Abstract
Haptoglobin is a hemoglobin-binding acute-phase protein which possesses anti-inflammatory and antioxidative properties. In this study, we investigated changes in protein expression of rat haptoglobin under diabetes-related inflammatory and oxidative stress conditions induced by an i.p. injection of streptozotocin. The progress of diabetes during an 8-week follow-up period was associated with the increased presence of haptoglobin in the serum and in the liver. This increase was most prominent during the first 2 weeks after which it started to decline. Temporary changes in haptoglobin expression strongly correlated with the serum levels of TNF-α and IL-6. Lower haptoglobin expression at the fourth week and thereafter correlated with a decrease in TNF-α concentration and changes in the TNF-α/IL-6 ratio. Based on the decrease of GSH/GSSG ratio and antioxidant enzyme activities in the liver until the end of fourth week, it was concluded that the liver was exposed to oxidative stress and injury which in the presence of the abovementioned inflammatory mediators lead to different haptoglobin expression profiles at different stages of diabetes. An inverse correlation was observed between the haptoglobin and free iron serum levels in diabetic rats. The higher levels of haptoglobin during the first 2 weeks were accompanied by a lower level of free iron. In view of the established function of haptoglobin, we discuss its possible role in decreasing oxidative stress during the early stage of diabetes.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Haptoglobin is a hemoglobin scavenger protein that is constitutively present in the serum. It belongs to the functionally heterogenous group of proteins that are referred to as positive acute-phase (AP) proteins whose synthesis and secretion by hepatocytes and subsequent serum concentrations undergo to an increase in the course of the AP response [12]. The AP response represents a major pathophysiological reaction in which the physiological, homeostatic mechanisms are modified to different set-points. This response is a major contributor to an organism’s defensive and adaptive capabilities against infection, inflammation, and oxidative stress. Haptoglobin possesses anti-inflammatory and immunomodulatory properties and functions as an antioxidant as a direct result of its ability to bind free hemoglobin and thereby prevent iron-driven oxidative tissue damage [12, 22]. Haptoglobin knockout mice are more prone to oxidative tissue damage and mortality following hemolysis, demonstrating the importance of haptoglobin in the physiological defense against hemoglobin toxicity [22]. This ability of haptoglobin is lost when hemoglobin becomes heavily glycosylated as occurs in the diabetic state [2].
It has been suggested that haptoglobin could serve as a predictive biomarker for virus-induced autoimmune type 1 diabetes and for pathogenesis of non-insulin-dependent type 2 diabetes since mutations in the human haptoglobin gene are associated with increased risk of diabetic complications [18]. Oxidative stress is an accepted important contributing factor in the development and progression of both types of diabetes [34]. The main factors that lead to increased production of reactive oxygen species (ROS) in the diabetic state are glucose autoxidation, nonenzymatic protein glycation, and formation of advanced glycation end-products. Other important factors include the imbalance of cellular oxidation/reduction processes, lower antioxidant defenses resulting from decreased levels of cellular antioxidant molecules, and reduced activities of antioxidant enzymes.
At the cellular level, oxidative stress leads to a spectrum of responses depending on the cell type, ROS levels, and duration of exposure [24]. High and/or sustained levels of ROS result in oxidative damage of cell components that leads to cell dysfunction and tissue injury. These events are implicated in pancreatic β-cell destruction and pathogenesis of type 1 diabetes as well as in liver injury [17], and are causal factors of the chronic inflammatory state. Chronic inflammation is typically assessed by measuring inflammatory biomarkers which in the systemic circulation include the inflammatory cytokines and the AP proteins such as haptoglobin. Since concentrations of AP proteins do not increase uniformly under different stress conditions, it was suggested that they are regulated by stressor-specific patterns of inflammatory mediators [12]. Interleukin (IL)-6 and tumor necrosis factor (TNF) α are the main proinflammatory cytokines driving liver production of haptoglobin [12]. Several studies have reported increases of IL-6 and TNF-α serum concentrations in diabetic individuals [10, 33]. Elevated plasma IL-6 and TNF-α concentrations are also observed in acute hyperglycemia in non-diabetic and subjects with impaired glucose tolerance [11]. This effect is abolished by infusion of the antioxidant glutathione (GSH), suggesting that hyperglycemia-induced cytokine production is mediated by ROS.
In humans, two alleles denoted as 1 and 2 have been reported for the haptoglobin gene, as well as three phenotypes, Hp 1–1, Hp 2–1, and Hp 2–2, of which the Hp 1–1 phenotype exhibits superior antioxidant activity [26]. Rats possess a single haptoglobin phenotype that is homologous to human Hp 1–1 [15]. Many reports have established a strong association between the haptoglobin phenotypes and complications of diabetes [2, 26]. However, data analyzing the relationship between protein expression of haptoglobin and oxidative stress status during progression of diabetes are limited. Thus, our study was partly aimed at clarifying whether changes in haptoglobin protein expression in the rat during progression of diabetes induced by streptozotocin (STZ) administration, correlate with pro- and antioxidative, as well as with the overall inflammatory status.
Haem-containing proteins are extremely susceptible to damage by glucose and glycation, with a resulting liberation of redox-active free iron which is capable of generating hydroxyl radicals and causing lipid peroxidation [8]. Physiologically, the ability of haem-containing proteins to catalyze iron-induced oxidative stress is counteracted by the binding of hemoglobin to haptoglobin, and of the haem prosthetic group to hemopexin, followed by clearance of the resulting complexes by the monocytes/macrophages and hepatocytes. Since diabetes is associated with disturbances in the iron status [38], correlation between diabetes-related haptoglobin expression and the free iron levels in the serum was also investigated.
Materials and methods
Animals and treatment
Animal procedures were approved by the Committee for Ethical Animal Care and Use of the Institute for Biological Research, Belgrade, which acts in accordance with the Guide for the Care and Use of Laboratory Animals, published by the US National Institute of Health (NIH Publication No. 85/23, revised in 1986). Male Wistar rats weighing approximately 220 g were used. Animals were housed three per cage at constant temperature (22 ± 2 °C), humidity (50 ± 5 %) and 12 h dark/light intervals. Tap water and standard laboratory chow were available ad libitum. Diabetes was induced by a single intraperitoneal injection of STZ (60 mg/kg body weight; Sigma, St. Louis, MO, USA) that was dissolved in freshly prepared 0.01 mol/l sodium citrate, pH 4.5. Control rats received only citrate buffer. Blood samples for glucose monitoring by a hand-held glucometer (GlucoSure Plus, Apex Biotechnology Corp, Taiwan) were obtained from the tail tip. Rats with a fasting blood glucose concentration greater than 20 mmol/l were considered as diabetic. All the experiments were replicated three times, each time with new groups of STZ-treated and control, untreated rats.
Tissue preparation
At the end of the first, second, fourth, and eighth weeks of STZ treatment, age-matched control and diabetic rats were weighed and killed by decapitation. For each week, the control and diabetic group were comprised of six rats each. Blood was rapidly collected in tubes without or with heparin for estimation of enzymes and other parameters. Serum, plasma, and erythrocytes were obtained after centrifugation at 2,000×g in an Eppendorf 5415 R centrifuge for 15 min, and stored at −20 °C. In samples used for assaying catalase (CAT) activity, the isolated erythrocytes were washed three times with three volumes of ice-cold 155 mmol/l NaCl and hemolysates containing about 50 g of hemoglobin (Hb) per liter (Hb/l) were used. For the assay of superoxide dismutase (SOD) activity the hemolysates were diluted with distilled water (1:7 v/v) and treated with chloroform–ethanol (0.6:1 v/v) in order to remove hemoglobin.
Livers were removed, weighed, frozen in liquid nitrogen, and kept at −80 °C. For assays of haptoglobin protein expression in the liver whole liver homogenates were prepared. Frozen rat liver tissue was homogenized at 4 °C in a Dounce tissue grinder in ice-cold homogenization buffer (10 mM Tris pH 7.6, 1 mM EDTA, 250 mM sucrose) containing a mixture of protease and phosphatase inhibitors (5 mg/ml aprotinin, 0.1 mM PMSF, 1 mM NaVO3, and 5 mg/ml leupeptin). The homogenate was centrifuged at 10,000×g for 20 min at 4 °C (Eppendorf 5415 R centrifuge), and the resulting supernatant was aliquoted and stored at −80 °C. For measuring the hepatic activities of antioxidative enzymes, a 10 % homogenate of fresh liver was prepared in sucrose buffer (0.25 M sucrose, 1 mM EDTA, 0.05 M Tris–HCl, pH 7.4). The homogenate was centrifuged (100,000×g for 90 min at 4 °C) and stored at −80 °C in aliquots until use.
Biochemical assessment
Serum glucose was estimated by the glucose oxidase procedure using a glucose GOD/PAP test kit (Randox Laboratories, UK). The total cholesterol level was measured by the enzymatic cholesterol esterase-cholesterol oxidase method using a CHOD-PAP test kit (Randox Laboratories, UK). Serum triglycerides were measured by the GPO-PAP method with an enzymatic kit (Randox Laboratories, UK). Quantitative determination of glycohemoglobin (HbA1c) in whole blood was performed with the hemoglobin A1c reagent test (Point Scientific Inc., USA). Activities of the alanine aminotransferases (ALT) and aspartate aminotransferases (AST) were estimated in the serum by measuring the produced oxaloacetate and pyruvate, respectively, using an optimized standard UV kinetic method kit (GPT (ALAT) IFCC mod., GOT (ASAT) IFCC mod). Serum albumin concentration was evaluated by the bromocresol kit (Randox Laboratories). Serum concentrations of rat insulin, TNF-α and IL-6 were measured by the ELISA method according to the protocol provided by the manufacturer (Mercodia, Uppsala, Sweden for insulin; eBioscience, San Diego, CA for TNF-α; and Bender MedSystems GmbH, Vienna, Austria for IL-6). Serum concentrations of free iron were measured spectrophotometrically according to the manufacturer’s instruction using the QuantiChromTM Iron Assay Kit (BioAssay Systems, USA).
Measurement of serum concentration of haptoglobin
The serum levels of haptoglobin were assessed by rocket immunoelectrophoresis with goat anti-human haptoglobin antibody (Sigma-Aldrich, Milwaukee, WI) which is cross-reactive with rat haptoglobin. The method is based on immunoprecipitation between the haptoglobin present in the serum and the polyspecific anti-haptoglobin antibody incorporated in the agarose gel in regions of their optimal concentrations, and is characterized by the formation of immunoprecipitation peaks. The area under the formed immunoprecipitation peak was used to quantify the relative haptoglobin concentration in the serum. The peaks were drawn out on tracing paper and their areas were quantified. The values obtained after quantification were expressed as the means ± SE from three separate experiments.
Western immunoblot analysis of haptoglobin protein expression in the liver
Proteins from the whole liver homogenate (40 μg) were separated by 12 % sodium-dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE) and transferred to nitrocellulose membranes (Hybond-C, Amersham Pharmacia Biotech, Uppsala, Sweden) and immunoblotted with goat anti-human haptoglobin antibody (Sigma-Aldrich, Milwaukee, WI). After incubation with blocking solution (0.2 % Tween 20, 50 mM Tris–HCl pH 7.6, 150 mM NaCl, and 5 % nonfat condensed milk), the membranes were incubated with primary antibody for 1.5 h at room temperature. After rinsing, the blots were incubated with horseradish peroxidase (HRP)-conjugated anti-goat immunoglobulin as a secondary antibody for 1 h. Immunoreactive bands were identified by an enhanced chemiluminescence detection system according to the manufacturer’s instructions (Santa Cruz Biotechnology, Santa Cruz, CA). The blots were scanned and the intensities of the signals were quantified using TotalLab (Phoretix, Newcastle upon Tyne, England) electrophoresis software (ver. 1.10).
Determination of the GSH/GSSG ratio in liver homogenate
Liver homogenates were deproteinated with 5 % 5-sulfosalicylic acid, centrifuged at 7,000×g for 5 min at 4 °C. The supernatants were neutralized with triethanolamine to a final concentration of 0.2 M. Supernatant fractions were assayed for total glutathione and oxidized glutathione (GSSG) by the standard recycling method. Fifty microliters of the deproteinated 50× diluted sample were transferred into microtiter plate wells. After the addition of 150 μl of the reaction mixture (0.1 M sodium phosphate buffer (pH 7.5) containing 1 mM EDTA, 0.3 mM DTNB, 0.4 mM NADPH, and 1 U/ml glutathione reductase I), and incubation for 30 min at room temperature, the reaction rate was monitored by measuring the absorption at 412 nm. The GSH content was evaluated using a calibration curve established with a series of dilutions of the glutathione standard which was processed as the samples. Samples for GSSG determination were incubated at room temperature with 2 μl 0.5 M 2-vinylpyridine solution (2VP) per 50 μl of a 50× diluted sample for 1 h. Incubation with 2-vinyl pyridine conjugates any reduced glutathione present in the sample so that only GSSG is recycled to GSH without interference by GSH. Standard amounts of GSSG were treated in the same way. After a 1-h incubation at room temperature, 2VP-treated samples and standards were assayed as described above using a calibration curve. The GSSG (as GSH × 2) was then subtracted from the total glutathione to determine the actual GSH level and GSH-to-GSSG ratio.
Determination of plasma superoxide anion radical concentration
The plasma concentration was estimated by the nitroblue tetrazolium (NBT) photometric assay which is based on the reduction of NBT by superoxide anion radicals to form diformazan, a dark insoluble precipitate that absorbs at 550 nm [3]. The first absorbance (A1) was detected spectrophotometrically at 550 nm in a reaction mixture containing 50 mM Tris–HCl buffer, pH 8.6 (with 0.1 mM EDTA, 0.1 mM NBT, and 0.1 mg/ml gelatin). To 0.19 ml of the reaction mixture was added 0.01 ml of the sample. The change in absorbance was monitored until stabilization was evidenced for 5 min (A2). The blank test contained 0.19 ml of the reaction mixture and 0.01 ml of dH2O. The concentration of O −2 was calculated from the differences in absorbance (A2 − A1) and the molar extinction coefficient for diformazan at 550 nm, and expressed in nanomoles of NBT per milliliter of plasma.
Determination of plasma hydrogen peroxide
Determination of the hydrogen peroxide (H2O2) concentration was performed according to Pick and Keisari [32]. The assay is based on HRP-mediated oxidation of phenol red by H2O2 which results in the formation of a compound demonstrating increased absorbance at 610 nm. The sample mixture, which included 0.1 ml of plasma and 1 ml of phenol red solution, was incubated for 10 min at room temperature. The reaction was stopped by adding 0.02 ml of 1 N NaOH, and the acidity of the contents of the test tubes was adjusted to pH 12.5. Absorbance of the sample was measured spectrophotometrically at 610 nm; 0.1 ml dH2O served as a blank. A linear relationship between absorbance at 610 nm and the concentration of H2O2 was established in the 1–60 μM (1–60 nmol/ml) range.
TBARS assay
The thiobarbituric acid reactive substances (TBARS) assay is based on the reaction of malondialdehyde (MDA) with thiobarbituric acid (TBA) at 95 °C. The level of TBARS was determined according to the method of Ohkawa et al. [31]. Briefly, 0.1 ml aliquots of the plasma were mixed with 0.2 ml of 8.1 % SDS, 1.5 ml of 20 % acetic acid (pH 3.5), 1.5 ml of 0.8 % TBA, and 0.7 ml H2O, and heated at 95°C for 60 min. After cooling to room temperature, 1 ml of H2O and 5 ml of n-butanol/pyridine (15:1, v/v) were added, mixed, and centrifuged at 3,000×g for 10 min. The absorbance of the organic layer was measured at 532 nm and the standard curve was plotted, using MDA as standard.
Determinations of antioxidative enzyme activities
Glutathione-S-transferase (GST) activity was determined spectrophotometrically by the method of Habig, Pabst, and Jakoby [13]. The method is based on the reaction of 1-chloro-2,4-dinitrobenzene (CDNB) with the –SH group of GSH, catalyzed by GST contained in the samples. The reaction mixture (3 ml) contained 0.6 ml 0.5 M sodium phosphate buffer, pH 6.5, 0.1 ml 30 mM CDNB, and twice-distilled water. After pre-incubating the reaction mixture at 37 °C for 5 min, the reaction was started by the addition of 100 μl of plasma or 20 μl of liver homogenates and 0.3 ml 20 mM GSH. The reaction mixture without the enzyme was used as blank. After mixing, the rate of increase in absorbance for 3 min was measured at 340 nm. Activity was expressed as nanomoles of GSH per minute per milliliter of plasma (units per milliliter of plasma) or nanomoles of GSH per minute per milligram of protein (units per milligram of protein) in liver homogenates. CAT activity was determined by the method of Beutler [5]. The reaction is based on the rate of H2O2 degradation by catalase contained in the examined samples. The reaction was performed in an incubation mixture containing 50 μl buffer (1 M Tris–HCl, 5 mM EDTA, pH 8.0), 5–10 μl sample, and 1 ml 10 mM H2O2 (A = 0.800–0.860). The rate of decrease in absorbance was measured at 230 nm. CAT activity is expressed as micromoles of H2O2 per minute per milliliter of blood (units per milliliter) in hemolysates or as micromoles of H2O2 per minute per milligram of protein (units per milligram of protein) in liver homogenates where one CAT activity unit is defined as 1 μmol of H2O2 decomposed per minute under the assay conditions. SOD activity, based on its capacity to inhibit auto-oxidation of epinephrine to adrenochrome, was determined by measuring the inhibition of the rate of autocatalytic adrenochrome formation at 480 nm, in a reaction medium containing 3 mM epinephrine dissolved in 0.1 N HCl and 0.05 M Na2CO3 with 1 mM EDTA, pH 10.2 [27]. The reaction was performed at a constant temperature (25 °C) for 3 min. The enzymatic activity was expressed as SOD units per gram of Hb (units per gram of Hb) in the hemolysates, or as SOD units per milligram of protein (units per milligram of protein) in the liver homogenates. One unit is defined as the amount of enzyme that inhibits the rate of adrenochrome formation by 50 %.
Statistical analysis
Results are expressed as means ± SE. All results were compared with control animals (C). Because no differences were found between control rats at the different time-points in any of the studied variables (except for body and liver weight), control data were pooled for statistical analysis. Data were analyzed using the non-parametric Mann–Whitney two-tailed test (Statistica v.5.0, StatSoft, Tulsa, OK). The level of significance was established at p < 0.05.
Results
Diabetic and inflammatory status of STZ-treated rats
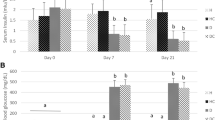
Treatment of rats with STZ produces a diabetic state characterized by a reduction of body weight (Fig. 1) and elevation of serum glucose levels (Table 1). One, 2, 4, and 8 weeks after treatment, the body weight of diabetic rats was 32.7, 34.6, 44.8, and 42.1 %, respectively, lower compared to age-matched control rats. The initial mean value for serum glucose concentration was 6.3 ± 0.5 mmol/l. Starting from the first week after STZ treatment, the serum glucose levels were about 5-fold higher (p < 0.05) in all diabetic groups compared to the control rats. Concomitant with the reduction in body weight and hyperglycemia, the diabetic state induced by STZ resulted in hypoinsulinemia, hypercholesterolemia, and hypertriglyceridemia at all time intervals (Table 1). In the diabetic groups, the reduction in serum insulin level was about 3-fold (p < 0.05). The serum cholesterol and triglyceride levels began to increase by the first week after STZ administration. The largest increase was observed at the end of fourth week when 2-fold (p < 0.05) and 9-fold (p < 0.05) increases of cholesterol and triglycerides were measured, respectively. The level of HbA1c, an important indicator of diabetes, progressively increased with diabetes progression. The most significant rise in the level of HbA1c in whole blood was observed 4 weeks after STZ administration (around 6-fold).
Changes in body weight, liver weight, and relative liver weight (liver to body weight ratio) of Wistar rats 1, 2, 4, and 8 weeks after diabetes induction by streptozotocin (STZ) administration. Data are given as percent change of respective control values (100 %). Values are shown as the mean ± SE from three independent experiments (*p < 0.05)
The liver is an insulin-sensitive organ that undergoes functional abnormalities in individuals with untreated diabetes [20]. Figure 1 shows that the diabetes-related reduction in body weight was accompanied by a relative increase (p < 0.05) in liver weight (expressed as a percentage of the body weight) at all time intervals. This finding suggests that the liver undergoes progressive hypertrophy that is most likely linked to changes of its functioning. This is partly confirmed by results showing a mild and chronic elevation of serum activities of AST and ALT, established markers of liver injury and inflammation (Table 1). The highest serum levels of both enzymes were observed at the end of the fourth week after STZ administration. The increases ranged from 1.4- to 2-fold (p < 0.05) for AST, and from 2.1- to 3.2-fold (p < 0.05) for ALT. During progression of STZ-induced diabetes, the liver underwent changes in synthetic activity, presented as a decrease in serum concentrations of albumin (1.2–1.4-fold, p < 0.05) at all post STZ intervals (Table 1). The decrease in serum albumin can be most likely attributed to the activation of inflammatory processes in the liver, since the inflammatory response of the liver is characterized by decreased synthesis and secretion of negative AP proteins such as albumin and transferrin, and increased synthesis and secretion of positive AP proteins, including haptoglobin.
Concentrations of TNF-α, IL-6, and haptoglobin during diabetes progression
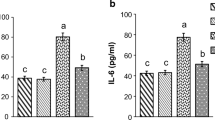
The diabetes-related changes in concentration of serum inflammatory markers TNF-α, IL-6, and haptoglobin are presented in Fig. 2. While their concentrations in the control group remained unchanged throughout the 8-week follow-up period, in diabetic rats the concentrations of all three markers were elevated. The concentration of IL-6 increased progressively with diabetes progression. The highest increase was observed at the end of the fourth week when it was 3.4-fold (p < 0.05) higher than in the control group (Fig. 2a). The highest increases in concentrations of TNF-α (Fig. 2b) and haptoglobin (Fig. 2c) were measured at the end of the second week (9.8-fold for TNF-α and 4.2-fold for haptoglobin). After the second week, their concentrations exhibited a downward trend. Compared to the control, at the end of eighth post STZ administration week, the concentrations of TNF-α and haptoglobin were 2.2-fold and 1.3-fold (p < 0.05) higher, respectively. The diabetes-related temporal changes in serum concentration of haptoglobin essentially followed a similar profile of increase as the change in haptoglobin protein levels in the liver (Fig. 2d). Scans of the Western immunoblots, revealed 2.8- (p < 0.05) and 4-fold (p < 0.05) increases of haptoglobin β-chain levels in total liver homogenates at the end of first and second weeks, respectively. Thereafter the degree of relative increase of the haptoglobin protein level in the liver declined progressively. At the end of eighth week the relative haptoglobin increase was 1.3-fold higher than in the controls.
Diabetes-related changes in the concentrations of inflammatory biomarkers TNF-α and IL-6, and haptoglobin (Hp). a Concentration of TNF-α and b IL-6 in the serum of non-diabetic and STZ-induced diabetic rats determined by ELISA. c The relative haptoglobin concentration in the serum of diabetic rats determined by rocket immunoelectrophoresis and expressed as percent changes with respect to the control value (100 %). d Relative haptoglobin levels in whole liver homogenates from control and STZ-induced diabetic rats assessed by Western immunoblot analysis with anti-haptoglobin antibody and expressed as percent changes with respect to the control value (100 %); β-actin was used as a loading control; the immunoblots were quantified using TotalLab Phoretix electrophoresis software ver. 1.10. The values represent the means ± SE from three independent experiments; *p < 0.05 significantly different from the control
Correlation between diabetes-related haptoglobin expression and the free iron levels in the serum
At the end of the first week after induction of diabetes when the serum level of Hp rose 3.3-fold (Fig. 2c), a 1.3-fold (p < 0.05) increase in the level of free iron compared to control rats (Fig. 3) was measured. At the end of the second week when the highest level of serum haptoglobin was observed, the free iron level was below that of the control group. The decrease in haptoglobin protein expression detected in the fourth and eighth weeks (Fig. 2c) were accompanied by 1.8- and 2.2-fold increases (p < 0.01) in free iron levels (Fig. 3), respectively.
Diabetes-related changes of free iron levels in the serum. Iron concentrations were determined with the QuantiChromTM Iron Assay Kit. The data are expressed as percent changes with respect to the control (C, 100 %). The values represent the means ± SE from three independent experiments; *p < 0.05 significantly different from the control
Diabetes-related oxidative stress status in blood and liver
To examine whether expression of haptoglobin in diabetic rats correlates with the oxidative stress status, the concentrations of ROS, products of peroxidation, and the activities of individual antioxidative enzymes were measured. As can be seen on Fig. 4a, progression of diabetes was accompanied by a progressive increase in superoxide anion content in the plasma. The time course of changes of the plasma levels of H2O2 and TBARS, the markers of free radical production and lipid peroxidation, are shown in Fig. 4b. The level of H2O2 and TBARS were significantly higher (p < 0.05) at all time intervals in comparison to the respective control groups. The highest increase of H2O2 (about 2.5-fold), was observed at the end of the second week after the induction of diabetes, while the maximal increase (about 5-fold) of TBARS was measured at the end of the fourth week.
Changes in oxidative stress parameters after 1, 2, 4, and 8 weeks after diabetes induction by STZ administration. a Plasma concentration of the superoxide anion radical determined by the NBT photometric assay; b the plasma concentrations of hydrogen peroxide (H 2 O 2 ) and thiobarbituric acid reactive substances (TBARS) were assessed spectrophotometrically, as described in section “Materials and methods”; c ratio of reduced and oxidized gluthatione in the liver homogenate (GSH/GSSG). The values represent the means ± SE from three independent experiments; *p < 0.05 significantly different from the control
The liver is the central organ of oxidative and detoxifying processes. In general, when mammalian cells are exposed to increased oxidative stress, the ratio of GSH/GSSG decreases as a consequence of GSSG accumulation [22]. The GSH/GSSG ratio served as an indicator of oxidative stress in the livers of diabetic rats (Fig. 4c). The GSH/GSSG ratio was 2.1 in control livers. It decreased significantly (p < 0.05) to 1.18, 1.16, and 1.43, 1, 2, and 4 weeks after the induction of diabetes, respectively. At the end of follow-up period, the GSH/GSSG ratio was normalized. Based on these findings we concluded that the liver is exposed to ROS-mediated injury during the development of diabetes.
SOD and CAT are the two major scavenging enzymes that remove free radicals in vivo. The liver showed an overall decrease in the activities of SOD and CAT between the first and fourth weeks (Fig. 5). At the end of the eighth week the activities of these enzymes returned to near control levels. The decrease in SOD activity in the liver of diabetic rats was significant (p < 0.05) at the end of the first (27.6 % lower compared to the control level that was assumed to be 100 %) and at the end of the fourth week (18.3 %). Liver CAT activity decreased (p < 0.05) in diabetic rats to about 25.6, 59.7, and 21.6 % at the end of the first, second, and fourth weeks, respectively.
The activities of antioxidative enzymes in the liver and blood of STZ-induced diabetic rats 1, 2, 4, and 8 weeks after diabetes induction: a superoxide-dismutase (SOD); b catalase (CAT); and c glutathione-S-transferase (GST) activities were assessed spectrophotometrically, as described in section “Materials and methods”. Statistical significance: *p < 0.05 with respect to the non-diabetic control values. The values represent the means ± SE from three independent experiments
The activity of SOD in the blood was increased at all time intervals compared to the control, however, this increase was significant only at the end of the first week (1.2-fold). At same time, CAT activity in the blood did not significantly change until the second week. Thereafter its activity decreased to about 18.4 % (p < 0.05) at the end of the fourth week, and 34.2 % (p < 0.05) at the end of the eighth week compared to the control value.
In diabetic rats, liver GST activity was significantly lower until the end of the fourth week compared to the control value. Thereafter, GST activity decreased by 27.5 % (p < 0.05) during the first 2 weeks and 15 % (p < 0.05). At the end of first week, we observed a 1.2-fold increase (p < 0.05) in plasma GST activity. At the end of the second, fourth, and eighth weeks after diabetes induction, the activity of GST significantly decreased and the lowest level was measured at the end of the second week (a decrease by 58.3 % (p < 0.05)).
Discussion
The diabetic state has a strong inflammatory component that includes oxidative stress and iron abnormalities which are related to diabetic complications [36, 40]. In this study we investigated whether the diabetic conditions correlated with the protein expression of haptoglobin, a hemoglobin-binding AP protein possessing anti-inflammatory and antioxidative properties. Increased serum levels of haptoglobin have been documented in diabetic patients and in individuals exhibiting glucose intolerance [25]. In the present study, the concentration of haptoglobin was found to be differentially increased in the serum and in the liver of STZ-treated rats throughout the 8-week follow-up period (Fig. 2c, d). Upregulation of haptoglobin protein in the serum and liver, progressively increased after the STZ injection, peaked at the end of second week, and remained elevated but at lower levels up to the end of eighth week. Haptoglobin is secreted primarily by the liver in response to proinflammatory cytokines IL-1, IL-6, and TNF-α [12]. Temporal changes in diabetes-related haptoglobin expression were followed by similar changes in the serum concentration of TNF-α and by progressive increase in the concentration of IL-6 with its maximal level detected at the end of the fourth week. Increased serum levels of haptoglobin and TNF-α and IL-6 during diabetes point to dose-related effects of these pro-inflammatory cytokines on haptoglobin expression which is in agreement with Baumman et al. [4]. The authors showed that IL-6 alone and in combination with IL-1β, TNF-α, and dexamethasone leads to a dose-dependent induction of production in cultured rat liver cells. Vadas et al. [39] also reported that the combined actions of TNF-α with IL-6 were additive for haptoglobin production in human hepatoma cells. Given that the maximal expression of haptoglobin correlated with the maximal concentration of TNF-α, we concluded that the lower expression of haptoglobin observed at the fourth week post diabetes induction and thereafter was the result of decreased concentration of TNF-α and changes in the TNF-α/IL-6 ratio.
Temporal changes in haptoglobin protein expression could also be related to changes in the synthetic capacity of hepatocytes during chronic diabetes. Diabetes is associated with structural and functional changes in the liver, in particular, hepatomegaly caused by hypertrophy, hyperplasia, and apoptosis imbalance [16]. Ultrastuctural analysis of liver biopsies from diabetic patients revealed that the protein-synthesizing compartment of the majority of hepatocytes is reduced during chronic diabetes [29]. According to Lenk et al. [21], the defect in secretory protein synthesis in diabetic liver is the consequence of inadequate insulin levels which are necessary to maintain the state of aggregation of the endoplasmatic reticulum-bound polysomes for secretory protein synthesis. In our study, hypoinsulinemia (Table 1), was observed from the first week after STZ treatment. Noorafshan et al. [30] reported that from the fourth week after STZ administration, the hepatocyte volume decreases in the periportal zone, the main area for protein metabolism and plasma protein synthesis [6]. This is followed by changes in nuclear size and consequently reduction in nuclear activity and protein synthesis. Using the comet assay we observed increased DNA damage in the hepatocytes on the fourth week of diabetes induction (unpublished data). Since expression of haptoglobin depends on the number of synthesizing hepatocytes [6], diabetes-related injury of hepatocytes and reduction of the liver’s protein-synthesizing compartment could be responsible for the reduction in haptoglobin protein expression that was observed at the fourth and eighth weeks of diabetes.
A high hyperglycemic level (Table 1), increased production of ROS, superoxide anion radical, and H2O2 and significant lipid peroxidation (Fig. 4a, b) lead to oxidative stress and liver injury, reflected by a progressive increase in serum ALT and AST levels (Table 1). Oxidative stress in the liver was demonstrated by a decrease in the GSH/GSSG ratio and lower activities of antioxidative enzymes GST, SOD, and CAT (Fig. 4c) between the first and fourth weeks after STZ administration. The obtained results are in agreement with the data showing that in the diabetic state, the redox state, and the level of antioxidative defenses in tissues are lower in comparison with the non-diabetic state [17]. According to Loven et al. [23], the decrease in liver GSH is due to its increased utilization by hepatic cells, which could be the result of decreased synthesis or increased degradation of GSH by oxidative stress. GSH is an endogenous antioxidant which belongs to the first line of defense, acting by scavenging potentially damaging free radical moieties and reducing H2O2. It is also a cofactor of several enzymes, including GST, which provides a second line of defense by primarily detoxifying noxious byproducts that are generated by ROS and by preventing the propagation of free radicals. Therefore, the reduced GST activity observed in diabetic liver (Fig. 5) might be the result of a reduced GSH content. This may result in a number of deleterious effects due to the accumulation of toxic products. In this context, other researchers also reported a decrease in the activity of SOD and CAT in livers of diabetic rats [14, 35]. The decreased activities of these enzymes in the liver reflect the increased production of reactive oxygen radicals that can themselves reduce the activity of these enzymes. We detected higher levels of haptoglobin in the livers of diabetic rats and lower antioxidative defense. This points to a cumulative influence of antioxidative disturbances as well as of inflammation on the level of haptoglobin expression.
Unexpected results were obtained in the livers of rats on the eighth week post STZ administration. From the fourth week, the diabetic livers exhibited improvement of the GSH/GSSG ratio and an increase in antioxidative enzyme activity, resulting in a normal GSH/GSSG ratio and antioxidative defense by the eighth week. This could be result of a compensatory adaptation observed in chronic diabetes [29] and the regenerative effects of IL-6. It was reported that IL-6, produced in response to circulating TNF-α or other cytokines is a critical component of the regenerative response [7]. Its high circulating levels were found in several liver disease. IL-6 can ameliorate the liver inflammatory response by downregulating TNF-α production [7]. This could be a reason for the observed decrease in serum concentration of TNF-α from the fourth week (Fig. 2a) and related haptoglobin expression. While these results point to an inverse relationship between protein expression of haptoglobin in the liver and its oxidative status, no significant association was observed between haptoglobin expression and oxidative stress parameters in the circulation. In diabetes there are controversial results concerning antioxidant enzyme activities. In our study, SOD activity was elevated in the circulation of diabetic rats probably as a result of increased oxidative stress. The decreased levels of CAT and GST activities in the diabetic groups may possibly result from a compensatory mechanism of the body.
The results presented in this study suggest that in rats, activation of the inflammatory response occurs in the early stage of diabetes development. This period is characterized by significant elevation of serum haptoglobin levels, suggesting that haptoglobin could be viewed as an early biomarker that could be used to predict the development of diabetes. This is in agreement with Kruger et al. [18] who observed the elevation of serum haptoglobin before type 1 diabetes or its complications appeared in biobreeding diabetes-resistant rats after virus infection. Haptoglobin is a multifunctional protein involved in the regulation of a variety of processes, including immune responses, angiogenesis, prostaglandin synthesis, and malignancy [12]. However, the primary function of haptoglobin is scavenging of hemoglobin that escapes into the circulation and its transport to the liver and macrophages, thus protecting the body from harmful, iron-related oxidative processes. Over 75 % of the iron in the body is found in hemoglobin inside the erythrocytes. Diabetes is associated with structural and functional modifications of the erythrocyte membrane and with accelerated endothelial cell injury [19] which results in increased amounts of hemoglobin and hemoglobin-derived redox active iron in the circulation [38]. According to Roy, Sen, and Chakraborti [37], the extent of glycation in hemoglobin, weaker heme-globin linkage in HbA1c, and increased formation of H2O2 that induces iron release from hemoglobin, could be responsible for enhanced iron levels in the diabetic state. We found a lower increase in serum levels of free iron at the end of the first week and its significant elevation in the fourth and eighth weeks (Fig. 3). We also observed an inverse correlation between haptoglobin expression and the amount of free iron in the serums of diabetic rats. The higher concentrations of haptoglobin in sera of diabetic rats observed at the end of the first and in particular at the end of the second week, were accompanied by lower amounts of free iron. Asleh et al. [1] showed that the presence of redox active iron is haptoglobin and glucose dependent. They found that in the absence of diabetes, iron concentration was markedly elevated in the plasma of haptoglobin knockout (Hp 0) and mice genetically modified to express the Hp 2–2 phenotype which is biologically less active in binding free hemoglobin than phenotype Hp 1–1 [2, 28]. The plasma level of iron was further elevated in STZ-induced diabetes. By applying the in vitro cell culture paradigm, these authors also showed a haptoglobin- and glucose-dependent increase in oxidative stress in cells. Similar findings were reported in the study of Delanghe et al. [9] where HIV-infected patients carrying the Hp 2–2 phenotype were observed to have lower haptoglobin concentration and excess serum iron. This in turns induces viral replication, probably as a result of a decreased protection against hemoglobin/iron-driven oxidative stress. In accordance with this finding, the results presented in our study implicate a potential antioxidative role of haptoglobin in the early phase of diabetes. This antioxidant effect of haptoglobin appears to be inhibited or is considerably lower at later stages, i.e., at 4 and 8 weeks after diabetes induction. This could be, partially related to insufficient amounts of haptoglobin, and/or its abrogated affinity for hemoglobin as a result of its glycosylation, as we observed at the end of the fourth week of diabetes induction and thereafter (Table 1). However, further studies are needed, particularly on diabetic patients in which haptoglobin strategies aimed at limiting iron accumulations and oxidative stress are applied and their potential benefit in delaying the onset of diabetic complications is evaluated.
References
Asleh R, Guetta J, Kalet-Litman S, Miller-Lotan R, Levy AP (2005) Haptoglobin genotype- and diabetes-dependent differences in iron-mediated oxidative stress in vitro and in vivo. Circ Res 96:435–441
Asleh R, Marsh S, Shilkrut M, Binah O, Guetta J, Lejbkowicz F, Enav B, Shehadeh N, Kanter Y, Lache O, Cohen O, Levy NS, Levy AP (2003) Genetically determined heterogeneity in hemoglobin scavenging and susceptibility to diabetic cardiovascular disease. Circ Res 92:1193–1200
Auclair C, Voisin E (1985) Nitroblue tetrazolium reduction. In: Greenwald RA (ed) Handbook of methods for oxygen radical research. CRC, Boca Raton, pp 123–132
Baumann H, Prowse KR, Marinković S, Won K-A, Jahreis GP (1989) Stimulation of hepatic acute phase response by cytokines and glucocorticoids. Ann N Y Acad Sci 557:280–295
Beutler E (1982) Catalase. In: Beutler E (ed) Red cell metabolism: a manual of biochemical methods. Grune and Stratton, New York, pp 105–106
Courtoy PJ, Lombart C, Feldmann G, Moguilevsky N, Rogier E (1981) Synchronous increase of four acute phase proteins synthesized by the same hepatocytes during the inflammatory reaction: a combined biochemical and morphologic kinetics study in the rat. Lab Invest 44:105–115
Cressman DE, Greenbaum LE, DeAngelis RA, Ciliberto G, Furth EE, Poli V, Taub R (1996) Liver failure and defective hepatocyte regeneration in interleukin-6-deficient mice. Science 274:1379–1383
Cussimanio BL, Booth AA, Todd P, Hudson BG, Khalifah RG (2003) Unusual susceptibility of heme proteins to damage by glucose during non-enzymatic glycation. Biophys Chem 105:743–755
Delanghe JR, Langlois MR, Boelaert JR, van Acker J, van Wanzeele F, van der Groen G, Hemmer R, Verhofstede C, De Buyzere M, De Bacquer D, Arendt V, Plum J (1998) Haptoglobin polymorphism, iron metabolism and mortality in HIV infection. AIDS 12:1027–1032
Dogan Y, Akarsu S, Ustundag B, Yilmaz E, Gurgoze MK (2006) Serum IL-1β, IL-2 and IL-6 in insulin-dependent diabetic children. Mediators Inflamm 2006:1–6
Esposito K, Nappo F, Marfella R, Giugliano G, Giugliano F, Ciotola M, Quagliaro L, Ceriello A, Giugliano D (2002) Inflammatory cytokine concentrations are acutely increased by hyperglicemia in humans. Role of oxidative stress. Circulation 106:2067–2072
Gabay C, Kushner I (1999) Acute-phase proteins and other systemic responses to inflammation. New Engl J Med 340:448–454
Habig WH, Pabst MJ, Jakoby WB (1974) Glutathione S-transferases: the first enzymatic step in mercapturic acid formation. J Biol Chem 249:7130–7139
Hamdena K, Carreaub S, Elfeki A (2009) Inhibitory effects of Zinc on hyperglycaemia and metabolic disorders in the liver of alloxan-induced diabetic rats. Asian Biomed 3:745–750
Hanley JM, Haugen TH, Health EC (1983) Biosynthesis and processing of rat haptoglobin. J Biol Chem 258:7858–7869
Herrman CE, Sanders RA, Klaunig JE, Schwarz LR, Watkins JB (1999) Decreased apoptosis as a mechanism for hepatomegaly in streptozotocin-induced diabetic rats. Toxicol Sci 50:146–151
Kakkar R, Mantha SV, Radhi J, Prasad K, Kalra J (1998) Increased oxidative stress in rat liver and pancreas during progression of streptozotocin-induced diabetes. J Clin Sci 94:623–632
Kruger JA, Yang C, Tam WS, Hinerfeld D, Evans EJ, Green MK, Leszyk J, Yang K, Guberski LD, Mordes PJ, Greiner LD, Rossini AA, Bortell R (2010) Haptoglobin as an early serum biomarker of virus-induced autoimmune type 1 diabetes in biobreeding diabetes resistant and LEW1.WR1 rats. Exp Biol Med 235:1328–1337
Laight DW, Carrier MJ, Anggard EE (1999) Endothelial cell dysfunction and the pathogenesis of diabetic macroangiopathy. Diabetes Metab Res Rev 15:274–282
Leclercq IA, Morais ADS, Schroyen B, Hul NV, Geerts A (2007) Insulin resistance in hepatocytes and sinusoidal liver cells: mechanisms and consequences. J Hepat 47:142–156
Lenk SE, Bhat D, Blakeney W, Dunn WA Jr (1992) Effects of streptozotocin-induced diabetes on rough endoplasmic reticulum and lysosomes of rat liver. Am J Physiol 263:856–862
Lim SK, Kim H, bin Ali A, Lim YK, Wang Y, Chong SM, Costantini F, Baumman H (1998) Increased susceptibility in Hp knockout mice during acute hemolysis. Blood 92:1870–1877
Loven D, Schedl H, Wilson H, Diekus M (1986) Effect of insulin and oral glutathione on glutathione levels and superoxide dismutase activities in organs of rats with streptozotocin induced diabetes. Diabetes 35:503–507
Martindale JL, Holbrook NJ (2002) Cellular response to oxidative stress: signaling for suicide and survival. J Cell Physiol 192:1–15
McMillan DE (1989) Increased levels of acute-phase serum proteins in diabetes. Metabolism 38:1042–1046
Melamed-Frank M, Lache O, Enav BI, Szafranek T, Levy NS, Ricklis RM, Levy AP (2001) Structure-function analysis of the antioxidant properties of haptoglobin. Blood 98:3693–3698
Misra HP, Fridovich I (1972) The role of superoxide anion in the autoxidation of epinephrine and a simple assay for superoxide dismutase. J Biol Chem 247:3170–3175
Moreira LRS, Miranda-Virela AL, Silva ICR, Akimoto AK, Klautau-Guimarӓes MN, Grisolia CK (2009) Antioxidant effect of haptoglobin phenotypes against DNA damage induced by hydrogen peroxide in human leukocytes. Gen Mol Res 8:284–290
Nepomnyashchikh GI, Pavlenko OA, Aidagulova SV, Nepomnyashchikh DL (2001) Ultrastructural analysis of liver biopsy specimens in diabetes mellitus associated with chronic opisthorchiasis. Bull Exp Biol Med 132:1190–1194
Noorafshan A, Esmail-Zadeh B, Bahmanpour S, Poost-Pasand A (2005) Early stereological changes in liver of Sprague–Dawley rats after streptozotocin injection. Indian J Gastroenterol 24:104–107
Ohkawa H, Ohishi N, Yagi K (1979) Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal Biochem 95:351–358
Pick E, Keisari Y (1980) A simple colorimetric method for the measurement of hydrogen peroxide produced by cells in culture. J Immunol Methods 38:161–170
Pickup JC, Chusney GD, Thomas SM, Burt D (2000) Plasma interleukin-6, tumour necrosis factor alpha and blood cytokine production in type 2 diabetes. Life Sci 67:291–300
Rahimi R, Nikfar S, Larijani B, Abdollahi M (2005) A review on the role of antioxidants in the management of diabetes and its complications. Biomed Pharmacother 59:365–373
Rekna N, Balaji R, Deecaraman M (2008) Effect of aqueous extract of Syzygium cumini pulp on antioxidant defense system in streptozotocin induced diabetic rats. IJPT 7:137–145
Rossi R, Dalle-Donne I, Milzani A, Giustarini D (2006) Oxidized forms of glutathione in peripheral blood as biomarkers of oxidative stress. Clin Chem 52:1406–1414
Roy M, Sen S, Chakraborti AS (2008) Action of pelargonidin on hyperglycemia and oxidative damage in diabetic rats: Implication for glycation-induced hemoglobin modification. Life Sci 82:1102–1110
Swaminathan S, Fonseca VA, Alam MG, Shah SV (2007) The role of iron in diabetes and its complications. Diabetes Care 30:1926–1933
Vadas P, Grouix B, Stefanski E, Wloch M, Pruzanski W, Schroeder J, Gauldie J (1997) Coordinate expression of group II phospholipase A2 and the acute-phase protein haptoglobin (HP) and α1-anti-chymotrypsin (ACH) by HepG2 cells. Clin Exp Immunol 108:175–180
Wolff SP (1993) Diabetes mellitus and free radicals. Free radicals, transition metals and oxidative stress in the aetiology of diabetes mellitus and complications. Br Med Bull 49:642–652
Acknowledgments
This work was supported by the Serbian Ministry of Education and Science, grant no. 173020.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Jelena, A., Mirjana, M., Desanka, B. et al. Haptoglobin and the inflammatory and oxidative status in experimental diabetic rats: antioxidant role of haptoglobin. J Physiol Biochem 69, 45–58 (2013). https://doi.org/10.1007/s13105-012-0186-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13105-012-0186-7