Abstract
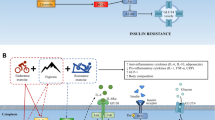
Obesity is an important public health problem worldwide and is a major risk factor for a number of chronic diseases such as type II diabetes, adverse cardiovascular events and metabolic syndrome-related features. Different treatments have been applied to tackle body fat accumulation and its associated clinical manifestations. Often, relevant weight loss is achieved during the first 6 months under different dietary treatments. From this point, a plateau is reached, and a gradual recovery of the lost weight may occur. Therefore, new research approaches are being investigated to assure weight maintenance. Pioneering investigations have reported that oxygen variations in organic systems may produce changes in body composition. Possible applications of intermittent hypoxia to promote health and in various pathophysiological states have been reported. The hypoxic stimulus in addition to diet and exercise can be an interesting approach to lose weight, by inducing higher basal noradrenalin levels and other metabolic changes whose mechanisms are still unclear. Indeed, hypoxic situations increase the diameter of arterioles, produce peripheral vasodilatation and decrease arterial blood pressure. Furthermore, hypoxic training increases the activity of glycolytic enzymes, enhancing the number of mitochondria and glucose transporter GLUT-4 levels as well as improving insulin sensitivity. Moreover, hypoxia increases blood serotonin and decreases leptin levels while appetite is suppressed. These observations allow consideration of the hypothesis that intermittent hypoxia induces fat loss and may ameliorate cardiovascular health, which might be of interest for the treatment of obesity. This new strategy may be useful and practical for clinical applications in obese patients.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Obesity: prevalence, causes and complications
Obesity is an important public health problem in most countries [63], which is characterized by an excess of body fat, when amounting values higher than 20% in men and 30% in adult women [20, 66].
The main etiological factors causing excess body weight for height are assigned to overeating and low physical activity, which lead to a positive energy balance, and result in a body fat increase [91]. Furthermore, there are other causes such as the genetic component, the distribution of energy intake throughout the day, the dietary composition of macronutrients, sleep rest, endocrine disruptions or the individual ability to oxidize energy substrates [26, 70, 93, 146]. Thus, obesity is a chronic multifactorial disease resulting from the interaction between genotype, environment and physical activity patterns [14, 91, 95].
Obesity is considered as one of the major risk factors in the onset of associated chronic diseases, such as hypercholesterolemia, type II diabetes (T2D), cardiovascular disorders and metabolic syndrome features [101]. On the other hand, the higher BMI and waist circumference are, the greater is the relative risk of comorbidities [118]. Indeed, obesity has become the second leading cause of premature and evitable death, just after tobacco [14, 49].
Treatments for obesity: diet, exercise, pharmacology and bariatric surgery
Low-calorie diets, programmes of physical activity (typically aerobic) and behavioural therapy are common strategies to lose weight in the obese patient [121]. These types of interventions are often the first step in any therapeutic approach to treat obesity. In general, it should be tested at least for 6 months before considering the introduction of any other therapeutic strategy [79]. The needs and characteristics of a treatment against obesity will be determined regarding the estimated risk in the obese patient and according to clinical criteria for therapeutic intervention [53]. Additionally, there are interindividual differences in the response to different dietetic interventions or to physical exercise due to the genetic predisposition [90, 112]. Thus, some attempts to set out differences depending on the genotype have been reported, although the information about the treatment success is relatively poor.
Other therapeutic approaches have been applied to induce weight loss, which may include exercise, psychological support, drug therapy and at last, surgery treatment [50], being the first two also prevention strategies. Therefore, long-term approaches to maintain weight loss are still sought.
Generally speaking, weight losses are achieved during the first 6 months of treatment. From this point, a plateau is often reached, and gradual recovery of the lost weight commonly occurs [39]. This outcome is facilitated by the fact that adherence to the diet and physical activity often declines and also due to a possible metabolic adaptation [10], therefore long-lasting therapies have been searched.
Dietetic and nutritional treatments
The most common treatment guidelines for obesity, currently in use, are dietary and nutritional approaches [1]. The restriction should not result in a caloric intake below 1,000–1,200 kcal/day in women and 1,200–1,600 kcal/day in men (except in case of morbid obesity), since those values are the minimum energy intake, which ensure a balanced intake of micronutrients in the requested amounts [2, 10, 103]. An energy restriction of 500–1,000 kcal/day compared to the usual diet results in weight loss of 0.5–1.0 kg/week, which represents an average of 8–10% of initial body weight in 6 months [1]. However, this weight loss is not completely linear [8].
There is some controversy regarding the distribution of macronutrients to be prescribed, being the classical approach, with respect to a 1,000–1,600 kcal/day, a distribution of 20% proteins, 50% carbohydrates and 25–35% fat [48], although moderate high-protein diets (30% energy) can also be applied [80]. Short-term weight reductions have been observed, but weight is regained in the long term [5]. It seems that one of the causes for the interruption in weight loss is a reduction in basal energy expenditure [106]. Protein intake is maintained high in some cases with the aim of slowing the loss of lean body mass and delaying the stabilization of weight [1, 28]. However, there are several unresolved issues, such as the prescription of moderately high-protein diets (20–35% of energy) during the weight maintenance phase or the beneficial effect of some foods with lower glycaemic index or glycaemic load on body weight [2, 36, 80, 144]. Considering high-protein or low-fat diets, it is important to increase the amount of fibre (more than 30 g/day) and the monounsaturated fat in order to facilitate the maintenance of a healthy circulating lipid profile [114]. In conclusion, one can notice that adherence is low in many of the extreme diets, with different proportions of macronutrients, being the cause that in the long term they are often not effective and obese people need other types of aid [39].
Physical activity programmes
Some guidelines state that 60–90 min of daily moderate exercise is requested for the treatment of obesity [67]. Traditionally, aerobic exercise has been recommended in order to lose body fat, but recent studies show that strength–endurance exercises (circuits of 6–10 exercises and 10–15 repetitions, with a frequency of 2–3 times/week) can be more effective [154], particularly to reduce abdominal fat [60, 97] and visceral adipose tissue [62].
The strength–endurance programmes tend to burn primarily abdominal fat, unlike fasting [141]. They also favour low appetite by stimulating the corticotrophin-releasing hormone [115]. The most effective treatment to improve health parameters as well as body composition appears to be strength training or strength training combined with aerobic endurance training, which seem to improve both health biomarkers and to reduce fat content [133, 135, 142, 145].
Since the energy cost of all exercise modes except walking seems to be beneficial, priority should be given to the obese preferences to promote long-term activity behavioural change [137]. Moreover, it is known that physical exercise increases the secretion of endorphins [74], improving mood, and helping the patient to maintain motivation increasing adherence to the treatment.
Strength–resistance training (SRT) is associated with great energy expenditure during the exercise session [30]. Some studies have reported that regular SRT is effective in promoting weight loss in obese subjects, decreasing fat mass and increasing lean body mass and thus has little influence or no effective change in total body weight [59, 120, 132]. SRT prevents the loss of lean body mass, secondary to dietary restriction [134], and reduces exercise-induced oxidative stress and homocysteine, regardless of adiposity [125]. A potential mechanism for this reduction could include contraction-induced antioxidant enzyme upregulation [139].
Several studies have demonstrated a decrease in visceral adipose tissue after SRT programmes [38, 139] which seem to reduce visceral fat depots through both immediate effects (during weight loss and weight maintenance) and delayed effects (during weight regain). Furthermore, it has been shown that SRT preferentially mobilizes the visceral and subcutaneous adipose tissue in the abdominal region [31, 38, 61, 105]. This kind of training procedure improves insulin-stimulated glucose uptake in patients with impaired glucose tolerance or T2D [41]. Subsequent increases in muscle mass, may regulate glucose levels and insulin responses to glucose load and therefore, improves insulin sensitivity [22, 132]. Also, high-intensity SRT decreases glycosylated haemoglobin (HbA1c) levels in diabetics, regardless of age [13, 127]. In addition, SRT is considered a potential coadjuvant to pharmacotherapy for the treatment of metabolic disorders, by decreasing some major risk factors of metabolic syndrome [46, 132, 133].
During the first 2 weeks of SRT, intensity should be kept to an adaptive level so that patients learn the exercise techniques and their muscle mass would probably not increase. From the third week, the objective of the training is hypertrophy [159]. Participants should start with three sets per muscle group per week, on three non-consecutive days of the week. One set should consist of 10–15 repetitions corresponding with 60–70% one repetition maximum lifting weight per 2–3 sessions per week, are likely to be beneficial for maximizing the health effects of increased skeletal muscle mass [155]. The number of training sets for each muscle per week should be increased progressively every 4 weeks by one set to a maximum of ten sets per week. The SRT programme should consist of exercises for all major muscle groups [132, 142].
Pharmacological treatments
If the goal of weight loss has not been achieved after 6 months of good adherence to a therapy combining diet and physical activity, prescribing a drug treatment can be considered, always with some limitations and considerations [45]. Regarding the pharmacological therapy, one may mention the appetite suppressants, the food absorption/digestion blockers and the stimulators of thermogenesis [16, 65, 82]. There is a long list of prospective drugs, including tesofensine and agonists and antagonists of satiety-inducing peptides and orexigens respectively, confirming a potential bright future for the treatment of the pandemic of obesity [119]. Nevertheless, they all entail well-known adverse effects and some of them show a very doubtful benefit/risk rate. Some other non-pharmacological alternative products or plants have been considered, but none of them has proved a long-term efficacy and/or safety [42, 107]. Indeed, in Europe, the only accepted pharmacological treatment is the prescription of Orlistat, an inhibitor of lipase, combined with a low-fat diet [71, 119].
Bariatric surgery
At last, surgery may be an effective long-term treatment of morbid obesity, by reducing some clinical manifestations associated with obesity [88]. This approach could be indicated in subjects with BMI >40 kg/m2 or BMI >35 kg/m2 in the presence of comorbidities or with an inadequate metabolic control [117]. Diabetes is the fastest comorbidity corrected after bariatric surgery, achieving significant improvements in glycaemic control or eradication of the disease between 80% and 95% in 10 years [23, 37]. This surgery entails serious postoperative complications directly related with the severity of the obesity; also male gender and increasing age were globally associated with an increased risk of complications [138, 140].
Hypoxic therapy and its applications
One of the alternatives to address high-prevalence diseases seems to be hypoxic therapy, which is commonly used in medical practice nowadays and well positioned in the field of traditional/alternative medicine, hypoxitherapy. The question remains open whether hypoxia or re-oxygenation is the responsible for the formation of adaptive signals and what the functional significance is. Experimental studies have not been appropriately focused on pheno- and genotypic features of the body’s response to hypoxia. Until now, what it is known is that appetite is suppressed, and a percentage of body mass is lost at high altitudes [126, 156]. Furthermore, oxygen variations in the organic system produce changes in body composition [111]. These findings can be of great interest for the treatment of obesity. Therefore, this review will focus on the methods of hypoxia and their potential applications.
Methods of hypoxic exposure and of hypoxic training
In general, there are two types of hypoxia stimuli: (1) intermittent hypoxic exposure (IHE)—passive exposure to hypoxia lasting from a few minutes to hours that is usually repeated over several days. These intermittent hypoxic exposures are interspersed with exposure to normoxia or lower levels of hypoxia. IHE raises the question of the minimum duration of exposure to induce erythropoiesis. It has been suggested that 180 min of IHE daily is necessary to increase endogenous erythropoietin (EPO), but in the vast majority of protocols, this appears to be inconsistent [19, 40, 75, 148]. Cerebral oxygenation decreases, whereas muscle oxygenation increases due to a greater ability to extract O2 in order to counteract reduced O2 availability, as shown by a decreased deoxyhaemoglobin during heavy exercise [92]. (2) Intermittent hypoxic training (IHT) consists of physical activity under hypoxic conditions (for short periods) and remaining at normoxic conditions for the rest of the time. This is another way to benefit from hypoxic stimulus without undergoing the detrimental effects of a prolonged exposure to hypoxia. This method induces specific molecular adaptations at muscular level that do not occur in normoxic conditions [116, 148].
Several methods of IHE and/or IHT are currently performed by elite athletes: traditional “live high-train high”, “live low-train high”, “live high-train low” or by using supplemental O2 when training in altitude (LH + TLO2) [148]. More recently, the interest on intermittent hypoxic methods has been investigated: IHE and IHT. The technological development of various hypoxic devices has also made possible to combine these methods [143]. In spite of the substantial differences between these forms of hypoxic training and/or exposure, all these methods have the same goal, inducing physiological adaptations and enhancement of athletic performance [158] (Table 1).
The experimentally used strategies differ greatly in cycle length, the number of hypoxic episodes per day and the number of exposure days. Thus, protocols vary from those that examine the effect of as few as 3–12 relatively short (2–10 min) bouts of hypoxia interspersed with 2–20-min episodes of normoxia on a single day [15] to those that examine longer daily exposures (1–12 h) over periods ranging from 2 to 90 days [19, 143] and those that consider short sinusoidal cycles of hypoxia/normoxia (30–90 s) 7–8 h daily for 30–70 days [75]. Regardless of the schedule, the compelling outcome is that these repeated episodes of hypoxia elicit persistent changes in a variety of physiological responses [27, 58, 92, 122, 124].
Intermittent hypoxia and its applications in physiological and pathophysiological states
Certainly, there are adaptations to chronic hypoxia that are not necessarily beneficial. Chronic intermittent hypoxia significantly increases right ventricular heart mass [25]. It has been occasionally associated with pulmonary vascular remodelling and pulmonary hypertension [96]. A number of investigations and trials identified physiological effects on different cells, tissues and systems and respiratory disorders (Table 2).
Intermittent hypoxic exposure
A significant percentage of the obese population suffers from obstructive sleep apnea (OSA) [94]. OSA is characterized by transient periods of oxygen desaturation followed by re-oxygenation and is a major cause of systemic damage (oxidative stress, inflammation, sympathetic activity, vasculature remodelling and endothelial dysfunction) and/or protective (preconditioning-like cardioprotective) effects. This condition (OSA) has rarely been given importance in obese patients, although many OSA-bearing subjects are obese, and obesity is an independent risk factor for many comorbidities associated with OSA [104].Thus, it has been speculated that the chronic intermittent hypoxia caused by OSA in obese patients might be one of the underlying mechanisms in the morbidity–mortality paradox of obesity. Moreover, OSA increased plasma neuropeptide Y levels, an appetite-stimulating peptide, independently of body weight [136]. This is a controversial feature since risks concerning endocrine health caused by sleep breathing disorders threaten to exacerbate the already compromised metabolic regulation and control of normal body weight in this obese population [102]. Experimental evidence from human models is required to elucidate such risks and to facilitate clinical decisions about whom to treat. Interestingly, IHE induces lessening of bronchial spasm, more uniform lung ventilation and increases ventilation sensitivity to hypoxia [9].
Watson et al. [152] explored genetic contributions of sleep disturbances in over 1,800 twin pairs and found that 10% of common additive gene effects accounted for 10% of the phenotypic association between obesity and insomnia. Similar gene model fitting studies are needed to assess the genetic contribution of reduced sleep to BMI [94].
Sleep intermittent hypoxia debt exerts profound effects on metabolic hormones and molecular signatures [4]. These changes are accompanied by an increased food intake and energy storage, which potentiates the development of insulin resistance, T2D, hypertension and heart disease [113]. Recent reports provide new insights about possible mechanisms to explain OSA effects on lipid and glucose metabolism by inducing sympathetic activation, increasing systemic inflammation, stimulating counter-regulatory hormones and fatty acids or causing direct pancreatic beta-cell injury [44].
Lately, it has been shown that hypoxia through hypoxia-inducible factor (HIF)-1 expression changes helps to regulate mitochondrial function. It is interesting to note that there are often defects in the mitochondrial function in many pathophysiological processes, which seem to improve with the stimuli of IHE [130].
Intermittent hypoxic training
The potential applications of IHT in health and in various pathophysiological states are numerous [9, 76], since it could be a non-pharmacological method for enhancing some physiological functions and rehabilitation in patients with different chronic diseases [63]. Hypoxic training increased physical work capacity of about 5% in the healthy elderly subjects and 10% in elderly subjects [155].
The ventilatory response to hypoxia increases during submaximal exercise and induces a smaller hypoxic pre-acclimation decrease in arterial oxygen saturation (SpO2) [54, 110]. Furthermore, IHT has been shown to raise baroreflex sensitivity to normal levels and to selectively increase hypercapnic ventilatory response, total exercise time, total haemoglobin blood levels and lung diffusion capacity for carbon monoxide in bronchial asthma and chronic obstructive pulmonary disease patients [149].
Some favourable effects of IHT are conditioned by triggering a long-term adaptation to hypoxia, leading to positive changes in internal organs. These phenomena have been well studied in young and middle-aged people. In the cardiovascular systems, reduced sympathetic-adrenal reaction to stress and the blood flow along the vessels of miocirculatory system were improved at the expense of increases on the diameter of arterioles [63]. In elderly people, IHT had a positive influence on the antioxidant system, along with an activation of the antiradical defence enzymes, and a decrease of lipid peroxidation products in tissues [18].
A few studies have investigated the influence of various IHT regimens on inflammation-related cytokine secretion caused by acute exercise [151]. Severe intermittent hypoxia (IH) or moderate IH (12–15% O2, 1 h/day, 5 days/week for 8 weeks) ameliorated the effects of severe exercise on IL-1β secretion. A possible reason for this outcome is that training increases circulatory anti-inflammatory cytokine levels, such as IL-6 and IL-10, with inhibited production of IL-1β during severe exercise [150].
The neuroprotective role of EPO has been proven [129] by showing that normobaric hypoxia reduced the risk of cardiovascular disease [99, 155, 164], improved the respiratory function [68] and produced CNS protection [89] and basal metabolism reprogramming [7]. On the other hand, it is known that training in hypoxia and in normoxia reduced the circulating concentration of free fatty acids, total cholesterol and HDL cholesterol. However, it did not reduce the levels of homocysteine, an acidic molecule implicated in heart disease [98].
At the same time, it has been demonstrated that IHT may normalize blood pressure in hypertensive patients [123, 124]. Moreover, it has been shown that systolic blood pressure is reduced after IHT, causing a hypotensive effect [43]. Thus, it has been confirmed that physical exercise in hypoxia decreases the risk of cardiovascular disease [12, 24].
On the other hand, aging is associated with changes in breathing regulation, particularly, in respiratory sensitivity to IHT. One theory of aging holds that reactive oxygen species play a key role in this process. These species have also been implicated in the carotid body O2 sensing. Studies have investigated hypoxic ventilatory responses (HVR) and antioxidant enzymes activity in healthy young and elderly people in adaptation to IHT [76]. The elderly demonstrated decreased HVR and blood catalase activity on a background of strong negative correlation between the levels of end-tidal CO2 tension and superoxide dismutase (SOD) activity. The adaptation to IHT resulted in increased HVR and SOD activity in both groups [76].
Furthermore, the results of clinical studies have shown that IHT in elderly patients could be useful and valuable, leading to reduction in clinical symptoms of angina and duration of daily myocardial ischemia, normalization of lipid metabolism, optimization of oxygen consumption and improvement of vasomotor endothelial function due to increased formation of nitric oxide, normalization of microcirculation and increased exercise tolerance [77, 78].
Intermittent hypoxia and insulin sensitivity
Acclimation to an altitude of 4,000 m in IHE has been found to decline blood glucose, parallel to a higher glucose turnover, both at rest and during exercise [21]. This increased muscle uptake of glucose is accompanied by enhanced insulin sensitivity [11, 131]. Likewise, it was reported that in high altitude (2,600 m) the HbA1c decreased significantly in obese people 4 weeks after the altitude stay [84].
An increase in body fat is linked with a decline in insulin sensitivity in both obese and elderly individuals, and a recent study showed that acute hypoxic training improved glucose tolerance, and that combined exercise in hypoxic state further ameliorated insulin sensitivity in T2D [87]. Other trial demonstrated that IHE glucose tolerance enhancement 4 h after exposure can be attributed to improvements in peripheral insulin sensitivity in sedentary males with T2D. Therefore, it may improve short-term glycaemic control [85].
Similar to the effects of exercise, increased epinephrine during IHE, which was reported in healthy humans may have contributed to glycogen utilization via increases in cyclic adenosine monophosphate concentrations, potentially leading to post-treatment increased on insulin sensitivity [72]. Similarly, it has been described that both IHE and IHT programmes carried out in hypoxic conditions increased glucose transporter GLUT-4 levels [33].
Hypoxia and exercise have shown an additive effect on insulin sensitivity [87], suggesting that insulin signalling and insulin-dependent glucose transport, might have been upregulated following hypoxic exercise [33, 34]. This additive improvement could also be attributed to an increase in the relative intensity of the practised exercise in hypoxia [56]. To sum up, acute hypoxic exercise could improve short-term glycaemic control in sedentary individuals with insulin resistance.
Intermittent hypoxia training and molecular mechanisms
In recent years, certain proteins involved in the process of sensing and regulating the concentrations of oxygen in tissues have been described. Such proteins may have a predominant role in the HIF and in the hydroxylase regulation [92].
There is a large demand of oxygen during physical activity, regardless of the type and intensity of exercise [73]. In spite of that, there is a general condition of ischemia–reperfusion due to the high voltage generated by the muscles during maximal or submaximal contractions. In other words, there are cycles of hypoxia–hyperoxia [35]. Physical activity leads to an increase in oxygen free radicals, but it is not entirely clear whether the phenomenon linked to free radicals increase is hypoxia or hyperoxia. However, it seems that hyperoxia is the most likely factor [157]. Other researchers have suggested that free radicals are possible mediators of the response to hypoxia [3]. However, it has not been possible to fully understand the interaction between hypoxia–hyperoxia, free radicals, HIF and intracellular signalling neither in the skeletal muscle nor in other cells yet [157].
There are reports showing that after 6 weeks of physical training, mRNA levels of HIF-1 rise (between 58% and 82%) only in subjects who trained in hypoxic environment (3,850 m), both at low and at high intensity, compared to other groups who trained in normoxia at low and high intensities of exercise [58, 148]. These studies suggest that there are no HIF-1 increases in normoxic environments and that in hypoxia only the effects achieved due to physical training in normoxia are relevant.
On the other hand, mRNA levels of phosphofructokinase gene, a key enzyme in glycolysis, are increased as it has been shown to occur only in high-intensity IHT [148]. This finding that the intensity of exercise in hypoxia may have different effects at the metabolic level, and that might be related to variations between the hypoxia and the hyperoxia produced, required to be further clarified.
There is an extensive list of genes regulated by hypoxia [122], many of which are involved in growth and differentiation [153]. Furthermore, it is also known that exercise regulates the expression of many genes, but the mechanisms are often unknown and the genes controlling this phenomenon (and favour cardiovascular health) are still under investigation. Regular bouts of physical activity may cause changes in gene expression that accumulate over time and, ultimately, affect phenotypes, such as body weight, blood lipid profile and tumour development. Furthermore, acute activity may affect gene expression and phenotypes differently depending on whether the individual is regularly inactive or active [128]. Knowledge of these genes would help to understand, for example, how exercise leads to muscle hypertrophy. This outcome suggests that further studies are needed to evaluate the relationship between hypoxia and the genes regulated by the HIF-1.
Adaptations to exercise or hypoxia are increasingly being applied preventively or as a treatment to different pathophysiological situations and, therefore, they begin to have global significance [27]. HIF expression is upregulated with exercise and it might be an important factor that regulates adaptive gene responses to exercise [86]. It is therefore important that only high-intensity training under hypoxic conditions leads to adaptations that include regulation and elevated HIF-1, as a mechanism to compensate the reduced availability of oxygen. It seems that intense muscle contraction and oxygen deficiency are essential to generate some adjustments. Thus, it has been noted that the muscle generates a series of major adaptations when training in hypoxia [58].
However, at the present time, there is scarce information about how different intensities and kinetic contraction of muscle affect the activation of HIF-1 and other proteins [58]. The only available information is that HIF-1 average life is less than 5 min [55]. For this reason, training protocols in intermittent hypoxia are usually of 5′–5′, hypoxia–normoxia. This situation may explain why training at sea level is not enough to alter the HIF-1 cascade [86]. Although it may be surprising, the studies developed by Lundby and his colleagues indicate that regular training reduces the hypoxia observed after acute exercise. Therefore, there are no major changes in the HIF-1, as they existed at the beginning of the training [100]. Although cellular hypoxia persists, the HIF-1 is not affected equally and, as a consequence, it becomes tolerant to hypoxia. According to Lundby and colleagues [86], there are other mechanisms explaining the effects produced by intermittent chronic training in hypoxia. Nitric oxide (a potent vasodilator) may be involved in this adaptive mechanism, but no studies proving this hypothesis have been found. Oxygen free radicals may be the other factor involved in this mechanism [29], since it is known that they increase in hypoxia and that, at the same time, they are mediators of muscular adaptations [57, 58, 64].
Intermittent hypoxia and exercise: possible applications in obesity
Oxygen plays an important role, as an electron acceptor in the long chain of reactions produced to obtain energy in the form of ATP, both in humans and in other higher organisms. Low oxygen levels lead to tissue hypoxia; high oxygen levels lead to inflammation hyperoxia [140]. The two states entail different effects, and body composition changes may occur because of them [111]. Studies show that appetite is suppressed and that there is body mass loss at high altitudes [126, 156]. Similarly, it has been noted that oxygen variations in the organic system produce changes in body composition [111].
Acute exposure to hypoxia, both in humans and in rats, produces an increase in blood serotonin levels [51]. Moreover, hypoxia causes an increase in the adrenergic system [6, 159]. Food intake, protein intake and carbohydrates selection, as well as body weight, are partially regulated by serotonin, a compound that after administration to rats produces anorexia [51].
Rats submitted to a high altitude (Cerro de Pasco, Perú, 4,340 m) for up to 84 days showed a physiological adaptive response with decreased body weight gain (−15%), increased right ventricle weight (+100%) and increased hematocrit (+40%) compared with sea level animals. These classical parameters of adaptation to high altitude were accompanied by an increase in heart mitochondrial enzymes: complexes I–III activity by 34% and mitochondrial nitric oxide synthase activity and expression by more than 75% [160].
Similarly, in obese subjects, it is known that leptin is involved in regulating body weight and controlling energy resources [12, 139]. It has been observed that acute hypoxia decreases blood glucose and basal leptin levels [72]. These observations allow us to consider the hypothesis that IHE defined as situations of exposure to low concentrations of ambient oxygen alternating with periods of normoxia [61] may result in decreased appetite and fat loss, which might be of interest for the treatment of obesity. On the other hand, IHE can reduce not only body weight by increasing leptin concentration and enhancing liver leptin expression, but also decrease serum glucose, blood cholesterol and meanwhile prevent steatosis in liver cells effectively, in obese mice [83]. Moreover, it has been described that obese individuals staying 1 week at 2,650 m of altitude (without exercise) lost weight and reduced blood pressure [84]. It can be hypothesized that adding exercise to the IHE stimulus could result in further cardiovascular changes and in an improved health and body composition.
Physical activity in hypoxia increased the number of mitochondria, the capillary density [107] and muscle oxidative enzymes and changed energy production pathways with potential for an increase in lipolysis [116]. Adaptive responses to the respiratory tract and cardiovascular system are produced, and at the same time, the mitochondrial efficiency, pH/lactate regulation [81, 164] and physical performance are increased [17, 108, 143].
There is scientific evidence suggesting that a lower degradation of hypoxia-inducible factor alpha (HIF-1α) caused by conditions of hypoxia, along with physical activity, can prove to be very effective and be part of major medical applications in the long term [27]. After 6-week training in IH, there was a dramatic increase in mRNA concentrations of many genes, such as HIF-1α (+104%), glucose transporter-4 (+32%), phosphofructokinase (+32%), citrate synthase (+28%), carbonic anhydrase-3 (+74%) or monocarboxylate transporter-1 (+44%) [148]. These results demonstrated that training in IH at high intensity is probably a good way to favour oxygen utilization within the muscle.
Thus, the hypoxic stimulus in addition to diet and exercise can be another powerful incentive to lose weight, which may have effects in the mid and long term (unlike diet therapy and physical activity alone), since effects of hypoxia are maintained for 1 month post-treatment, due to increases in basal noradrenalin and other possible changes that remain to be clarified [84].
Proposed new hypoxic training model
The interval hypoxic training protocol comprises repeated exposures to hypoxic air breathing, alternated with breathing environment (normoxic) air. During the training course, strength–resistance exercises (20′) and high-intensity aerobic exercises (30–40′) are performed. The hypoxic air decreases gradually from 16.7% to 11.2% (SpO2, 89–75%) in order to provide a stepwise adaptation and to avoid overtraining. Sessions are repeated three to four times per week for periods of 3–6 weeks, and session duration is in the range of 40–60′ (Table 3).
The physiological response is monitored by a pulsioxymeter, a device that measures SpO2 and heart rate. Other physiological parameters can also be monitored, for instance blood pressure and cardiac activity by an electrocardiogram. Monitoring of the user’s physiological parameters allows us to avoid the undesirable effects of overdosing. Advanced biofeedback-controlled hypoxicators are capable of adjusting the oxygen concentration in the inhaled hypoxic air automatically, compensating for individual variability [15, 143].
Hypoxia stimulus charge (HSc = SpO2% × time in minutes) parameter provides an objective measure of the hypoxic stress delivered during the IHT session, compared to simple recording of the inhaled fraction of oxygen (FiO2). HSc provides the dosage received by a person at the end of the session and compensates for individual variability in scientific studies to ensure that subjects are correctly controlled for individual exposure. It is accepted that tissue hypoxia develops only when SpO2 drops to 90% or below. This is due to the oxyhaemoglobin dissociation curve. SpO2 above 90% produce very little effects or decrease of arterial partial pressure. Due to all of these data, our protocol of IHT comprises ranges of SpO2 between 75% and 89%.
Concluding remarks
High-intensity exercise and intermittent hypoxia in the short and long term may have important medical applications in pathophysiological problems with metabolic–muscular disorders, such as obesity. However, nowadays, there are few studies and clinical initiatives to use stimuli of intense physical activity or intermittent hypoxia, with the uncertainty that patients may not bear this type of training or that the training may not result very pleasant for them. Although it is not possible to stay at high altitude and to remain there in many countries—due to orographic conditions—current technology allows to simulate the effects of altitude (hypoxia) at sea level, through instruments that reduce the content of oxygen on inspired air (normobaric hypoxia) (Fig. 1).
Normobaric–intermittent hypoxic tent equipped for research [143] and used to trained climbers for an Everest expedition, 2011 (unpublished results)
Intermittent hypoxia may have many applications for improving cardiovascular health, the great public health concern in Western societies [24]. Similarly, staying in altitude or hypoxic situations may help to lose weight and normalize arterial blood pressure. Summing up, the IHT is a non-pharmacological method for enhancing the functional resources of a healthy organism and the rehabilitation in patients with different chronic diseases. The IHT stimulus could be of great tool and very practical for clinical use in obese patients by reducing appetite and regulating fat deposition.
References
Abete I, Astrup A, Martínez JA, Thorsdottir I, Zulet MA (2010) Obesity and the metabolic syndrome: role of different dietary macronutrient distribution patterns and specific nutritional components on weight loss and maintenance. Nutr Rev 68:214–2131
Abete I, Parra MD, Zulet MA, Martínez JA (2006) Different dietary strategies for weight loss in obesity: role of energy and macronutrient content. Nutr Res Rev 19:5–17
Acker H (2005) The oxygen sensing signal cascade under the influence of reactive oxygen in skeletal muscle. Philos Trans R Soc Lond B Biol Sci 360:2201–2210
Amardottir ES, Mackiewicz M, Gislason T, Teff KL, Pack AI (2009) Molecular signatures of obstructive sleep apnea in adults: a review and perspective. Sleep 32(4):447–470
Anderson JW, Konz EC, Frederich RC, Wood CL (2001) Long-term weight-loss maintenance: a meta-analysis of US studies. Am J Clin Nutr 74:579–584
Antezana AM, Kacimi R, Letrong JL, Marchal M, Aboushal I, Dubrai C et al (1994) Adrenergic status of humans during prolonged exposure to the altitude of 6,542 m. J Apply Physiol 76:1055–1059
Aragones J, Scheneider M, Van Geyte K, Kraisl P, Dresselaers T, Mazzone M et al (2008) Deficiency or inhibition of oxygen sensor Phd1 induces hypoxia tolerance by reprogramming basal metabolism. Nat Genet 40:170–180
Aranceta J, Pérez C, Serra LI, Ribas L, Quiles J, Vioque J, et al y Grupo Colaborativo SEEDO (2003) Prevalencia de obesidad en España. Resultados del estudio SEEDO 2000. Med Clin (Barc) 608–612.
Asanov AO (2006) Changes in the ventilation function of the lungs in elderly people during adaptation to periodic hypoxia. Ukr Pulm J 2:68–69
Avenell A, Grant AM, McGee M, McPherson G, Campbell MK, McGee MA et al (2004) The effects of an open design on trial participant recruitment, compliance and retention—a randomized controlled trial comparison with a blinded, placebo-controlled design. Clin Trials 1(6):490–498
Azevedo JL, Carey JO, Pories WJ, Morris TG, Dohm-Lynid G (1995) Hypoxia stimulates glucose transport in insulin-resistant human skeletal muscle. Diabetes 44:695–698
Bailey DM, Davies B, Baker J (2000) Training in hypoxia: modulation of metabolic and cardiovascular risk factors in men. Med Sci Sport Exerc 32:1058–1066
Baldi JC, Snowling N (2003) Resistance training improves glycaemic control in obese type 2 diabetic men. Int J Sports Med 24:419–423
Banegas JR, López-García E, Gutiérrez-Fisac JL, Guallar-Castillón J, Rodríguez-Artalejo F (2003) A simple estimate of mortality attributable to excess weight in the European Union. Eur J Clin Nutr 57:201–208
Bassovitch O (2007). Training manual for fully biofeedback controlled Go2Altitude hypoxicator ONEPLUS. Biomedtech Australia Pty Ltd. www.go2altitude.com.
Basulto J, Baladia E, Manera M (2009) Posicionamiento del GREP-AEDN: complementos alimenticios para la pérdida de peso. Actividad dietética 13(1):41–42
Beidleman BA, Muza SR, Fulco CS, Cymerman A, Sawka MN, Lewis SF, Skrinar GS (2008) Seven intermittent exposures to altitude improves exercise performance at 4300 m. Med Sci Sports Exerc 40:141–148
Belikova MV, Asanov EO (2006) Effects of intermittent normobaric hypoxic training of the lipid peroxidation intensity and antioxidant system state in the blood plasma in essentially healthy people of different ages. Probl Aging Longevity 2:128–131
Bernardi L (2001) Interval hypoxic training. Adv Exp Med Biol 502:377–399
Bray G, Bouchard C, James WPT (1998) Definitions and proposed current classifications of obesity. In: Bray G, Bouchard C, James WPT (eds) Handbook of obesity. Marcel Dekker, New York, pp 31–40
Brooks GA, Butterfield GE, Wolfé RR, Groves BM, Mazzeo RS, Suston JR et al (1991) Increased dependence on blood glucose after acclimatization to 4300 m. J Appl Physiol 70:919–923
Brooks N, Layne JE, Gordon PL, Roubenoff R, Nelson ME, Castaneda-Sceppa C (2007) Strength training improves muscle quality and insulin sensitivity in Hispanic older adults with type 2 diabetes. Int J Med Sci 4:19–27
Buchwald H, Avidor Y, Braunwald E, Jensen MD, Pories W, Fahrbach K et al (2004) Bariatric surgery: a systematic review and meta-analysis. JAMA 292:1724–1737
Burtscher M, Pachinger O, Ehrenbourg I, Mitterbauer G, Faulhaber M, Pühringer R et al (2004) Intermittent hypoxia increases exercise tolerance in elderly men with and without coronary artery disease. Int J Cardiol 96:247–254
Calbet JA, Robach P, Lundby C (2009) The exercising heart at altitude. Cell Mol Life Sci 66(22):3601–3613
Campión J, Milagro F, Martínez JA (2010) Epigenetics and obesity. Prog Mol Biol Transl Sci 94:291–347
Caramelo C, Peña JJ, Castilla A, Justo S, De Solis AJ, Neria F et al (2006) Respuesta a la hypoxia. Un mecanismo sistémico basado en el control de la expresión génica. Medicina (BA) 66:155–164
Carmeliet P (2005) Angiogenesis in life, disease and medicine. Nature 438:932–936
Casey DP, Walker BG, Curry TB, Joyner MJ (2011) Ageing reduces the compensatory vasodilatation during hypoxic exercise: the role of nitric oxide. J Physiol 15(6):1477–1488
Cauza E, Hanusch-Enserer U, Strasser B (2005) The relative benefits of endurance and strength training on the metabolic factors and muscle function of people with type 2 diabetes mellitus. Arch Phys Med Rehabil 86:1527–1533
Cauza E, Strehblow C, Metz-Schimmerl S, Strasser B, Hanusch-Enserer U, Kostner K et al (2009) Effects of progressive strength training on muscle mass in type 2 diabetes mellitus patients determined by computed tomography. Wien Med Wochenschr 159(5–6):141–147
Cerretelli P, Samaja M (2003) Acid-base balance at exercise in normoxia and in chronic hypoxia. Revisiting the “lactate paradox”. Eur J Appl Physiol 90:431–448
Chiu LL, Chou SW, Cho YM, Ho HY, Ivy JL, Hunt D et al (2004) Effect of prolonged intermittent hypoxia and exercise training on glucose tolerance and muscle GLUT4 protein expression in rats. J Biomed Sci 11:838–846
Chou SW, Chiu LL, Cho YM, Ho HY, Ivy JL, Ho CF et al (2004) Effect of systemic hypoxia on GLUT-4 protein expression in exercise rat heart. Jpn J Physiol 54:357–363
Clanton TL, Zuo L, Klawiter P (1999) Oxidants and skeletal muscle function: physiologic and pathophysiologic implications. Proc Soc Exp Biol Med 222:253–262
Cocate PG, Pereira LG, Marins JC, Cecon PR, Bressan J, Alfenas RC (2011) Metabolic responses to high glycemic index and low glycemic index meals: a controlled crossover clinical trial. Nutr J 5(10):1–11
Colucci RA (2011) Bariatric surgery in patients with type 2 diabetes: a viable option. Postgrad Med 123:24–33
Cuff DJ, Meneilly GS, Martin A, Ignaszewski A, Tildesley HD, Frohlich JJ (2003) Effective exercise modality to reduce insulin resistance in women with type 2 diabetes. Diabetes Care 22:2977–2982
Dansinger ML, Gleason JA, Griffith JL, Selker HP, Schaefer EJ (2005) Comparison of the Atkins, Ornish, Weight Watchers, and Zone diets for weight loss and heart disease risk reduction: a randomized trial. JAMA 293:43–53
Daussin FN, Zoll J, Ponsot E, Dufour SP, Doutreleau S, Lonsdorfer E et al (2008) Training at high exercise intensity promotes qualitative adaptations of mitochondrial function in human skeletal muscle. J Appl Physiol 104:1436–1441
Dela F, Kjaer M (2006) Resistance training, insulin sensitivity and muscle function in the elderly. Essays Biochem 42:75–88
De la Garza AL, Milagro FI, Boque N, Campión J, Martínez JA (2011) Natural inhibitors of pancreatic lipase as new players in obesity treatment. Planta Med 77(8):773–785
Donina ZhA, Lavrova IN, Tikhonov MA (2008) Effects of intermittent hypoxic training on orthostatic reactions of the cardiorespiratory system. Bull Exp Biol Med 145:661–664
Drager LF, Jun JC, Polotsky VY (2010) Metabolic consequences of intermittent hypoxia: relevance to obstructive sleep apnea. Best Pract Res Clin Endocrinol Metab 24:843–851
Elfhag K, Rössner S (2005) Who succeeds in maintaining weight loss? A conceptual review of factors associated with weight loss maintenance and weight regain. Obes Rev 16:67–85
Fenkci S, Sarsan A, Rota S, Ardic F (2006) Effects of resistance or aerobic exercises on metabolic parameters in obese women who are not on a diet. Adv Ther 23(3):404–413
Ferrara N, Kerbel RS (2005) Angiogenesis as a therapeutic target. Nature 438:967–974
Finer N (2001) Low-calorie diets and sustained weight loss. Obes Res 9:s290–s294
Franks PW, Hanson RL, Knowler WC, Sievers ML, Bennett PH, Looker HC (2010) Childhood obesity, other cardiovascular risk factors, and premature death. N Engl J Med 362:485–493
Garaulet M, Pérez de Heredia F (2009) Behavioural therapy in the treatment of obesity (I): new directions for clinical practice. Nutr Hosp 24:629–639
Gonzales GF (1980) Serotonin blood levels under several physiological situations. Life Sci 27:647–650
Gonzalez NC, Clancy RL, Moue Y, Richalet JP (1998) Increasing maximal heart rate increases maximal O2 uptake in rats acclimatized to simulated altitude. J Appl Physiol 84:164–168
Heiat A, Vaccarino V, Krumholz HM (2001) An evidence-based assessment of federal guidelines for overweight and obesity as they apply to elderly persons. Arch Intern Med 18:1194–1203
Hetzler RK, Stickley CD, Kimura IF, LaBotz M, Nichols AW, Nakasone KT et al (2009) The effect of dynamic intermittent hypoxic conditioning on arterial oxygen saturation. Wilderness Environ Med Spring 20:26–32
Hirota K, Semenza GL (2001) Regulation of hypoxia sensing. Curr Opin Cell Biol 13:167–171
Holloszy JO (2005) Exercise-induced increase in muscle insulin sensitivity. J Appl Physiol 99:338–343
Hoppeler H, Klossner S, Vogt M (2008) Training in hypoxia and its effects on skeletal muscle tissue. Scand J Med Sci Sports 18:38–49
Hoppeler H, Vogt M (2001) Muscle tissue adaptations to hypoxia. J Exp Biol 204:3133–3139
Hunter GR, Bryan DR, Wetzstein CJ, Zuckerman PA, Bamman MM (2002) Resistance training and intraabdominal adipose tissue in older men and women. Med Sci Sports Exerc 34:1023–1028
Ibáñez I (2005) La aplicación del preacondicionamiento hipóxico en Medicina Anti-Aging. Trabajo de Investigación presentada en la Universidad de Barcelona.
Ibañez J, Izquierdo M, Argüelles I, Forga L, Larrión JL, García-Unciti M, Idoate F, Gorostiaga EM (2005) Twice-weekly progressive resistance training decreases abdominal fat and improves insulin sensitivity in older men with type 2 diabetes. Diabetes Care 28:662–667
Idoate F, Ibañez J, Gorostiaga EM, García-Unciti M, Martínez-Labari C, Izquierdo M (2011) Weight-loss diet alone or combined with resistance training induces different regional visceral fat changes in obese women. Int J Obes 35:700–713
Ishchuk VO (2007) Safety and efficacy of the intermittent normobaric hypoxic training of elderly patients with ischemic heart disease. J Acad Med Sci Ukraine 13:374–384
Jackson MJ, Pye D, Palomero J (2007) The production of reactive oxygen and nitrogen species by skeletal muscle. J Appl Physiol 102:1664–1670
James WP, Astrup A, Finer N, Hilsted J, Kopelman P, Rossner S et al (2000) Effect of sibutramine on weight maintenance after weight loss: a randomised trial. STORM Study Group (Sibutramine Trial of Obesity Reduction and Maintenance). Lancet 356:2119–2125
James WP (2005) Assessing obesity: are ethnic differences in body mass index and waist classification criteria justified? Obes Rev 6(3):179–181
Jeffery RW, Wing RR, Sherwood NE, Tate DF (2003) Physical activity and weight loss: does prescribing higher physical activity goals improve outcome? Am J Clin Nutr 78:684–689
Katayama K, Ishida K, Iwasaki K, Kiyamura M (2009) Effect of two duration of short-term intermittent hypoxia exposures on ventilatory response in humans. Higt Alt Med Biol 105:815–821
Katayama K, Smith CA, Henderson KS, Dempsey JA (2000) Chronic intermittent hypoxia increases the CO2 reserve in sleeping dogs. J Appl Physiol 103:1942–1949
Keich SW, Redden DT, Katzmarzyk PT, Boggiano MM, Hanlon EC, Benca RM et al (2006) Putative contributors to the secular increase in obesity: exploring the roads less travelled. Int J Obes 30:1585–1594
Kelley DE, Kuller LH, McKolanis TM, Harper T, Mancino J, Kalhan S (2004) Orlistat on insulin resistance, regional adiposity, and fatty acids in type 2 diabetes mellitus. Diabetes Care 33–40.
Kelly KR, Williamson DL, Fealy CE, Kriz DA, Krishnan RK, Huang H et al (2010) Acute altitude-induced hypoxia suppresses plasma glucose and leptin in healthy humans. Metab 59:200–205
Killgore GL, Coste SC, O’ Meara SE, Konnecke CJ (2010) A comparison of the physiological exercise intensity differences between shod and barefoot submaximal deep-water running at the same cadence. J Strength Cond Res 24(12):3302–3312
Koehl M, Meerlo P, Gonzales D, Rontal A, Turek FW, Abrous DN (2008) Exercise-induced promotion of hippocampal cell proliferation requires beta-endorphin. FASEB J 22:2253–2262
Kolchinskaya AZ, Tsyganova NT, Ostapenko LA (2003) Normobaric interval hypoxic training in medicine and sports: manual for physicians. Meditsina, Moscow
Kolesnikova EE, Safronova OS, Serebrovskaya TV (2003) Age-related peculiarities of breathing regulation and antioxidant enzymes under intermittent hypoxic training. J Physiol Pharmacol 54:20–24
Korkushko OV, Shatilo VB, Ishchuk VA (2010) Effectiveness of intermittent normobaric hypoxic trainings in elderly patients with coronary artery disease. Adv Gerontol 23:476–482
Korkushko OV, Pysaruk AV, Lyshnevs’ka VIu, Asanov EO, Chebotar’ov MD (2005) Age peculiarities of cardiorespiratory system reaction to hypoxia. Fiziol Zh 51:11–17
Kushner RF (2010) Obesity and therapeutic approaches to weight loss. Contemp Cardiol 1:91–106
Larsen TM, Dalskov SM, van Baak M, Jebb SA, Papadaki A, Pfeiffer AF et al (2010) Diets with high or low protein content and glycemic index for weight-loss maintenance. N Engl J Med 363:2102–2113
Lecoultre V, Boss A, Tappy L, Borrani F, Tran C, Schneiter P et al (2010) Training in hypoxia fails to further enhance endurance performance and lactate clearance in well-trained men and impairs glucose metabolism during prolonged exercise. Exp Physiol 95:315–330
Li Z, Maglione M, Tu W, Mojica W, Arterburn D, Shugarman LR et al (2005) Meta-analysis: pharmacologic treatment of obesity. Ann Intern Med 142:532–546
Ling Q, Sailan W, Ran J, Zhi S, Cen L, Yang X et al (2008) The effect of intermittent hypoxia on bodyweight, serum glucose and cholesterol in obesity mice. Pak J Biol Sci 11:869–875
Lippl FJ, Neubauer S, Schipfer S, Lichter N, Tufman A, Otto B et al (2010) Hypobaric hypoxia causes body weight reduction in obese subjects. Obesity 184:675–681
Louis M, Punjabi NM (2009) Effects of acute intermittent hypoxia on glucose metabolism in awake healthy volunteers. J Appl Physiol 106:1538–1544
Lundby C, Gassmann M, Pilegaard H (2006) Regular endurance training reduces the exercise induces HIF-1 alpha and HIF-2 alpha mRNA expresion in human eskeletal muscle in normoxic conditions. Eur J Appl Physiol 96:363–969
Mackenzie R, Maxwell N, Castle P, Brickley G, Watt P (2011) Acute hypoxia and exercise improve insulin sensitivity (S(I) (2*)) in individuals with type 2 diabetes. Diabetes Metab Res Rev 27:94–101
Maggard MA, Shugarman LR, Suttorp M, Maglione M, Sugerman HJ, Livinston EH et al (2005) Meta-analysis: surgical treatment of obesity. Ann Intern Med 142:547–559
Margail I, Plotkine M, Lerouet D (2005) Antioxidant strategies in the treatment of stroke. Free Radic Biol Med 39:429–443
Marti A, Goyenechea E, Martinez JA (2010) Nutrigenetics: a tool to provide personalized nutritional therapy to the obese. World Rev Nutr Diet 101:21–33
Marti A, Martinez-Gonzalez MA, Martinez JA (2008) Interaction between genes and lifestyle factors on obesity. Proc Nutr So 67:1–8
Marxwell PH (2005) Hypoxia-inducible factor as a physiological regulator. Exp Physiol 90:791–797
McAllister EJ, Dhurandhar NV, Keith SW, Aronne LJ, Barger J, Baskin M et al (2009) Ten putative contributors to the obesity epidemic. Crit Rev Food Sci Nutr 49:868–913
McCallister JW, Adkins EJ, O’Brien JM (2009) Obesity and acute lung injury. J Clin Chest Med 30:495–508
McCarthy MI (2010) Genomics, type 2 diabetes, and obesity. N Engl J Med 363:2339–2350
McGuire M, Bradford A (1999) Chronic intermittent hypoxia increases haematocrit and causes right ventricular hypertrophy in the rat. Respir Physiol 117:53–58
Mcinnis KJ, Frankilin BA, Rippe JM (2003) Counseling for physical activity in overweight and obese patients. Am Fam Physician 67:1249–1256
Milano G, Corno AF, Lippa S, Von Segesser LK, Samaja M (2002) Chronic and intermittent hypoxia induce different degrees of myocardial tolerance to hypoxia-induced dysfunction. Exp Biol Med 227:389–397
Milano W, Petrella C, Casella A, Capasso A, Carrino S, Milano L (2005) Use of sibutramine, an inhibitor of the reuptake of serotonin and noradrenaline, in the treatment of binge eating disorder: a placebo-controlled study. Adv Ther 22:25–31
Millet GP, Roels B, Schmitt L, Woorons X, Richalet JP (2010) Combining hypoxic methods for peak performance. Sports Med 40(1):1–25
Misra A, Khurana L (2008) Obesity and the metabolic syndrome in developing countries. J Clin Endocrinol Metab 93:S9–S30
Morgan BJ (2009) Intermittent hypoxia: keeping it real. J Appl Physiol 107:1–3
Mustajoki P, Pekkarinen T (2001) Very low energy diets in the treatment of obesity. Obes Res 2:61–72
Ozeke O, Ozer C, Gungor M, Celenk MK, Dincer H, Ilicin G (2011) Chronic intermittent hypoxia caused by obstructive sleep apnea may play an important role in explaining the morbidity-mortality paradox of obesity. Med Hypotheses 76:61–63
Park SK, Park JH, Kwon YC, Kim HS, Yoon MS, Park HT (2003) The effect of combined aerobic and resistance exercise training on abdominal fat in obese middle-aged women. J Physiol Anthropol Appl Human Sci 22:129–135
Pirozzo S, Summerbell C, Cameron C, Glasziou P (2003) Should we recommend low-fat diets for obesity? Obes Rev 4(2):83–90
Pittler MH, Ernst E (2005) Complementary therapies for reducing body weight: a systematic review. Int J Obes 25:1030–1038
Ponsot E, Dufour SP, Zoll J, Doutrelau S, Guessan N, Geny B et al (2006) Exercise training in normobaric hypoxia in endurance runners. Improvement of mitochondrial properties in skeletal muscle. J Appl Physiol 100:1249–1257
Prabbhakar NR (2001) Oxygen sensing during intermittent hypoxia: cellular to molecular mechanisms. J Appl Physiol 90:1986–1994
Prommer N, Henicke K, Viola T, Cajigal J, Behn C, Smichdt WF (2007) Long-term intermittent hypoxia increases O2-trasport capacity but not VO2max. Hight Alt Med Biol 8:225–235
Quintero P, Milagro FI, Campion MJA (2009) Impact of oxygen availability on body weight management. Med Hypothesis 74:901–907
Ramel A, Arnarson A, Parra D, Kiely M, Bandarra NM, Martinéz JA et al (2010) Gender difference in the prediction of weight loss by leptin among overweight adults. Ann Nutr Metab 56:190–197
Rasche K, Keller T, Tautz B, Hader C, Hergenc G, Antosiewicz J et al (2010) Obstructive sleep apnea and type 2 diabetes. Eur J Med Res 4:152–156
Riccardi G, Giaccob R, Rivellese AA (2004) Dietary fat, insulin sensitivity and the metabolic syndrome. Clin Nutr 23:447–456
Rivest S, Richard D (1990) Involvement of corticotrophin-releasing factor in the anorexia induced by exercise. Brain Res Bull 25:169–172
Roels B, Thomas C, Bentley DJ, Mercier J, Hayot M, Millet G (2007) Effects of intermittent hypoxic training on amino and fatty acid oxidative combustion in human permeabilized muscle fibers. J Appl Physiol 102:79–86
Runkel N, Colombo-Benkmann M, Hüttl TP, Tigges H, Mann O, Flade-Kuthe R et al (2011) Evidence-based German guidelines for surgery for obesity. Int J Colorectal Dis 12:120–139
Salas-Salvadó J, Rubio MA, Barmany M, Moreno B, y Grupo colaborativo de SEEDO (2007) Consenso SEEDO 2007 para la evaluación del sobrepeso y la obesidad y establecimientos de criterios de intervención terapéutica. Rev Esp Obes 5:135–175
Salvador FJ (2008) Actualizaciones en el tratamiento farmacológico de la obesidad. Revista de la Sociedad Española de Medicina y Seguridad del Trabajo 3:162–173
Sarsan A, Ardic F, Ozgen M, Topuz O, Sermez Y (2006) The effects of aerobic and resistance exercises in obese women. Clin Rehabil 20:773–782
Sarwer DB, Von Sydow GA, Vetter ML, Wadden TA (2009) Behavior therapy for obesity: where are we now? Curr Opin Endocrinol Diabetes Obes 16:347–352
Semenza G (2002) Signal transduction to hypoxia-inducible factor 1. Biochem Pharmacol 64:993–998
Serebrovscaya TV, Manukhina EB, Smith ML, Dwney HF, Mallet RT (2008) Intermittent hypoxia: cause of or therapy for systemic hypertension? Exp Biol Med 233:627–650
Shatilo VB, Korkushko OV, Ischuk VA, Downey HF, Serebrovscaya TV (2008) Effects of intermittent hypoxia training on exercise performance, hemodynamics and ventilation in healthy senior men. Hight Alt Med Biol 9:43–52
Shaw I, Shaw BS (2006) Consequence of resistance training on body composition and coronary artery disease risk. Cardiovasc J S Afr 17:111–116
Shukla V, Singh SN, Vats P, Singh VK, Singh SB, Banerjee PK (2005) Ghrelin and leptin levels of sojourners and acclimatized lowlanders at high altitude. Nutr Neurosci 8:161–165
Sigal RJ, Kenny GP, Boul’e NG et al (2007) Effects of aerobic training, resistance training, or both on glycemic control in type 2 diabetes: a randomized trial. Ann Intern Med 147:357–669
Simonsen ML, Alessio HM, White P, Newsom DL, Hagerman AE (2010) Acute physical activity effects on cardiac gene expression. Exp Physiol 95:1071–1080
Siren AL, Ehrenreich H (2001) Erythopoetin: a novel concept for neuroprotection. Eur Arch Psychiatry Clin Neurosci 251:179–184
Solaini G, Baracca A, Lenaz G, Sgarbi G (2010) Hypoxia and mitochondrial oxidative metabolism. Biochim et Biophys Acta Bioenerg 1797:1171–1177
Steil G, Volund A, Kahn S, Bergman RN (1995) Reduced sample number for calculation of insulin sensitivity and glucose effectiveness from the minimal model. Diabetes 96:431–442
Strasser B, Schobersberger W (2011) Evidence for resistance training as a treatment therapy in obesity. J Obes. doi:10.1155/2011/482564
Strasser B, Siebert U, Schobersberger W (2010) Resistance training in the treatment of the metabolic syndrome: a systematic review and meta-analysis of the effect of resistance training on metabolic clustering in patients with abnormal glucose metabolism. Sports Med 40:397–415
Sweeney ME, Hill JO, Heller PA, Baney R, DiGirolamo D (1993) Severe vs moderate energy restriction with and without exercise in the treatment of obesity: efficiency of weight loss. Am J Clin Nutr 57:127–134
Tambalis KA, Panagiotakos DB, Kavouras SA, Sidossis LS (2009) Responses of blood lipids to aerobic, resistance, and combined aerobic with resistance exercise training: a systematic review of current evidence. Angiology 60:614–632
Tasali E, Van Cauter E (2002) Sleep-disordered breathing and the current epidemic of obesity: consequence or contributing factor? Am J Respir Crit Care Med 165:562–563
Thiel C, Vogt L, Claußnitzer G, Banzer W (2011) Energy cost of youth obesity exercise modes. Int J Sports Med 32:142–146
Tiwari MM, Goede MR, Reynoso JF, Tsang AW, Oleynikov D, McBride CL (2011) Differences in outcomes of laparoscopic gastric bypass. Surg Obes Relat Dis 7(3):277–282
Trayhurn P, Hoggard N, Mercer JG, Rayner DV (1999) Leptin: fundamental aspects. Int J Obes 23:22–28
Trayhurn P, Wang B, Wood IS (2008) Hypoxia in adipose tissue: a basis for the dysregulation of tissue function in obesity? Br J Nutr 100:227–235
Tremblay A, Simoneau JA, Bouchard C (1994) Impact of exercise intensity on body fatness and skeletal muscle metabolism. Metabolism 43:814–818
Tresierras MA, Balady GJ (2009) Resistance training in the treatment of diabetes and obesity: mechanisms and outcomes. J Cardiopulm Rehabil Prev 29:67–75
Urdampilleta A, Gomez-Zorita S, Martínez-Sanz JM, Roche E (2011) Eficacia de un programa de ejercicio físico en hypoxia intermitente en la mejora de la fuerza-resistencia aeróbica específica e inespecífica. Revista Española de Educación Física y Deportes 2:7–14
Van Baak MA, Astrup A (2009) Consumption of sugars and body weight. Obes Rev 10:9–23
Villareal DT, Smith GI, Sinacore DR, Shah K, Mittendorfer B (2011) Regular multicomponent exercise increases physical fitness and muscle protein anabolism in frail, obese, older adults. Obesity 19:312–318
Vimaleswaran KS, Loos RJ (2010) Progress in the genetics of common obesity and type 2 diabetes. Expert Rev Mol Med 12:e7
Vincent HK, Bourguignon C, Vincent KR (2006) Resistance training lowers exercise-induced oxidative stress and homocysteine levels in overweight and obese older adults. Obesity 14:1921–1930
Vogt M, Puntschart A, Geiser J, Zuleger C, Billeter R, Hoppeler H (2001) Molecular adaptations in human skeletal muscle to endurance training under simulates hypoxic conditions. J Appl Physiol 91:173–182
Vogtel M, Michels A (2010) Role of intermittent hypoxia in the treatment of bronchial asthma and chronic obstructive pulmonary disease. Curr Opin Allergy Clin Immunol 10:206–213
Wang B, Wood IS, Trayhurn P (2007) Dysregulation of the expression and secretion of inflammation-related adipokines by hypoxia in human adipocytes. Pflugers Arch 455:479–492
Wang JS, Lin HY, Cheng ML, Wong MK (2007) Chronic intermittent hypoxia modulates eosinophil and neutrophil platelet aggregation and inflammatory cytokine secretion caused by strenuous exercise in men. J Appl Physiol 103:305–314
Watson NF, Goldberg J, Arguelles L, Buchwald D (2006) Genetic and environmental influences on insomnia, daytime sleepiness, and obesity in twins. Sleep 29:645–649
Werger RH (2000) Mammalian oxygen sensing, signalling and gene regulation. J Exp Biol 203:1253–1263
Wernbom M, Augustsson J, Thome’e R (2007) The influence of frequency, intensity, volume and mode of strength training on whole muscle cross-sectional area in humans. Sports Med 37:225–264
West MB, Rocosh G, Obal D, Yelayuthan M, Xan YT, Hill BG et al (2008) Cardiac myocyte-specific expression on inducible nitric oxide synthase protects against ischemia/reperfusion injury by preventing mitochondrial permeability transmission. Circulation 118:1970–1978
Westerterp KR, Kayser B, Wouters L, Le Trong JL, Richalet JP (1994) Energy balance at high altitude of 6542 m. J Appl Physiol 77:862–866
White CW (2006) Commentary on “Hypoxia, hyposic signaling, tissue damage, and detection of reactive oxygen species (ROS)”. Free Radic Biol Med 40:923–927
Wilber RL (2007) Application of altitude/hypoxic training by elite athletes. Med Sci Sports Exerc 39:1610–1624
Williams MA, Haskell WL, Ades PA, Amsterdam EA, Bittner V, Franklin BA et al (2007) Resistance exercise in individuals with and without cardiovascular disease: 2007 update: a scientific statement from the American Heart Association Council on Clinical Cardiology and Council on Nutrition, Physical Activity, and Metabolism. Circulation 116:572–584
Zaobornyj T, Valdez LB, Iglesias DE, Gasco M, Gonzales GF, Boveris A (2009) Mitochondrial nitric oxide metabolism during rat heart adaptation to high altitude: effect of sildenafil, l-NAME, and l-arginine treatments. Am J Physiol Heart Circ Physiol 296:H1741–H1747
Zhu LL, Zhao T, Li H, Zhao H, Wu L, Ding A et al (2005) Neurogenesis in the adult rat brain after intermittent hypoxia. Brain Res 1005:1–6
Zhu LL, Wu L, Yew DT, Fan M (2005) Effects of hypoxia on the proliferation and differentiation of SNCs. Mol Neurobiol 31:231–242
Zoll J, Ponsot E, Dufour S, Doutreleau S, Ventura-Clapier R, Vogt M et al (2006) Exercise training in normobaric hypoxia in endurance runners. Muscular adjustments of selected gene transcripts. J Appl Physiol 100:1258–1266
Zong P, Setty W, Sun R, Martinez JD, Tune IV, Ehrenburg EN et al (2004) Intermittent hypoxic training protects canine myocardium from inafrction. Esp Biol Med 229:806–812
Acknowledgements
We are grateful to fellowship research training at the University of Basque Country (UPV-EHU), to the EXPLORA Subprogramme, MICINN, Spain (SAF2010-11630-E) for grants, to the University of Navarra for financial support through the linea especial of Nutrición, Obesidad y Salud (LE/97), as well as to BIOLASTER for technical support on the hypoxic systems and RETICS (PREDIMED) and CIBERobn from the Ministry of Health of Spain.
Conflicts of interest
The authors declare not having any personal or financial support or involvement with organizations with financial interest in the subject matter or any actual or potential conflicts of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Urdampilleta, A., González-Muniesa, P., Portillo, M.P. et al. Usefulness of combining intermittent hypoxia and physical exercise in the treatment of obesity. J Physiol Biochem 68, 289–304 (2012). https://doi.org/10.1007/s13105-011-0115-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13105-011-0115-1