Abstract
Background
Acute extracranial internal carotid artery (ICA) occlusion by a huge cardiogenic embolus is rare, but can be catastrophic.
Methods
Seven patients with acute ischemic stroke due to embolic occlusion of extracranial ICA who underwent emergent cervical surgical embolectomy were retrospectively reviewed. Diagnosis was made in six patients with magnetic resonance imaging (MRI) with optional digital subtraction angiography (DSA), while computed tomography (CT) and DSA were used in one patient with an implanted pacemaker. Clinical outcomes, including recanalization rate, recanalization time, complications, modified Rankin scale (mRS) at 3 months, and National Institute of Health Stroke Scale (NIHSS) score improvement at 1 month were evaluated.
Results
Complete recanalization was obtained in seven patients (100 %). Median recanalization time from symptom onset and from start of surgery was 402 and 40 min, respectively. All seven patients showed severe left ventricular hypertrophy (LVH) according to an increased cardiothoracic ratio (CTR) ≥50 %. Complications included recurrence of cardioembolic stroke with the right middle cerebral artery occlusion, minimal expansion of infarction, and aggravation of heart failure, each in one patient, respectively. Four (57.1 %) patients had a history or postoperative recurrence of cardioembolic stroke. Median NIHSS at 1 month was 2 (range, 0–30). Median mRS at 3 months was 2 (range, 0–5). Five patients (71.4 %) had a favorable outcome (mRS2).
Conclusions
Cervical surgical embolectomy for acute extracranial ICA occlusion resulted in a high complete recanalization rate with an acceptable safety profile. A possible association between severe cardiac illness and huge embolus occluding proximal large artery was suggested.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Acute ischemic stroke due to the internal carotid artery (ICA) occlusion is associated with dense neurological deficits, and intravenous administration of tissue plasminogen activator (IV t-PA) often fails to achieve recanalization [6, 21]. Recently, the effectiveness of a combination of IV t-PA and endovascular treatments has been reported [7, 8, 12]. However, most studies of extracranial ICA occlusion include various pathophysiologies such as atherosclerosis, dissection, and cardiogenic embolism. Among them, extracranial ICA occlusion due to cardiogenic embolism is rare, and a study of numerous cases is lacking. We present our experience with seven consecutive patients of extracranial ICA occlusion due to cardiogenic embolism, all of whom underwent emergent cervical surgical embolectomy.
Materials and methods
Patient selection
Between November 2007 and October 2014, 2382 patients who suffered ischemic stroke (887 embolic and 1495 atherosclerotic stroke) were admitted to Fuji Brain Institute and Hospital. We defined any cardiogenic embolic ischemia and major intracranial arterial occlusion by artery-to-artery emboli from unstable carotid plaque as embolic stroke. We also defined lacunar stroke, atherosclerotic major arterial stroke, and microembolic spray from unstable carotid plaque as atherosclerotic stroke.
We identified seven patients with diagnosis of cardioembolic extracranial ICA occlusion out of 2382 acute ischemic stroke patients. All the seven patients underwent emergent cervical surgical embolectomy. Under institutional review board approval, we retrospectively reviewed the medical charts of the seven consecutive patients. Clinical and radiographic data were analyzed in detail.
National Institute of Health Stroke Scale (NIHSS) scores upon initial presentation and 1 month after the ictus were then evaluated. We proceeded with surgical embolectomy according to the magnetic resonance angiography (MRA)-diffusion-weighted imaging (DWI) mismatch [14]. In a patient with an implanted pacemaker, occlusion of the extracranial ICA was confirmed with digital subtraction angiography (DSA) as well as negative early computed tomography (CT) sign [10]. The decision to perform cervical surgical embolectomy was based on a clinical diagnosis of acute embolic occlusion of the extracranial ICA with a dense focal deficit, such as severe hemiparesis and severe aphasia, and the absence of acute hyperintensity on DWI or low density area on CT involving more than one-third of the middle cerebral artery (MCA) territory. Informed consent to undergo cervical surgical embolectomy was obtained from the patient’s family in all cases.
Operation
The actual surgical procedure is described in detail in a case report [13]. In short, an S-shaped curvilinear skin incision was made to expose the carotid bifurcation. As soon as the common carotid artery (CCA) was exposed, we immediately clamped the proximal portion of the CCA to prevent distal migration of the embolus. A longitudinal incision was made to resect the embolus, and a FURUI’s Double-Balloon Internal Shunt device (Inter Medical Co., Ltd, Japan) was selected. The arteriotomy was sutured with 6–0 nylon. Continuous administration of heparin 5000 units/day was started immediately after the admission to the intensive care unit after the procedure, and warfarin was started on postoperative day 1 with continuation of heparin 10,000 units/day until the prothrombin time-international normalization ratio (PT-INR) was in the range of 2 to 3.
Postoperative evaluation and follow-up
CT was performed several times to assess for hemorrhagic events or expansion of ischemic lesions, usually immediately after the operation, on postoperative days 1, 2, and 7. Follow-up magnetic resonance imaging (MRI) studies including DWI and MRA were performed immediately after the operation in five cases and on day 1 in one case. In a patient with an implanted pacemaker, we performed CT and DSA immediately after the procedure instead. Final recanalization status was assessed according to the Thrombolysis In Myocardial Infarction (TIMI) scale [9]. Functional outcomes were evaluated at approximately 1 month with NIHSS score, and at approximately 3 months via the modified Rankin scale (mRS) after a review of medical records. Warfarin was continued on an outpatient basis to all patients achieving a PT-INR at 2–3.
Results
Patient characteristics are summarized in Table 1. The seven patients included four women and three men with a median age of 78 years (range, 63–86 years). The median presenting NIHSS score was 8 points (range, 0–26). A patient with an initial NIHSS score of 0 presented with monocular blindness only (patient 5). A detailed clinical description of this case was reported in a case report [13]. Six of seven patients had atrial fibrillation (Af). Two patients had a history of cardiac surgery for atrial septal defect (ASD), one of whom also had an implanted pacemaker. One patient required cardiac surgery for severe valvular disease after discharge (patient 3). All seven patients showed left ventricular hypertrophy (LVH) in their chest X-ray as suggested by a cardiothoracic ratio (CTR) above 50 %. Median CTR was 62 (range, 53–81). All patients experienced complete recanalization (TIMI 3) as confirmed by postoperative MRA or DSA. Time to recanalization from onset varied with a median of 402 min (range, 255–1089 min). Median time from the start of the surgery until the recanalization was 40 min (range, 19–131 mins).
There were three instances of major complications (three cases, 42.9 %): recurrence of infarction by cardiogenic embolism, expansion of infarction, and aggravation of heart failure (one patient [14.3 %] with each respective complication). There was no postoperative intracranial bleeding. Patient 1, who presented with an initial NIHSS score of 26, regained consciousness without apparent paresis according to bedside examination, but this patient acutely deteriorated secondary to recurrent cardiogenic embolism in the right MCA on postoperative day 5 despite anticoagulation with warfarin. This patient had been bedridden (mRS 5), and she died 3 years later due to stomach cancer. Postoperative DWI in patient 3 showed minute expansion of high-intensity lesions. Patient 4 developed dyspnea on the night on postoperative day 2. Aggravation of heart failure necessitated transfer to the cardiology service.
Four (57.1 %) of seven patients had a previous history or postoperative recurrence of stroke attributed to cardiogenic embolism. As described above in patient 1, cardiogenic embolus occluded the right MCA, and resulting symptoms were severe (mRS 5). Patient 3 had a past history of emergent surgical embolectomy for right ICA terminus occlusion 17 months prior. Patient 4 had a history of cerebral infarction 20 years previously. Patient 5 also had a three-time history of cerebral infarction. All of these patients were medicated with antithrombotic agents when the ictus occurred: three (75 %) of four patients were medicated with warfarin, and the other patient was on ticlopidine.
Median NIHSS at 1 month was 2 (range, 0–30). Median mRS at 3 months was 2 (range, 1–5). Five patients (71.4 %) had favorable outcomes (mRS≤2).
Pathological studies were performed on resected emboli from five patients. None of the specimens contained the intima. Three specimens (patients 2, 4, and 6) were observed to be simple fibrin thrombi. The other two specimens (patients 1 and 5) were fresh mixed thrombus of aggregated platelets, polymorphonuclear leukocytes, fibrin, and red blood cells without organization.
Actual case illustration is shown in Figs. 1 and 2.
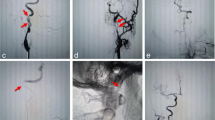
Case illustration of patient 4 (a–e). An 85-year-old woman with a history of surgery for atrial septal defect, pacemaker implantation for bradycardia, and cerebral infarction presented with National Institute of Health Stroke Scale (NIHSS) 9. Computed tomography (CT) denied massive low-density areas (a). Digital subtraction angiography (DSA) shows occlusion of the left internal carotid artery (ICA) (b), and fair collateral flow via the anterior communicating artery (c). Postoperative DSA shows complete recanalization (d). Postoperative CT shows no obvious expansion of low-density areas (e), and she made modified Rankin scale (mRS)-0 recovery
Case illustration of patient 6 (a–e). An 86-year-old woman with a history of surgery for atrial septal defect presented with left hemiparesis with her NIHSS score was 16. Magnetic resonance images (MRI) showed only minimal diffusion-high lesions (a), and occlusion of the right extracranial ICA (b and c). Postoperative MRI showed complete recanalization (d) and no expansion of ischemic lesions (e). She made mRS-2 recovery
Discussion
Extracranial ICA occlusion due to cardiogenic embolism
This study showed that cervical surgical embolectomy was associated with a high TIMI 3 recanalization rate (100 %). In patients with acute ischemic stroke with proximal vessel occlusion, recanalization is the strongest predictor of good outcome [2, 3]. However, a more proximal the site of occlusion is associated with a lower the rate of recanalization in response to IV t-PA. Indeed, the rate of recanalization in the proximal or terminal internal carotid artery is less than 10 % [6, 20]. Especially in the setting of the extracranial ICA occlusion, IV t-PA reduces the proportion of patients dependent in activities of daily living but also increases the probability of death and any intracranial bleeding [17]. It is not yet clear whether endovascular treatments are superior to IV t-PA, and neither a combination of endovascular treatments and IV t-PA is [18]. Further, most studies regarding ICA occlusion do not distinguish the treatment or the results according to whether the pathophysiology is atherosclerotic or cardioembolic. Acute extracranial ICA occlusion of cardioembolic origin is quite rare, and there are only limited studies of carotid recanalization in patients with cardiogenic emboli [16].
Association of a huge embolus with a heart disease
In our study, six (85.7 %) of seven patients had a history of Af, which was detected on electrocardiogram at admission or which had been described by the medical record or the patients’ family. The other patient had a history of aortic valve replacement, and medicated with dabigatran etexilate, both of which suggested a possibility of cardiogenic embolism. Four (57.1 %) of seven patients had remarkable cardiac illness, such as a history of valve replacement, surgery for ASD, or severe combined valvular disease that necessitated elective cardiac surgery. Further, all seven patients had a CTR > 50 %, suggesting the present of severe LVH. Our suggestion is that more severe heart illness correlates with a larger embolus. This notion is supported by observations from several studies. Indeed, one echocardiographic study concluded that the size of the left ventricle is the strongest predictor of left atrial appendage thrombus [4]. Such thrombus could have migrated to the carotid arteries in our series. In the diagnosis and treatment of patients with Af and LVH, clinicians should consider whether intracardiac emboli may have occluded the carotid arteries. In their study of cardioembolic stroke due to non-valvular atrial fibrillation, Sadahiro et al. reported that patients with distal artery occlusion had a significantly higher rate of detection of left atrial thrombus than those with more proximal main trunk occlusion [19]. They concluded that fragmental embolization is associated with distal artery occlusion, and therefore, remnant emboli in the left atrium are more commonly detected. Their findings are consistent with our assumption that larger embolus is less likely to have been fragmented and can lodge in more proximal, large arteries. Indeed, intracardiac thrombus was detected in nobody in the present study, implying no fragmentation of the thrombus. All of the patients in our study had severe heart illness, as demonstrated by their CTR, which may be associated with giant embolus that can lodge in the extracranial ICA.
Association of recurrence of cardiogenic embolic stroke with heart disease
The present series showed a high rate of stroke recurrence (four patients; 57.1 %). Moreover, all four of these patients had been taking antithrombotic agents when the recurrence occurred (warfarin, n = 3; ticlopidine, n = 1). Although unsatisfactory achievement of therapeutic PT-INR might have led to stroke recurrence, the recurrence rate even under anticoagulation therapy is quite high compared with previous studies (1–22 %) [1]. Generally, risk factors for cardioembolic stroke recurrence include alcohol abuse, hypertension with valvular heart disease, Af, nausea and vomiting, and previous cerebral infarction [1]. Especially in a study of Af patients without mitral stenosis or prosthetic valves, female sex, underlying heart disease, and left atrial size greater than or equal to 4.0 cm were associated with an increase in the risk of embolization, including cerebral and peripheral embolic events [5]. Left atrial enlargement is a pathophysiologic response to volume and pressure overload that is associated with various cardiovascular disorders leading to left ventricular dysfunction [15]. Thus, excessive LVH as evaluated with significant increase in CTR accompanying Af may be a strong predictor for cardioembolic stroke recurrence.
Possible advantages of surgical embolectomy compared to endovascular treatments
Endovascular treatment with proximal protection should be an adequate alternative treatment for extracranial ICA occlusion, but no study has focused on endovascular embolectomy in the extracranial ICA. Some studies of endovascular recanalization of extracranial ICA occlusion report that successful recanalization was obtained in 77.3–94 % [7, 11, 12]. Simple comparison of these recanalization rates with that of the present study is not suitable for at least two reasons. First, most of these studies included various stroke pathophysiology types (e.g., atherosclerosis, dissection, or cardiogenic embolism). Further, recanalization endpoints in these studies often included both partial and complete recanalization.
Nevertheless, we obtained satisfactory results compared to that of endovascular treatments from the standpoints of high rate of complete recanalization and patients’ favorable outcome possibly for the following reasons. First, surgical embolectomy could be advantageous in obtaining complete recanalization than endovascular treatments because rigid and swift clamp of the proximal portion without touching the more distal emolus-related dangerous part can prevent distal migration or fragmentation of the embolus more safely. Successful complete recanalization without distal embolism might have contributed to higher proportion of the favorable outcome of the patients. Second, these seven patients might have had satisfactory collaterals to endure ischemia until the treatment and recanalization. Indeed, head MRA or DSA of all the patients demonstrated the MCA in the diseased side, implying fair collateral blood flow via the anterior communicating artery and/or the posterior communicating artery.
Meanwhile, endovascular treatments might be advantageous when compared with open embolectomy from the standpoint of time, as treatments can be done as soon as the diagnosis is made. Either open embolectomy or endovascular treatments should be considered according to the circumstances of man power, endovascular staff, and so on.
Limitations of the present study
This study has several limitations. For example, this is a retrospective study, but extracranial ICA occlusion due to cardiogenic embolus is quite rare, making it difficult to perform a randomized controlled study. Due to the same reason, a simple comparison between open embolectomy and endovascular treatments is unrealistic.
Conclusions
Emergent cervical embolectomy in extracranial ICA occlusion was feasible in seven patients with a satisfactory complete recanalization rate (100 %) and an acceptable safety profile (no intracranial hemorrhage). All the patients demonstrated severe LVH. A potential association between severe cardiac illness and proximity of the occluded site and/or cardioembolic stroke recurrence is suggested.
References
Arboix A, Alio J (2012) Acute cardioembolic cerebral infarction: answers to clinical questions. Curr Cardiol Rev 8:54–67
Bhatia R, Hill MD, Shobha N, Menon B, Bal S, Kochar P, Watson T, Goyal M, Demchuk AM (2010) Low rates of acute recanalization with intravenous recombinant tissue plasminogen activator in ischemic stroke: real-world experience and a call for action. Stroke 41:2254–2258
Bhatia R, Shobha N, Menon BK, Bal SP, Kochar P, Palumbo V, Wong JH, Morrish WF, Hudon ME, Hu W, Coutts SB, Barber PA, Watson T, Goyal M, Demchuk AM, Hill MD (2014) Combined full-dose IV and endovascular thrombolysis in acute ischaemic stroke. Int J Stroke 9:974–979
Boyd AC, McKay T, Nasibi S, Richards DA, Thomas L (2013) Left ventricular mass predicts left atrial appendage thrombus in persistent atrial fibrillation. Eur Heart J Cardiovasc Imaging 14:269–275
Cabin HS, Clubb KS, Hall C, Perlmutter RA, Feinstein AR (1990) Risk for systemic embolization of atrial fibrillation without mitral stenosis. Am J Cardiol 65:1112–1116
Christou I, Felberg RA, Demchuk AM, Burgin WS, Malkoff M, Grotta JC, Alexandrov AV (2002) Intravenous tissue plasminogen activator and flow improvement in acute ischemic stroke patients with internal carotid artery occlusion. J Neuroimaging 12:119–123
Dalyai RT, Chalouhi N, Singhal S, Jabbour P, Gonzalez LF, Dumont AS, Rosenwasser R, Ghobrial G, Tjoumakaris SI (2013) Stent-assisted endovascular recanalization of extracranial internal carotid artery occlusion in acute ischemic stroke. World Neurosurg 79:143–148
Duijsens HM, Spaander F, van Dijk LC, Treurniet FE, Keunen RW, Mosch A, Majoie CB, van Overhagen H (2014) Endovascular treatment in patients with acute ischemic stroke and apparent occlusion of the extracranial internal carotid artery on CTA. J Neurointerv Surg. doi:10.1136/neurintsurg-2014-011297
Ganz W (1985) The thrombolysis in myocardial infarction (TIMI) trial. N Engl J Med 313:1018
Hacke W, Kaste M, Fieschi C, von Kummer R, Davalos A, Meier D, Larrue V, Bluhmki E, Davis S, Donnan G, Schneider D, Diez-Tejedor E, Trouillas P (1998) Randomised double-blind placebo-controlled trial of thrombolytic therapy with intravenous alteplase in acute ischaemic stroke (ECASS II). Second European-Australasian Acute Stroke Study Investigators. Lancet 352:1245–1251
Hauck EF, Natarajan SK, Ohta H, Ogilvy CS, Hopkins LN, Siddiqui AH, Levy EI (2011) Emergent endovascular recanalization for cervical internal carotid artery occlusion in patients presenting with acute stroke. Neurosurgery 69:899–907, discussion 907
Jovin TG, Gupta R, Uchino K, Jungreis CA, Wechsler LR, Hammer MD, Tayal A, Horowitz MB (2005) Emergent stenting of extracranial internal carotid artery occlusion in acute stroke has a high revascularization rate. Stroke 36:2426–2430
Kiyofuji S, Inoue T, Shigeeda T, Sugiura T, Tamura A, Saito I (2015) Emergent cervical surgical embolectomy to rescue total monocular blindness due to simultaneous cervical internal and external carotid artery occlusion by cardiogenic emboli. Surg Neurol Int 6:29
Lansberg MG, Thijs VN, Bammer R, Olivot JM, Marks MP, Wechsler LR, Kemp S, Albers GW (2008) The MRA-DWI mismatch identifies patients with stroke who are likely to benefit from reperfusion. Stroke 39:2491–2496
Lupu S, Mitre A, Dobreanu D (2014) Left atrium function assessment by echocardiography—physiological and clinical implications. Med Ultrason 16:152–159
Murata T, Horiuchi T, Nitta J, Sakai K, Ogiwara T, Kobayashi S, Hongo K (2010) Urgent open embolectomy for cardioembolic cervical internal carotid artery occlusion. Neurosurg Rev 33:341–348, discussion 348
Paciaroni M, Balucani C, Agnelli G, Caso V, Silvestrelli G, Grotta JC, Demchuk AM, Sohn SI, Orlandi G, Leys D, Pezzini A, Alexandrov AV, Silvestrini M, Fofi L, Barlinn K, Inzitari D, Ferrarese C, Tassi R, Tsivgoulis G, Consoli D, Baldi A, Bovi P, Luda E, Galletti G, Invernizzi P, DeLodovici ML, Corea F, Del Sette M, Monaco S, Marcheselli S, Alberti A, Venti M, Acciarresi M, D’Amore C, Macellari F, Lanari A, Previdi P, Gonzales NR, Pandurengan RK, Vahidy FS, Sline M, Bal SS, Chiti A, Gialdini G, Dumont F, Cordonnier C, Debette S, Padovani A, Cerqua R, Bodechtel U, Kepplinger J, Nesi M, Nencini P, Beretta S, Trentini C, Martini G, Piperidou C, Heliopoulos I, D’Anna S, Cappellari M, Donati E, Bono G, Traverso E, Toni D (2012) Systemic thrombolysis in patients with acute ischemic stroke and Internal Carotid ARtery Occlusion: the ICARO study. Stroke 43:125–130
Paciaroni M, Inzitari D, Agnelli G, Caso V, Balucani C, Grotta JC, Sarraj A, Sung-Il S, Chamorro A, Urra X, Leys D, Henon H, Cordonnier C, Dequatre N, Aguettaz P, Alberti A, Venti M, Acciarresi M, D’Amore C, Zini A, Vallone S, Dell’Acqua ML, Menetti F, Nencini P, Mangiafico S, Barlinn K, Kepplinger J, Bodechtel U, Gerber J, Bovi P, Cappellari M, Linfante I, Dabus G, Marcheselli S, Pezzini A, Padovani A, Alexandrov AV, Shahripour RB, Sessa M, Giacalone G, Silvestrelli G, Lanari A, Ciccone A, De Vito A, Azzini C, Saletti A, Fainardi E, Orlandi G, Chiti A, Gialdini G, Silvestrini M, Ferrarese C, Beretta S, Tassi R, Martini G, Tsivgoulis G, Vasdekis SN, Consoli D, Baldi A, D’Anna S, Luda E, Varbella F, Galletti G, Invernizzi P, Donati E, De Lodovici ML, Bono G, Corea F, Sette MD, Monaco S, Riva M, Tassinari T, Scoditti U, Toni D (2014) Intravenous thrombolysis or endovascular therapy for acute ischemic stroke associated with cervical internal carotid artery occlusion: the ICARO-3 study. J Neurol. doi:10.1007/s00415-014-7550-1
Sadahiro H, Inamura A, Ishihara H, Kunitsugu I, Goto H, Oka F, Shirao S, Yoneda H, Wada Y, Suzuki M (2014) Fragmental or massive embolization in cardiogenic stroke caused by nonvalvular atrial fibrillation. J Stroke Cerebrovasc Dis 23:63–68
Saqqur M, Uchino K, Demchuk AM, Molina CA, Garami Z, Calleja S, Akhtar N, Orouk FO, Salam A, Shuaib A, Alexandrov AV (2007) Site of arterial occlusion identified by transcranial Doppler predicts the response to intravenous thrombolysis for stroke. Stroke 38:948–954
Smith WS, Lev MH, English JD, Camargo EC, Chou M, Johnston SC, Gonzalez G, Schaefer PW, Dillon WP, Koroshetz WJ, Furie KL (2009) Significance of large vessel intracranial occlusion causing acute ischemic stroke and TIA. Stroke 40:3834–3840
Conflicts of interest
None.
Author information
Authors and Affiliations
Corresponding author
Additional information
Comment
The authors describe the open surgical embolectomy of extra-cranial ICA after acute occlusion due to cardiac source large embolus. From a large cohort of stroke patients, seven patients were selected who met this specific criteria. They achieved 100 % re-canalization rate and four patients had recurrent emboli with a median NHISS score of 2 and median mRS score of 2. Considering the fact that without this intervention, the only chance of a good outcome in a symptomatic patient is a robust collateral circulation, this procedure should be considered among the treatment arm for this particular subset of stroke patients. A direct comparison of surgical embolectomy to endovascular treatment is difficult due to limited number of patients in this specific setting but ultimately one could argue that all of these patients would benefit from a rapid endovascular recanalization even in very proximal ICA occlusions.
Amir Dehdashti
NY, USA
Rights and permissions
About this article
Cite this article
Kiyofuji, S., Inoue, T., Tamura, A. et al. Emergent cervical surgical embolectomy for extracranial internal carotid artery occlusion. Acta Neurochir 157, 1313–1319 (2015). https://doi.org/10.1007/s00701-015-2478-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00701-015-2478-5