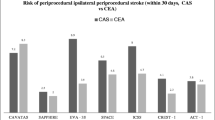
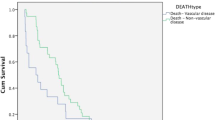
Abstract
The current literature indicates carotid endarterectomy (CEA) as the preferred treatment for symptomatic, moderate to severe carotid artery stenosis. However, recommendations for the management of acute tandem stenosis and complete occlusion, as well as postintervention restenosis of the carotid artery, remain controversial. Here, we review the literature evaluating these conditions and provide suggestions for clinical decision-making. Acute tandem stenosis or occlusion of the common and internal carotid arteries may be treated with angioplasty alone, reserving carotid artery stenting (CAS) or CEA for severe and complex cases. Patients who underwent CEA and developed ipsilateral restenosis may be subjected to angioplasty followed by CAS, which carries a lower risk of cranial nerve injury and subsequent restenosis of the artery. For post-CAS restenosis, current evidence recommends angioplasty and CAS for the management of moderate stenosis and CEA for severe stenosis of the carotid artery. Given the lack of level 1 evidence for the management of these conditions, the abovementioned recommendations may assist clinical decision-making; however, each case and its unique risks and benefits need to be assessed individually. Future studies evaluating and defining the risks and benefits of specific treatment strategies, such as CEA and CAS, in patients with acute tandem stenosis, occlusion, and postintervention restenosis of the carotid artery need to be conducted.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Carotid artery stenosis remains an unresolved medical problem with a substantial socioeconomic impact. It affects approximately 10% of the general population by the eighth decade of life and is the underlying cause of stroke in approximately 10% of all ischemic events [1, 2]. Carotid artery disease is caused by a buildup of atherosclerotic plaques inside the arterial wall, which may reduce blood flow to the brain, especially in moderate (50–69%) and severe (70–99%) stenosis of the common or internal carotid arteries. Several clinical trials have evaluated and compared different treatment strategies for this condition, including carotid endarterectomy (CEA) and carotid artery stenting (CAS). Results of these trials are relatively consistent, and it is generally well accepted that, unless general anesthesia is contraindicated secondary to the patients’ comorbidities or frailty, CEA is preferred over CAS [2, 3]. This conclusion was made based on clinical studies evaluating de novo carotid artery disease, without including patients presenting with either complete artery occlusion or restenosis after successful primary intervention (CEA or CAS). Thus, there is a lack of consensus among treating physicians for the management of patients with complete vessel occlusion or restenosis. Recently, various prospective and retrospective clinical studies were conducted, with the aim of identifying ideal treatment strategies for these special conditions.
The purpose of this current article is to review treatment strategies of carotid artery disease, particularly CEA and CAS, in the setting of de novo artery stenosis but also in the setting of acute tandem stenosis, complete artery occlusion, and postintervention restenosis.
Pathophysiology of Carotid Artery Stenosis
The common carotid artery bifurcation is the most common location in the cerebral vascular system for stenosis secondary to atherosclerosis, normally with an hourglass configuration. The atherosclerosis is believed to be caused by entry of low-density lipoprotein (LDL) particles into the subintimal space followed by migration of inflammatory cells, such as monocytes [4, 5]. The atherosclerotic plaque is comprised of a fibrous cap composed of vascular smooth muscle cells embedded in a matrix of collagen fibers and a core that is rich in cellular debris and cholesterol crystals. Factors influencing the morphology and progression of atherosclerotic plaques include hypertension, dyslipidemia, diabetes mellitus, and smoking. Restenosis can occur after initial removal of the plaque by CEA. The pathogenesis of early carotid restenosis involves an inflammatory reaction leading to the formation of a plaque composed of fibroblasts and smooth muscle cells, a phenomenon known as myointimal hyperplasia [6]. Late carotid restenosis is mainly attributed to recurrence and/or progression of carotid atherosclerotic disease, which is similar to the initial disease process [7]. Similar to post-CEA restenosis, myointimal hyperplasia and recurrent atherosclerosis have been identified as the major mechanisms of in-stent restenosis [4, 7,8,9]. In addition to reduction in vessel diameter, formation of a superimposed thrombus or rupture of the plaque can occur resulting in further vessel narrowing. Thus, the underlying mechanism of stroke may be embolic, thrombotic, or secondary to low flow with inadequate collateral compensation [5, 10, 11]. Presentation of carotid artery disease includes transient ischemic attack (TIA) and stroke. TIAs may be due to either low flow or emboli. Low-flow type TIAs are usually short in duration with multiple similar episodes [5]. These symptoms are “warning signs” of acute stroke. In comparison, embolic TIAs usually cause single yet prolonged episodes of transient neurological deficits.
In the past, risk stratification of patients with carotid artery disease was accomplished by measuring the degree of carotid stenosis. However, with recent improvements of vascular imaging techniques, radiological risk factors have gained much attention, specifically for risk stratification of patients [12, 13]. These imaging features include intraplaque hemorrhage, plaque ulceration, plaque neovascularity, fibrous cap thickness, and the presence of a lipid-rich necrotic core [12]. Using these imaging features in clinical practice is the focus of ongoing and future studies.
At the time when most carotid endarterectomy trials were conducted (late 1980s to mid 1990s), the medical therapy of choice for carotid artery stenosis was considered to be the antiplatelet agent aspirin. Since then, combination therapies have emerged and proven successful, including antiplatelet medications combined with statins and antihypertensive medications. Dual antiplatelet treatment was found to be effective for prevention of recurrent stroke [14, 15]. Furthermore, phosphodiesterase-3 inhibitors such as cilostazol have been associated with a favorable outcome in patients with carotid artery in-stent restenosis [16].
Treatment Recommendations for Symptomatic De Novo Carotid Stenosis
In 2011, the American Heart Association/American Stroke Association (AHA/ASA) released guidelines for the treatment of symptomatic carotid artery stenosis [2]. The recommendations read as follows: “For patients who suffered a transient ischemic attack (TIA) or ischemic stroke within the past 6 months, and ipsilateral moderate to severe (50–99%) carotid artery stenosis has been diagnosed by noninvasive imaging, CEA is recommended if the perioperative morbidity and mortality risk is estimated to be less than 6%. However, if the degree of carotid stenosis is less than 50%, neither CEA nor CAS are recommended (Class III; Level of Evidence A).” If revascularization is indicated for patients who suffered a TIA or a minor, nondisabling ischemic stroke, it is reasonable to perform the procedure within 2 weeks of the index event as long as no contraindications exist for an early revascularization. CAS is indicated as an alternative to CEA for patients with symptomatic carotid artery stenosis, who present with anatomic or medical conditions, which greatly increase the risk for surgery; or when other specific circumstances exist such as radiation-induced stenosis or restenosis after prior CEA [2, 3]. U.S. physicians have followed these general guidelines and no significant changes or additions have been made to the AHA/ASA recommendations in recent years. In fact, the number of clinical studies evaluating treatment modalities for symptomatic carotid artery stenosis has significantly decreased after the release of the AHA/ASA recommendations in 2011.
Several guidelines for the treatment of symptomatic carotid artery stenosis have been made outside the USA; however, the major conclusions are compatible with the AHA/ASA recommendations, as demonstrated by Abbott et al. [1]. Specifically, 31 of 33 international guidelines (94%) for the treatment of symptomatic carotid artery stenosis recommended CEA for patients with moderate to severe stenosis (50–99%) as the preferred treatment modality. Furthermore, 19 guidelines (58%) endorsed CAS, whereas 9 (27%) opposed CAS as suitable therapy for symptomatic carotid artery stenosis. These international guidelines were based on clinical trials conducted 12 to 34 years ago, which according to the authors rarely reflected clinical improvements in patients and oftentimes understated potential risks and hazards associated with CAS. Thus, CAS has oftentimes been reserved for high-risk patients who did not seem suitable to undergo general anesthesia needed for CEA [1]. The feasibility of CAS for carotid artery stenosis in patients with increased surgical risks was adopted by the Centers for Medicare and Medicaid Services after reviewing results of large CAS studies, such as the SAPPHIRE (Stenting and Angioplasty with Protection in Patients at High Risk for Endarterectomy) trial, which demonstrated that among patients with advanced carotid artery stenosis and preexisting high-risk surgical comorbidities, CAS was not inferior to CEA. In fact, patients who underwent CAS required significantly fewer revascularization procedures within 1 year of the initial treatment [17]. While CAS has been frequently chosen as the treatment modality for patients deemed to be at high risk for CEA, it still needs to be determined whether CAS is indeed safer than CEA in these specific circumstances. Previous studies have suggested that in asymptomatic high-risk CEA patients, CEA and CAS carry similar incidences for stroke, myocardial infarction (MI), and death; however, in symptomatic high-risk CEA patients, CAS was actually found to carry higher periprocedural risks [18, 19].
Most clinical society recommendations are either based on randomized control trials, case series, or expert opinions and may not reflect real-world scenarios where clinicians are often faced with challenging cases that are excluded in clinical trials or not discussed in any society guidelines. Hence, treatment for carotid artery disease needs to be tailored to the particular patient’s needs in view of the pathophysiology of the disease process that is closely consistent with existing literature.
Developments and Controversies Regarding Acute and Chronic Carotid Artery Occlusions
Carotid artery occlusion is a completely different entity from stenosis. The treatment rationales are different from the carotid stenosis. There are also significant differences between acute and chronic carotid occlusion. Table 1 summarizes the major studies of carotid occlusion including both acute and chronic occlusion.
Acute Tandem Occlusion of the Carotid Artery
Acute tandem occlusion involving the proximal (cervical) internal carotid artery and concomitant thromboembolism involving the distal (intracranial) internal carotid artery or the ipsilateral middle cerebral artery occur in approximately 15% of large-vessel ischemic stroke cases [31]. To date, the optimal management of such acute tandem occlusions remains controversial. Patients with acute tandem occlusions involving the ipsilateral extra- and intracranial segments of the internal carotid artery or its main branches (middle cerebral artery) usually present with acute stroke symptoms secondary to intracranial hypoperfusion. These patients frequently require instant revascularization utilizing mechanical thromboembolectomy techniques. Acute tandem occlusions are usually treated with balloon angioplasty and stenting followed by thromboembolectomy using an anterograde or retrograde approach. Indeed, intracranial mechanical thromboembolectomy followed by angioplasty and/or stenting of the proximal occlusion, or vice versa, is frequently applied [31, 32]. A recent meta-analysis demonstrated no statistically significant differences in successful revascularization, neurological outcomes, mortality, and incidence of symptomatic intracranial hemorrhage between patients treated first with an extracranial or intracranial approach [33]. Furthermore, similar results between these two groups were demonstrated for the procedure duration, rates of successful revascularization, and procedure-related complications.
Another controversial issue regarding the management of carotid artery acute tandem occlusion is whether angioplasty alone is sufficient for an adequate treatment or whether angioplasty should be followed by CAS. The benefits and potential risks of subjecting patients to CAS after angioplasty have not yet been evaluated. The risk of intracranial hemorrhage associated with dual antiplatelet treatment, which is the standard treatment after stenting procedures, is an important concern in patients who remain stenotic after completed angioplasty. Indeed, the rate of symptomatic intracranial hemorrhage after stenting of acute tandem occlusions was found to be approximately 8%, which is higher than the risk associated with mechanical thromboembolectomy [32]. Heck and Brown demonstrated a significantly increased rate of intracerebral hemorrhages (22%) in stented patients, which the authors attributed to the periprocedural loading dose of glycoprotein IIb/IIIa receptor antagonist, abciximab [34].
The authors recommended balloon angioplasty of the extra- and intracranial vessels in cases of acute tandem occlusions and suggested stenting only for cases where sufficient patency cannot be obtained in the acute setting. In a recent meta-analysis, no statistically significant differences in clinical outcome were seen between patients subjected to extracranial stenting and angioplasty-only approaches, although this interpretation was limited due to the small sample size of patients undergoing angioplasty alone (without stenting) [33]. Dual antiplatelet therapy as compared to mono antiplatelet therapy has been shown to decrease the risk of TIA/stroke after elective CAS as demonstrated in a recent meta-analysis [35]. But no study to date has been conducted to investigate the rate of acute stent occlusion with mono versus dual antiplatelet therapy. Because acute tandem occlusion may be complicated by acute cerebral ischemia and dual antiplatelet therapy increases the risk of hemorrhagic events, it is worthwhile to investigate the effectiveness of mono antiplatelet therapy (clopidogrel versus aspirin versus newer generation antiplatelet medications) following CAS in future clinical trials.
Based on the abovementioned studies, stenting of acute tandem occlusion following angioplasty should be avoided or delayed, if not urgently required. However, even if CAS can be avoided at the time of thromboembolectomy, the risk for future strokes as a consequence of the persistent carotid artery stenosis remains and will need to be addressed as soon as safely possible. Currently, delayed stenting is preferred by most physicians, especially since CAS in the setting of an acute stroke is associated with higher complication rates. Carotid artery re-occlusion can occur rapidly and may necessitate an emergent intervention [36]. Emergent CEA can be performed in the same manner as elective CEA in patients with progressive carotid stenosis. Slawski et al. showed that emergent CEA had lower rates of symptomatic intracerebral hemorrhage and mortality than CAS [36]. The authors suggested that both CEA and CAS should be considered in cases of life-threatening acute tandem occlusion.
Considering the abovementioned studies, the following approach may be considered for the management of acute tandem occlusion: first, attempt to perform an angioplasty or direct aspiration to obtain cervical access, then perform intracranial thromboembolectomy. Avoid stenting if possible and use stents only for cases where sufficient patency cannot be obtained in the acute setting. Emergent CEA should be used for extracranial occlusions.
Acute Proximal Carotid Occlusion Without Intracranial Involvement
Symptomatic occlusion of the proximal internal carotid artery without the associated large-vessel involvement may have variable presentations such as minor neurological deficits with either mild or no visible cerebral perfusion abnormalities on magnetic resonance imaging (MRI), acute onset of severe hemodynamic ischemic symptoms with recurrent transient episodes, or progressive symptomatology. Clinical decision-making regarding the appropriate treatment in these situations remains quite challenging. Intravenous thrombolysis has a low rate of recanalization. Thus, surgical or endovascular treatment strategies are often required, which carry the risk of distal embolic infarctions. The most effective and safe treatment approach for acute occlusion of the internal carotid artery has not yet been identified. Clinical investigations evaluating endovascular interventions for the treatment of acute carotid artery occlusion have demonstrated variable recanalization rates and patient outcomes [27, 30, 37,38,39].
In the largest case series to date, Jadhav et al. studied 107 patients with occlusion of the internal carotid artery who were subjected to endovascular angioplasty/stenting [30]. The authors demonstrated recanalization rates of 92%. Good clinical outcomes were reported in 65% of all patients; however, significant periprocedural risks and high rates of restenosis were noted. The most common complication was distal embolization, with rates as high as 22%, of which 16% required intra-arterial treatment. The rate of significant restenosis (≥ 70%) was 15% at 1 year after the initial treatment. Reynolds et al. reported their experience of seven consecutive patients with extracranial occlusion of the internal carotid artery secondary to cardiogenic embolisms, all of whom underwent emergent surgical embolectomy with a 100% recanalization rate [28]. Furthermore, 71.4% of the patients had good clinical outcomes and no evidence of distal embolic infarctions on imaging. As demonstrated by studies evaluating the management of de novo carotid artery stenosis, CEA is likely to produce a better outcome with less chance of distal embolic infarct and a high rate of recanalization.
A recent report has demonstrated promising results of emergent CEA as a treatment option for acute carotid artery occlusion [40]. Specifically, three patients who presented with large acute strokes were radiographically confirmed to have symptomatic extracranial internal carotid artery occlusive lesions without intracranial thromboembolic occlusions. All patients underwent emergent CEA and had relatively good clinical outcomes with improved National Institutes of Health Stroke Scale (NIHSS) and modified Rankin Scale (mRS) scores. The possible benefit of emergent CEA has also been demonstrated by Slawski at al. [36]. The authors suggested that emergent CEA for acute tandem carotid occlusion has a smaller risk of symptomatic ICH when compared with CAS in the setting of acute stroke after endovascular embolectomy for intracranial large-vessel occlusion. However, multicenter randomized comparison studies are needed to prove the superiority of emergent CEA over CAS in the setting of acute extracranial carotid occlusion.
Chronic Carotid Artery Occlusion
Chronic internal carotid artery occlusion (CICAO) is an important cause of transient ischemic attacks and cerebral infarction. When effective compensatory collateral circulation exists, these patients may be asymptomatic and have a benign prognosis. However, if the cerebral vascular reserve is diminished or when collateral blood flow is insufficient to meet the intracerebral demand, transient ischemic symptoms may develop or embolic infarction can occur, usually originating from the distal internal carotid artery [41]. CICAO causes approximately 10% of transient ischemic attacks and 15 to 25% of ischemic strokes within the anterior cerebral circulation [23, 42]. The prognosis for symptomatic CICAO is poor and treatment is required according to prior investigations [43]. Revascularization with superficial temporal artery to middle cerebral artery (STA–MCA) bypass was tested with high expectation of good clinical outcomes; however, high perioperative stroke rates seen in patients included in the Carotid Occlusion Surgery Study (COSS) prevented bypass surgery from being used routinely [44]. Accordingly, the 2011 AHA/ASA guideline for preventing stroke did not recommend STA–MCA bypass surgery for stroke prophylaxis in asymptomatic CICAO [2]. However, in the 2014 update of the AHA/ASA guideline, this procedure was recommended for patients with CICAO and neurological symptoms lasting for more than 6 months [45]. The Japanese external carotid to internal carotid (EC–IC) bypass trial (JET study) showed promising preliminary findings, but the final results have yet to be published [44].
With suboptimal efficacy of bypass surgery, direct revascularization has also been studied as an alternative treatment approach for CICAO. As mentioned above, CEA is a well-established method for the treatment of internal carotid artery occlusion and may also be performed in patients with retrograde filling to the skull base [46]. However, in the setting of a chronic occlusion, CEA remains a technically challenging procedure. Thompson et al. demonstrated successful recanalization in only 41% of 118 patients undergoing CEA for chronic CICAO [47]. Therefore, based on available literature, neither arterial bypass surgery nor CEA should be recommended for stable chronic carotid occlusion.
Endovascular treatment was also evaluated as a possible option for CICAO treatment; however, similar to CEA, technical challenges were encountered. Lin et al. reported a revascularization rate of 65% in a series of 54 patients [21]. Chen et al. showed a technical success rate of 61.6% in 138 consecutive patients with CICAO [48]. A nontapered stump, distal internal carotid artery reconstitution via contralateral injection, and distal internal carotid artery reconstitution at communicating or ophthalmic segments were identified as independent negative predictors of technical success during endovascular recanalization. A prominent drawback of intravascular interventional therapy is that the thrombus may dislodge and enter the circulation during the procedure causing complete occlusion of distal intracranial vessels.
Hybrid surgery incorporates performing a CEA first, followed by endovascular manipulation. The endovascular intervention can be done under direct view of the occlusion through the arteriotomy or as a regular endovascular intervention after the arteriotomy has been closed [26, 49]. As indicated by Shih et al. hybrid surgery has several advantages over endovascular treatment alone [25]. The hybrid technique allows for insertion of the endovascular catheter/sheath into the true lumen with direct visualization, possibly saving operating time otherwise spent on attempting blind recanalization in the occluded segment. Creation of an artificial stump during surgical CEA facilitates wire passing through the distal occlusion site during the endovascular intervention [26, 49]. Hybrid surgery also allows for avoidance of distal embolic strokes given that antegrade blood flow is hindered by clamping of the common carotid artery. Indeed, the incidence of distal embolic infarction is negligible as demonstrated by Li et al. [49]. The same study demonstrated significantly higher rates of complete recanalization when utilizing a hybrid approach as compared to regular endovascular interventions alone [49]. A limiting factor for the hybrid technique is the level of the distal internal cerebral artery reconstitution. It has been shown that recanalization success rates are lower if the reconstitution occurs at the level of the communicating or ophthalmic segments of the distal internal cerebral artery (ICA) [48, 49].
Developments and Controversies Regarding Restenosis After Initial Treatment
Restenosis remains an important complication after CEA and CAS. Current treatment recommendations are not as clear as those for de novo stenosis. Table 2 summarizes the major studies examining restenosis after CEA and CAS.
In-Stent Restenosis
Similar to post-CEA restenosis, myointimal hyperplasia and recurrent arthrosclerosis have been identified as the major mechanisms of this occurrence [7,8,9]. Restenosis was also associated with a fourfold increase in the risk of stroke in the Carotid Revascularization Endarterectomy versus Stenting Trial (CREST) [62]. Specifically, the incidences of restenosis of 70% or more following CAS and CEA were similar, 6 versus 6.3%, respectively, at 2 years after the initial treatment. Other studies have reported restenosis rates of 1.7 to 21% with a wide variation in follow-up and the threshold of restenosis on imaging [63,64,65,66]. CAS after failed CEA or after radiation therapy to the neck is associated with high rates of in-stent stenosis [54]. Criteria for the extent of in-stent stenosis are controversial and difficult to standardize. This leads to difficulty in defining specific indications for treatment. Unlike de novo carotid artery stenosis, no treatment guidelines exist for treating in-stent stenosis. In current practice, physicians make decisions using clinical judgment based on the extent of stenosis, severity of symptoms, and the presence of contralateral carotid artery stenosis or occlusion. Currently, the most common treatment for in-stent stenosis is angioplasty with or without restenting. Angioplasty alone was found to be inferior to angioplasty and stenting in de novo carotid stenosis. But for in-stent restenosis, balloon angioplasty alone has been reported to show satisfactory results in some case series but with generally short durability and high rates of restenosis [65, 67,68,69].
Since CAS is used as an alternative treatment for patients with moderate carotid stenosis who are high-risk candidates for surgical recanalization, CEA rarely becomes an option for in-stent stenosis. However, for patients without increased surgical risk, CEA may be a reasonable choice. Reichmann et al. reported their case series consisting of 15 patients subjected to CEA for the treatment of in-stent stenosis with removal of the stent [50]. A successful recanalization rate of 100% was reached. Only one patient suffered minor and recoverable periprocedural neurological deficits. There was no restenosis in any of these 15 patients at 21 months follow-up. Zhang et al. reported a case series of 10 patients who underwent CEA for in-stent stenosis with a recanalization rate of 100%, a surgical complication rate of 30%, and a restenosis rate of 10% at 1 year follow-up [57].
Although few studies have directly compared surgical and endovascular treatments for in-stent stenosis, Arhuidese et al. have compared a total of 645 carotid artery interventions (CEA 134 and redo CAS 511) in patients with prior ipsilateral CAS [9]. The authors reported no statistically significant difference in perioperative and 1-year outcomes between the two groups [9]. Generally, CEA is offered to patients who are more severely ill since redo CAS resulted in a significantly higher absolute mortality in this subgroup. It is difficult to provide confident guidance regarding which approach is optimal, and a randomized control study is needed to answer this important question. Currently, a reasonable strategy would include angioplasty and stenting for moderate stenosis, and CEA with stent explantation for severe stenosis, especially in patients with progressive disease.
Post-CEA Restenosis
The published incidence of carotid artery restenosis (defined as stenosis of greater than 50%) after an initial CEA is between 6 and 36% [70]. Early carotid restenosis is normally a result of myointimal hyperplasia [6]. Late carotid restenosis is believed to occur due to recurrence or progression of carotid atherosclerotic disease [7]. According to the guidelines from the AHA and Society of Vascular Surgery, post-CEA restenosis was determined a contraindication for redo CEA and considered an indication for CAS [2, 3]. However, several studies evaluating redo CEA in patients with post-CEA stenosis have been conducted. Dorigo et al. reported a retrospective single center matched case–control study of 148 patients comparing CAS (80 cases) and redo CEA (68 cases) for post-CEA stenosis [71]. After propensity matching, 32 CAS interventions were matched with 32 redo CEAs. CAS and redo CEA in patients with post-CEA restenosis provided similar perioperative results in a sample of comparable patients. Furthermore, CAS patients had better outcomes in terms of secondary restenosis and the need for further redo interventions. Freedom from secondary restenosis at 4 years was 100% in the CAS group and 72.5% in the redo CEA group. A meta-analysis by Texakalidis et al. including 13 studies totaling 4163 patients with carotid artery restenosis after initial CEA demonstrated similar risks of periprocedural stroke, TIA, MI, and death in CAS and CEA [8]. However, patients treated with CAS had a lower risk for a new restenosis and periprocedural cranial nerve injury. Thus, there is consistent result of CAS being more beneficial than redo CEA in the setting of restenosis and redo intervention. This supports the guideline for CAS treatment of post-CEA stenosis by the AHA and Society of Vascular Surgery.
Complications Associated with CEA and CAS
Complications Associated with CEA
Based on the results of the CREST trial [72], there was a higher risk of stroke within the periprocedural period after CAS (4.1% following CAS versus 2.3% following CEA) and a higher risk of MI after CEA (1.1% following CAS versus 2.3% following CEA). A recent meta-analysis of 5 studies evaluating and comparing CEA and CAS, which included the CREST trial and involved a total of 3901 patients (1585 subjected to CEA; 2316 subjected to CAS), demonstrated significantly increased risk of stroke during the periprocedural period following CAS as compared to those that underwent CEA (2.6% risk of stroke following CAS and 1.3% following CEA) [73]. Interestingly, there was no significant difference in the incidence of major debilitating strokes between CAS and CEA (CAS 0.5%, CEA 0.3%). However, there were significantly more minor strokes in the CAS group when compared to CEA patients (CAS 2.2%; CEA 1.0%). This study also showed a higher rate of MI in the CEA group, but no statistically significant difference was achieved (CAS 0.7%; CEA 1.5%). In the North American Symptomatic Carotid Endarterectomy Trial (NASCET) [74], wound bleeding after CEA occurred in 5.5% of all patients, making this a more frequent complication than major stroke (2.1%) or MI (0.9%). Indeed, the incidence of neck hematoma has been reported between 1.5 and 12% [75]. However, it is generally not associated with an increased risk of stroke or death. Previous studies have identified antiplatelet treatment with clopidogrel as a main risk factor for postoperative neck hematoma [75, 76]. Other factors associated with this complication were a reintervention and postoperative arterial hypertension after CEA. In a recent prospective case series, 188 consecutive patients on dual antiplatelet therapy underwent CEA, and no hemorrhagic complications were observed in this patient population [58].
Postoperative cranial nerve injury (CNI) remains a critical complication of CEA occurring in 4.6 to 8.6% of cases [77]. CNI is most often transient and not disabling; permanent deficits lasting longer than 1 year are rare. The marginal mandibular branch of the facial nerve was most frequently involved (52%), followed by the hypoglossal (29%), the vagus (13%), and the spinal accessory nerve (6%). The vast majority (94%) of these CNIs were transient in nature. The arteriotomy during CEA can be closed primarily or with the use of a synthetic or biologic patch. Early reviews showed that carotid patch angioplasty may reduce the risk of perioperative arterial occlusion, restenosis, and ipsilateral stroke [78]. However, more recent studies showed no difference in clinical outcome regarding the rate of stroke and arterial occlusion [79,67,68,82]. In a recent meta-analysis involving over 3000 patients, closure of the arteriotomy with different patch materials showed no statistical significance in terms of short- and long-term outcomes [83].
The incidence of cerebral hyperperfusion syndrome has been reported as 1–3% [84,85,86], often presenting at first with headache, followed by seizure and intracerebral hemorrhage. Cerebral hyperperfusion syndrome usually occurs in severe carotid stenosis (≥ 80% stenosis) and/or post-CEA for recent cerebral infarction [87, 88]. High volume operator and high hospital volume have been associated with decreased mortality and stroke after CEA and CAS indicating that aiming for a high volume may reduce procedural complications [89]. Interestingly, a review of carotid artery disease management showed that left-sided CEA is consistently associated with higher postoperative adverse events. The etiology of these side-specific adverse events is not well explained [90].
CAS-Associated Complications
The most common CAS-associated complications include stroke, myocardial infarction, renal failure, and access-related problems. Periprocedural stroke rates are higher for CAS than CEA as mentioned above. Combined 30-day stroke and mortality rates for CAS in randomized trials are 6–9% for symptomatic patients and 2–4% for asymptomatic patients [91,92,93]. Periprocedural strokes related to CAS may be due to stent thrombosis and/or thromboembolism [94]. Stroke rates can be reduced with perioperative treatment including dual antiplatelet therapy (aspirin and clopidogrel), optimal intraoperative anticoagulation, and possibly, use of embolic protection devices. Periprocedural bradycardia and hypotension are other common problems associated with CAS, occurring in up to 68% of patients [95,96,97]. This is believed to be secondary to carotid baroreceptor stimulation during inflation of the poststent angioplasty balloon. Hypotension and bradycardia are more prevalent in patients with contralateral high-degree carotid artery stenosis. These symptoms are usually self-limited and do not cause any major vascular events but have resulted in longer hospital stays [98]. Cerebral hyperperfusion syndrome is an uncommon sequela following CAS, but its occurrence is otherwise similar to the hyperperfusion syndrome in CEA patients [99]. Periprocedural myocardial infarction rates following CAS are 1–4% [72], lower than those seen in CEA. Renal dysfunction following CAS is most likely due to contrast-induced nephropathy and/or renal emboli; it is more common in patients with preexisting moderate-to-severe renal insufficiency and diabetes. Access-related complications include groin hematoma/bleeding, pseudoaneurysm formation, and atheroembolization to the lower extremities. Approximately 3% of CAS patients develop access site complications (hematoma) [100].
Conclusion
The current literature supports CEA as standard therapy for symptomatic carotid artery stenosis. Treatment recommendations are less clear for acute tandem carotid artery stenosis/occlusion. Here, we have reviewed the literatures on these specific conditions and attempted to provide suggestions for clinical decision-making regarding treatment. For acute tandem occlusion of the internal and common carotid arteries, angioplasty is suggested for most cases, reserving CAS or CEA for severe or complicated cases. For post-CEA restenosis, current literature suggests CAS, while for poststenting restenosis, current evidence is lacking; however, angioplasty and redo CAS for moderate stenosis and CEA for severe stenosis might be a reasonable treatment approach (Fig. 1). Most clinical society recommendations are either based on randomized control trials, case series, or expert opinions and may not reflect real-world scenarios where clinicians are often faced with challenging cases that are excluded in clinical trials or not discussed in any society guidelines. Hence, treatment for carotid artery disease needs to be tailored to the particular patient’s need in view of pathophysiology of the disease process that is closely consistent with existing literature. Future studies evaluating and defining the risks and benefits of specific treatment strategies, such as CEA and CAS, in patients with acute tandem stenosis, occlusion, and postintervention restenosis of the carotid artery need to be conducted.
References
Abbott AL, Paraskevas KI, Kakkos SK, Golledge J, Eckstein HH, Diaz-Sandoval LJ, et al. Systematic review of guidelines for the management of asymptomatic and symptomatic carotid stenosis. Stroke. 2015;46(11):3288–301. https://doi.org/10.1161/STROKEAHA.115.003390.
Furie KL, Kasner SE, Adams RJ, Albers GW, Bush RL, Fagan SC, et al. Guidelines for the prevention of stroke in patients with stroke or transient ischemic attack: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2011;42(1):227–76. https://doi.org/10.1161/STR.0b013e3181f7d043.
Hobson RW 2nd, Mackey WC, Ascher E, Murad MH, Calligaro KD, Comerota AJ, et al. Management of atherosclerotic carotid artery disease: clinical practice guidelines of the Society for Vascular Surgery. J Vasc Surg. 2008;48(2):480–6. https://doi.org/10.1016/j.jvs.2008.05.036.
Dai Z, Xu G. Restenosis after carotid artery stenting. Vascular. 2017;25(6):576–86. https://doi.org/10.1177/1708538117706273.
Mohr JP. Transient ischemic attacks and the prevention of strokes. N Engl J Med. 1978;299(2):93–5. https://doi.org/10.1056/NEJM197807132990209.
Hunter GC, Edgar J. Poth Memorial/W.L. Gore and Associates, Inc. Lectureship. The clinical and pathological spectrum of recurrent carotid stenosis. Am J Surg. 1997;174(6):583–8.
AbuRahma AF, Abu-Halimah S, Hass SM, Nanjundappa A, Stone PA, Mousa A, et al. Carotid artery stenting outcomes are equivalent to carotid endarterectomy outcomes for patients with post-carotid endarterectomy stenosis. J Vasc Surg. 2010;52(5):1180–7. https://doi.org/10.1016/j.jvs.2010.06.074.
Texakalidis P, Giannopoulos S, Jonnalagadda AK, Kokkinidis DG, Machinis T, Reavey-Cantwell J, et al. Carotid artery endarterectomy versus carotid artery stenting for restenosis after carotid artery endarterectomy: a systematic review and meta-analysis. World Neurosurg. 2018;115:421–9 e1. https://doi.org/10.1016/j.wneu.2018.02.196.
Arhuidese IJ, Nejim B, Chavali S, Locham S, Obeid T, Hicks CW, et al. Endarterectomy versus stenting in patients with prior ipsilateral carotid artery stenting. J Vasc Surg. 2017;65(5):1418–28. https://doi.org/10.1016/j.jvs.2016.11.041.
Kistler JP, Ropper AH, Heros RC. Therapy of ischemic cerebral vascular disease due to atherothrombosis (1). N Engl J Med. 1984;311(1):27–34. https://doi.org/10.1056/NEJM198407053110105.
Kistler JP, Ropper AH, Heros RC. Therapy of ischemic cerebral vascular disease due to atherothrombosis. (2). N Engl J Med. 1984;311(2):100–5. https://doi.org/10.1056/NEJM198407123110206.
Brinjikji W, Huston J 3rd, Rabinstein AA, Kim GM, Lerman A, Lanzino G. Contemporary carotid imaging: from degree of stenosis to plaque vulnerability. J Neurosurg. 2016;124(1):27–42. https://doi.org/10.3171/2015.1.JNS142452.
Bulwa Z, Gupta A. Embolic stroke of undetermined source: the role of the nonstenotic carotid plaque. J Neurol Sci. 2017;382:49–52. https://doi.org/10.1016/j.jns.2017.09.027.
Wang Y, Wang Y, Zhao X, Liu L, Wang D, Wang C, et al. Clopidogrel with aspirin in acute minor stroke or transient ischemic attack. N Engl J Med. 2013;369(1):11–9. https://doi.org/10.1056/NEJMoa1215340.
Johnston SC, Easton JD, Farrant M, Barsan W, Conwit RA, Elm JJ, et al. Clopidogrel and aspirin in acute ischemic stroke and high-risk TIA. N Engl J Med. 2018;379(3):215–25. https://doi.org/10.1056/NEJMoa1800410.
Miyazaki Y, Mori T, Iwata T, Aoyagi Y, Tanno Y, Kasakura S, et al. Continuous daily use of cilostazol prevents in-stent restenosis following carotid artery stenting: serial angiographic investigation of 229 lesions. Journal of NeuroInterventional Surgery. 2016;8(5):471–5.
Yadav JS, Wholey MH, Kuntz RE, Fayad P, Katzen BT, Mishkel GJ, et al. Protected carotid-artery stenting versus endarterectomy in high-risk patients. N Engl J Med. 2004;351(15):1493–501. https://doi.org/10.1056/NEJMoa040127.
Spangler EL, Goodney PP, Schanzer A, Stone DH, Schermerhorn ML, Powell RJ, et al. Outcomes of carotid endarterectomy versus stenting in comparable medical risk patients. J Vasc Surg. 2014;60(5):1227–31, 31 e1. https://doi.org/10.1016/j.jvs.2014.05.044.
Yoshida S, Bensley RP, Glaser JD, Nabzdyk CS, Hamdan AD, Wyers MC, et al. The current national criteria for carotid artery stenting overestimate its efficacy in patients who are symptomatic and at high risk. J Vasc Surg. 2013;58(1):120–7. https://doi.org/10.1016/j.jvs.2012.12.075.
Kao H-L, Lin M-S, Wang C-S, Lin Y-H, Lin L-C, Chao C-L, et al. Feasibility of endovascular recanalization for symptomatic cervical internal carotid artery occlusion. J Am Coll Cardiol. 2007;49(7):765–71.
Lin MS, Lin LC, Li HY, Lin CH, Chao CC, Hsu CN, et al. Procedural safety and potential vascular complication of endovascular recanalization for chronic cervical internal carotid artery occlusion. Circ Cardiovasc Interv. 2008;1(2):119–25. https://doi.org/10.1161/CIRCINTERVENTIONS.108.772350.
Shojima M, Nemoto S, Morita A, Miyata T, Namba K, Tanaka Y, et al. Protected endovascular revascularization of subacute and chronic total occlusion of the internal carotid artery. Am J Neuroradiol. 2010;31(3):481–6.
Powers WJ. Atherosclerotic carotid artery occlusion. Curr Treat Options Cardiovasc Med. 2003;5(6):501–9.
Jiao L, Song G, Hua Y, Ma Y, Chen Y, Wang Y, et al. Recanalization of extracranial internal carotid artery occlusion: a 12-year retrospective study. Neural Regen Res. 2013;8(23):2204–6. https://doi.org/10.3969/j.issn.1673-5374.2013.23.011.
Shih YT, Chen WH, Lee WL, Lee HT, Shen CC, Tsuei YS. Hybrid surgery for symptomatic chronic total occlusion of carotid artery: a technical note. Neurosurgery. 2013;73(1 Suppl Operative):onsE117–23; discussion onsE23. https://doi.org/10.1227/NEU.0b013e31827fca6c.
Zanaty M, Samaniego EA, Teferi N, Kung DK, Nakagawa D, Hudson J, et al. Hybrid surgery for internal carotid artery revascularization. World Neurosurg. 2019;121:137–44. https://doi.org/10.1016/j.wneu.2018.09.230.
Hauck EF, Natarajan SK, Ohta H, Ogilvy CS, Hopkins LN, Siddiqui AH, et al. Emergent endovascular recanalization for cervical internal carotid artery occlusion in patients presenting with acute stroke. Neurosurgery. 2011;69(4):899–907; discussion. https://doi.org/10.1227/NEU.0b013e31821cfa52.
Kiyofuji S, Inoue T, Tamura A, Saito I. Emergent cervical surgical embolectomy for extracranial internal carotid artery occlusion. Acta Neurochir. 2015;157(8):1313–8; discussion 8-9. https://doi.org/10.1007/s00701-015-2478-5.
Gunka I, Krajickova D, Lesko M, Jiska S, Raupach J, Lojik M, et al. Emergent carotid thromboendarterectomy for acute symptomatic occlusion of the extracranial internal carotid artery. Vasc Endovasc Surg. 2017;51(4):176–82.
Jadhav A, Panczykowski D, Jumaa M, Aghaebrahim A, Ranginani M, Nguyen F, et al. Angioplasty and stenting for symptomatic extracranial non-tandem internal carotid artery occlusion. J Neurointerv Surg. 2018;10:1155–60. https://doi.org/10.1136/neurintsurg-2018-013810.
Rangel-Castilla L, Rajah GB, Shakir HJ, Shallwani H, Gandhi S, Davies JM, et al. Management of acute ischemic stroke due to tandem occlusion: should endovascular recanalization of the extracranial or intracranial occlusive lesion be done first? Neurosurg Focus. 2017;42(4):E16. https://doi.org/10.3171/2017.1.FOCUS16500.
Akpinar CK, Gurkas E, Aytac E. Carotid angioplasty-assisted mechanical thrombectomy without urgent stenting may be a better option in acute tandem occlusions. Interv Neuroradiol. 2017;23(4):405–11. https://doi.org/10.1177/1591019917701113.
Wilson MP, Murad MH, Krings T, Pereira VM, O'Kelly C, Rempel J, et al. Management of tandem occlusions in acute ischemic stroke - intracranial versus extracranial first and extracranial stenting versus angioplasty alone: a systematic review and meta-analysis. J Neurointerv Surg. 2018;10:721–8. https://doi.org/10.1136/neurintsurg-2017-013707.
Heck DV, Brown MD. Carotid stenting and intracranial thrombectomy for treatment of acute stroke due to tandem occlusions with aggressive antiplatelet therapy may be associated with a high incidence of intracranial hemorrhage. J Neurointerv Surg. 2015;7(3):170–5. https://doi.org/10.1136/neurintsurg-2014-011224.
Barkat M, Hajibandeh S, Hajibandeh S, Torella F, Antoniou GA. Systematic review and meta-analysis of dual versus single antiplatelet therapy in carotid interventions. Eur J Vasc Endovasc Surg. 2017;53(1):53–67. https://doi.org/10.1016/j.ejvs.2016.10.011.
Slawski DE, Jumaa MA, Salahuddin H, Shawver J, Humayun MJ, Russell T, et al. Emergent carotid endarterectomy versus stenting in acute stroke patients with tandem occlusion. J Vasc Surg. 2018;68:1047–53. https://doi.org/10.1016/j.jvs.2017.12.077.
Gliem M, Lee JI, Barckhan A, Turowski B, Hartung HP, Jander S. Outcome and treatment effects in stroke associated with acute cervical ICA occlusion. PLoS One. 2017;12(1):e0170247. https://doi.org/10.1371/journal.pone.0170247.
Cohen JE, Gomori JM, Leker RR, Eichel R, Itshayek E. Emergency revascularization of acute internal carotid artery occlusion: follow the spike, it guides you. J Clin Neurosci. 2016;29:95–9. https://doi.org/10.1016/j.jocn.2015.12.013.
Rahme R, Abruzzo TA, Ringer AJ. Acute ischemic stroke in the setting of cervical carotid occlusion: a proposed management strategy. World Neurosurg. 2011;76(6 Suppl):S60–5. https://doi.org/10.1016/j.wneu.2011.08.016.
Devlin TG, Phade SV, Hutson RK, Fugate MW, Major GR 2nd, Albers GW, et al. Computed tomography perfusion imaging in the selection of acute stroke patients to undergo emergent carotid endarterectomy. Ann Vasc Surg. 2015;29(1):125.e1–11. https://doi.org/10.1016/j.avsg.2014.07.023.
Cote R, Barnett HJ, Taylor DW. Internal carotid occlusion: a prospective study. Stroke. 1983;14(6):898–902.
Flaherty ML, Flemming KD, McClelland R, Jorgensen NW, Brown RD Jr. Population-based study of symptomatic internal carotid artery occlusion: incidence and long-term follow-up. Stroke. 2004;35(8):e349–52. https://doi.org/10.1161/01.STR.0000135024.54608.3f.
Kuroda S, Kawabori M, Hirata K, Shiga T, Kashiwazaki D, Houkin K, et al. Clinical significance of STA-MCA double anastomosis for hemodynamic compromise in post-JET/COSS era. Acta Neurochir. 2014;156(1):77–83. https://doi.org/10.1007/s00701-013-1961-0.
Reynolds MR, Derdeyn CP, Grubb RL Jr, Powers WJ, Zipfel GJ. Extracranial-intracranial bypass for ischemic cerebrovascular disease: what have we learned from the Carotid Occlusion Surgery Study? Neurosurg Focus. 2014;36(1):E9. https://doi.org/10.3171/2013.10.FOCUS13427.
Kernan WN, Ovbiagele B, Black HR, Bravata DM, Chimowitz MI, Ezekowitz MD, et al. Guidelines for the prevention of stroke in patients with stroke and transient ischemic attack: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2014;45(7):2160–236. https://doi.org/10.1161/STR.0000000000000024.
Greiner C, Wassmann H, Palkovic S, Gauss C. Revascularization procedures in internal carotid artery pseudo-occlusion. Acta Neurochir. 2004;146(3):237–43; discussion 43. https://doi.org/10.1007/s00701-004-0216-5.
Thompson JE, Austin DJ, Patman RD. Carotid endarterectomy for cerebrovascular insufficiency: long-term results in 592 patients followed up to thirteen years. Surg Clin North Am. 1986;66(2):233–53.
Chen YH, Leong WS, Lin MS, Huang CC, Hung CS, Li HY, et al. Predictors for successful endovascular intervention in chronic carotid artery total occlusion. JACC Cardiovasc Interv. 2016;9(17):1825–32. https://doi.org/10.1016/j.jcin.2016.06.015.
Li J, Wang C, Zou S, Liu Y, Qu L. Hybrid surgery for nontaper or nonstump lesions in symptomatic subacute or chronic internal carotid occlusion: a better solution. World Neurosurg. 2018;122:e1416–25. https://doi.org/10.1016/j.wneu.2018.11.075.
Reichmann BL, van Laanen JH, de Vries JP, Hendriks JM, Verhagen HJ, Moll FL, et al. Carotid endarterectomy for treatment of in-stent restenosis after carotid angioplasty and stenting. J Vasc Surg. 2011;54(1):87–92. https://doi.org/10.1016/j.jvs.2010.11.118.
Donas KP, Eisenack M, Torsello G. Balloon angioplasty for in-stent stenosis after carotid artery stenting is associated with an increase in repeat interventions. J Endovasc Ther. 2011;18(5):720–5.
Montorsi P, Galli S, Ravagnani PM, Trabattoni D, Fabbiocchi F, Lualdi A, et al. Drug-eluting balloon for treatment of in-stent restenosis after carotid artery stenting: preliminary report. J Endovasc Ther. 2012;19(6):734–42.
Gandini R, Del Giudice C, Da Ros V, Sallustio F, Altobelli S, D'Onofrio A, et al. Long-term results of drug-eluting balloon angioplasty for treatment of refractory recurrent carotid in-stent restenosis. J Endovasc Ther. 2014;21(5):671–7.
Moon K, Albuquerque FC, Levitt MR, Ahmed AS, Kalani MY, McDougall CG. The myth of restenosis after carotid angioplasty and stenting. J Neurointerv Surg. 2016;8(10):1006–10. https://doi.org/10.1136/neurintsurg-2015-011938.
Arhuidese IJ, Nejim B, Chavali S, Locham S, Obeid T, Hicks CW, et al. Endarterectomy versus stenting in patients with prior ipsilateral carotid artery stenting. J Vasc Surg. 2017;65(5):1418–28.
Stilo F, Sirignano P, Montelione N, Mansour W, Capoccia L, Catanese V, et al. Bypass for symptomatic in-stent carotid restenosis. Int J Cardiol. 2017;249:392–5.
Yu LB, Yan W, Zhang Q, Zhao JZ, Zhang Y, Wang R, et al. Carotid endarterectomy for treatment of carotid in-stent restenosis: long-term follow-up results and surgery experiences from one single centre. Stroke Vasc Neurol. 2017;2(3):140–6. https://doi.org/10.1136/svn-2017-000089.
Illuminati G, Schneider F, Pizzardi G, Masci F, Calio FG, Ricco JB. Dual antiplatelet therapy does not increase the risk of bleeding after carotid endarterectomy: results of a prospective study. Ann Vasc Surg. 2017;40:39–43. https://doi.org/10.1016/j.avsg.2016.09.012.
Attigah N, Külkens S, Deyle C, Ringleb P, Hartmann M, Geisbüsch P, et al. Redo surgery or carotid stenting for restenosis after carotid endarterectomy: results of two different treatment strategies. Ann Vasc Surg. 2010;24(2):190–5.
Fokkema M, de Borst GJ, Nolan BW, Lo RC, Cambria RA, Powell RJ, et al. Carotid stenting versus endarterectomy in patients undergoing reintervention after prior carotid endarterectomy. J Vasc Surg. 2014;59(1):8–15.e2.
Marques de Marino P, Martinez Lopez I, Hernandez Mateo MM, Cernuda Artero I, Cabrero Fernandez M, Reina Gutierrez MT, et al. Open versus endovascular treatment for patients with post-carotid endarterectomy restenosis: early and long-term results. Ann Vasc Surg. 2016;36:159–65.
Lal BK, Beach KW, Roubin GS, Lutsep HL, Moore WS, Malas MB, et al. Restenosis after carotid artery stenting and endarterectomy: a secondary analysis of CREST, a randomised controlled trial. Lancet Neurol. 2012;11(9):755–63. https://doi.org/10.1016/S1474-4422(12)70159-X.
Arquizan C, Trinquart L, Touboul PJ, Long A, Feasson S, Terriat B, et al. Restenosis is more frequent after carotid stenting than after endarterectomy: the EVA-3S study. Stroke. 2011;42(4):1015–20. https://doi.org/10.1161/STROKEAHA.110.589309.
Zhou W, Lin PH, Bush RL, Peden EK, Guerrero MA, Kougias P, et al. Management of in-sent restenosis after carotid artery stenting in high-risk patients. J Vasc Surg. 2006;43(2):305–12. https://doi.org/10.1016/j.jvs.2005.10.040.
Lal BK, Hobson RW 2nd, Goldstein J, Geohagan M, Chakhtoura E, Pappas PJ, et al. In-stent recurrent stenosis after carotid artery stenting: life table analysis and clinical relevance. J Vasc Surg. 2003;38(6):1162–8; discussion 9. https://doi.org/10.1016/j.jvs.2003.08.021.
Christiaans MH, Ernst JM, Suttorp MJ, van den Berg JC, Overtoom TT, Kelder JC, et al. Restenosis after carotid angioplasty and stenting: a follow-up study with duplex ultrasonography. Eur J Vasc Endovasc Surg. 2003;26(2):141–4.
Setacci C, Pula G, Baldi I, de Donato G, Setacci F, Cappelli A, et al. Determinants of in-stent restenosis after carotid angioplasty: a case-control study. J Endovasc Ther. 2003;10(6):1031–8. https://doi.org/10.1177/152660280301000602.
Chakhtoura EY, Hobson RW 2nd, Goldstein J, Simonian GT, Lal BK, Haser PB, et al. In-stent restenosis after carotid angioplasty-stenting: incidence and management. J Vasc Surg. 2001;33(2):220–5; discussion 5-6. https://doi.org/10.1067/mva.2001.111880.
Setacci C, de Donato G, Setacci F, Pieraccini M, Cappelli A, Trovato RA, et al. In-stent restenosis after carotid angioplasty and stenting: a challenge for the vascular surgeon. Eur J Vasc Endovasc Surg. 2005;29(6):601–7. https://doi.org/10.1016/j.ejvs.2005.01.033.
Vos JA, de Borst GJ, Overtoom TT, de Vries JP, van de Pavoordt ED, Zanen P, et al. Carotid angioplasty and stenting: treatment of postcarotid endarterectomy restenosis is at least as safe as primary stenosis treatment. J Vasc Surg. 2009;50(4):755–61 e1. https://doi.org/10.1016/j.jvs.2009.04.060.
Dorigo W, Fargion A, Giacomelli E, Pulli R, Masciello F, Speziali S, et al. A propensity matched comparison for open and endovascular treatment of post-carotid endarterectomy restenosis. Eur J Vasc Endovasc Surg. 2018;55(2):153–61. https://doi.org/10.1016/j.ejvs.2017.11.015.
Brott TG, Hobson RW 2nd, Howard G, Roubin GS, Clark WM, Brooks W, et al. Stenting versus endarterectomy for treatment of carotid-artery stenosis. N Engl J Med. 2010;363(1):11–23. https://doi.org/10.1056/NEJMoa0912321.
Cui L, Han Y, Zhang S, Liu X, Zhang J. Safety of stenting and endarterectomy for asymptomatic carotid artery stenosis: a meta-analysis of randomised controlled trials. Eur J Vasc Endovasc Surg. 2018;55(5):614–24. https://doi.org/10.1016/j.ejvs.2018.02.020.
North American Symptomatic Carotid Endarterectomy Trial C, Barnett HJM, Taylor DW, Haynes RB, Sackett DL, Peerless SJ, et al. Beneficial effect of carotid endarterectomy in symptomatic patients with high-grade carotid stenosis. N Engl J Med. 1991;325(7):445–53. https://doi.org/10.1056/NEJM199108153250701.
Baracchini C, Gruppo M, Mazzalai F, Lorenzetti R, Meneghetti G, Ballotta E. Predictors of neck bleeding after eversion carotid endarterectomy. J Vasc Surg. 2011;54(3):699–705. https://doi.org/10.1016/j.jvs.2011.03.262.
Morales Gisbert SM, Sala Almonacil VA, Zaragoza Garcia JM, Genoves Gasco B, Gomez Palones FJ, Ortiz ME. Predictors of cervical bleeding after carotid endarterectomy. Ann Vasc Surg. 2014;28(2):366–74. https://doi.org/10.1016/j.avsg.2013.04.011.
Chisci E, Rehring TF, Pigozzi C, Colon S, Borgheresi A, Tramacere L, et al. Cranial nerve injury is associated with dual antiplatelet therapy use and cervical hematoma after carotid endarterectomy. J Vasc Surg. 2016;64(4):985–9 e2. https://doi.org/10.1016/j.jvs.2016.04.029.
Rerkasem K, Rothwell PM. Patch angioplasty versus primary closure for carotid endarterectomy. Cochrane Database Syst Rev. 2009;4:CD000160. https://doi.org/10.1002/14651858.CD000160.pub3.
Maertens V, Maertens H, Kint M, Coucke C, Blomme Y. Complication rate after carotid endarterectomy comparing patch angioplasty and primary closure. Ann Vasc Surg. 2016;30:248–52. https://doi.org/10.1016/j.avsg.2015.07.045.
Avgerinos ED, Chaer RA, Naddaf A, El-Shazly OM, Marone L, Makaroun MS. Primary closure after carotid endarterectomy is not inferior to other closure techniques. J Vasc Surg. 2016;64(3):678–83 e1. https://doi.org/10.1016/j.jvs.2016.03.415.
Cheng I, Vyas KS, Velaga S, Davenport DL, Saha SP. Outcomes of carotid endarterectomy with primary closure. Int J Angiol. 2017;26(2):83–8. https://doi.org/10.1055/s-0037-1601053.
Harrison GJ, How TV, Poole RJ, Brennan JA, Naik JB, Vallabhaneni SR, et al. Closure technique after carotid endarterectomy influences local hemodynamics. J Vasc Surg. 2014;60(2):418–27. https://doi.org/10.1016/j.jvs.2014.01.069.
Texakalidis P, Giannopoulos S, Charisis N, Giannopoulos S, Karasavvidis T, Koullias G, et al. A meta-analysis of randomized trials comparing bovine pericardium and other patch materials for carotid endarterectomy. J Vasc Surg. 2018;68(4):1241–56 e1. https://doi.org/10.1016/j.jvs.2018.07.023.
Youkey JR, Clagett GP, Jaffin JH, Parisi JE, Rich NM. Focal motor seizures complicating carotid endarterectomy. Arch Surg. 1984;119(9):1080–4.
Reigel MM, Hollier LH, Sundt TM Jr, Piepgras DG, Sharbrough FW, Cherry KJ. Cerebral hyperperfusion syndrome: a cause of neurologic dysfunction after carotid endarterectomy. J Vasc Surg. 1987;5(4):628–34.
Naylor AR, Ruckley CV. The post-carotid endarterectomy hyperperfusion syndrome. Eur J Vasc Endovasc Surg. 1995;9(4):365–7.
Kablak-Ziembicka A, Przewlocki T, Pieniazek P, Musialek P, Tekieli L, Roslawiecka A, et al. Predictors of cerebral reperfusion injury after carotid stenting: the role of transcranial color-coded Doppler ultrasonography. J Endovasc Ther. 2010;17(4):556–63. https://doi.org/10.1583/09-2980.1.
Bouri S, Thapar A, Shalhoub J, Jayasooriya G, Fernando A, Franklin IJ, et al. Hypertension and the post-carotid endarterectomy cerebral hyperperfusion syndrome. Eur J Vasc Endovasc Surg. 2011;41(2):229–37. https://doi.org/10.1016/j.ejvs.2010.10.016.
Poorthuis MHF, Brand EC, Halliday A, Bulbulia R, Bots ML, de Borst GJ. High operator and hospital volume are associated with a decreased risk of death and stroke after carotid revascularization: a systematic review and meta-analysis. Ann Surg. 2018. https://doi.org/10.1097/SLA.0000000000002880.
Girard LP, Feasby TE, Eliasziw M, Quan H, Kennedy J, Barnett HJ, et al. Complication rates after left- versus right-sided carotid endarterectomy. Circ Cardiovasc Qual Outcomes. 2009;2(6):642–7. https://doi.org/10.1161/CIRCOUTCOMES.109.850842.
Group SC, Ringleb PA, Allenberg J, Bruckmann H, Eckstein HH, Fraedrich G, et al. 30 day results from the SPACE trial of stent-protected angioplasty versus carotid endarterectomy in symptomatic patients: a randomised non-inferiority trial. Lancet. 2006;368(9543):1239–47. https://doi.org/10.1016/S0140-6736(06)69122-8.
Mas JL, Chatellier G, Beyssen B, Branchereau A, Moulin T, Becquemin JP, et al. Endarterectomy versus stenting in patients with symptomatic severe carotid stenosis. N Engl J Med. 2006;355(16):1660–71. https://doi.org/10.1056/NEJMoa061752.
International Carotid Stenting Study i, Ederle J, Dobson J, Featherstone RL, Bonati LH, van der Worp HB, et al. Carotid artery stenting compared with endarterectomy in patients with symptomatic carotid stenosis (International Carotid Stenting Study): an interim analysis of a randomised controlled trial. Lancet. 2010;375(9719):985–97. https://doi.org/10.1016/S0140-6736(10)60239-5.
Moulakakis KG, Mylonas SN, Lazaris A, Tsivgoulis G, Kakisis J, Sfyroeras GS, et al. Acute carotid stent thrombosis: a comprehensive review. Vasc Endovasc Surg. 2016;50(7):511–21. https://doi.org/10.1177/1538574416665986.
Mendelsohn FO, Weissman NJ, Lederman RJ, Crowley JJ, Gray JL, Phillips HR, et al. Acute hemodynamic changes during carotid artery stenting. Am J Cardiol. 1998;82(9):1077–81.
Gupta R, Abou-Chebl A, Bajzer CT, Schumacher HC, Yadav JS. Rate, predictors, and consequences of hemodynamic depression after carotid artery stenting. J Am Coll Cardiol. 2006;47(8):1538–43. https://doi.org/10.1016/j.jacc.2005.08.079.
Lin PH, Zhou W, Kougias P, El Sayed HF, Barshes NR, Huynh TT. Factors associated with hypotension and bradycardia after carotid angioplasty and stenting. J Vasc Surg. 2007;46(5):846–53; discussion 53-4. https://doi.org/10.1016/j.jvs.2007.07.020.
Gokcal E, Niftaliyev E, Deniz C, Ergelen M, Guzel V, Goktekin O, et al. Prolonged hypotension after carotid artery stenting: incidence, predictors and consequences. Acta Neurochir. 2017;159(11):2081–7. https://doi.org/10.1007/s00701-017-3295-9.
Timaran CH, Veith FJ, Rosero EB, Modrall JG, Valentine RJ, Clagett GP. Intracranial hemorrhage after carotid endarterectomy and carotid stenting in the United States in 2005. J Vasc Surg. 2009;49(3):623–8; discussion 8-9. https://doi.org/10.1016/j.jvs.2008.09.064.
Taha MM, Sakaida H, Asakura F, Maeda M, Toma N, Sano T, et al. Access site complications with carotid angioplasty and stenting. Surg Neurol. 2007;68(4):431–7. https://doi.org/10.1016/j.surneu.2006.11.036.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
Runqi Wangqin, Paul R. Krafft, Keaton Piper, Jay Kumar, and Kaya Xu declare that they have no conflict of interest. Maxim Mokin serves as consultant for Cerebrotech and Imperative Care. Zeguang Ren serves as consultant for Penumbra, Inc.
Ethical Approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Wangqin, R., Krafft, P.R., Piper, K. et al. Management of De Novo Carotid Stenosis and Postintervention Restenosis—Carotid Endarterectomy Versus Carotid Artery Stenting—a Review of Literature. Transl. Stroke Res. 10, 460–474 (2019). https://doi.org/10.1007/s12975-019-00693-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12975-019-00693-z