Abstract
Background
Since diabetes mellitus type-1 (DM-1) induces testicular oxidative and inflammatory damage with finally an ultimate male infertility, and as fenofibrate (FEN) plays an important antioxidant and anti-inflammatory role, the aim of the present study was to investigate the effects of FEN on diabetes-induced reproductive damage and clarifying the underlying related mechanisms.
Methods
DM-1 was induced in male Wistar rats by a single intraperitoneal injection of streptozotocin (50 mg/kg). FEN (100 mg/kg/day, orally) was administrated to diabetic rats for 4 weeks. Testicular damage was detected by estimation of both testicular and body weights, assessment of serum testosterone, testicular oxidative stress parameters (malondialdehyde and nitric oxide levels) and testicular oxidant defenses (reduced glutathione, superoxide dismutase and hemeoxygenase-1). Expressions of the inflammatory markers (inducible nitric oxide synthase, p38 mitogen-activated protein kinase (MAPK), tumor necrosis factor alpha, interleukin-6 and apoptotic marker (caspase-3) were evaluated in testicular tissue. Our results were confirmed by histopathological examination of testicular tissues.
Results
Diabetic testicular damage was proved by both biochemical and histopathological examinations. FEN treatment reversed diabetic testicular damage; normalized the serum testosterone level, improved anti-oxidative capacity, ameliorated the pro-inflammatory cytokine expression in testicular tissue with the down regulation of p38 MAPK mediated-testicular apoptosis.
Conclusion
FEN treatment exerted a protective effect against streptozotocin-induced diabetic reproductive dysfunction not only through its powerful antioxidant and hypoglycemic effects, but also through its anti-inflammatory and anti-apoptotic effect via down-regulation of testicular p38 MAPK expression in diabetic rats.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Diabetes mellitus (DM) is a prevalent chronic endocrinal disease, considered as a principal cause for morbidity and mortality in both developed and developing countries and is strongly related to infertility and reproductive dysfunction in males, especially in adolescents and young adults [1].
DM type1(DM-1) is an autoimmune disease characterized by an immune response against islet, specifically destroys islet β cells, results in an abnormality in insulin synthesis and secretion, causes disorder in glucose metabolism which is highly correlated with pathological damages, leading to a series of complications, and even death [2].
DM-1can affect the spermatogenesis by oxidative damage via the generation of reactive oxygen species (ROS) which either macerate the cellular antioxidant defense mechanisms resulting in damage to cell proteins, lipids, and DNA or directly stimulate the inflammatory signaling pathways such as p38 mitogen-activated protein kinase (MAPK) activation and inducible nitric oxide synthase (iNOS) production along with cytokines release initiating testicular cells inflammation with disruption of spermatogenesis and steroidogenic dysfunction ending by testicular apoptosis [3,4,5].
Peroxisome proliferator-activated receptors (PPARs) are nuclear receptors including three isotypes which are encoded by variable genes: PPAR α, β, γ, δ. PPARs receptors. PPARs are well established to be involved in lipid metabolism, glucose homeostasis, cellular proliferation and differentiation and are also involved in the immune response. So, PPARs are paramount objectives in the management of different types of inflammatory or autoimmune disease as DM-1and also in metabolic disorders such as DM type 2 [4, 6].
In testis, PPARα; one of the PPARs subtypes, is mainly expressed in Leydig cells and at lower levels in Sertoli cells [7]. Most notably, PPARα regulates the cholesterol metabolism; the precursor of sex hormones synthesis. So, any agonist of PPARα could possibly influence sex hormone synthesis [8]. PPARα is a target for fibrates, PPARα stimulation increase the genes expression of superoxide dismutase (SOD), glutathione reductase (GSH) and hemeoxygenase-1 (HO-1) augmenting the redox state [9, 10].
Furthermore, PPARα activation inhibit the expression of iNOS and p38 MAPK signaling pathways resulting in decrease in the levels of tumor necrosis factor alpha (TNF-α) and interleukin-6 (IL-6) and consequently the expression of caspase-3 which suppress the inflammatory response, oxidative stress, and cellular apoptosis [11,12,13].
So, we aimed in the present study to evaluate the role of fenofibrate (FEN); a PPARα agonist on DM-1 induced testicular damage in rats, and the underlying mechanisms of action will be clarified.
Materials and methods
Chemicals
FEN powder and Streptozotocin (STZ) were obtained from Mina Pharm Company and Sigma-Aldrich Co., (St. Louis, MO, USA), respectively. The ready to use iNOS (Catalog number: PA3-030A; dilution 1:200) and caspase-3 (Catalog number: RB-1197-R7) rabbit polyclonal antibodies were obtained from Thermo Fisher Scientific Inc./Lab Vision (Fremont, CA, USA).
Animals
Adult male Wistar rats of average weight (250–300 g), aged 8–10 weeks were obtained from National Research Center, Giza, Egypt. Commercial rat chow (El-Nasr Co., Cairo, Egypt) with tap water was used for animals feeding and the rats were left for 1 week before inclusion in the experiment to acclimatize to the laboratory environment. The study was maintained at 24 ± 2 °C with a 12-h dark: light cycle. Animals were arranged in polypropylene autoclavable rat cage (300 L × 200 H × 180 W mm) with stainless steel cover lid and built in food feeder. Procedures including animals complied with the ARRIVE guidelines and according to the U.K. Animals Act, 1986 and the board of Faculty of medicine, Minia university and the EU Directive which approved the procedures of animal experiment protocol(148:1/2019).
Experimental design
A diabetic rat model was constructed by single intraperitoneal (ip) injection of STZ which was freshly prepared in 0.01 mM citrate buffer, pH 4.5 in a dose 50 mg/kg [14]. All STZ injected rats were fasted overnight before STZ administration, then after STZ injection they were received 20% sucrose dissolved in water in the first 48 h to prohibit the initial STZ-induced hypoglycemia. Blood glucose levels were detected after 72 h using blood glucose meter (ACCU-CHEK® Active, Roche Diagnostics, Germany). Rats considered as diabetic and implicated in our study when the blood glucose levels were higher than 250 mmol/l.
Rats were arranged randomly into 4 groups (8/group):
- Group 1:
-
Control group; received vehicle (1% carboxymethylcellulose) orally once daily and a single ip saline injection [15].
- Group 2:
-
FEN group; received FEN (100 mg/kg) once daily po and a single ip saline injection [16].
- Group 3:
-
Diabetic group; received vehicle (1% carboxymethylcellulose) once daily po and received STZ (50 mg/kg) a single ip injection [14].
- Group 4:
-
STZ-FEN treated group; received FEN (100 mg/kg) and a single ip injection of STZ (50 mg/kg).
Sample collection and storage
Oral drugs were administered by intra-gastric tube after establishment of DM-1. At the end of the experimental period (4 weeks), rats were sacrificed by decapitation under anesthesia using urethane hydrochloride (1 g/kg) ip injection. Blood samples were collected from abdominal aorta, and then centrifuged for 10 min at 5000 rpm. Clear serum was obtained and stored at − 80 °C to be used for the determination of biochemical parameters. One testis was immersed in Bouin's fixative and entrenched in paraffin for histopathological and immunohistochemical assessment and the other testis was frozen using liquid nitrogen and then stored at − 80 °C. Testes were either homogenized in cold potassium phosphate buffer (0.01 M, pH 7.4) for use in different biochemical analyses, or processed for RNA extraction.
Biochemical analysis
Assessment of serum levels of testosterone and glucose
Serum testosterone was assessed using testosterone enzyme-linked immunosorbent assay (ELISA) kit (Cayman Chemicals., USA). Serum glucose was colorimetrically assessed using a commercial kit.
Assessment of testicular tissue antioxidant enzymes (SOD and GSH)
Antioxidant enzymes in testicular tissue was evaluated by measuring SOD activity and GSH level.
Superoxide dismutase (SOD) activity was chemically measured as previously described by Marklund and Marklund in 1974 [17] who reported that one SOD unit is equal to the percent of enzyme which inhibit pyrogallol autoxidation by 50%. SOD activity in testicular tissue was evaluated by spectrophotometry at 420 nm.
Testicular GSH level was measured by spectrophotometry on the base of the reduction of Ellman's reagent by thiol (–SH) groups of GSH to produce a yellow color measured at 412 nm [18].
Assessment of testicular oxidative stress parameters (MDA and NO)
Testicular Malondialdehyde (MDA) level was biochemically evaluated by spectrophotometric method which based on the use of thiobarbituric acid method and the sample absorption was evaluated at 535 nm [19].
Total nitrite was evaluated in testicular tissue homogenate depending on the Griess reaction which is based on the interaction of nitrite with a blend of naphthylethylenediamine and sulfanilamide; NO level was measured at 540 nm [20].
ELISA assay
TNF-α, IL-6, HO-1 and p38 MAPK levels in testicular homogenate were measured using ELISA kits according to the instructions of the manufacturer. TNF-α ELISA kit was from Sigma-Aldrich Co. (St. Louis, MO, USA), Catalog No: E-EL-R0019. IL-6 ELISA kit was purchased from Invitrogen ThermoFisher Scientific USA (LOT 192587043). HO-1 ELISA kit from (ELISA Genie Co., Dublin, Ireland), Catalog No: SKU: RTFI00859. p38 MAPK ELISA kit purchased from Assay biotechnology Co. (USA), Catalog No: FLUO-CBP1641, respectively.
Real-time reverse transcription polymerase chain reaction (RT-PCR)
Extraction of total RNA from testicular tissue using RiboZol reagent (Amresco, Solon, USA) in accordance with the manufacturer instructions was done. 5 µg of total RNA was used for RT-PCR according to manufacture instructions (Thermo Scientific Verso sybr green one step qRT-PCR kits plus ROX Vial, code no AB-4104/A) using primers as mentioned below in the thermal cycler (Applied Biosyst 7500 fast, Techne (Cambridge) LTD., UK). RT-PCR cycling parameters were 50 °C at 15 min, 95 °C at 15 min and 40 cycles of denaturation at 95 °C for 15 s, annealing at 60 °C for 30 s and extension at 72 °C for 30 s. Relative expression of` HO-1, PPARα and p38 MAPK genes were calculated using the comparative threshold cycle method (Ct). All values were normalized to the β-actin gene.
The sets of primers used were as follows:
HO-1 primer sequence (5′–3′)
Forward primer: 5′-TTAAGCTGGTGATGGCCTCCs-3′
Reverse primer: 5′- GTGGGGCATAGACTGGGTTC-3′
PPARα primer sequence (5′–3′)
Forward primer: 5′-TCGGCGAACTATTCGGCTG-3′
Reverse primer: 5′-GCACTTGTGAAAACGGCAGT-3′
p38 MAPK primer sequence (5′–3′)
Forward primer: 5′-TTCCCAGCAGTCCTATCC-3′
Reverse primer: 5′-GTCAGATGGCAAGGGTTC-3′
β-actin primer sequence (5′–3′)
Forward: 5′-GTCGTACCACTGGCATTGTG-3′
Reverse: 5′-CAGCATGGTGACCGTAACA-3′
By the use of the formula 2 (− ΔΔCt) reported by [21], the relative expression of each gene was detected. They were scaled in comparison to controls. The control samples were settled at a value of 1. So, the sample results were graphed as the relative expression in comparison to the control.
Histopathological and immunohistochemical studying
Testes were rapidly fixed in Bouin’s solution for 24 h, cleaned with xylene, and then prepared by paraffin processing then tissue slides 7 μm were cut by Leitz 1512 Microtome. Sections were stained by Hematoxylin and Eosin stain. Histological findings of the testicular tissue was assessed by a histologist that was blinded to study treatment. Olympus light microscope (Olympus, Japan) was used for examining and capturing images for the histological and immunohistochemical sections. Slides were photographed using an Olympus digital camera. Images were saved as jpg.
In the current study, Cosentino score was used for semi- quantitation of pathological changes on different seminiferous tubules in the whole groups [22] (Table 1) by grading the tubules between 1 to 4 and the mean score was calculated by dividing the totality/the whole number of testicular tubules. Johnsen’s scoring system was used to evaluate the effect of DM-1 on the spermatogenesis. The grades ranged from 1 to 10 and the total scores were calculated by dividing on the whole number of testicular tubules [23] (Table 2).
Immunostaining was done using the standard method (avidin biotin peroxidase). Paraffin blocks were cut into 5 μm thick and mounted on positively charged glass slides for immunostaining with polyclonal rabbits antibody (iNOS and caspase-3antibody). The sections were incubated with the primary antibody rabbit polyclonal anticaspase-3 ready-to use antibody, and anti-iNOS antibody.
The intensity of the staining was evaluated as mild: 1, moderate: 2, and strong: 3. This evaluation was done in at least 10 tubuli/ testicular section, in 5 sections from each rat at 400× [24].
Statistical analysis
Results are presented as the means ± SEM and were analyzed by the use of Kruskal–Wallis test followed by Dunns multiple comparison test. Graph Pad Prism software (version 5) was used. Differences with p value < 0.05 were considered significant.
Results
Effect of FEN on serum levels of glucose and testosterone, body weight, testicular weight and mortality rate
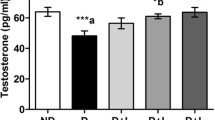
Serum glucose level showed a significant increase in diabetic group as compared to control group. Diabetic rats treated with FEN showed a significant decrease in serum glucose level as compared to diabetic rats. There was no significant difference in serum glucose level between FEN and control group (Table 3 and Fig. 1a).
Effect of FEN on non-fasting serum glucose and testosterone levels. Data are presented as mean ± SEM (n = 6–8); a,bSignificant difference from control group and diabetic group, respectively. FEN fenofibrate; p value: < 0.0001, F (df): 135.3 (3); p value: 0.0004, F (df): 20.43 (3) for a, b, respectively
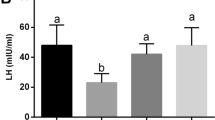
Diabetic rats showed a significant decrease in serum testosterone level and both body weight and testicular weight as compared to control group. Diabetic rats treated with FEN showed a significant improvement in serum testosterone level and both body weight and testicular weight as compared to diabetic rats. There was no significant difference in serum testosterone level and both body weight and testicular weight between FEN and control group.
Mortality rate of control and FEN groups were 0%. On the other hand, mortality rates in diabetic and diabetic/FEN were 30% and 20%, respectively (Table 3 and Fig. 1b).
Effect of FEN on testicular spermatogenesis scoring
There was a significant decrease in a spermatogenesis in the testicular tissue of diabetic rats as compared to the control group using the Johnson’s scoring. While, in rats treated with FEN there was a significant spermatogenesis improvement as compared to diabetic group. There was no significant difference in a spermatogenesis between FEN and control groups (Table 3).
Effect of FEN on testicular oxidative stress parameters
Testicular tissue of diabetic rats showed significantly a higher MDA concentration, lower GSH level and SOD activity compared with control rats. There was a significant reduction in testicular MDA level along with significant increase in GSH level and SOD activity in FEN treated group as compared with diabetic rats (Table 4).
There was a significant reduction in HO-1 level and a significant increase in NOx levels in testicular tissue of diabetic rats. In diabetic rats treated with FEN; HO-1 level was significantly increased with attenuation of NOx levels as compared to diabetic group. There was no significant difference in testicular oxidative stress parameters between FEN and control group (Table 4).
Effect of FEN on testicular inflammatory parameters
In diabetic rats, there was a significant increase in testicular levels of TNF-α, IL-6 and p38 MAPK when compared to control group. However, the diabetic rats treated with FEN showed a significant decline in testicular levels of TNF-α, IL-6 and p38/MAPK as compared to diabetic group. There was no significant difference in testicular inflammatory parameters between FEN and control groups (Table 5).
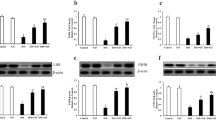
Effect of FEN on testicular mRNA expression of PPAR-α, HO-1, p38 MAPK
In diabetic rats, mRNA expression of p38/MAPK was increased significantly in testicular tissue with a significant reduction in PPAR-α and HO-1 mRNA expression as compared to control rats. Diabetic group treated with FEN showed a significant decrease in mRNA expression of p38/MAPK with a significant increase in PPAR-α and HO-1 mRNA expression as compared to diabetic group (Fig. 2a–c). There was a significant increase in testicular mRNA expression of PPAR-α in FEN group as compared to control group. There was no significant difference in testicular mRNA expression of HO-1, p38 MAPK between FEN and control groups.
Effect of FEN on mRNA expression of PPAR-α, HO-1, and p38/MAPK. Data are presented as mean ± SEM (n = 6–8); a,bSignificant difference from control group and diabetic group, respectively. FEN fenofibrate, PPAR-α peroxisome proliferator-activated receptors alpha, HO-1 hemeoxygenase-1, p38 MAPK p38 mitogen-activated protein kinase. p value: < 0.0001, F (df): 29.93 (3); p value:0.0004, F (df): 17.97 (3); p value: < 0.0001, F (df): 50.93 (3) for a–c, respectively
Histopathological study of testicular tissue
Hematoxylin and Eosin slides of the control and FEN groups showed normal morphology of testicular tissue. The seminiferous tubules were lined with stratified testicular epithelium rested on basement membranes. The interstitial tissues contained groups of Leydig cells (Fig. 3a, b). While sections from the diabetic group, Some tubules showed disruption of epithelium with disorganized germ cells, other showed testicular atrophy with absent of spermatogenesis and congestion of blood vessels (Fig. 3c1, c2). In FEN treated group, an obvious recovery of the all previous morphological findings was present (Fig. 3d). These results were in line with the improvement of the histopathological finding of Cosentino's scoring (Fig. 3e).
Photomicrographs of testicular sections of control and FEN groups (a, b), respectively, showing normal structure of the seminiferous tubules with normal spermatogenesis (Circle: spermatocytes, arrow: elongated spermatids, triangle: leydig cells). In diabetic testicular sections (c1, c2) there was disruption of the epithelial lining (arrow), distorted seminiferous tubules lined by disorganized darkly stained epithelium, congested blood vessels (star) with absent of spermatogenesis only spermatogonia is present (arrow). FEN treated group (d) shows more or less normal testicular structure; apparent normal germinal cells with normal seminiferous tubules. H and E × 200. a,bSignificant difference from control group and diabetic group, respectively. FEN fenofibrate; p value: 0.0356, F (df): 20.33 (3)
Immunohistopathological study of iNOS and caspase-3 in testicular tissue
For iNOS immunohistopathological study of control and FEN groups, negative cytoplasmic immunoreactions in interstitial and germ cells were found. Section from diabetic rats showed an intense cytoplasmic reaction in interstitial cells which had been significantly ameliorated in FEN treated group (Fig. 4).
Photomicrographs of sections of rat testis from control and FEN groups (a, b), respectively, showing negative iNOS immunoreactivity in the germinal cell cytoplasm. In diabetic testicular group (c), an extensive cytoplasmic reaction in interstitial cells (arrows) was present. FEN treated group (d) showing little cytoplasmic expression in the interstitial and germinal cells. Immunostaining iNOS × 400. a,bSignificant difference from control group and diabetic group, respectively. FEN fenofibrate; p value: < 0.0001, F(df): 32.77 (3)
Immunohistopathological examination of both the control and FEN groups revealed nearly negative caspase-3 immunoreactivity in the germinal cell cytoplasm. However, in diabetic group, extensive positive cytoplasmic expression of caspase-3 was found in germ cells. In contrast, the FEN treated rats exhibited a little cytoplasmic reaction in the germ cells compared to diabetic rats (Fig. 5).
Photomicrographs of sections of rat testis from control and FEN groups (a, b), respectively, showing negative caspase-3 immunoreactivity in the germinal cell cytoplasm. In diabetic testicular group (c), an intense cytoplasmic reaction in interstitial cells and germinal cells (arrows) was present. FEN treated group (d) showing little cytoplasmic expression in the interstitial and germinal cells. Immunostaining caspase-3 × 400. a,bSignificant difference from control group and diabetic group, respectively. FEN fenofibrate; p value: < 0.0001, F(df): 27.69 (3)
Discussion
DM is one of the most important public health problems, with a yearly increased in DM-1 patients. In 2017, the International Diabetes Federation's eighth edition of the Diabetes Map showed there was about 1.1 millon young DM-1 patients (< 20 years) around the world, which were twice as many as that of 2015 [2].
Results of the current study had demonstrated different forms of testicular injury in STZ-induced DM-1, manifested by the alteration in blood glucose, body weight, high mortality rate, testicular inflammation, oxidative damage, and testicular cells apoptosis which was proved biochemically and confirmed by histopathological examination reflecting that the DM-1 model was successfully established. FEN treatment reversed the diabetic testicular damage; normalized the serum testosterone level, improved anti-oxidative capacity, ameliorated the pro-inflammatory cytokine expression in testicular tissue with the down regulation of p38 MAPK mediated-testicular apoptosis.
As testicular structural and functional deterioration is a critical complication in diabetic male patient which leads to fertility impairment in adulthood [25], Therefore, the development of appropriate strategies to prevent the testicular germ cells loss and restore the integrity of testicular tissue structures will present a fundamental approach to preserve or improve the fertility of adult male patients.
In this study, the classical method of ip injection of STZ; a genotoxic agent, was used to establish DM-1 rat model; ip injection of STZ enters the pancreatic β-cells, disrupts the balance between antioxidant and oxidant systems damaging the insulin-producing islet β-cells leading to hyperglycemia and a significant decrease in insulin secretion within 48 after its injection [26].
Testicular tissue contain a highly proliferating cells; sperm cell is the most differentiated mammalian cell which needs an undue amount of glucose for their metabolism and their spermatogenic function, DM-1 complication with an expanding oxidative damage and sustained inflammation, testicular dysfunction with altered steroidogenesis and spermatogenesis for sure will occur [27].
In the current study, STZ-induced diabetic rats, revealed an increase in serum glucose level with decrease in both body and testicular weights with an obvious reduction in serum testosterone level in comparison to control animals matching with a previous result of Heeba and Hamza [28].
Low testosterone level in diabetic rats could be assigned to the high estradiol concentrations derived from testosterone metabolism by aromatase enzyme in hypertrophied adipose tissue which suppresses luteinizing hormone release from the pituitary gland leading to a lower plasma testosterone level [1], and in addition, it has been reported that hyperglycemia caused sustained Leydig cells dysfunction with inhibition in testosterone production [28].
Matching with other previous studies [29,30,31], diabetic testicular damage in the current work was accompanied by enhanced lipid peroxidation product; MDA, and depletion of antioxidant defenses involving GSH content, SOD activity and HO-1level in testicular tissues. These results can be based on that mammalian sperm cells have high levels of lipids and unsaturated fatty acids [32] and in DM-1, glucose autoxidation can produce lipid peroxidation, leading to free radicals formation and lipid oxidative modification with consumption of the natural antioxidants, producing a state of oxidative stress [33].
Thus, attenuation of ROS is important for the treatment of reproductive damage in diabetic patients. The pivotal role of inflammation is well clarified in diabetic testicular complications, as overexpression of inflammatory cytokines can significantly inhibit the testosterone synthesis and suppress the spermatogonial differentiation with gonadal dysfunction especially the steroidogenic potential of Leydig cells [34].
In the present study there is a high testicular level of different cytokines, including IL-6 and TNF-α which will promote an inflammatory responses and create a state of severe oxidative stress, these results in agreement with Rashid and Sil [35]. In addition, we revealed an over production of testicular tissue level of NO as a result of up regulation of iNOS expression in Sertoli cells and Leydig cells which was also proved in our study by immunohistopathological study matching with previous reported results [36, 37].
The biologically active NO and the pro-inflammatory mediators mediate the assorted reproductive dysfunction by motivating testicular damage with considerable germ cells loss, testicular atrophy and apoptosis, exploring there is a cross links between inflammation, oxidative stress, sperm cell apoptosis and testicular injury [38]. p38 MAPK signaling plays a key role in various cellular events and is involved in multiple different male reproductive processes, In DM-1-induced testicular dysfunction, increased intracellular ROS can activate p38 MAPK which plays an important role in the induction of testicular apoptosis [35, 39].
In the present study there is a significant increase in p38 MAPK testicular level along with a significant increase in caspase-3 immunoexpression matching with Vera et al. [40] who reported that germ cell apoptosis was induced after GnRH antagonist treatment through p38MAPKactivation, followed by caspase activation. In the present study, there are histopathological modalities in testis of diabetic rats in the form of germinal cell derangement; atrophy with necrosis which is significantly different from the control group in accordance with the used Cosentino's score. Moreover, the spermatogenesis process was significantly suppressed in comparison to the control group by the use of Johnsen's score which confirmed the structural damage. These results match with previously published results [3].
FEN; the most widely used PPARɑ agonist, which has a highly efficient lipid-lowering effect, and afforded a protective effects in different animal models including STZ induced-DM-1, acts independent on its effect on plasma lipid profile but via its anti-inflammatory, antioxidant and anti-apoptotic properties [41].
In the current study diabetic rats treated with FEN had a significant higher body and testicular weight than control group level along with a decrease in fasting blood glucose and a decrease in mortality rate, these results were accompanied by restoration of the normal histopathological patterns of testicular tissue and the spermatogenesis process as compared to diabetic non-treated group and these results are in consistent with previous study [4].
Finding of our study can be based upon its ability to preserve the testicular tissue against DM-1 induced tissue damage, in contrast to another study which reported that FEN may reduce the body weight, which was assigned to decrease the fat accumulation in subcutaneous tissue through inhibition of STAT3 signaling which plays a key role in body weight regulation and glucose homeostasis [42]. The improvement of testicular weight loss in FEN group may be due to the improvement in insulin resistance through PPAR‐α dependent pathway which improved the testicular metabolic pattern [43].
In the present study diabetic rats treated with FEN significantly increased serum testosterone level in pointing to the beneficial effects on steroidogenesis and Leydig cell functions matching with others [4]. Additionally, diabetic rats treated with FEN provide antioxidant effect which can be demonstrated through a significant increase in testicular levels of GSH and HO-1 and SOD activities along with a significant decrease in MDA and NO levels via its protective effect against membrane lipid peroxidation and nitric oxide formation via PPARα agonist dependent effect. These finding are in line with previous studies [44, 45].
In the current study, It is worthy to note that FEN administration alone or in diabetic rats was associated with a significant up-regulation in PPARα receptor gene expression in testicular tissue as compared to control or diabetic non-treated group, via its PPARα agonistic effect. These finding are in line with previous study proved that FEN improved testicular damage in rats testicular ischemia reperfusion via its agonistic action and upregulation of PPARα receptor [4].
FEN also suppress iNOS immunoexpression; a well-known biological marker of inflammatory responses, and p38 MAPK activation in testicular tissue, exerting anti-inflammatory action; proved by a significant inhibition of cytokine production; IL-6 and TNF-α, these results were in line with other previous results [4, 46, 47].
FEN also was able to inhibit the activation of p38 MAPK in testicular tissue resulting in reduction of testicular tissue caspase-3 immunoexpressions which indicates the anti-apoptotic effect of FEN on diabetes-induced germ cell death in line with Thongnuanjan et al. [48] who reported that cisplatin-induced apoptosis was attenuated by co-treatment with FEN and Refaie [4] who mentioned that FEN had the ability to ameliorate the testicular damage induced by testicular ischemia reperfusion injury via its anti-apoptotic effect. Results of our study proved that FEN has a beneficial and a promising effect in attenuating STZ-induced diabetic testicular damage in rats.
Conclusions
Results of the current study proved that FEN ameliorates STZ-induced DM-1 testicular damage in rats, its potential protective effect was attributed to various mechanisms as up regulation of PPARα receptor, anti-inflammatory, antioxidant and anti-apoptotic action, mediated via up regulation of HO-1, down regulation of iNOS signaling pathways, and down regulation of p38 MAPK. These findings indicate the promising beneficial role of FEN in STZ-induced DM-1 testicular damage.
Limitation of our study
Of note, further immunocytochemical studies of the testicular cells basement membrane components are required to verify the possible variations in different mammalian species during development.
References
Hasan MM, El-Shal AS, Mackawy AMH, Ibrahim EM, Abdelghany EMMA, Saeed AA, et al. Ameliorative effect of combined low dose of pioglitazone and omega-3 on spermatogenesis and steroidogenesis in diabetic rats. J Cell Biochem. 2020;121:1524–40.
Ma Q, Li Y, Wang J, Li P, Duan Y, Dai H, et al. Investigation of gut microbiome changes in type 1 diabetic mellitus rats based on high-throughput sequencing. Biomed Pharmacother. 2020;124:109873.
Mohamed MZ, Hafez HM, Zenhom NM, Mohammed HH. Cilostazol alleviates streptozotocin-induced testicular injury in rats via PI3K/Akt pathway. Life Sci. 2018;198:136–42.
Refaie MMM. Upregulation of peroxisome proliferator activated receptor alpha by fenofibrate in induced testicular ischemia reperfusion. Biomed Pharmacother. 2018;98:507–15.
Jiang YP, Ye RJ, Yang JM, Liu N, Zhang WJ, Ma L, et al. Protective effects of salidroside on spermatogenesis in streptozotocin induced type-1 diabetic male mice by inhibiting oxidative stress mediated blood-testis barrier damage. Chem Biol Interact. 2020;315:108869.
Ibarra-Lara L, Sánchez-Aguilar M, Sánchez-Mendoza A, Del Valle-Mondragón L, Soria-Castro E, Carreón-Torres E, et al. Fenofibrate therapy restores antioxidant protection and improves myocardial insulin resistance in a rat model of metabolic syndrome and myocardial ischemia: the role of angiotensin II. Molecules. 2016;22:E31.
Harada Y, Tanaka N, Ichikawa M, Kamijo Y, Sugiyama E, Gonzalez FJ, et al. PPARα-dependent cholesterol/testosterone disruption in Leydig cells mediates 2,4-dichlorophenoxyacetic acid-induced testicular toxicity in mice. Arch Toxicol. 2016;90:3061–71.
Li Y, Ramdhan DH, Naito H, Yamagishi N, Ito Y, Hayashi Y, et al. Ammonium perfluorooctanoate may cause testosterone reduction by adversely affecting testis in relation to PPARα. Toxicol Lett. 2011;205:265–72.
Wang Y, Yu M, Ma Y, Wang R, Liu W, Xia W, et al. Fenofibrate increases heme oxygenase 1 expression and astrocyte proliferation while limits neuronal injury during intracerebral hemorrhage. Curr Neurovasc Res. 2017;14:11–8.
Liu Q, Zhang F, Zhang X, Cheng R, Ma JX, Yi J, et al. Fenofibrate ameliorates diabetic retinopathy by modulating Nrf2 signaling and NLRP3 inflammasome activation. Mol Cell Biochem. 2018;445:105–15.
Losey P, Ladds E, Laprais M, Guevel B, Burns L, Bordet R, et al. The role of PPAR activation during the systemic response to brain injury. J Neuroinflamm. 2015;12:99.
Lin C, Chen PY, Chan HC, Huang YP, Chang NW. Peroxisome proliferator-activated receptor alpha accelerates neuronal differentiation and this might involve the mitogen-activated protein kinase pathway. Int J Dev Neurosci. 2018;71:46–51.
Wang F, Jiang J, Xia L, Lyu J, Cai C. Xin B [4i, a novel PPARγ ligand, inhibits the production of inflammatory cytokines in murine macrophages by blocking NF-κB and MAPK pathway]. Int J Dev Neurosci. 2018;71:46–51.
Taghizadeh M, Rashidi AA, Taherian AA, Vakili Z, Mehran M. The protective effect of hydroalcoholic extract of rosa canina (Dog Rose) fruit on liver function and structure in streptozotocin-induced diabetes in rats. J Diet Suppl. 2018;15:624–35.
Yousaf AM, Kim DW, Oh YK, Yong CS, Kim JO, Choi HG. Enhanced oral bioavailability of fenofibrate using polymeric nanoparticulated systems: physicochemical characterization and in vivo investigation. Int J Nanomed. 2015;10:1819–30.
Oidor-Chan VH, Hong E, Pérez-Severiano F, Montes S, Torres-Narváez JC, Del Valle-Mondragón L, et al. Fenofibrate plus metformin produces cardioprotection ina type 2 diabetes and acute myocardial infarction model. PPAR Res. 2016;2016:8237264.
Marklund S, Marklund G. Involvement of the superoxide anion radical in the autoxidation of pyrogallol and a convenient assay for superoxide dismutase. Eur J Biochem. 1974;47:469–7.
Moron MS, Depierre JW, Mannervik B. Levels of glutathione, glutathione reductase and glutathione S-transferase activities in rat lung and liver. Biochim Biophys Acta. 1979;582:67–78.
Buege J, Aust S. Microsomal lipid peroxidation. Methods Enzymol. 1978;52:302–10.
Ridnour LA, Sim JE, Hayward MA, Wink DA, Martin SM, Buettner GR, et al. A spectrophotometric method for the direct detection and quantitation of nitric oxide, nitrite, and nitrate in cell culture media. Anal Biochem. 2000;281:223–9.
VanGuilder HD, Vrana KE, Freeman WM. Twenty-five years of quantitative PCR for gene expression analysis. Biotechniques. 2008;44:619–26.
Cosentino MJ, Nishida M, Rabinowitz R, Cockett AT. Histological changes occurring inthe contralateral testes of prepubertal rats subjected to various durations of unilateral spermatic cord torsion. J Urol. 1985;133:906–11.
Johnsen SG. Testicular biopsy score count-a method for registration of spermatogenesis in human testes: normal values and results in 335 hypogonadal males. Hormones. 1970;1:2–25.
Bashir N, Shagirtha K, Manoharan V, Miltonprabu S. Biosci Rep. 2019;39:BSR20180515.
Zhu X, Guo F, Tang H, Huang C, Xie G, Huang T, et al. Islet transplantation attenuating testicular injury in type 1 diabetic rats is associated with suppression of oxidative stress and inflammation via Nrf-2/HO-1 and NF-κB pathways. J Diabetes Res. 2019;2019:8712492.
El-Baz FK, Salama A, Salama RAA. Dunaliella salina attenuates diabetic neuropathy induced by STZ in rats: involvement of thioredoxin. Biomed Res Int. 2020;2020:1295492.
Ding GL, Liu Y, Liu ME, Pan JX, Guo MX, Sheng JZ, et al. The effects of diabetes on male fertility and epigenetic regulation during spermatogenesis. Asian J Androl. 2015;17:948–53.
Heeba GH, Hamza AA. Rosuvastatin ameliorates diabetes-induced reproductive damage via suppression of oxidative stress, inflammatory and apoptotic pathways in male rats. Life Sci. 2015;141:13–9.
Mohasseb M, Ebied S, Yehia MA, Hussein N. Testicular oxidative damage and role of combined antioxidant supplementation in experimental diabetic rats. J Physiol Biochem. 2011;67:185–94.
Rochette L, Zeller M, Cottin Y, Vergely C. Diabetes, oxidative stress and therapeutic strategies. Biochim Biophys Acta. 2014;1840:2709–29.
AboElGheit R, Emam MN. Targeting heme oxygenase-1 in early diabetic nephropathy in streptozotocin-induced diabetic rats. Physiol Int. 2016;103:413–27.
Esmaeili V, Shahverdi AH, Moghadasian MH, Alizadeh AR. Dietary fatty acids affect semen quality: a review. Andrology. 2015;3:450–61.
Hunt JV, Smith CC, Wolff SP. Autoxidative glycosylation and possible involvement of peroxides and free radicals in LDL modification by glucose. Diabetes. 1990;39:1420–4.
Coştur P, Filiz S, Gonca S, Çulha M, Gülecen T, Solakoğlu S, et al. Êxpression of inducible nitric oxide synthase (iNOS) in the azoospermic human testis. Andrologia. 2012;44(Suppl 1):654–60.
Rashid K, Sil PC. Curcumin enhances recovery of pancreatic islets from cellular stress induced inflammation and apoptosis in diabetic rats. Toxicol Appl Pharmacol. 2015;282:297–310.
Guo J, Jia Y, Tao SX, Li YC, Zhang XS, Hu ZY, et al. Expression of nitric oxide synthase during germ cell apoptosis in testis of cynomolgus monkey after testosterone and heat treatment. J Androl. 2009;30:190–9.
Soskić SS, Dobutović BD, Sudar EM, Obradović MM, Nikolić DM, Djordjevic JD, et al. Regulation of inducible nitric oxide synthase (iNOS) and its potential role in insulin resistance, diabetes and heart failure. Open Cardiovasc Med J. 2011;5:153–63.
Kushwaha S, Jena GB. Telmisartan ameliorates germ cell toxicity in the STZ-induced diabetic rat: studies on possible molecular mechanisms. Mutat Res. 2013;755:11–23.
Ranawat P, Bansal MP. Apoptosis induced by modulation in selenium status involves p38 MAPK and ROS: implications in spermatogenesis. Mol Cell Biochem. 2009;330:83–95.
Vera Y, Erkkilä K, Wang C, Nunez C, Kyttänen S, Lue Y, et al. Involvement of p38 mitogenactivated protein kinase and inducible nitric oxide synthase in apoptotic signaling of murine and human male germ cells after hormone deprivation. Mol Endocrinol. 2006;20:1597–609.
Lian X, Gu J, Gao B, Li Y, Damodaran C, Wei W, et al. Fenofibrate inhibits mTOR-p70S6K signaling and simultaneously induces cell death in human prostate cancer cells. Biochem Biophys Res Commun. 2018;496:70–5.
Hua H, Yang J, Lin H, Xi Y, Dai M, Xu G, et al. PPARα-independent action against metabolic syndrome development by fibrates is mediated by inhibition of STAT3 signalling. J Pharm Pharmacol. 2018;70:1630–42.
Neschen S, Morino K, Dong J, Wang-Fischer Y, Cline GW, Romanelli AJ, et al. n-3 Fatty acids preserve insulin sensitivity in vivo in a peroxisome proliferator-activated receptor-alpha-dependent manner. Diabetes. 2007;56:1034–41.
Ibrahim MA, El-Sheikh AA, Khalaf HM, Abdelrahman AM. Protective effect of peroxisome proliferator activator receptor (PPAR)-α and -γ ligands against methotrexate-induced nephrotoxicity. Immunopharmacol Immunotoxicol. 2014;36:130–7.
Helmy MM, Helmy MW, El-Mas MM. Additive renoprotection by pioglitazone and fenofibrate against inflammatory, oxidative and apoptotic manifestations of cisplatin nephrotoxicity: modulation by PPARs. PLoS ONE. 2015;10:e0142303.
Cheng SM, Chu KM, Lai JH. The modulatory mechanisms of fenofibrate on human primary T cells. Eur J Pharm Sci. 2010;40:316–24.
Xuan AG, Chen Y, Long DH, Zhang M, Ji WD, Zhang WJ, et al. PPARα agonist fenofibrate ameliorates learning and memory deficits in rats following global cerebral ischemia. Mol Neurobiol. 2015;52:601–9.
Thongnuanjan P, Soodvilai S, Chatsudthipong V, Soodvilai S. Fenofibrate reduces cisplatin-induced apoptosis of renal proximal tubular cells via inhibition of JNK and p38 pathways. J Toxicol Sci. 2016;41:339–49.
Funding
This research did not receive any specific Grant from funding agencies in the public, commercial or not-for-profit sectors.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
There is no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Abdel-Aziz, A.M., Abozaid, S.M.M., Yousef, R.K.M. et al. Fenofibrate ameliorates testicular damage in rats with streptozotocin-induced type 1 diabetes: role of HO-1 and p38 MAPK. Pharmacol. Rep 72, 1645–1656 (2020). https://doi.org/10.1007/s43440-020-00096-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s43440-020-00096-0