Abstract
Adults of some butterfly species have odors, several components of which are known to have pheromonal or defensive functions. Little is known about the odors of hesperiid butterflies, however. Erynnis montanus (Hesperiidae), especially male adults, emit a pungent odor that is detectable by the human nose. Chemical analysis has revealed that crude extracts of wild individuals contained 10 volatile substances, of which docosane and heneicosane were the main components. Because males contain a significantly larger amount of p-cresol than females, this aromatic compound is characterized as a strong male odor. At the end of the adult occurrence period, p-cresol decreased substantially in each male. Benzothiazole, identified for the first time in lepidopteran adults, was present in both sexes in almost equal amounts. Among the 10 volatile substances detected in males, biased distribution in the wings rather than the body was observed for benzothiazole, heptanal, and p-cresol. Male adults have androconial organs in the costal part of the forewing on which benzothiazole and p-cresol tended to concentrate. However, these compounds were detected not only in other parts of the forewing but also in the hindwing and body, suggesting the presence of undiscovered scent-producing organs.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Adults of several butterfly species, especially males, emit odors that are detectable by the human nose. These odors are usually associated with morphologically specialized structures called androconia (Boppré 1989). Androconia are diverse in terms of structure from wing patches to abdominal hair pencils, in which modified scales with glandular bases exist (Boppré 1993). Both the chemical nature and biological significance of androconial odors have been revealed in a limited number of Pieridae and Danaidae species, in which some male-specific substances serve as sex pheromones at close range (Honda 2008; Yildizhan et al. 2009). Moreover, the phenolic odors from some butterfly species are thought to function as feeding deterrents to potential predators (Eisner and Eisner 1990; Nishida et al. 1996).
The family of Hesperiidae, consisting of about 4000 species distributed among 567 genera, is commonly known as the skipper family (Warren et al. 2008). Thirty-seven hesperiid species are found in Japan (Shirôzu 2006). In many hesperiid species, male adults have different types of androconial organs, for example the stigmata (patches of characteristic scales) and tufts of hair-like scales (Warren et al. 2009). The purpose of androconial organs has been clarified in a few hesperiid species only. Thymelicus lineola, T. acteon, and Hesperia comma have forewing stigmata enclosing tubular scales within a hollow medulla (Pivnick et al. 1992; Wüest 1998). These specialized scales can break into small pieces called osmophores, which serve as pheromone dispensers. Adult female T. lineola had strong electroantennographic responses to the androconial odor of conspecific males, and copulation frequency significantly decreased on removal of the male stigmata (Pivnick et al. 1992). These results demonstrate the presence of a male sex pheromone in the T. lineola stigmata and the first evidence of it in the Hesperiidae family. Nevertheless, the androconial substances of T. lineola have not yet been revealed, and little knowledge of the odor components has been obtained from hesperiid butterflies (El-Sayed 2009).
The spring flat Erynnis montanus Bremer is a montane hesperiid species that feeds on a variety of host plants belonging to the Fagaceae family. This skipper is univoltine and occurs from the end of March to the middle of May in Hiroshima prefecture. Male adults actively fly around in sunny open areas, for example mountain paths and meadows, whereas female adults tend to be found in sparse woodland areas (Fukuda et al. 1984). Because male courtship behavior is rarely observed in the field, details of the mating behavioral sequence remain unclear. Adult skippers have a characteristic pungent odor that is stronger in males than in females. Interestingly, the male odor changes within the period of occurrence of adults, with the odor being fainter for aging males than for adolescent males. This skipper is sexually dimorphic, especially in the forewing. Whereas females have a unique white band in the forewing color pattern, males have a distinct structure called a forewing costal fold, which encloses characteristic scales that are smaller than normal wing scales (Shirôzu 2006). This alar structure is specific to males; other characteristic organs, for example hairpencils have never been found in males. The forewing costal fold is regarded as the androconial organ of E. montanus. However, it remains unclear whether the pungent odor is associated with it. In addition, the function of the odor of E. montanus remains unknown. As a first step in elucidating the biological significance of odors emitted from adult E. montanus, we examined:
-
1
the chemical composition of the odoriferous components;
-
2
quantitative alteration of these components within the adult occurrence period; and
-
3
the component distribution in male adults.
Materials and methods
Insects
Wild E. montanus adults were collected in Higashihiroshima in April and May in the years 2008–2010. Captured butterflies were placed individually in cylindrical plastic cases (75 mm high, 80 mm ID) and kept quiet for at least 1 h before extraction.
Extraction
Butterflies were frozen to death at −20°C and subjected to 3-min extraction with purified (twice-distilled) dichloromethane. We conducted the following sample preparation. First, we examined sex-based differences in the chemical composition of odor using 25 males and 7 females collected in 2008. Within 5 weeks after the first collection of adult butterflies, 5 males were collected every week for brief examination of quantitative component changes, i.e., 25 males were divided into 5 groups (5 males each). Because females were seldom seen in open areas and rarely collected within the occurrence period, the 7 females constituted 1 group. Each individual was soaked in 1 mL solvent. Second, the distribution of volatile compounds in male adults was examined using 10 males collected on April 2 and 5, 2009. One male was divided into 3 parts (forewings, hindwings, and body), and each part obtained from 2 males was soaked in 1 mL solvent. Five samples of each part were prepared in this manner. Third, the distribution of 2 characteristic volatiles on male wings was examined using 10 males collected on April 3 and 4, 2010. The forewings and hindwings obtained from 2 males were separated into costal, basal, and distal parts (Fig. 1). The 6 wing parts were individually soaked in 1 mL solvent. Five samples of each part were prepared in this manner.
These resulting extracts were filtered and concentrated to 100 μL under a stream of nitrogen at 10°C. The concentrated extracts were stored at −20°C until the chemical analysis was performed.
Chemical analysis
The concentrated extracts were examined by gas chromatography–mass spectrometry (GC–MS). GC–MS analysis was performed at 70 eV using a Shimadzu (Kyoto, Japan) QP5000 mass spectrometer and a Shimadzu GC-17A gas chromatograph equipped with a Varian CP-Wax 58CB fused-silica capillary column (0.25 mm ID × 25 m, 0.20 μm film thickness). The column oven temperature was programmed from 40°C (held initially for 2 min) to 200°C at 5°C/min. The interface temperature was maintained at 250°C. Splitless injection of samples was performed at 220°C using helium as carrier gas. Compound identification was based on comparison of retention times and mass spectral data with those of authentic samples obtained commercially. Heneicosane and docosane were obtained from Sigma–Aldrich (St Louis, USA), and heptanal, nonanal, decanal, acetic acid, octanoic acid, nonanoic acid, benzothiazole, and p-cresol were obtained from Tokyo Chemical Industry (Tokyo, Japan). Quantification of the compounds was based on comparison of peak intensities in total-ion chromatograms with those from 100 ng of the authentic samples.
Results
Chemical composition of the odor from hesperiid adults
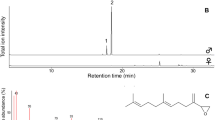
Dichloromethane extracts of the whole body of the skipper yielded 10 highly volatile substances: 2 aliphatic hydrocarbons, 3 aliphatic aldehydes, 3 aliphatic acids, and 2 aromatic compounds (Fig. 2; Table 1). Of these 10 components, docosane was the most abundant in both sexes, and the average amount was >1 μg/individual. Heneicosane was the second most abundant compound in both sexes and was present at 300–1000 ng/individual. In terms of abundance, the compounds following these alkanes were p-cresol in males and nonanoic acid in females, average amounts of which were approximately 200 ng/individual. Other components were present in amounts <100 ng/individual. Docosane and p-cresol were significantly more abundant in males (N = 25) than in females (N = 7) (P < 0.05 for docosane and P < 0.001 for p-cresol, Mann–Whitney U test).
Typical total-ion chromatograms obtained from crude extracts from 1 adult Erynnis montanus. Chromatograms were run on a Varian CP-Wax 58CB capillary column (0.25 mm ID × 25 m, programmed from 40°C (held initially for 2 min) to 200°C at 5°C/min). Compounds 1 heptanal, 2 nonanal, 3 acetic acid, 4 decanal, 5 benzothiazole, 6 octanoic acid, 7 p-cresol, 8 heneicosane, 9 nonanoic acid, and 10 docosane
Among the males collected over 5 weeks of 2008, the maximum quantity of each compound was found in those collected in the second week (Table 1). Of the 10 compounds identified, a substantial decrease in the average amount per male was observed for p-cresol only; in fact, the quantity was significantly lower in the fifth week than in the first and second weeks (P < 0.05, Mann-Whitney U test).
Distribution of adult male volatile components
The 10 compounds were detected in all 3 amputated parts of the male adults: the forewings, hindwings, and body (Table 2). To assess possible biased component distribution on either the wings or the body, the wing/body ratio in amount per individual was calculated for each compound. The ratio for benzothiazole (the highest value among the 10 components) was 5.78, indicating this compound emanated primarily from the wings. For heptanal and p-cresol the ratios were 2.43 and 1.95, respectively, demonstrating that the content of each was approximately twice as high in the wings as in the body. The other 7 compounds were almost evenly distributed between wings and body (ratios 0.83–1.70). Equal amounts of benzothiazole and octanoic acid were present in the forewings and hindwings, whereas the other 8 compounds were somewhat more abundant in the hindwings. Although p-cresol and benzothiazole were detected in all 3 (costal, basal, and distal) parts of the male forewings or hindwings, in terms of amount per area these aromatic compounds tended to be most abundant in the costal part (Table 3).
Discussion
To the best of our knowledge (El-Sayed 2009), this study is the first to report on the odor of hesperiid butterflies. Ten volatile compounds were identified in adult E. montanus extracts. Whereas 8 of these were present in almost equal amounts in both sexes, p-cresol and docosane were significantly more abundant in males than in females. Among the 10 components identified, docosane was the most abundant, followed by heneicosane. Because these aliphatic hydrocarbons were present in significantly higher amounts than the other components, they seemed to be the cuticular hydrocarbons (CHCs) of this skipper. n-Alkanes with 21–31 carbon atoms have been identified as the CHCs of the adult butterflies Danaus erippus and D. plexippus (Hay-Roe et al. 2007). Compounds present in moderate quantity (ca. 200 ng/individual) were p-cresol in males and nonanoic acid in females. Benzothiazole was detected at 80 ng/individual in both sexes. These three compounds are regarded as the major substances of the odor of E. montanus adults. In particular, p-cresol is regarded as the characteristic compound of the strong male odor. Quantitative decreases of this phenolic compound are responsible for most of the male odor changes within the occurrence period.
Several volatile compounds found in adult E. montanus have been reported in other lepidopterans also. p-Cresol is one of the compounds secreted from the hairpencils of the male giant danaid butterfly Idea leuconoe (Nishida et al. 1996). The sex pheromone of the adult male cabbage looper Trichoplusia ni contains p-cresol to attract conspecific females (Heath et al. 1992). Nonanoic acid is found in several danaid butterflies (Schulz et al. 1993), and heptanal, nonanal, decanal, and acetic acid have been identified in Papilionidae (Honda 1980; Ômura et al. 2001; Ômura and Honda 2005). On the other hand, this study is the first to identify benzothiazole as a volatile compound in lepidopteran adults (El-Sayed 2009), suggesting that this compound is one of the characteristics of this skipper’s scent.
Many insect species discharge a secretion in response to disturbance. The secretion is usually regarded a chemical weapon against potential predators, because of the presence of repellent and irritating compounds. Of the volatile compounds identified in this study, p-cresol and benzothiazole have been reported as effective repellents against predatory ants (Eisner et al. 1978; Drilling and Dettner 2010). In addition, p-cresol, benzothiazole, and nonanoic acid have strong antimicrobial activity (Smith and Grula 1982; Drilling and Dettner 2010). This evidence suggests that the pungent odor of adult E. montanus has the potential function of chemical defense.
Some butterfly species produce male-specific volatile compounds that serve as sex pheromones, e.g., citral in Pieris napi (Andersson et al. 2007) and danaidone in several danaid species (Honda 2008). Moreover, sexual dimorphism has been found in CHCs of several butterfly species (Ômura and Honda 2005; Dapporto 2007). In this study, amounts of p-cresol and docosane were significantly higher for male adults than for female adults. These compounds are likely to have potential roles in chemical communication of E. montanus between the sexes.
The several compounds identified in this study were localized in both the forewings and hindwings rather than in the body. The wing/body ratio was highest for benzothiazole, and p-cresol, the male-specific compound, was relatively concentrated in the wings. These results suggest that biosynthetic or storage organs for these compounds are present in both wings. In Atrophaneura alcinous (Papilionidae) and several species of Danaidae, male-specific volatiles are discharged from the androconia organs in the hindwing (Honda 1980, 2008). Male adults of E. montanus and Pyrgus maculatus (Hesperiidae) have forewing costal folds that enclose fine scales with a string-like upper part (Kawazoé and Wakabayashi 1976). The presence of morphologically peculiar scales indicates that this alar structure is the androconial organ of E. montanus. Our current results support the theory that the forewing costal folds are related to the discharge of benzothiazole and p-cresol. On the other hand, these aromatic compounds were detected not only in other parts of the forewing but also in the hindwing and body. Moreover, volatile substances were found in females despite the lack of costal folds in the forewings. These results indicate the possibility that hitherto undiscovered scent-producing organs are present. Despite our extensive examinations of male wings using optical and scanning electron microscopy, the existence of androconia has not been detected in locations other than the forewing costal folds.
With regard to quantitative changes of the compounds, a significant decrease within the occurrence period of male adults was observed for p-cresol only. Similar quantitative changes have been reported for the sex pheromone compounds of the moth species Sesamia nonagrioides and Helicoverpa assulta (Babilis and Mazomenos 1992; Kamimura and Tatsuki 1993). The distinct quantitative change of p-cresol in E. montanus males suggests a possible biological function that differs from that of other compounds. Further studies are needed to elucidate the biological significance of the scent substances emitted by adult E. montanus.
References
Andersson J, Borg-Karlson A-K, Vongvanich N, Wiklund C (2007) Male sex pheromone release and female mate choice in a butterfly. J Exp Biol 210:964–970. doi:10.1242/jeb.02726
Babilis NA, Mazomenos BE (1992) Pheromone production in Sesamia nonagrioides: diel periodicity and effect of age and mating. J Insect Physiol 38:561–564. doi:10.1016/0022-1910(92)90106-N
Boppré B (1989) Chemically mediated interactions between butterflies. In: Vane-Wright RI, Ackery PR (eds) The biology of butterflies. Princeton University Press, Princeton, pp 259–275
Boppré B (1993) The American monarch: courtship and chemical communication of a peculiar danaine butterfly. In: Malcolm SB, Zalucki MP (eds) Biology and conservation of the monarch butterfly. Natural History Museum of Los Angeles County, Los Angeles, pp 29–41
Dapporto L (2007) Cuticular lipid diversification in Lasiommata megera and Lasiommata paramegaera: the influence of species, sex, and population (Lepidoptera: Nymphalidae). Biol J Linn Soc 91:703–710. doi:10.1111/j.1095-8312.2007.00833.x
Drilling K, Dettner K (2010) First insights into the chemical defensive system of the erotylid beetle, Tritoma bipustulata. Chemoecology 20:243–253. doi:10.1007/s00049-010-0054-2
Eisner T, Eisner M (1990) p-Methoxyphenol: chemical basis of stench of a female butterfly. Naturwissenschaften 77:33. doi:10.1007/BF01131794
Eisner T, Alsop D, Hicks K, Meinwald J (1978) Defensive secretions of millipedes. In: Bettini S (ed) Arthropod venoms. Handbook of experimental pharmacology, vol 48. Springer, Berlin, pp 41–72
El-Sayed AM (2009) The Pherobase. http://www.pherobase.net/
Fukuda H, Hama E, Kuzuya T, Takahashi A, Takahashi M, Tanaka B, Tanaka H, Wakabayashi M, Watanabe Y (1984) Erynnis montanus Bremer. In: The life histories of butterflies in Japan, vol IV. Hoikusha, Osaka, pp 184–193 (in Japanese)
Hay-Roe MM, Lamas G, Nation JL (2007) Pre- and postzygotic isolation and Haldane rule effects in reciprocal crosses of Danaus erippus and Danaus plexippus (Lepidoptera: Danainae), supported by differentiation of cuticular hydrocarbons, establish their status as separate species. Biol J Linn Soc 91:445–453. doi:10.1111/j.1095-8312.2007.00809.x
Heath RR, Landolt PJ, Dueben BD, Murphy RE, Schneider RE (1992) Identification of male cabbage looper sex pheromone attractive to females. J Chem Ecol 18:441–453. doi:10.1007/BF00994243
Honda K (1980) Odor of a papilionid butterfly: odoriferous substances emitted by Atrophaneura alcinous alcinous (Lepidoptera: Papilionidae). J Chem Ecol 6:867–873. doi:10.1007/BF00994243
Honda K (2008) Addiction to pyrrolizidine alkaloids in male danaine butterflies: a quest for the evolutionary origin of pharmacophagy. In: Maes RP (ed) Insect physiology: new research. Nova Science Publishers, New York, pp 73–118
Kamimura M, Tatsuki S (1993) Diel rhythms of calling behavior and pheromone production of oriental tobacco budworm moth, Helicoverpa assulta (Lepidoptera: Noctuidae). J Chem Ecol 19:2953–2963. doi:10.1007/BF00980595
Kawazoé A, Wakabayashi M (1976) Erynnis Schrank, 1801. In: Colored illustrations of the butterflies of Japan. Hoikusha, Osaka, pp 301–302 (in Japanese)
Nishida R, Schulz S, Kim CS, Fukami H, Kuwahara Y, Honda K, Hayashi N (1996) Male sex pheromone of a giant danaine butterfly, Idea leuconoe. J Chem Ecol 22:949–972. doi:10.1007/BF02029947
Ômura H, Honda K (2005) Chemical composition of volatile substances from adults of the swallowtail, Papilio polytes (Lepidoptera: Papilionidae). Appl Entomol Zool 40:421–427. doi:10.1303/aez.2005.421
Ômura H, Honda K, Hayashi N (2001) Identification of odoriferous compounds from adults of a swallowtail butterfly, Papilio machaon (Lepidoptera: Papilionidae). Z Naturforsch 56c:1126–1134
Pivnick KA, Lavoie-Dornik J, McNeil JN (1992) The role of the androconia in the mating behaviour of the European skipper, Thymelicus lineola, and evidence for a male sex pheromone. Physiol Entomol 17:260–268. doi:10.1111/j.1365-3032.1992.tb01020.x
Schulz S, Boppre M, Vane-Wright RI (1993) Specific mixtures of secretions from male scent organs of African milkweed butterflies (Danainae). Philos Trans R Soc Lond B 342:161–181. doi:10.1098/rstb.1993.0144
Shirôzu T (2006) Erynnis montanus. In: The standard of butterflies in Japan. Gakken, Tokyo, pp 301 (in Japanese)
Smith RJ, Grula EA (1982) Toxic components of the larval surface of the corn earworm (Heliothis zea) and their effects on germination and growth of Beauveria bassiana. J Invertebr Pathol 39:15–22. doi:10.1016/0022-2011(82)90153-7
Warren AD, Ogawa JR, Brower AVZ (2008) Phylogenetic relationships of subfamilies and circumscription of tribes in the family Hesperiidae (Lepidoptera: Hesperioidea). Cladistics 24:642–676. doi:10.1111/j.1096-0031.2008.00218.x
Warren AD, Ogawa JR, Brower AVZ (2009) Revised classification of the Hesperiidae (Lepidoptera: Hesperioidea) based on combined molecular and morphological data. Syst Entomol 34:467–523. doi:10.1111/j.1365-3113.2008.00463.x
Wüest J (1998) The pheromone dispersing apparatus in some Hesperiinae (Lepidoptera: Hesperiidae). Rev Suisse Zool 105:813–822
Yildizhan S, van Loon J, Sramkova A, Ayasse M, Arsene C, ten Broeke C, Schulz S (2009) Aphrodisiac pheromones from the wings of the small cabbage white and large cabbage white butterflies, Pieris rapae and Pieris brassicae. Chem Bio Chem 10:1666–1677. doi:10.1002/cbic.200900183
Acknowledgments
We greatly thank Professor Takao Itoh for initiating this study and Dr Akihiro Yoshida for providing valuable comments about androconia morphology. This study was supported in part by a Grant-in-Aid for Scientific Research from the Japan Society for the Promotion of Science (JSPS) to H.O. (No. 22780047).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ômura, H., Honda, K. Pungent odor of the adult skipper butterfly Erynnis montanus (Lepidoptera: Hesperiidae). Appl Entomol Zool 46, 281–286 (2011). https://doi.org/10.1007/s13355-011-0039-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13355-011-0039-9