Abstract
The present study was designated to assess oxidative damage and its effect on germ cell apoptosis in testes of streptozotocin (STZ)-induced diabetic rats. The role of antioxidant supplementation with a mixture of vitamins E and C and alpha lipoic acid for protection against such damage was also evaluated. Forty-five adult male rats were randomly divided into three groups: group I, control, non-diabetic rats; group II, STZ-induced, untreated diabetic rats; group III, STZ-induced diabetic rats supplemented with a mixture of vitamins E and C and alpha lipoic acid. Glycated hemoglobin (HbA1C), glucose, and insulin levels were estimated in blood samples. Malondialdehyde (MDA), the activities of the enzymes superoxide dismutase (SOD), glutathione peroxidase (GPx), and caspase-3 in addition to testosterone (T) level were all determined in testicular tissues. Histopathological studies using H&E stain, as well as, immunohistochemical detection of apoptosis using (TUNEL) method were also performed. Blood glucose and HbA1c were significantly increased while insulin was significantly decreased in STZ-induced diabetic rats as compared with controls. In rat testicular tissues, MDA, and caspase-3 activity were significantly elevated while SOD and GPx enzymatic activities as well as T level were significantly decreased in diabetic rats as compared with control group. Antioxidant supplementation to diabetic rats restored the testicular enzymatic activities of SOD and GPx to almost control levels, in addition, MDA and caspase-3 activity decrease while T increase significantly as compared with untreated diabetic group. Prominent reduction of germ cell apoptosis was found in diabetic rats supplemented with antioxidants. An important role of testicular oxidative damage and germ cell apoptosis in diabetes-induced infertility could be suggested, treatment with antioxidants has a protective effect by restoring SOD and GPx antioxidant enzymatic activity.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Diabetes mellitus is a degenerative disease that has deleterious effects on male reproductive function [21]. Streptozotocin-induced diabetes in rats provides a relevant model for studying reproductive dysfunction as they exhibit a number of deficits that resemble those seen in human diabetes [42]. Gnodos and his colleagues [22] reported that spermatogenesis is severely altered in diabetic mice and the authors suggested a direct causal effect of hyperglycemia on testicular alteration.
Diabetes-associated hyperglycemia produces intracellular oxidative stress, defined as an imbalance between the production of reactive oxygen species (ROS) and antioxidant defense systems [24]. Although many enzymes can act as antioxidants, SOD and GPx are the two major enzymes that scavenge harmful ROS in male reproductive organs [18].
It has been demonstrated that reactive oxygen species and the resulting oxidative stress play a pivotal role in apoptosis [30]. Apoptosis or programmed cell death is a cellular suicide program in which individual cells are destroyed while the integrity and architecture of surrounding tissue is preserved. It is involved in many physiological processes including tissue homeostasis, embryonic development, and the immune response [15].
Spermatogenic cells at various stages of differentiation are prone to undergo natural apoptosis; however, excess apoptosis would cause infertility due to germ cell loss [38].
Given the involvement of oxidative stress in the pathogenesis of diabetic complications, antioxidant micronutrients can be proposed as adjunct therapy in diabetic patients. It has been suggested that antioxidant supplements may show interdependency and may have effects only if given in combination. Moreover, supplements are expected to be more effective when a deficiency in these micronutrients exists [3].
Vitamins E and C and alpha lipoic acid are naturally occurring antioxidants, working in synergy with each other and against different types of free radicals [17]. Their levels are known to be suppressed in diabetes [43].
This study is designated to assess the effect of diabetes on oxidative stress in testicular tissues and its effect on germ cell apoptosis in experimental rats. The role of antioxidant supplementation with a mixture of vitamins E and C and alpha lipoic acid for protection against such damage was also evaluated.
Material and methods
Animals
Forty-five adult male albino rats (170–200 g) obtained from Medical Research Institute, Alexandria, Egypt were used in the present study. The animals were housed in cages at room temperature (22–25°C) and in a photoperiod of 14-h light/10-h dark/day. Rats were maintained on standard laboratory balanced commercial diet and water ad libitum. All experiments were performed in line with the ethical considerations, recommended by the Alexandria University, Egypt.
Induction of experimental diabetes
Experimental diabetes was induced by a single intra-peritoneal injection of STZ (sigma, USA) (60 mg/kg) in sodium citrate buffer (pH = 4.5) to an overnight fasted animals [41]. Three days after administration of STZ the tail vein blood glucose levels was measured in all animals using Gluco-meter (Roche Diagnostic, Manheim, Germany). Blood glucose levels of 270 mg/dl and above were considered diabetic. The experiment was carried out 3 days after the confirmation of diabetes.
Experimental design
The experimental animals were divided into three groups, each group comprising of 15 rats as detailed below:
-
Group I
Control rats, were injected intraperitoneally with buffer alone.
-
Group II
STZ-induced untreated diabetic rats, received an equivalent volume of olive oil.
-
Group III
STZ-induced, diabetic rats supplemented with a mixture of vitamin E (3 mg/kg body weight) [39], vitamin C (30 mg/kg body weight) [10] and alpha lipoic acid (100 mg/kg body weight) [31], by gavages once daily for a period of 8 weeks. This duration completes one spermatogenic cycle, as in most rodent species for which data are available, the duration of the cycle of the seminiferous epithelium is between 8 and 13 days [35] and approximately four cycles of the seminiferous epithelium elapse between the initial spermatogonial division and spermiation [37].
At the end of the 8th week, all rats were anesthetized and killed. Blood samples were collected into a tube containing ethylenediaminetetraacetic acid (EDTA) for assaying glycated hemoglobin (HbA1C) and plasma was separated for estimation of glucose and insulin. The testes were separated and one testis was transferred to Bouin fixative to be used for histopathological and immunohistochemical studies. The other testis was washed with ice cold 0.9% NaCl, blotted dry, decapsulated, thereafter, testis homogenization in 0.05 M phosphate buffer saline pH 7.4 (10% w/v) was performed using a glass homogenizer with a loose filling Teflon pestle, in an ice bath (4°C). Total homogenate was used for the determination of MDA and testicular testosterone. The remaining homogenate was centrifuged at 10,000×g for 10 min at 4°C and the supernatant was used for assaying the enzymatic activities of SOD, GPx, and protein content, and the pellet was used for caspase-3 determination.
Hormonal assessment
Immunoenzymatic assay for the quantitative measurement of plasma insulin was performed using Medgenix-Ins-Easia kit (Medgenix. Diagnostics) according to manufacturer’s instructions.
Testicular testosterone was determined using RIA coat-A-count kit from Diagnostic Products Corporation, Los Angelo’s CA 90045-5597, USA. For testosterone extraction, the homogenate was extracted with diethyl ether before hormonal assay [12].
Biochemical assessment
HbA1C was assayed using Stanbio kit, San Antonio, Texas. Determination of MDA level, an end product of tissue lipid peroxidation, was measured as described by Ohkawa et al. [32].The reaction mixture contained 0.1 ml of 10% tissue homogenate (0.2 ml of 8.1% sodium dodecyl sulfate, 1.5 ml of a 20% acetic acid solution, and 1.5 ml of a 0.8% aqueous solution of TBA). The mixture was made up to 4 ml with water and heated at 95◦C for 1 h. After cooling, the mixture was added to 1.0 ml of water and 5.0 ml of n-butanol and then shaken vigorously. After centrifugation at 1,200×g for 10 min, the absorbance of the organic layer was measured at 532 nm. The standard curve was plotted using, 1,1,3,3-tetramethoxypropane as standard. Values were expressed as nanomoles per milligram of protein.
Superoxide dismutase activity was determined by the pyrogallol method of Marklund and Maklund [29]. In a cuvette, 1 ml of tris HCL buffer was mixed with 10 μl of pyrogallol and 10 μl of diluted supernatant (1/10). After mixed the rate of increase in absorbance was measured at 420 nm. The enzyme specific activity was expressed as units per milligram protein.
Glutathione peroxidase activity was determined by the method of Flohe and Gunzler [16]. The assay mixture contained 500 μL 0.1 M phosphate buffer (pH 7.0), 100 μL of enzyme sample, 100 μL of glutathione (GSH) reductase (0.24 U/mL), 100 μL of 10 mM GSH and 100 μL nicotinamide adenine dinucleotide phosphate (NADPH). The reaction was started by adding 100 μL of 12 mM t-butyl hyroperoxide. Conversion of NADPH to NADP + was monitored continuously using a spectrophotometer at 340 nm for 3 min. GPx specific activity was expressed as nanomoles of NADPH oxidized per minute per milligram of protein using an extinction coefficient (6.22 × 103/M/cm).
Caspase-3 activity was measured spectrophotometrically using kit (Caspase-3 Cellular Activity Assay Kit, Calbiochem, San Diego, CA) according to the manufacturer’s protocol. Testicular pellets were lysed using the lyses buffer provided in the kit. Lysates were centrifuged at 15,000 rpm at 4°C for 20 min and the clear supernatants were used for caspase-3 activity and protein determination [5]. The assay for caspase-3 activity was carried out in a 96-well plate. Briefly, 45 μl of sample and 45-μl assay buffer (100 mM NaCl, 50 mM HEPES, 10 mM DTT, 1 mM EDTA, 10% glycerol, 0.1% CHAPS, pH 7.4) and 10 μl of caspase-3 colorimetric substrate DEVD-pNA were added to wells. The plate was incubated at 37°C, during which time caspase in the sample was allowed to cleave the chromophore pNA from the substrate molecule. Absorbance was recorded at 10-min intervals for 2 h at 405 nm using a micro plate reader (Vmax, Molecular Devices, Sunnyvale, CA). Data were normalized to total protein, and caspase-3 activity was expressed as picomoles pNA per hour per milligram of protein.
Histopathological and immunohistochemical methods
The testis transferred to Bouin fixative was used for histopathological studies for detecting spermatogenesis using haematoxylin and eosin stain, as well as immunohistochemical detection of apoptosis using TdT-mediated dUTP—biotin nick end labeling (TUNEL) method. In situ apoptosis detection kit (TACS™ TdT kit), purchased from R&D systems Inc, USA, was used.
The immunohistochemical procedure followed in the present study for TUNEL labeling was described by Gavrieli et al. [19]. Twenty micrograms per millilliter of proteinase-K was placed on the section and kept at room temperature for 15 min. After washing twice in distilled water, the sections were treated with DNA probs/PBS for 2 h and washed with PBS. After drying the sections with filter paper, they were covered with terminal deoxynucleotidyl transferase (TdT) and then placed in a humidified chamber overnight at −4°C. The reaction was stopped by placing the sections in stop/wash buffer. The sections were washed thrice with PBS, and treated with anti-digoxigenin-peroxidase conjugate and incubated in a humidified chamber for 1 h at room temperature. After washing the sections four times in PBS, and stained with diaminobenzidine tetra-hydrochloride, which was used as chromogen reagent to develop the characteristic color (dark brown). Control slides were processed in an identical manner except that TdT was omitted. The sections were counterstained with methyl green, dehydrated with ethanol and xylene, cover slipped, and images viewed and recorded using Optus microscope equipped with Spot digital camera (Histochemistry and Cell Biology department, Medical Research Institute, Alex. University, Egypt.)
Statistical analysis
All data are presented as mean ± SEM. All analyses were carried out using the SPSS software. One-way analysis of variance (ANOVA) was utilized for comparison between the means of the three groups and the least significant difference test was used for comparison of each two individual means. P < 0.05 was considered statistically significant.
Results
Biochemical and hormonal results
Table 1 shows mean ± SEM of HbA1C, plasma glucose, and insulin levels. By ANOVA test significant difference were found between the three studied groups.
The mean values of HbA1C and plasma glucose were significantly elevated while insulin was significantly lower in diabetic rats (groups II and III) as compared with control group (group I). In diabetic rats supplemented with antioxidants (group III), the mean value of HbA1C and plasma glucose were significantly lower, while insulin level was significantly higher than that in the untreated diabetic group (group II).
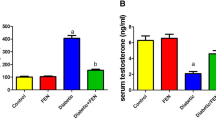
The mean ± SEM of MDA, SOD, GPx, and caspase enzymatic activities and T level in testicular tissues of control and diabetic rats with and without antioxidant supplementation are shown in Table 2. Significant differences were detected between the three groups using ANOVA test.
The mean values of MDA level and caspase activity were significantly elevated in both the untreated and treated diabetic rats (groups II and III) versus the control group (group I). Antioxidant supplementation significantly lowers both MDA and caspase activity in treated diabetic group (group III) than that in untreated diabetic group (group II).
As regards SOD and GPx specific activities, their levels were significantly lower in untreated diabetic rats (group II) than that of control group (group I). Their levels were significantly higher in diabetic rats supplemented with antioxidants (group III) as compared with untreated diabetic rats (group II), and they were within the levels of control group.
In diabetic rats (groups II and III), the mean values of testosterone were significantly lower than that in the control group. Significantly higher levels were detected in treated diabetic rats (group III) as compared with untreated diabetic rats (group II).
Histopathological results
Paraffin sections from (group I) non-diabetic control rats’ testes revealed compact-variable-sized semineferous tubules lined by a thick layer of spermatogenic cells in variable stages of maturation beginning from the primary spermatogonia till final sperm production filling the lumen. Basement membrane of all tubules was thin with no thickening and no peritubular fibrosis. Occasional apoptotic figures were encountered in some of the spermatogenic cells. Interstitium was scanty, slightly edematous, and included scattered small groups of Leydig cells having small round monotonous nuclei and prominent tiny nucleoli (Fig. 1).
In (group II) untreated diabetic rats’ testes, about 30% of the tubules showed incomplete spermatogenesis with arrest at the secondary spermatogenic stage with occasional sperm production. Quite frequent apoptotic figures were encountered at both primary and secondary spermatogenic stage forming small dense nuclei with clumped chromatin and occasional fragmentation surrounded by clear hallow. Interstitium was edematous vascular and congested with normal Leydig cells (Fig. 2).
Treated diabetic rats’ testes (group III) revealed restoration of normal spermatogenesis in almost all semineferous tubules with good number of terminal sperm production. Interstitium was edematous and slightly vascular with normal Leydig cells (Fig. 3).
Immunohistochemical results
In situ hybridization using (TUNEL) technique detected apoptotic cells with fragmentation of DNA which was observed as intra nuclear black granules (TUNEL-positive cells). Testes of (group I) non-diabetic control rats revealed scanty scattered positive nuclei (Fig. 4).
Paraffin section photograph of (group I) non-diabetic control rat testis, showing scanty scattered and occasional black nuclear granules (arrows). Primary spermatocyte (1ry), secondary serpmatocytes (2nd) and spermatogonia (SP). Green stain was observed in spermatid nuclei and flagella (SD) (DAB X1000)
The untreated (group II) diabetic rats’ testes revealed evident increase in TUNEL-positive cells seen as round black intra nuclear granules especially at the final maturation stage cells. In some testicular sections, TUNEL-positive cells were evident all through the stages of maturation beginning from the spermatogonia till the spermatid stage (Fig. 5), and other section showed some Sertoli cells to be TUNEL positive (Fig. 6).
Diabetic rats supplemented with antioxidants (group III), revealed TUNEL-positive cells in some tubules mainly at primary spermatocytes, and negative reaction was shown in Sertoli and secondary spermatocytes. Proportion of apoptotic cells was decreased than their corresponding proportion in diabetic group but was still higher than that of the control group. In some tubules there was complete absence of staining nearly simulating the control group (Fig. 7).
Paraffin section of (group II) untreated diabetic rat’s testis showed negative TUNEL cells under the absence of TdT enzyme (Fig. 8).
Discussion
Streptozotozin is a short acting beta cell toxin, its structure facilitates its preferential uptake into the pancreatic β-cells, probably via the low-affinity glucose transporter, GLUT2 [45]. Studies have indicated that detrimental effects of streptozotocin mediated persistent hyperglycemia is a proximate cause of inexorable deterioration of β-cell function, which is mediated and complicated through the enhanced formation of reactive free radicals [36]. In the present study, blood glucose level in STZ-diabetic rats was significantly higher as compared with control rats, this occurred as a result of insulin deficiency, the basic pathogenic factor responsible for hyperglycemia.
Increased oxidative stress, which contributes to the pathogenesis of diabetic complications, is the consequence of either enhanced ROS production or attenuated ROS scavenging capacity, resulting in tissue damage that is most easily assessed by measuring lipid peroxide levels [13]. Superoxide dismutase and GPx are two major enzymes that scavenge harmful ROS in male reproductive organs, [18] Superoxide dismutase is considered as a first line of defence against oxygen toxicity and central regulator of ROS levels by catalyzing the dismutation of superoxide radicals to H2O2 and molecular oxygen. [2] Glutathione peroxidase catalyzes the reduction of a variety of hydroperoxides (ROOH and H2O2) using GSH, thereby protecting mammalian cells against oxidative damage [6].
In the present study, induction of diabetes resulted in marked testicular oxidative impact as evidenced by significantly higher levels in MDA, an end product of lipid peroxidation, and significantly lowers SOD and GPx enzymatic activities in testicular tissues of untreated diabetic rats than that in control group. These results are in agreement with those of Shrilatha et al. [41]; they provided significant evidence in favor of the hypothesis that diabetes induction is associated with significant oxidative stress in rat testis and epididymal sperm during early (2 weeks) as well as progressive (6 weeks) phase.
The decrease in SOD and GPx enzymatic activities in the untreated diabetic group can be attributed to protein glycation reactions which may affect amino acids close to the active sites of the enzymes leading to its inactivation [20]. In the present study HbA1c, which is used as a marker for estimating the degree of protein glycation in diabetes mellitus [7], was found to be significantly elevated in diabetic rats as compared with control group. Thus, oxidative impact in testis of diabetic rats in the present study could be suggested, which may render testicular cells susceptible to oxidative insult and apoptosis.
Caspases are considered as a central player for the apoptotic process and cascade of proteolytic cleavage event. Caspase-3, in particular, is associated with the execution of apoptosis and induces the large number of morphological changes characteristic of cells undergoing apoptosis including nuclear fragmentation at the final step of apoptosis [40]. In the present study, testicular caspase-3 activity was significantly higher in diabetic rats as compared with control group. This result was confirmed by immunohistochemical findings which revealed fragmentation of DNA (TUNEL-positive cells) in semineferous tubules. TUNEL-positive cells were mainly in primary and secondary spermatocytes. In an experimental study in mice [28], it was found that exposure of semineferous tubules to various apoptotic inducers, as heat and certain chemicals, led to similar morphological pattern indicating that germ cells might be more vulnerable to apoptotic insult.
Testosterone may function as a cell survival factor, in some way protecting germ cell from apoptotic death [14]. In the present study significantly lower level in testicular T was found in diabetic rats as compared with the control group. This finding is in agreement with Sudha et al. [44], who reported significantly lower levels of T in serum and seminal fluid of diabetic rats. Low T levels may result from the effect of lipid peroxidation on T biosynthesis [26].
Diabetic patients represent a population in whom oxidative stress is much higher than in the general population. Several mechanisms seem to be involved in the development of oxidative stress in presence of elevated glucose levels [4]. Regimes that counter hyperglycemia such as oral anti-diabetic drugs and insulin do not completely prevent the risk of developing diabetic complications [1]. No drug, including insulin, is capable of keeping glucose levels in the normal range all the time. No drug can replace the exquisitely tuned rates of insulin secretion from the native β-cell that finely control glucose levels. Attempts with drugs to control blood glucose levels within the normoglycemic range often force it into the hypoglycemic range.
The use of antioxidants has been widely proposed to assist diabetics in avoiding complications, by reducing oxidative stress, either indirectly by improving glycemic control and/or directly by scavenging free radicals or stimulating the antioxidant defenses [3]. Agents such as vitamins E and C and alpha lipoic acid have shown promise in diabetes. Vitamin E is a well-known lipophilic vitamin, with antioxidant properties. It essentially reacts with peroxyl free radicals and protects membranes and lipoproteins. Tocopherol was reported to decrease hyperglycemia-induced protein kinase C activation which is responsible for many pathological changes observed in diabetes [9]. Vitamin E also has beneficial effects on glycemic control in experimental diabetes [23]. Vitamin C has been shown to inhibit some of the pathways that lead to late complications of diabetes, including the polyol pathway and protein glycation; moreover, it participates in vitamin E regeneration [25]. Alpha lipoic acid is a co-enzyme of pyruvate and α-ketoglutarate dehydrogenase multienzyme complex of the tricarboxylic acid cycle. Its properties as an antioxidant have been reviewed by Paker et al. [34]. Lipoic acid or its reduced form, dihydrolipoic acid, quenches a number of oxygen-free radical species in both lipid and aqueous phase, chelates transition metals, and prevents membrane lipid peroxidation and protein damage. It participates in the recycling of vitamins C and E, increases cellular levels of glutathione, and suppresses non-enzymatic glycation. Lipoic acid also increases glucose uptake through recruitment of glucose transporter-4 to the plasma membrane, which is also the mechanism of insulin stimulated glucose uptake [27].
Since antioxidants interact with each other in a biochemical chain of defense against free radicals, and the use of high doses of a single antioxidant poses potential risks because it could perturb the antioxidant-prooxidant balance in antioxidant-depleted subjects, it has been suggested that combination supplements may prevent this potentially adverse effect of antioxidant supplementation [33]. It was found that triple antioxidant therapy (vitamins E and C and alpha lipoic acid) for a period of 6 weeks significantly reduced HbA1c in the diabetic volunteers, which returned back to its initial values 4 weeks after the end of supplementation. In addition, total antioxidant status measurement indicated that diabetic plasma antioxidant capacity was significantly improved during antioxidant supplementation [11].
In the present study, treatment of diabetic rats with combined antioxidants yielded promising results by significantly reducing plasma glucose, HbA1C, and testicular MDA levels and caspase-3 activity, while plasma insulin and testicular SOD and GPx enzymatic activities as well as T level in testes were significantly elevated as compared with untreated diabetic group. Furthermore, histopathological and immunohistochemical studies in diabetic rats’ testis supplemented with antioxidants showed prominent reduction in germ cell apoptosis, as compared with untreated diabetic rats.
Chronic oxidative stress in diabetes milieu is a central mechanism for glucose toxicity in pancreatic β-cells which in turn causes suppression of insulin gene expression and insulin secretion as well as increased β-cell apoptosis that ultimately results in the relentless deterioration of pancreatic β-cells [36]. In the present study, antioxidant supplementation to diabetic rats significantly improved plasma insulin levels which may occur as a result of reducing oxidative impact by scavenging free radicals and stimulating antioxidant defenses, thus allowing the residual pancreatic β-cell to synthesize and secrete more insulin. In agreement with the present study, it was found that quercetin, which contains antioxidant properties, had the beneficial effects in decreasing blood glucose concentration, promoting regeneration of the pancreatic islets and increasing insulin release in STZ-induced diabetic rats [46]. In addition, it was found that alpha lipoic acid supplementation to diabetic rats significantly lower blood glucose and HbA1C and significantly increased plasma SOD activity than non-supplemented rats [8].
Although glucose level in treated diabetic rats was still significantly higher than that in the control group, HbA1C in diabetic rats treated with antioxidants was statistically decreased as compared with untreated diabetic rats. These findings could be interpreted in view of Brownlee [7], who stated that antioxidant supplementation could inhibit glycosylation independently of any effect they may have on blood glucose level.
In conclusion, an important role of germ cell apoptosis in diabetes-induced infertility could be speculated. The present study further suggested that supplementation with antioxidants vitamins C and E and alpha lipoic acid can effectively attenuate hyperglycaemia-induced testicular oxidative damage and exert a protective role against germ cell apoptosis.
References
Amos AF, Mccarty DJ, Zimmet P (1997) The rising global burden of diabetes and its complications: estimates and projections to the year 2010. Diabet Med 14:S1–S85
Bannister JU, Bannister WH, Rotilio G (1987) Aspects of the structure, function, and applications of superoxide dismutase. CRC Crit Rev Biocheme 22:111–180
Bonnefont R (2004) The role of antioxidant micronutrients in the prevention of diabetic complications. Treat Endocrinol 3:41–52
Bonnefont R, Bastard JP, Jaudon MC, Delattre J (2000) Consequences of diabetic status on the oxidant/antioxidant balance. Diab Metab 26:163–176
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254
Brigelius FR (1999) Tissue specific functions of individual glutathione peroxidases. Free Radic Biol Med 27:951–965
Brownlee M (1995) The pathological implications of proteins glycation. Clin Invest Med 18:275–281
Budin SB, Othman F, Louis SR, Abu Bakar M, Radzi M, Osman K (2009) Effect of alpha lipoic acid on oxidative stress and vascular wall of diabetic rats. RJME 50:23–30
Bursell SE, King GL (1999) Can protein kinas C inhibition and vitamin E prevent the development of diabetic vascular complications? Diab Res Clin Pract 5:169–182
Cay M, Nazıro M, Sim sek H, Aydilek N, Aksakal M, Demirci M (2001) Effects of intraperitoneally administered vitamin C on antioxidative defence mechanism in streptozotocin-induced diabetic rats. Res Exp Med 200:205–213
Coleman MD, Fernandes S, Khanderia L (2003) A preliminary evaluation of a novel method to monitor a triple antioxidant combination (vitamins E, C and alpha-lipoic acid) in diabetic volunteers using in vitro methaemoglobin formation. Environ Toxicol Pharmacol 14:69–75
D’ Aniello A, Cosmo AD, Cristo CD, Assisi L, Virgilo B, Fiore MD (1996) Occurrence of sex steroid hormones and their binding proteins in octopus vulgaris lam. Biochem Biophys Res Commun 277:782–788
Dickinson PJ, Carrington AL, Frost GS, Boulton AJM (2002) Neurovascular disease, antioxidants and glycation in diabetes. Diab Metab Res Rev 18:260–272
Erkkilä K, Henriksén K, Hirvonen V, Rannikko S, Salo J, Parvinen M, Dunkel L (1997) Testosterone regulates apoptosis in adult human seminiferous tubules in vitro. J Clin Endocrinol Metab 82:2314–2321
Fadeel B, Orrenius S (2005) Apoptosis: a basic biological phenomenon with wide-ranging implications in human disease. J Intern Med 58:479–517
Flohe L, Gunzler WA (1984) Assay of glutathione peroxidase. Meth Enzymol 105:114–121
Flora SJ (2007) Role of free radicals and antioxidants in health and disease. Cell Mol Biol 53:1–2
Fujii J, Luchi Y, Matsuki S, Lshii T (2003) Cooperative function of antioxidant and redox systems against oxidative stress in male reproductive tissues. Asian J Androl 5:231–242
Gavrieli Y, Sherman Y, Ben-Sasson SA (1992) Identification of programmed cell death in situ via specific labeling of nuclear DNA fragmentation. J Cell Biol 119:493–501
Gillery P (2006) Oxidative stress and protein glycation in diabetes mellitus. Ann Biol Clin Paris 64:309–314
Glenn DR, McClure N, Lewis SE (2003) The hidden impact of diabetes on male sexual dysfunction and fertility. Hum Fertil Camb 6:174–179
Gnodos B, Rivikind Y, Jovaniivic L (1998) Effect of increasing glucose concentration on sertoli cell viability in the non obese diabetic mouse (NOD mouse). Ann Clin Lab Sci 28:236–241
Ihara Y, Yamada Y, Toyokuni S, Miyawaki K, BanN AT, Kuroe A, Iwakura T, Kubota A, Hiai H, Seino Y (2000) Antioxidant alpha-tocopherol ameliorates glycemic control of GK rats, a model of type 2 diabetes. FEBS Lett 473:24–26
Jones DP (2006) Redefining oxidative stress. Antioxid Redox Signal 8:1865–1879
Kagan VE, Serbinova EA, Forte T, Scita G, Paker L (1992) Recycling of vitamin E in human low density lipoproteins. J Lipid Res 33:385–397
Khokha AM, Kashko MF, Voronov PP (1993) Effect of ethanol and lipid peroxidation on testosterone biosynthesis by interstitial cells of testes. Ukr Biochem Zh 65:111–115
Konrad D, Somwar R, Sweeney G, Yaworsky K, Hayashi M, Ramlal T, Klip A (2001) The antihyperglycemic drug α-lipoic acid stimulates glucose uptake via both GLUT4 translocation and GLUT4 activation: potential role of p38 mitogen-activated protein kinase in GLUT4 activation. Diabetes 50:1464–1471
Lee JS, Ahn SS, Jung KC, Kim Y-W, Lee SK (2004) Effects of 60 Hz electromagnetic field exposure on testicular germ cell apoptosis in mice. Asian J Androl 6:29–34
Marklund S, Marklund G (1974) Involvement of superoxide anion radical in autoxidation of pyrogallol and convenient assay for superoxide dismutase. Eur J Biochem 47:469–474
Mostafa MH, Sharma RK, Thornton J, Mascha E, Abdel-Hafez MA, Thomas AJ (2004) Relationship between ROS production, apoptosis and DNA denaturation in spermatozoa from patients examined for infertility. Hum Reprod 19:129–138
Nagamatsu M, Nickander KK, Schmelzer JD, Raya A, Wittrock DA, Tritschler H, Low PA (1995) Lipoic acid improves nerve blood flow, reduces oxidative stress, and improves distal nerve conduction in experimental diabetic neuropathy. Diab Care 18:1160–1167
Ohkawa H, Ohishi N, Yagi K (1979) Assay of lipid peroxides in animal tissues by thiobarbituric acid reaction. Analyt Biochem 95:351–358
Opara EC (2002) Oxidative stress, micronutrients, diabetes mellitus and its complications. J R Soc Health 122:28–34
Packer L, Witt EH, Tritschler HJ (1995) Alpha-lipoic acid as a biological antioxidant. Free Radic Biol Med 19:227–250
Peirce EJ, Breed WG (2001) A comparative study of sperm production in two species of Australian arid zone rodents (Pseudomys australis, Notomys alexis) with marked differences in testis size. Reproduction 121:239–247
Robertson RP (2004) Chronic oxidative stress as a central mechanism for glucose toxicity in pancreatic islet beta cells in diabetes. J Biol Chem 279:42351–42354
Rosiepen G, Weinbauer GF, Schlatt S, Behre HM, Nieschlag E (1994) Duration of the cycle of the seminiferous epithelium estimated by the 5-bromodeoxyuridine technique, in laboratory and feral rats. J Reprod Fertil 100:299–306
Shaha C (2007) Modulators of spermatogenic cell survival. Soc Reprod Fertil Suppl 63:173–186
Shalaby MA, El Zorba HY, Kamel GM (2004) Effect of α-tocopherol and simvastatin on male fertility in hypercholesterolemic rats. Pharmacol Res 50:137–142
Shi Y (2002) Mechanisms of caspase activation and inhibition during apoptosis. Molec Cell 9:459–470
Shrilatha B, Muralidhara (2007) Occurrence of oxidative impairments, response of antioxidant defenses and associated biochemical perturbations in male reproductive milieu in streptozotocin-diabetic rat. Int J Androl 30:508–518
Soudamani S, Yuvaraj S, Malini T, Balasubramanian K (2005) Experimental diabetes has adverse effects on the differentiation of ventral prostate during sexual maturation of rats. Anat Rec Discov Mol Cell Evol Biol 287:1281–1289
Starin J (1991) Disturbances of micronutrients and antioxidant status in diabetes. Proc Nutr Soc 50:591–604
Sudha S, Valli G, Julie PM, Arunakaran J, Govindarajulu P, Balasubramanian K (2000) Influence of streptozotocin-induced diabetes and insulin treatment on the pituitary-testicular axis during sexual maturation in rats. Exp Clin Endocrinol Diab 108:14–20
Thulesen J, Orskov C, Holst JJ, Poulsen SS (1997) Short-term insulin treatment prevents the diabetogenic action of streptozotocin in rats. Endocrinology 138:62–68
Vessal M, Hemmati M, Vasei M (2003) Antidiabetic effects of quercetin in streptozotocin-induced diabetic rats. Comp Biochem Physiol C Toxicol Pharmacol 135C(3):357–364
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Mohasseb, M., Ebied, S., Yehia, M.A.H. et al. Testicular oxidative damage and role of combined antioxidant supplementation in experimental diabetic rats. J Physiol Biochem 67, 185–194 (2011). https://doi.org/10.1007/s13105-010-0062-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13105-010-0062-2