates a comprehensive comparison of morphological characters across our specimen and known Cuthona species. Nevertheless, Cuthona sp. fits well to the diagnosis of the genus Cuthona: wide body, cerata in rows non-elevated and numerous, rhinophores smooth, rachidian tooth with strong cusp (Fig. 13). The coloration and overall appearance resemble the morphology of Cuthona methana Valdés, Lundsten & N. G. Wilson, 2018, which was described recently from methane seeps in deep sea off California. The rachidian tooth of C. methana also possesses a strong cusp but has less denticles. Therefore, we suggest our Cuthona sp. may represent a sister species to C. methana. Unfortunately, no molecular data is available for the latter species.
-
Family Paracoryphellidae M. C. Miller, 1971
-
Genus Ziminella Korshunova, Martynov, Bakken, Evertsen, Fletcher, Mudianta, Saito, Lundin, Schrödl & Picton, 2017
-
Ziminella vrijenhoeki Valdés, Lundsten & N. G. Wilson, 2018
-
(Fig. 15)
Ziminella vrijenhoeki Valdés et al. 2018, MIMB 42255, external morphology and SEM micrographs of internal morphology. A, dorsal view. B, ventral view. C, living specimen in natural environment. D, jaw masticatory border. E, details of denticulation of masticatory border. F, radula. G, middle radular portion. H, rachidian tooth. I, lateral tooth. Scale bars: A, B, 5 mm. D, I, 20 µm; E, 10 µm; F, 500 µm; G, H, 50 µm. Living photos by Nadezhda Sanamyan and team of ROV “Komanch”
Material studied: MIMB 42255, Bering Sea, 60°50′3.5″ N, 174°22′21.4″ E, depth 660 m, July 03, 2018, R/V Akademik Lavrentyev, 1 specimen.
External morphology (Fig. 15A–C): Body length 21 mm. Body elongate, broad, with well-developed continuous notal edge. Cerata in dense indistinct rows. Oral tentacles short, wide. Foot corners short and wide. Rhinophores conical, smooth. Anal opening dorso-lateral, posterior to pericardium. Reproductive opening under notal edge on right anterior part of body.
Color (Fig. 15A–C): Dorsal side of body, head and oral tentacles light orange. Rhinophores intense orange. Digestive gland diverticula grayish orange to light brown. Cnidosac region dotted by white pigment. Dorsal cerata with orange subapical rings, lateral cerata lack this pigmentation.
Anatomy: Buccal mass large, muscular. Jaws elongate triangular plates, with up to 15 rows of rounded denticles on masticatory border (Fig. 15D, E). Radular formula 25 × 1.1.1 (Fig. 15F). Rachidian tooth with large central conical cusp and up to eight denticles on each side (Fig. 15G, H). Lateral teeth triangular, narrow, with pointed cusp and up to 14 denticles on inner side (Fig. 15I). Reproductive system diaulic. Ampulla very long, convoluted, forming loops. Prostatic vas deferens long and convoluted. Penis slightly curved, elongate. Receptaculum seminis oval.
Molecular data: On our phylogenetic reconstruction of four markers (COI, 16S, H3, 28S) here studied specimen forms a single clade with the type specimen of Z. vrijenhoeki (PP = 1; ML = 100) (Fig. 14B). This species is sister to the rest of Ziminella species in the ML analysis (ML = 100), but shows unresolved relationships with both Ziminella and Chlamylla in the BI analysis.
Biology: This species was found in seep communities with Calyptogena as a dominant species. Local seepage occurs through cracks in carbonate plates; such places are marked with thick bacterial mats and Calyptogena settlements. In the vicinity, several large buccinid gastropods, pogonophorans, and terebellid tubeworms were noted and various small ophiuroids are also an abundant component of the community. Cnidarian fauna is represented by abundant soft coral Caryophyllia and rare Hormatiidae anemones.
Distribution: Known from the deep sea off California (Valdés et al., 2018) and from the Bering Sea (present study).
-
Superfamily Aeolidioidea Gray, 1827
-
Family Aeolidiidae Gray, 1827
-
Genus Zeusia Korshunova, Zimina & Martynov, 2017
-
Zeusia herculea (Bergh, 1894)
-
= Aeolidia grandis Volodchenko, 1941 syn. nov.
-
(Fig. 16)
Zeusia herculea (Bergh, 1894), MIMB 42254, external morphology and SEM micrographs of internal morphology. A, dorsal view. B, ventral view. C, living specimen in natural environment. D, posterior radular portion. E, anterior radular portion. F, G, details of denticulation on rachidian tooth. Scale bars: A, B, 5 mm; D, 500 µm; E, 200 µm; F, 100 µm; G, 50 µm. Living photos by Nadezhda Sanamyan and team of ROV “Komanch”
Material studied: MIMB 42254, Bering Sea, 61°10′40.1″ N, 174°52′14.9″ E, depth 536 m, June 28, 2018, R/V Akademik Lavrentyev, 1 specimen.
External morphology (Fig. 16A–C): Body length 29 mm. Body wide, possessing numerous elongate, flattened cerata in distinct dense rows. Smooth conical oral tentacles. Rhinophores slightly tuberculated. Anus pleuroproctic. Genital openings on right side, at 1/3 of body length.
Color (Fig. 16A–C): Body, head, oral tentacles, and foot bright orange. Rhinophores intense orange, with lighter tubercles. Cerata semitransparent, with brownish digestive gland seen through body wall and numerous white dots. Cnidosac region dotted by white pigment, and wide orange ring below. Lateral cerata lack orange pigmentation.
Anatomy: Buccal mass large, muscular. Jaws elongate oval, lacking denticles on masticatory border. Radular formula 23 × 0.1.0 (Fig. 16D, E). Rachidian tooth wide, lacking central cusp, with up to 25 long sharp denticles on each side (Fig. 16F, G). Reproductive system diaulic. Ampulla long, convoluted. Prostatic vas deferens long and winding, forming several loops. Penile sheath broad. Receptaculum seminis oval.
Molecular data: On the three-marker phylogenetic tree (COI, 16S, H3) our specimen forms a single clade with Z. herculea (PP = 1; ML = 100), collected in the NE Pacific, near the type locality. Zeusia herculea clade is sister to Z. hyperborea Korshunova, Zimina & Martynov, 2017 (PP = 1; ML = 100). Within the family Aeolidiidae, the genus Aeolidia is sister to Zeusia (PP = 1; ML = 100).
Biology: This specimen was found in an ophiuroid community, far from methane seeps. Crustacean benthic fauna is a common component, with many hermit crabs, shrimps, and other decapods found in the area. Large buccinid gastropods, Myxoderma sea stars and Achorax bivalves also occur. Cnidarian fauna is represented by numerous species of Sagartiidae, Liponema and rarely large anemones.
Distribution: North Pacific deep-sea areas from California to the Sea of Okhotsk (Korshunova et al., 2017b; Valdés et al., 2018; Volodchenko, 1941; present study).
Discussion
Taxonomy
Due to their scarcity and low information baseline, studies of deep-sea biodiversity often significantly improve existing taxonomical and phylogenetic knowledge (Borisanova et al., 2018; Cannon et al., 2009; Chaban et al., 2019b; Kano, 2008; Kano et al., 2016; Leduc et al., 2018; and many others). In this study, we described two new species, proposed revisions for several groups, and confirmed previously suggested taxonomic changes based on new molecular data.
The new species Bathydoris antoni sp. nov. is described from the Sea of Okhotsk. This is the first member of the genus Bathydoris recorded for Russian fauna and only the second Bathydoris species described from the Northwest Pacific in addition to B. japonensis (see Hamatani & Kubodera, 2010). Another new species of the genus Dendronotus is described, a group for which an astonishing cryptic diversity in the boreal and Arctic regions has been revealed during the last decade (Ekimova et al., 2015, 2016a, b, 2019; Korshunova et al., 2016, 2017c, 2020a; Martynov et al., 2015, 2020; Stout et al., 2011). Dendronotus kurilensis sp. nov. together with D. claguei represent a particularly interesting lineage of narrow-bodied deep-sea Dendronotus, sister to the wide-bodied and deep-sea Dendronotus species of the so-called “robustus” species group (Ekimova et al., 2019). Both D. kurilensis sp. nov. and D. claugei display paedomorphic characteristics in their radular morphology (Ekimova & Malakhov, 2016; Ekimova et al., 2019; Korshunova et al., 2020b), i.e., possess a highly denticulated rachidian tooth of the “frondosus” radular type (Fig. 12F–H) that matches the rachidian tooth morphology of juvenile specimens in the “robustus” species group (Ekimova et al., 2019). It was suggested that heterochrony in Dendronotus may have evolved following the environmental changes and climatic fluctuation in the shallow North Pacific during the Miocene and Pliocene (Ekimova et al., 2019); however, data on those changes in the deep waters are not well documented (Briggs, 1995).
We also provide evidence of two potentially new nudibranch species of the genera Cadlina and Cuthona. The taxonomic composition of both genera has recently undergone a series of revisions based on integrative analysis (Cadlina: Do et al., 2020; Korshunova et al., 2020b. Cuthona: Chichvarkhin et al., 2016; Cella et al., 2016; Korshunova et al., 2017d, 2018) which resulted in the description of several new species in the Northwest Pacific: Cadlina paninae, C. umiushi, C. olgae, C. koreana, C. kamchatica, and Cuthona hermitophila. All this diversity was found in shallow waters, and our results suggest that several new taxa may occur in the deeper parts of the Northwest Pacific as well. A specimen of Cuthona sp. also represents an important finding, as our phylogenetic analysis based on three markers (COI, 16S, and H3) shows that it is a sister to a clade comprising the shallow-water Cuthona nana species complex and the brackish-water Bohuslania matsmichaeli. This unexpected result raises questions on the generic placement of B. matsmichaeli. Also, data from the COI haplotypes study (Fig. 14A) shows that at least the Northwest Pacific Cuthona hermitophila is conspecific to North Atlantic C. nana, which agrees with previous research (Cella et al., 2016; Chichvarkhin et al., 2016) and suggests that species diversity within the genus Cuthona may be overestimated. On the other hand, our internal morphology data of Cuthona sp. is quite limited, and another deep-water species, Cuthona methana, lacks molecular data and a description of its reproductive system. Filling this knowledge gap is a prospect for further studies clarifying the taxonomic status of the genera and species included in this group.
This paper is the first to report molecular data for Northwest Pacific specimens of Colga pacifica, Dendronotus patricki, Ziminella vrijenhoeki, and Zeusia herculea. The Northwest Pacific species Dendronotus patricki is represented by several color morphs (Fig. 10A–D), which led to the description of a new species in this area, Dendronotus bathyvela, based only on morphological data (Martynov et al., 2020). Here, we show that contemporary geneflow probably exists among distant populations of D. patricki in the Arctic and both sides of the North Pacific (Fig. 9C), and that these populations display identical internal morphological characters which allow us to regard D. bathyvela as a junior synonym of D. patricki. We also confirm the species identity of Tritonia psoloides and its distinctness from the sympatric T. tetraquetra. Our T. psoloides specimen was collected near the type locality of this species in the Bering Sea (Fig. 1) and matches the external morphology of the type material perfectly (Fig. 7C). Molecular data for the Northwest Pacific Zeusia herculea obtained in this study (Fig. 14D) supports a recent suggestion that this species has a transpacific range (Korshunova et al., 2017d) and thus confirms that the morphologically similar Northwest Pacific Aeolidia grandis should be regarded as a junior synonym of Z. herculea.
North Pacific deep-water nudibranch distribution
The distribution patterns of deep-water benthic organisms are understudied — sampling from such communities is limited both in size and frequency. Many species have only been recorded a few times (or even once, at first discovery), hence rendering even a preliminary estimation of their habitat area impossible. Biodiversity estimates indicate that with current sampling coverage, many species are erroneously considered endemic as a result of undersampling and low population density (Tittensor et al., 2010). Recovered material is often damaged and thus morphological characteristics are distorted. This can pose a serious problem for the study of species with high levels of morphological plasticity. The factors stated above, coupled with cases of unique deep-water vent communities, create a biased impression of obligate deep-water fauna as being extremely fragmented (McClain & Hardy, 2010). However, as knowledge on deep water benthic communities expands, we can construct an increasingly nuanced picture. While data is still insufficient in many cases, our findings show that many nudibranch species are widely distributed in deep bathymetric ranges of the North Pacific and indicate that several types of fauna connections exist between populations from different depths and geographic regions. These include species with extremely wide bathymetric ranges. Such a distribution pattern should be expected in species with wide environmental tolerance that allows them to expand into greater depths. For example, some species inhabiting upper subtidal depths can be found much deeper, when biotopes and conditions fit their preferences. In our study, this seems to be the case for Colga pacifica, Tritonia tetraquetra, Dendronotus dalli, and D. zakuro. Colga pacifica was found in a rich community with many sponges and echinoderms on gravel and sand at 285–304 m. This species was previously reported in many northwestern Pacific communities at ranges from 14 to 1000 m (Martynov & Baranets, 2002; Martynov et al., 2016). Both Dendronotus species were found deeper than at 200 m with D. zakuro at almost 300 m and in the same community as C. pacifica, though they are most often found in the 10–20 m range. These records are on gravel-sand as a part of species-rich communities with prominent and diverse benthic epifauna. Tritonia tetraquetra also occupies an extremely wide range of depths and can be found in both rocky communities and on soft silty bottoms surrounding methane seeps.
The second type of connectivity involves presumably new species that are sister to described coastal species from the same regions. This could be the result of depth range expansions followed by speciation, as the result of either (1) the deep-water populations specialization and divergence from their shallow water sister taxa in a parapatric model or (2) total separation from the original population and allopatric divergence. In the lineages from the Northwest Pacific examined here, we observe these patterns in species of the genus Cadlina, which is represented by a number of phylogenetically close shallow water species (Korshunova et al., 2020a). Cadlina sp. is genetically similar to C. laevis, C. paninae, and C. kamchatica (Fig. 4C); the latter two inhabit shallow bottoms of the same region, but our specimen shows several molecular differences from these species. The same pattern is found on an intraspecific level within Dendronotus dalli, where shallow- and deep-water specimens represent two haplogroups separated by seven substitutions (Fig. 9D).
The third and probably most interesting faunal connection is between deep water communities on each side of the North Pacific. We found several species from different groups, which have been reported or initially described from deep water habitats near the California coast. First and most abundant is Tritonia tetraquetra, already mentioned above. It is not only found on the deep shelf, where it is abundant in rich rocky and sandy communities, but it is also quite common on silt near methane seeps. Ziminella vrijenhoeki was initially described from silt bottoms surrounding whale carcass communities (Valdés et al., 2018); however, our data suggests they are not strictly tied to whale fall sites. In our study, it was found in the Bering Sea in seep communities with prominent bacterial mats at 660 m in depth. The aeolid Zeusia herculea was described from the deep sea off California (Bergh, 1894) and later found in the same locality on whale carcass communities, but it is not exclusive to them (Valdés et al., 2018). In the North Pacific, this species is known from the Bering Sea (present study; Korshunova et al., 2017b) and the Sea of Okhotsk (Korshunova et al., 2017b; Volodchenko, 1941). In our study, it was found at a 536-m depth far from methane seeps on muddy sand in ophiuroid communities with many decapods. Finally, Dendronotus patricki, described from the deep sea of California, was found at a depth range 261–282 m at two stations. Despite the fact that these sites are relatively shallow, they represent quite different substrates (muddy sand) from common communities of the deep Sea of Okhotsk shelf.
The cases discussed above clearly show close ties exist not only between shallow coastal nudibranch faunas and their continental slope counterparts, but also between communities on both sides of North Pacific, suggesting that they are connected by gene flow and that there is an ongoing exchange between these two regions. We found two general types of deep shelf and bathyal communities in the Northwest Pacific, each characterized by a certain type of fauna and a connectivity with different bathymetric and geographic areas. The first one is an extension of rocky and gravel communities characteristic of the North Pacific. These communities start from upper subtidal depths and continue down to steep continental slopes down to the point where sediments accumulate. Such biotopes are characterized by filter-feeding organisms and associated epifauna, where typical subtidal nudibranch species can be found to depths up to 400 m (i.e., Dendronotus dalli, D. zakuro, Colga pacifica, Tritonia tetraquetra). The second type of biotopes is represented by communities existing on muddy sand and silty substrates. The environmental parameters of these biotopes are quite similar on both sides of the North Pacific and were therefore assigned to a single bathyal province of the North Pacific (Watling et al., 2013) based on water mass characteristics (temperature and salinity) and particulate organic flux to the seafloor. We found a significant overlap between the nudibranch fauna from these biotopes on eastern and western parts of the North Pacific (i.e., Tritonia tetraquetra, Dendronotus patricki, Ziminella vrijenhoeki and Zeusia herculea), which supports the prediction of the biogeographic province. Our data shows that nudibranchs follow different pathways, which predicted and found in other groups. Ziminella vrjenhoecki and Zeusia herculea recorded from whale carcass sites in NE Pacific were found on soft grounds (Ziminella for the first time). This agrees with the generally accepted hypothesis, which wood and carcass sites serve as evolutionary launchpads for the transition from hard ground slope communities to silt planes and seeps (Shank et al., 1998; Kiel & Goedert, 2006).
Conclusions
Our study confirms the expected undersampling and undescribed diversity of the North Pacific deep-water nudibranch fauna and their association to predicted bathyal provinces. Novel data on distribution of several species reveals connections between the Northeast and Northwest Pacific, and between different types of communities. Further study of bathyal molluscs in this region is needed, as it will enhance our understanding of both deep-water communities and ways of their formation.
Availability of data and material
All studied specimens were deposited to the official collection of the National Scientific Center of Marine Biology, Far Eastern Branch RAS. All newly obtained sequences were submitted to GenBank. Unedited trees are provided as Supplementary material.
Code availability
Not applicable.
References
Afonso, P., McGinty, N., Graça, G., Fontes, J., Inácio, M., Totland, A., & Menezes, G. (2014). Vertical migrations of a deep-sea fish and its prey. PLoS ONE, 9(5), e97884. https://doi.org/10.1371/journal.pone.0097884
Altschul, S. F., Gish, W., Miller, W., Myers, E. W., & Lipman, D. J. (1990). Basic local alignment search tool. Journal of Molecular Biology, 215(3), 403–410.
Baco, A. R., Smith, C. R., Peek, A. S., Roderick, G. K., & Vrijenhoek, R. C. (1999). The phylogenetic relationships of whale-fall vesicomyid clams based on mitochondrial COI DNA sequences. Marine Ecology Progress Series, 182, 137–147. https://doi.org/10.3354/meps182137
Bergh, R. (1894). Die Opisthobranchien. Reports on the dredging operations off the west coast of Central America to the Galapagos, to the west coast of Mexico, and in the Gulf of California, in charge of Alexander Agassiz, carried on by the U. S. Fish Commission steamer “Albatross”, during 1891, Lieut. Commander Z.L. Tanner, U.S.N., commanding. Bulletin of the Museum of Comparative Zoology, 25 (10), 125–233.
Borisanova, A. O., Chernyshev, A. V., & Ekimova, I. A. (2018). Deep-sea Entoprocta from the Sea of Okhotsk and the adjacent open Pacific abyssal area: New species and new taxa of host animals. Deep Sea Research Part II: Topical Studies in Oceanography, 154, 87–98.
Brandt, A., Gooday, A. J., Brandao, S. N., Brix, S., Brökeland, W., Cedhagen, T., Choudhury, M., Cornelius, N., Danis, B., De Mesel, I., Diaz, R. J., Gillan, D. C., Ebbe, B., Howe, J. A., Janussen, D., Kaiser, S., Linse, K., Malyutina, M., Pawlowski, J., Raupach, M. & Vanreusel, A. (2007a). First insights into the biodiversity and biogeography of the Southern Ocean deep sea. Nature, 447(7142), 307–311.
Brandt, A., Brix, S., Brökeland, W., Choudhury, M., Kaiser, S., & Malyutina, M. (2007b). Deep-sea isopod biodiversity, abundance, and endemism in the Atlantic sector of the Southern Ocean—results from the ANDEEP I-III expeditions. Deep Sea Research Part II: Topical Studies in Oceanography, 54(16–17), 1760–1775.
Brandt, A., & Malyutina, M. V. (2015). The German-Russian deep-sea expedition KuramBio (Kurile Kamchatka biodiversity studies) on board of the RV Sonne in 2012 following the footsteps of the legendary expeditions with RV Vityaz. Deep Sea Research Part II: Topical Studies in Oceanography, 111, 1–9.
Brandt, A., Elsner, N. O., Malyutina, M. V., Brenke, N., Golovan, O. A., Lavrenteva, A. V., & Riehl, T. (2015). Abyssal macrofauna of the Kuril-Kamchatka Trench area (Northwest Pacific) collected by means of a camera–epibenthic sledge. Deep Sea Research Part II: Topical Studies in Oceanography, 111, 175–187.
Brandt, A., Alalykina, I., Brix, S., Brenke, N., Błażewicz, M., Golovan, O. A., & Lins, L. (2019). Depth zonation of Northwest Pacific deep-sea macrofauna. Progress in Oceanography, 176, 102–131.
Briggs, J. C. (1995). Global biogeography. Elsevier.
Briggs, J. C. (2003). Marine centers of origin as evolutionary engines. Journal of Biogeography, 30, 1–18.
Cannon, J. T., Rychel, A. L., Eccleston, H., Halanych, K. M., & Swalla, B. J. (2009). Molecular phylogeny of hemichordata, with updated status of deep-sea enteropneusts. Molecular Phylogenetics and Evolution, 52(1), 17–24.
Cella, K., Carmona, L., Ekimova, I., Chichvarkhin, A., Schepetov, D., & Gosliner, T. M. (2016). A radical solution: The phylogeny of the nudibranch family Fionidae. PloS ONE, 11(12), e0167800.
Chaban, E. M., Ekimova, I. A., Schepetov, D. M., & Chernyshev, A. V. (2019a). Meloscaphander grandis (Heterobranchia: Cephalaspidea), a deep-water species from the North Pacific: Redescription and taxonomic remarks. Zootaxa, 4646(2), 385–400.
Chaban, E. M., Ekimova, I. A., Schepetov, D. M., Kohnert, P. C., Schrödl, M., & Chernyshev, A. V. (2019b). Euopisthobranch mollusks of the order Cephalaspidea (Gastropoda: Heterobranchia) of the Kuril-Kamchatka Trench and the adjacent Pacific abyssal plain with descriptions of three new species of the genus Spiraphiline (Philinidae). Progress in Oceanography, 178, 102–185.
Chichvarkhin, A. (2016). Shallow water sea slugs (Gastropoda: Heterobranchia) from the northwestern coast of the Sea of Japan, north of Peter the Great Bay, Russia. PeerJ, 4, e2774.
Chichvarkhin, A. Y., Ekimova, I. A., Egorova, E. L., & Chichvarkhina, O. V. (2016). Identity of nudibranch mollusk of the genus Cuthona (Gastropoda: Tergipedidae), associated with hermit crabs in the Sea of Japan. Russian Journal of Marine Biology, 42(6), 449–457.
Clement, M., Snell, Q., Walker, P., Posada, D., & Crandall, K. (2002). TCS: Estimating gene genealogies. Parallel and Distributed Processing Symposium, International, 3, 0184–0184.
Colgan, D. J., McLauchlan, A., Wilson, G. D. F., Livingston, S. P., Edgecombe, G. D., Macaranas, J., Cassis, G., & Gray, M. R. (1998). Histone H3 and U2 snRNA DNA sequences and arthropod molecular evolution. Australian Journal of Zoology, 46(5), 419–437.
Darriba, D., Posada, D., Kozlov, A.M., Stamatakis, A., Morel, B., & Flouri, T. (2020). ModelTest-NG: A new and scalable tool for the selection of DNA and protein evolutionary models. Molecular Biology and Evolution, 37(1), 291–294. https://doi.org/10.1093/molbev/msz189
Do, T. D., Jung, D. W., Kil, H. J., & Kim, C. B. (2020). A report of a new species and new record of Cadlina (Nudibranchia, Cadlinidae) from South Korea. Zookeys, 996, 1–18. https://doi.org/10.3897/zookeys.996.54602
Edgar, R. C. (2004). MUSCLE: Multiple sequence alignment with high accuracy and high throughput. Nucleic Acids Research, 32(5), 1792–1797.
Ekimova, I., Korshunova, T., Schepetov, D., Neretina, T., Sanamyan, N., & Martynov, A. (2015). Integrative systematics of northern and Arctic nudibranchs of the genus Dendronotus (Mollusca, Gastropoda), with descriptions of three new species. Zoological Journal of the Linnean Society, 173(4), 841–886.
Ekimova, I. A., Schepetov, D. M., Chichvarkhina, O. V., & Chichvarkhin, A. Y. (2016a). Nudibranch molluscs of the genus Dendronotus Alder et Hancock, 1845 (Heterobranchia: Dendronotina) from Northwestern Sea of Japan with description of a new species. Invertebrate Zoology, 13(1), 15–42.
Ekimova, I., Valdés, Á., Schepetov, D., & Chichvarkhin, A. (2016b). Was Gordon Robilliard right? Integrative systematics suggest that Dendronotus diversicolor (multicolor frond-aeolis) is a valid species. Canadian Journal of Zoology, 94(11), 793–799.
Ekimova, I. A., & Malakhov, V. V. (2016). Progenesis in the evolution of the nudibranch mollusks genus Dendronotus (Gastropoda: Nudibranchia). Doklady Biological Sciences, 467(1), 86–88.
Ekimova, I., Valdés, Á., Chichvarkhin, A., Antokhina, T., Lindsay, T., & Schepetov, D. (2019). Diet-driven ecological radiation and allopatric speciation result in high species diversity in a temperate-cold water marine genus Dendronotus (Gastropoda: Nudibranchia). Molecular Phylogenetics and Evolution, 141, 106609. https://doi.org/10.1016/j.ympev.2019.106609
Folmer, O., Black, M., Hoeh, W., Lutz, R., & Vrijenhoek, R. (1994). DNA primers for amplification of mitochondrial cytochrome c oxidase subunit I from diverse metazoan invertebrates. Molecular Marine Biology and Biotechnology, 3(5), 294–299.
Flouri, T., Izquierdo-Carrasco, F., Darriba, D., Aberer, A. J., Nguyen, L. T., Minh, B. Q., von Haeseler, A., & Stamatakis, A. (2014). The Phylogenetic Likelihood Library. Systematic Biology, 64(2), 356–362. https://doi.org/10.1093/sysbio/syu084
Giribet, G., Carranza, S., Baguñà, J., Riutort, M., & Ribera, C. (1996). First molecular evidence for the existence of a Tardigrada + Arthropoda clade. Molecular Biology and Evolution, 13(1), 76–84.
Gordeeva, N. V. (2014). Phylogeography, genetic isolation, and migration of deep-sea fishes in the South Atlantic. Journal of Ichthyology, 54(9), 642–659.
Hallas, J. M., Chichvarkhin, A., & Gosliner, T. M. (2017). Aligning evidence: Concerns regarding multiple sequence alignments in estimating the phylogeny of the Nudibranchia suborder Doridina. Royal Society Open Science, 4(10), 171095.
Hamatani, I., & Kubodera, T. (2010). A new species of abyssal opisthobranch belonging to the genus Bathydoris Bergh, 1884 (Opisthobranchia: Nudibranchia: Doridoidea) from Japan. Venus, 68(3–4), 113–120.
Horne, D. J. (1999). Ocean circulation modes of the phanerozoic: Implications for the antiquity of deep-sea benthonic invertebrates. Crustaceana, 72, 999–1018. https://doi.org/10.1163/156854099503906
Ivanova, N. V., deWaard, J., & Hebert, P. D. N. (2006). An inexpensive, automation-friendly proto-col for recovering high-quality DNA. Molecular Ecology Notes, 6, 998–1002.
Jablonski, D., & Bottjer, D. J. (1991). Environmental patterns in the origins of higher taxa: The post-Palaeozoic fossil record. Science, 252, 251–253. https://doi.org/10.1126/science.252.5014.1831
Jablonski, D., Sepkoski, J. J., Jr., Bottjer, D. J., & Sheehan, P. M. (1983). Onshore-offshore patterns in evolution of Phanaerozoic shelf communities. Science, 22, 1123–1125. https://doi.org/10.1126/science.222.4628.1123
Jacobs, D. K., & Lindberg, D. R. (1998). Oxygen and evolutionary patterns in the sea: Onshore/offshore trends and recent recruitment of deep-sea faunas. Proceedings of the National Academy of Sciences of the United States of America, 95, 9396–9401. https://doi.org/10.1073/pnas.95.16.9396
Jeppsson, L. (1990). An oceanic mode for lithological and faunal changes tested on the Silurian record. Journal of the Geological Society, 147, 663–674. https://doi.org/10.1144/gsjgs.147.4.0663
Jumars, P. A., Mayer, L. M., Deming, J. W., Baross, J. A., & Wheatcroft, R. A. (1990). Deep-sea deposit-feeding strategies suggested by environmental and feeding constraints. Philosophical Transactions of the Royal Society of London. Series A, Mathematical and Physical Sciences, 331, 85–101. https://doi.org/10.1098/rsta.1990.0058
Kano, Y. (2008). Vetigastropod phylogeny and a new concept of Seguenzioidea: Independent evolution of copulatory organs in the deep-sea habitats. Zoologica Scripta, 37(1), 1–21.
Kano, Y., Brenzinger, B., Nützel, A., Wilson, N. G., & Schrödl, M. (2016). Ringiculid bubble snails recovered as the sister group to sea slugs (Nudipleura). Scientific Reports, 6(1), 1–11.
Kearse, M., Moir, R., Wilson, A., Stones-Havas, S., Cheung, M., Sturrock, S., & Drummond, A. (2012). Geneious Basic: An integrated and extendable desktop software platform for the organization and analysis of sequence data. Bioinformatics, 28(12), 1647–1649.
Kiel, S., & Goedert, J. L. (2006). Deep-sea food bonanzas: Early Cenozoic whale-fall communities resemble wood-fall rather than seep communities. Proceedings of the Royal Society B: Biological Sciences, 273, 2625–2631. https://doi.org/10.1098/rspb.2006.3620
Korshunova, T., & Martynov, A. (2020). Consolidated data on the phylogeny and evolution of the family Tritoniidae (Gastropoda: Nudibranchia) contribute to genera reassessment and clarify the taxonomic status of the neuroscience models Tritonia and Tochuina. PLoS ONE, 15(11), e0242103.
Korshunova, T., Sanamyan, N., Zimina, O., Fletcher, K., & Martynov, A. (2016). Two new species and a remarkable record of the genus Dendronotus from the North Pacific and Arctic oceans (Nudibranchia). ZooKeys, 630, 19.
Korshunova, T., Martynov, A., Bakken, T., Evertsen, J., Fletcher, K., Mudianta, W. I., Saito, H., Lundin, K., Schrödl, M., & Picton, B. (2017a). Polyphyly of the traditional family Flabellinidae affects a major group of Nudibranchia: Aeolidacean taxonomic reassessment with descriptions of several new families, genera, and species (Mollusca, Gastropoda). ZooKeys, 717, 1–139.
Korshunova, T., Zimina, O., & Martynov, A. (2017b). Unique pleuroproctic taxa of the nudibranch family Aeolidiidae from the Atlantic and Pacific Oceans, with description of a new genus and species. Journal of Molluscan Studies, 83(4), 409–421.
Korshunova, T., Martynov, A., Bakken, T., & Picton, B. (2017c). External diversity is restrained by internal conservatism: New nudibranch mollusc contributes to the cryptic species problem. Zoologica Scripta, 46(6), 683–692.
Korshunova, T., Martynov, A., & Picton, B. (2017d). Ontogeny as an important part of integrative taxonomy in Tergipedid aeolidaceans (Gastropoda: Nudibranchia) with a description of a new genus and species from the Barents Sea. Zootaxa, 4324(1), 1–22.
Korshunova, T., Lundin, K., Malmberg, K., Picton, B., & Martynov, A. (2018). First true brackish-water nudibranch mollusc provides new insights for phylogeny and biogeography and reveals paedomorphosis-driven evolution. PLoS ONE, 13(3), e0192177.
Korshunova, T., Fletcher, K., Picton, B., Lundin, K., Kashio, S., Sanamyan, N., & Martynov, A. (2020a). The Emperor’s Cadlina, hidden diversity and gill cavity evolution: New insights for the taxonomy and phylogeny of dorid nudibranchs (Mollusca: Gastropoda). Zoological Journal of the Linnean Society, 189(3), 762–827.
Korshunova, T., Bakken, T., Grøtan, V. V., Johnson, K. B., Lundin, K., & Martynov, A. (2020b). A synoptic review of the family Dendronotidae (Mollusca: Nudibranchia): A multilevel organismal diversity approach. Contributions to Zoology, 90(1), 93–153.
Kumar, S., Stecher, G., & Tamura, K. (2016). MEGA7: Molecular evolutionary genetics analysis version 7.0 for bigger datasets. Molecular Biology and Evolution, 33(7), 1870–1874.
Lê, H. L., Lecointre, G., & Perasso, R. (1993). A 28S rRNA-based phylogeny of the gnathostomes: First steps in the analysis of conflict and congruence with morphologically based cladograms. Molecular Phylogenetics and Evolution, 2(1), 31–51.
Leduc, D., Zhao, Z. Q., Verdon, V., & Xu, Y. (2018). Phylogenetic position of the enigmatic deep-sea nematode order Rhaptothyreida: A molecular analysis. Molecular Phylogenetics and Evolution, 122, 29–36.
Leigh, J. W., & Bryant, D. (2015). Popart: Full-feature software for haplotype network construction. Methods in Ecology and Evolution, 6(9), 1110–1116.
Linder, A., Cairns, S. D., & Cunningham, C. W. (2008). From offshore to onshore: Multiple origins of shallow-water corals from deep-sea ancestors. PLoS ONE, 3(6), e2429. https://doi.org/10.1371/journal.pone.0002429
Mahguib, J., & Valdés, Á. (2015). Molecular investigation of the phylogenetic position of the polar nudibranch Doridoxa (Mollusca, Gastropoda, Heterobranchia). Polar Biology, 38(9), 1369–1377.
Malyutina, M. V., & Brandt, A. (2012). The joint Russian-German deep-sea research projects SoJaBio (Sea of Japan Biodiversity Studies) and KuramBio (Kurile Kamchatka Deep-Sea Biodiversity). Marine Ecosystems under the Global Change in the Northwestern Pacific, 11.
Malyutina, M. V., & Brandt, A. (2013). Introduction to SoJaBio (Sea of Japan biodiversity studies). Deep Sea Research Part II: Topical Studies in Oceanography, 86, 1–9.
Malyutina, M. V., Chernyshev, A. V., & Brandt, A. (2018). Introduction to the SokhoBio (Sea of Okhotsk Biodiversity Studies) expedition 2015. Deep Sea Research Part II: Topical Studies in Oceanography, 154, 1–9.
Martynov, A. V. (1993). Subclassis Opisthobranchia. In: B. I. Sirenko (Ed.), List of species of the fauna of invertebrates of continental slope of the Kurile Islands String. Issledovaniya Fauny Morei, 46(54), 198.
Martynov, A. V. (2013). Morphology, taxonomic status and distribution of the opisthobranch mollusc Coryphella (s. l.) japonica from the central deep water basin of the Sea of Japan. Deep-Sea Research, Part II: Topical Studies in Oceanography, 86–87, 111–118. https://doi.org/10.1016/j.dsr2.2012.08.012
Martynov, A. V., & Baranets, O. N. (2002). A revision of the genus Colga Bergh (Opisthobranchia, Polyceridae), with description of a new species from the North Pacific. Ruthenica, 12(1), 23–43.
Martynov, A. V., & Roginskaya, I. S. (2005). A new species of the genus Doridunculus G.O. Sars. (1878). (Mollusca, Nudibranchia): A hydroid-feeding dorid from the abyssal depths of the Sea of Japan. Ruthenica, 14(2), 135–145.
Martynov, A. V., Sanamyan, N. P., Korshunova, T. A. (2015). New data on the opisthobranch molluscs (Gastropoda: Opisthobranchia) of waters of Commander Islands and Far-Eastern seas of Russia. In: Conservation of biodiversity of Kamchatka and coastal waters. Proceedings of XV international scientific conference Petropavlovsk-Kamchatsky. Kamchat Press, Petropavlovsk-Kamchatsky, 55–69.
Martynov, A. V., Sanamyan, N. P., & Korshunova, T. A. (2016). Review of the opisthobranch mollusc fauna of Russian Far Eastern seas: Pleurobranchomorpha, Doridida and Nudibranchia. Bulletin of Kamchatka State Technical University, 34, 62–87.
Martynov, A., Fujiwara, Y., Tsuchida, S., Nakano, R., Sanamyan, N., Sanamyan, K., Fletcher, K., & Korshunova, T. (2020). Three new species of the genus Dendronotus from Japan and Russia (Mollusca, Nudibranchia). Zootaxa, 4747(3), 495–513.
McClain, C. R. (2007). Guest editorial: Seamounts: Identity crisis or split personality? Journal of Biogeography, 34, 2001–2008. https://doi.org/10.1111/j.1365-2699.2007.01783.x
McClain, C. R., Rex, M. A., & Etter, R. J. (2009). Deep-sea macroecology. In J. D. Witman & K. Roy (Eds.), Marine macroecology (pp. 65–100). University of Chicago Press.
McClain, C. R., & Hardy, S. M. (2010). The dynamics of biogeographic ranges in the deep sea. Proceedings of the Royal Society B: Biological Sciences, 277(1700), 3533–3546.
Menzies, R. J., & Imbrie, J. (1958). On the antiquity of the deep-sea bottom fauna. Oikos, 9, 192–210. https://doi.org/10.2307/3564764
Milligan, R. J., Scott, E. M., Jones, D. O., Bett, B. J., Jamieson, A. J., & O’brien, R., & Bailey, D. M. (2020). Evidence for seasonal cycles in deep-sea fish abundances: A great migration in the deep SE Atlantic? Journal of Animal Ecology, 89(7), 1593–1603.
Moles, J., Berning, M. I., Hooker, Y., Padula, V., Wilson, N. G., & Schrödl, M. (2021). Due South: The evolutionary history of sub-Antarctic and Antarctic Tritoniidae nudibranchs. Molecular Phylogenetics and Evolution, 107209.
Orlov, A. M., Rabazanov, N. I., & Nikiforov, A. I. (2020). Transoceanic migrations of fishlike animals and fish: Norm or exclusion? Journal of Ichthyology, 60, 242–262.
Orlova, S. Y., Schepetov, D. M., Mugue, N. S., Smirnova, M. A., Senou, H., Baitaliuk, A. A., & Orlov, A. M. (2019). Evolutionary history told by mitochondrial markers of large teleost deep-sea predators of family Anoplopomatidae Jordan & Gilbert 1883, endemic to the North Pacific. Journal of the Marine Biological Association of the UK, 99(7), 1683–1691. https://doi.org/10.1017/S0025315419000572
Osterburg, H. H., Allen, J. K., & Finch, C. E. (1975). The use of ammonium acetate in the precipitation of ribonucleic acid. Biochemical Journal, 147(2), 367–368. https://doi.org/10.1042/bj1470367
Palumbi, S. R. (1996). Nucleic acids II: The polymerase chain reaction. In D. M. Hillis, C. Moritz, & B. K. Mable (Eds.), Molecular systematics (pp. 205–247). Sinauer & Associates Inc.
Pearse, J. S., & Lockhart, S. J. (2004). Reproduction in cold water: Paradigm changes in the 20th century and a role for cidaroid sea urchins. Deep Sea Research Part II: Topical Studies in Oceanography, 51(14–16), 1533–1549. https://doi.org/10.1016/j.dsr2.2004.06.023
Pradillon, F., & Gaill, F. (2007). Pressure and life: Some biological strategies. Reviews in Environmental Science and Bio/technology, 6, 181–195. https://doi.org/10.1007/s11157-006-9111-2
Puslednik, L., & Serb, J. M. (2008). Molecular phylogenetics of the Pectinidae (Mollusca: Bivalvia) and effect of increased taxon sampling and outgroup selection on tree topology. Molecular Phylogenetics and Evolution, 48(3), 1178–1188.
Raupach, M. J., Mayer, C., Malyutina, M., & Wagele, J.-W. (2009). Multiple origins of deep-sea Asellota (Crustacea: Isopoda) from shallow waters revealed by molecular data. Proceedings of the Royal Society B: Biological Sciences, 276(1658), 799–808. https://doi.org/10.1098/rspb.2008.1063
Ronquist, F., & Huelsenbeck, J. P. (2003). MrBayes 3: Bayesian phylogenetic inference under mixed models. Bioinformatics, 19(12), 1572–1574. https://doi.org/10.1093/bioinformatics/btg180
Rogers, A. D. (2000). The role of the oceanic oxygen minima in generating biodiversity in the deep sea. Deep Sea Research Part II: Topical Studies in Oceanography, 47(1–2), 119–148. https://doi.org/10.1016/S0967-0645(99)00107-1
Saeedi, H., Simões, M., & Brandt, A. (2020). Biodiversity and distribution patterns of deep-sea fauna along the temperate NW Pacific. Progress in Oceanography, 183, 102296.
Shank, T. M. (1998). Miocene radiation of deep-sea hydrothermal vent shrimp (Caridea: Bresiliidae): Evidence from mitochondrial cytochrome oxidase subunit I. Molecular Phylogenetics and Evolution, 13(2), 244–254. https://doi.org/10.1006/mpev.1999.0642
Sibuet, M., & Olu, K. (1998). Biogeography, biodiversity and fluid dependence of deep-sea cold-seep communities at active and passive margins. Deep Sea Research Part II: Topical Studies in Oceanography, 45(1–3), 517–567. https://doi.org/10.1016/S0967-0645(97)00074-X
Smith, A. B., & Stockley, B. (2005). The geological history of deep-sea colonization by echinoids: Roles of surface productivity and deep-water ventilation. Proceedings of the Royal Society B: Biological Sciences, 272, 865–869. https://doi.org/10.1098/rspb.2004.2996
Stamatakis, A. (2014). RAxML version 8: A tool for phylogenetic analysis and post-analysis of large phylogenies. Bioinformatics, 30(9), 1312–1313.
Stout, C. C., Wilson, N. G., & Valdés, Á. (2011). A new species of deep-sea Dendronotus Alder & Hancock (Mollusca: Nudibranchia) from California, with an expanded phylogeny of the genus. Invertebrate Systematics, 25(1), 60–69.
Sukumaran, J., & Holder, M. T. (2010). DendroPy: A Python library for phylogenetic computing. Bioinformatics, 26(12), 1569–1571.
Talavera, G., & Castresana, J. (2007). Improvement of phylogenies after removing divergent and ambiguously aligned blocks from protein sequence alignments. Systematic Biology., 56(4), 564–577.
Tittensor, D. P., Schlacher, T., Smith, C. R., & Susko, E. (2010). Endemism at low sampling effort: Real or artifact. International Deep-Sea Biology Symposium, 12th, Reykjavik, Iceland (pp. 84–85). Southhampton, UK: InterRidge.
Valdés, Á. (2002). Phylogenetic systematics of “Bathydoris” sl Bergh, 1884 (Mollusca, Nudibranchia), with the description of a new species from New Caledonian deep waters. Canadian Journal of Zoology, 80(6), 1084–1099.
Valdés, Á., & Bertsch, H. (2000). Redescription and range extension of Bathydoris aioca Marcus, & Marcus, 1962 (Nudibranchia: Gnathodoridoidea). Veliger, 43(2), 172–178.
Valdés, Á., Lundsten, L., & Wilson, N. G. (2018). Five new deep-sea species of nudibranchs (Gastropoda: Heterobranchia: Cladobranchia) from the Northeast Pacific. Zootaxa, 4526(4), 401–433.
Van Dover, C. L. (2000). The ecology of deep-sea hydrothermal vents. Princeton University Press.
Vinogradova, N. G. (1997). Zoogeography of the abyssal and hadal zones. Advances in Marine Biology, 32, 325–387. https://doi.org/10.1016/S0065-2881(08)60019-X
Volodchenko, N. I. (1941). New nudibranchiate molluscs from seas of the far-east of the U.S.S.R. Investigations of the Far Eastern Seas of the USSR, 1, 53–72.
Waelbroeck, C., Labeyrie, L., Michel, E., Duplessy, J. C., McManus, J. F., Lambeck, K., Balbon, E., & Labracherie, M. (2001). Sea-level and deep water temperature changes derived from benthic foraminifera isotopic records. Quaternary Science Reviews, 21, 295–305. https://doi.org/10.1016/S0277-3791(01)00101-9
Watling, L., Guinotte, J., Clark, M. R., & Smith, C. R. (2013). A proposed biogeography of the deep ocean floor. Progress in Oceanography, 111, 91–112.
Whiting, M. F., Carpenter, J. M., Wheeler, Q. D., & Wheeler, W. C. (1997). The Strepsiptera problem: Phylogeny of the holometabolous insect orders inferred from 18S and 28S ribosomal DNA sequences and morphology. Systematic Biology, 46, 1–68.
Wilson, G. (1999). Some of the deep-sea fauna is ancient. Crustaceana, 72(8), 1019–1030. https://doi.org/10.1163/156854099503915
Zardus, J. D., Etter, R. J., Chase, M. R., Rex, M. A., & Boyle, E. E. (2006). Bathymetric and geographic population structure in the pan-Atlantic deep-sea bivalve Deminucula atacellana (Schenck, 1939). Molecular Ecology, 15(3), 639–651. https://doi.org/10.1111/j.1365-294X.2005.02832.x
Zenkevitch, L. A., & Birstein, J. A. (1953). On the problem of the antiquity of the deep-sea fauna. Deep Sea Research, 7(1), 10–23.
Acknowledgements
We are deeply grateful to all friends and colleagues who kindly collected and supplied specimens for this study: Dr. Anastassya Maiorova (NSCMB RAS), and team of 82nd cruise of R/V “Akademik M.A. Lavrentyev, including Dr. Vladimir Mordukhovich (NSCMB RAS) and Dr. Elena Krylova (IO RAS). The authors gratefully acknowledge the National Scientific Center of Marine Biology FEBRAS for providing nudibranch molluscs collection for this study and the Team of ROV “Komanch” and Dr. Nadezhda Sanamyan for providing photos of nudibranchs collected from seeps. We thank Valentina Tambovceva for assistance with Sanger sequencing. We also want to thank the staff of Joint Usage Center “Instrumental methods in ecology at the A.N. Severtsov Institute of Ecology and Evolution RAS” and the staff of the scanning electron microscopic laboratory of the Moscow State University for providing SEM facilities. Sanger sequencing was conducted using equipment of the Core Centrum of Institute of Developmental Biology RAS.
Funding
This study is uniting proceeding made during research on the nudibranch families Fionidae, Dendronotidae, Flabellinidae, and Doridina in frame of scientific project of the State Order of the Russian Federation Government to Lomonosov Moscow State University No. 121032300121-0 with financial support of Russian Science Foundation (grant no. 19-74-00144 for the family Fionidae to DS and grant no. 20-74-10012 for the families Flabellinidae and Dendronotidae to IE, AM, MS, TA and DS). Depository of specimens used in this study was supported by the Moscow State University Grant for Leading Scientific Schools “Depository of the Living Systems” in the frame of the MSU Development Program. Specimen collection and fixation was supported by the Grant of the Ministry of Science and Higher Education of the Russian Federation (agreement number 075-15-2020-796, grant number 13.1902.21.0012).
Author information
Authors and Affiliations
Contributions
Conceptualization: IE, DS. Investigation: IE, AV, DS. Methodology: IE. Software: IE, DS. Validation: IE, DS. Formal analysis: IE, MS, AM, TA, TN, OC, DS. Resources: IE. Data curation: IE. Writing — original draft: IE, AV, DS. Writing — review and editing: IE, AV, MS, AM, TA, TN, OC, DS. Visualization: IE, AM, TA, DS. Supervision: IE. Project administration: IE, DS. Funding acquisition: IE, DS.
Ethics declarations
Ethics approval
Not applicable.
Consent to participate
All authors voluntarily agree to participate in this research study.
Consent for publication
All authors approved the final version of the manuscript.
Competing interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
13127_2021_526_MOESM2_ESM.docx
Supplementary file2 Table S1. List of specimens used in this study. Voucher numbers, collection data and collectors are given. (DOCX 20 KB)
13127_2021_526_MOESM3_ESM.xlsx
Supplementary file3 Table S2. Specimens used for molecular analyses. Voucher numbers, collection localities and GenBank accession numbers are given (sequences obtained for this study are pending accession numbers). Sequences obtained for this study are highlighted in bold. (XLSX 55 KB)
Rights and permissions
About this article
Cite this article
Ekimova, I., Valdés, Á., Stanovova, M. et al. Connected across the ocean: taxonomy and biogeography of deep-water Nudibranchia from the Northwest Pacific reveal trans-Pacific links and two undescribed species. Org Divers Evol 21, 753–782 (2021). https://doi.org/10.1007/s13127-021-00526-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13127-021-00526-8