Abstract
Motor impairment is the most common complication after stroke, and recovery of motor function has been shown to be dependent on the extent of lesion in the ipsilesional corticospinal tract (iCST) and activity within ipsilesional primary and secondary motor cortices. However, work from neuroimaging research has suggested a role of the contralesional hemisphere in promoting recovery after stroke potentially through the ipsilateral uncrossed CST fibers descending to ipsilateral spinal segments. These ipsilateral fibers, sometimes referred to as “latent” projections, are thought to contribute to motor recovery independent of the crossed CST. The aim of this paper is to evaluate using cumulative evidence from animal models and human patients on whether an uncrossed CST component is present in mammals and conserved through primates and humans, and whether iCST fibers have a functional role in hemiparetic/hemiplegic human conditions. This review highlights that an ipsilateral uncrossed CST exists in human during development, but the evidence on a functionally relevant iCST component in adult humans is still elusive. In addition, this review argues that whereas activity within the ipsilesional cortex is essential for enhancing motor recovery after stroke, the role of iCST projections specifically is still controversial. Finally, conclusions from current literature emphasize the importance of activity in the ipsilesional cortex and the integrity of crossed CST fibers as major determinants of motor recovery after brain injury.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
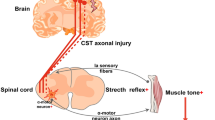
The outflow of the motor cortex to the spinal cord—the corticospinal tract (CST)—drives voluntary motor function predominantly through contralateral projections. Fibers from the primary motor cortex descend through the posterior limb of the internal capsule into the cerebral peduncles forming the pyramidal tract (PT), decussate at the level of the caudal medulla, and descend primarily in the dorsolateral funiculi of the spinal cord (Fig. 1a). Anatomical studies mapping CST connectivity in mammals provided evidence of ipsilateral CST (iCST) projections that descend in the lateral or ventral funiculi of the spinal cord of cats [1, 2] and monkeys [3, 4] (Fig. 1b–d), or that are components of re-crossed contralateral fibers [4] (Fig. 1e). iCST projections are sometimes referred to as “latent” projections with the implicit hypothesis that these projections do not contribute to motor function in the presence of intact contralateral projections, but their role in cortical motor control may arise after a lesion to the crossed CST projections [5]. In fact, unilateral lesions of the CST are associated with significant motor recovery which may suggest the existence or emergence of iCST projections in mammalian models [1,2,3,4] and human patients [6, 7]. However, this concept is challenged by evidence implicating subcortical brainstem descending pathways in the restoration of function after CST lesion especially in the event of bilateral pyramidotomy in monkeys [8, 9]. A potential role of iCST in recovery from injury would provide a rationale for targeting these fibers to modulate the recovery process.
Probable trajectories for contralateral ipsilateral corticospinal projections into spinal interneurons. a Most of CST fibers cross at the caudal medulla and descend through dorsolateral column. b A smaller component of crossed CST fibers descend in the ventral column. c Ipsilateral CST fibers uncrossed at the level of caudal medulla and descend through ipsilateral anterior and dorsolateral columns. d Some crossed CST projections may re-cross the midline and descend ipsilaterally different spinal levels. e Ipsilateral axonal innervation from crossed CST fibers through axonal collaterals that re-cross the midline and innervate the opposite hemicord
Using a variety of neuroanatomical and neurophysiological techniques, studies in rodents, cats, monkeys, and humans have significantly advanced our understanding of the neuroanatomical trajectory of the CST. However, no recent studies have reviewed and evaluated both the preclinical and clinical evidence for the presence and functional relevance of iCST projections. The aim of this manuscript is to review the neuroanatomical trajectory and functional relevance of iCST fibers in mammalian species, including human, and how these findings are implicated in neurorehabilitation after brain injury, mainly in the context of cerebral palsy and stroke. It is necessary to emphasize that discussing the role of the ipsilateral hemisphere in recovery from stroke is beyond the scope of this review and has been extensively reviewed [10, 11].
Major Theories on iCST
A common concept in neurodevelopment is that spinal neurons are innervated by bilateral CST projections that get refined during motor development creating a dominant contralateral system [12, 13]. A remnant of ipsilateral projections persists during adulthood and may still contribute to motor functions [3, 14,15,16,17]. In children with congenital hemiplegia, the leading hypothesis is that the developmental machinery is adaptively altered to favor bilateral innervation of spinal neurons from the intact hemisphere and a functional contribution of iCST fibers [18]. Similarly, after brain injury in adults, it is possible that the loss of contralateral CST fibers results in the strengthening of existing iCST fibers or emergence of new iCST collaterals that may contribute to recovery [19,20,21]. However, this possibility is still controversial with the counter-argument suggesting minimal or no role of iCST fibers in normal or compensatory motor activity after brain injury [7, 22,23,24,25,26,27,28].
iCST in non-primates
Preclinical studies provide a unique opportunity to use invasive approaches such as lesion studies and fiber tracing, to determine whether an iCST is evolutionary conserved, and to closely investigate the adaptation of CST projections after injury. Early studies by Armand et al. reported on subtotal transection in the cat spinal cord that spared the lateral or ventral funiculus from one side followed by HRP injection at cortical or spinal levels to anterogradely and retrogradely map motor cortical projections [1, 29]. Anterograde labeling of sections at cervical and lumbar enlargements demonstrated that regions within area 4 (M1) either projected contralaterally through the lateral funiculus or bilaterally through both lateral and ventral funiculi [1]. Around 92% of fibers in the dorsolateral funiculus were derived from contralateral area 4 while the remaining fibers were originating ipsilaterally [29]. Subsequent evidence using WGA-conjugated HRP confirmed that the sensorimotor cortex of cats projects to the bilateral ventral and lateral funiculi [2, 30]. A similar pattern of dominant contralateral CST and a minor iCST was also described in guinea pigs and rats [31]. However, conclusive evidence from these studies is challenged by several limitations. First, HRP may bleed into un-intended spinal segments after injection resulting in false positive findings. Additionally, the use of WGA-HRP, a trans-synaptic tracer, does not eliminate the possibility that the origin of ipsilateral labeling is subcortical nuclei rather than an iCST or that ipsilateral projections are “re-crossed” branching collaterals of initially crossed fibers [2, 9]. Another major concern in animal studies is that the separation of sensory and cortical motor components is difficult to establish [32].
In a thorough investigation of the iCST in adult rats, biotinylated dextran amine (BDA) injection into the right sensorimotor cortex bilaterally labeled terminals in the thalamic, mesencephalic, and pontine nuclei [33]. At the spinal level, BDA was predominantly present in the contralateral dorsolateral funiculus with a smaller ipsilateral fraction in the dorsolateral funiculus, and, occasionally, in the ventral funiculus. No bifurcating axons were detected on sagittal sections of the medulla, suggesting that uncrossed components cannot be explained by bifurcation of crossed CST axons [33]. The majority of iCST fibers terminated in Rexed lamina III-VI, covering predominantly interneurons and sensory nuclei [34]; however, this pattern is similar to that of contralateral CST in rodents that do not terminate directly at motor neurons (lamina IX).
Neurophysiological techniques including intra-cortical micro-stimulation and transcranial magnetic stimulation (TMS) were used to probe the physiological relevance of the iCST; however, they fail to establish evidence of an anatomically distinct iCST [35, 36] (Table 1).
iCST in non-human Primates
Non-human primates provide a valuable model to test whether an iCST is conserved in the mammalian hierarchy. Early observations by Hoff et al. showed that unilateral lesions to the PT resulted in extensive bouton degeneration in the contralateral spinal gray matter; however, minor degeneration was observed within the dorsal and ventral horns of ipsilateral gray suggesting the presence of ipsilateral pyramidal projections [37].
Subsequent neuroanatomical and neurophysiological studies characterized those ipsilateral projections regarding quantity, trajectory, termination, and functional relevance. Fiber tracing of spinal neurons in rhesus monkeys mapped cortical projections from area 4 and indicated the presence of iCST fibers that terminated predominantly at spinal laminae VIII [4, 15, 38].
Studies in rhesus monkeys tracing fibers descending from M1 showed that around 85–98% of CST fibers descended contralaterally in the dorsolateral funiculus, <1% descended contralaterally in the ventromedial funiculus, 2–15% descended ipsilaterally in the dorsolateral funiculus, and 2% descended ipsilaterally in the ventromedial funiculus (Table 2, Fig. 2) [3, 14, 39,40,41]. Interestingly, evidence for the crossing of ipsilateral fibers at spinal levels was also observed in multiple studies [3, 39, 40]; therefore, the presence of ipsilateral fibers suggested by BDA only indicates that these fibers descend ipsilaterally and there is a high likelihood that these fibers will re-cross and end up innervating contralateral neurons. When the termination patterns of these ipsilateral fibers were assessed, they were found to cover spinal laminae V-IX, with the highest density (~80%) of innervation in ipsilateral lamina VIII, and very sparse termination in ipsilateral lamina IX (Table 2, Fig. 2) [3, 14, 39,40,41]. The consistent finding that the majority of iCST fibers terminate at lamina VIII challenges the functional significance of iCST fibers since lamina VIII mainly harbors commissural interneurons that project through the midline to the contralateral cord, thus largely contributing to contralateral movement control [46]. In addition, few fibers labeled interneurons that project to motor neurons controlling proximal muscles (lamina VII), and only sparse labeling was found to potentially label motor neurons (lamina IX).
Termination patterns of CST fibers descending from the left M1 cortex in monkeys mapped using anterograde tracing. The majority of descending fibers descend contralaterally through the dorsolateral funiculus. Around 2–16% of fibers descend ipsilaterally and terminate predominantly at lamina VII and VIII of the spinal gray
Anterograde tracing of WGA-HRP from the supplementary motor cortex (SMA) in rhesus monkeys revealed that 23% of descending fibers were ipsilateral, descended through the dorsolateral funiculus, and terminated in similar patterns to ipsilateral M1 projections (mainly laminae VII-VIII) [14]. Findings in rhesus monkeys were replicated in marmoset monkeys where 11% of M1 CST fibers descended ipsilaterally through the dorsolateral funiculus and terminated in laminae VII-VIII [42]. These studies suggest that ipsilateral M1 may partake in the control of proximal and potentially distal muscles. A major limitation of quantitative studies with labeled tracers is the inability to label all fibers at the injection sites, which may result in variability of the relative proportions of different CST components.
The functional relevance of iCST fibers was investigated using in vivo micro-stimulation of the M1 cortex, SMA, and pyramidal neurons. Stimulation of M1 or SMA cortices while recording from both distal and proximal forearm muscles revealed that the vast majority of M1 and SMA outputs were contralateral [43,44,45]. However, a significant ipsilateral response was predominantly recorded in proximal or truncal muscles upon stimulation of SMA [44, 45], and, occasionally, M1 [43]. In vivo cortical stimulation studies performed in rhesus [24] and marmoset monkeys [42] showed that ipsilateral M1 projections are not monosynaptic. Although the lack of monosynaptic connection is not unanticipated given that the majority of contralateral fibers also do not monosynaptically connect to motor neurons, the presence of polysynaptic connections does not rule out the involvement of the contralateral hemisphere or contralateral brainstem nuclei upon stimulation of ipsilateral cortical regions. In fact, stimulation of the PT in rhesus monkeys elicited clear excitatory post-synaptic potentials in contralateral forearm spinal motor neurons, but failed to induce any ipsilateral responses [24]. Similarly, EMG recordings in forearm muscles demonstrated only contralateral MEPs in response to PT stimulation prior to the decussation. Stimulation of the forearm sites of M1 in awake monkeys resulted in EMG responses and behavioral movements in contralateral forearm muscles only [24]. These results have emphasized that the physiological role of motor cortical stimulation in eliciting responses in ipsilateral muscles is predominantly mediated by polysynaptic connections that do not necessarily involve ipsilateral PT.
Evidence of Reorganization of CST after Injury in Animal Models
Following unilateral lesion to the motor cortex or the PT proximal to decussation in rodents, sprouting axons from the crossed contralateral fibers re-innervate denervated neurons in a compensatory mechanism [47, 48]. This compensatory mechanism is not due to originally uncrossed CST at the pyramidal level that remained unchanged after injury, but due to re-crossing of contralateral fibers at the spinal level [47]. Supporting these findings, unilateral lesions proximal to the CST decussation failed to induce deficit at the ipsilateral limb, and iCST fibers failed to rescue limb function after contralateral pyramidal lesions [49]. Simultaneously, electrical stimulation of affected hemisphere after stroke in rodent model demonstrated improved recovery of skilled functions through promoting axonal sprouting at the subcortical (red nucleus) and spinal levels [9, 50]. However, controversial findings still suggest a role of iCST in recovery from injury. For instance, damage to the motor cortex in rodents can cause deficits in skilled movements ipsilaterally that was associated with increased synaptic plasticity in the undamaged hemisphere [51]. In addition to the sprouting of crossed CST fibers from undamaged neurons in ipsilateral hemisphere, axonal sprouting from the intact motor systems to cover denervated neurons after injury has been described in rodents [52]. Recently, Wahl et al. demonstrated that originally crossed CST fibers from the undamaged hemisphere re-cross at the spinal level to cover denervated neurons initially innervated by the damaged hemisphere [53]. This study used a unique pharmacogenomics approach to silence CST fibers terminating ipsilaterally and demonstrated a significant reduction in EMG response in the ipsilateral limbs and reappearance of functional deficits originally ameliorated by rehabilitation-induced plasticity [53]. Although these findings support a role of the undamaged hemisphere in plasticity after stroke that includes axonal sprouting to cover the ipsilateral neuronal territory, it does not support the presence of latent iCST that gets engaged after stroke.
Studies in primates have supported the contribution of axonal sprouting to recovery after stroke and showed that inhibition of neurite outgrowth inhibitor (NoGo) enhanced compensatory sprouting and improvement motor recovery [54, 55]. Compensatory recovery mechanisms are most prominent in brainstem circuitry. For example, after bilateral pyramidectomy, monkeys were still able to carry out proximal and distal muscle movement [8], pointing to the role of non-pyramidal projections in the recovery of motor performance after an injury to PT in primates. These early findings were further supported by recent work from Zaaimi et al. showing that after a unilateral lesion to the PT, PT stimulation did not result in activity in ipsilateral motor neurons indicating a negligible remodeling in the iCST system 6 months after a unilateral PT lesion [56]. The study demonstrated a limited role for iCST fibers in recovery after unilateral PT lesions and a significant compensatory role of reticulospinal systems in recovery [56].
Evidence of iCST in Healthy Human Subjects
Similar to observations in monkeys, Nathan et al. studied post-mortem spinal cord sections from subjects with supra-spinal lesions and examined the location of degenerating axons in the spinal cord [57]. In one subject with large right middle cerebral artery infarct, degenerating axons were observed both in the left CST and in the right anterior CST, but barely observed in the left anterior CST hinting that an anterior iCST might be present in humans [57]. However, due to limitations of the specificity of this approach, solid evidence supporting the presence of iCST that do not decussate at the medulla in humans was still lacking.
Beyond early neuroanatomical studies, studies investigating the presence of an iCST in healthy human subjects consistently used transcranial magnetic stimulation (TMS) or intra-cortical micro-stimulation (ICMS) to suggest the presence of these fibers. It was suggested that an iCST is more likely to control proximal and truncal muscles rather than the distal muscles since the latter tend to be more severely affected by stroke involving the motor systems and tend to recover last [58]. However, both proximal and distal muscles have been investigated using cortical stimulation and recording of MEP to assess the presence, relative delay, and relative amplitude of ipsilateral muscle responses compared to the contralateral responses (Table 3). Findings from these studies have shown that after TMS stimulation of the dominant hemisphere, bilateral MEPs were detected predominantly in proximal and truncal muscles and were delayed (2–9 ms) and lower in amplitude (10–80%) when compared to contralateral responses [23, 59,60,61,62,63,64, 66]. Similar findings were also reported after ICMS of motor cortex during functional mapping in epileptic patients [67] or intra-operative monitoring during spinal surgeries [68]. In one study, Muller et al. studied the development of the CST innervation and demonstrated that children ≤9 years of age have bilateral MEPs in the FDI, biceps brachii, and brachioradialis muscles after unilateral motor cortical stimulation; however, adults showed only contralateral MEPs in the same muscles [6].
Although this approach was most commonly adopted in human studies of healthy subjects, several significant challenges limit the utility of this method to establish the presence of an anatomically independent iCST. Studies using TMS to detect ipsilateral MEPs in muscles are limited by the inability to control focal stimulation of the motor cortex unilaterally without influencing the contralateral hemisphere or subcortical structures. Although the use of small coils has provided better resolution, this limitation is still of major concern, especially in studies that reported very short or absent latency between ipsilateral and contralateral responses [59, 60]. In contrast, a delay that reaches up to 9 ms in ipsilateral MEPs indicates a polysynaptic innervation and may involve either interhemispheric facilitation of the contralateral hemisphere or recruitment of bilateral brainstem circuitry rather than a direct projection of uncrossed ipsilateral fibers. This concern is particularly relevant after the finding that TMS stimulation of M1 in monkeys was able to activate the brainstem directly and independent of M1, a phenomenon that is likely to occur in humans [69]. Thus, TMS studies provide little insight into the neuroanatomical substrate of ipsilateral MEPs detected in muscles. An interesting subject in Ziemann’s study showed complete agenesis of the corpus callosum while still exhibiting bilateral MEPs in his FDI after unilateral motor cortical stimulation [64]. This finding indicates that these bilateral MEPs are independent of inter-hemispheric connections despite that they may still relate to direct stimulation of or bilateral cortical projections to brainstem nuclei. It is also noteworthy that in studies reporting bilateral MEPs after cortical stimulation, a subset of subjects showed only contralateral responses, pointing to the presence of individual variation in the laterality of cortical output [23, 59,60,61,62,63,64, 66].
Re-organization of Motor Cortical Projections in Hemiplegic Human Conditions
Although the contribution of iCST to motor control is controversial, disordered organization of CST connectivity, including abnormal decussation or bilateral projections, has been described in several pathological conditions such as essential mirror movement syndrome, Klippel-Feil disease, and progressive scoliosis [22]. In this section, we review the evidence on whether iCST projections play a functional role after brain injury.
One key determinant of motor cortical re-organization after brain injury is the stage of maturation of CST projections during development [70]. In contrast to adult stroke, children with cerebral palsy (CP) show substantial evidence of bilateral cortical innervation from the undamaged cortex to limb muscles [71]. Stimulation of intact motor cortex in children with CP resulted in bilateral contraction of hand muscles with high synchrony on cross-correlograms that sometimes manifested as mirror movements, suggesting that the two muscles may have received the same presynaptic inputs [72, 73]. Based on studies in cats and monkeys [8, 74], this could potentially be explained by the maintenance of dense bilateral CST projections that are otherwise retracted during development, or by bilateral cortical projections into brainstem nuclei [9, 12, 56, 75].
Studies on the neurodevelopment of the corticospinal in neonates have shown that motor cortical stimulation is associated with fast, short latency ipsilateral responses that occur at a similar threshold to contralateral responses [13]. Ipsilateral responses become smaller and more delayed after 18 months of age; however, short latency ipsilateral responses are only preserved in cerebral palsy (CP) patients who had ischemic insults at various gestational stages [76], but not in adults recovering from brain injury [12, 13, 77]. This points to a developmental shift in CST innervation from bilateral to contralateral innervation during early neonatal developmental window that may be inhibited in neonates with CP, but not in adult subjects with ischemic stroke [13, 77]. Thus, it is hypothesized that ipsilateral innervation after CP is likely due to persistent ipsilaterally descending fibers rather than sprouting of contralateral CST. This hypothesis is supported by findings in animals showing preferential withdrawal of iCST fibers during development of the CST [13, 78]. In addition, neuroanatomical data from post-mortem analysis of children showed significantly faster growth of contralateral compared to iCST [12, 79], and a larger number of iCST fibers with larger axons at the pyramidal level of subjects with CP but not those with unilateral lesions that occur during childhood or adulthood [12, 79]. This data indicates that in CP patients, ipsilaterally projecting fibers are not likely due to spinal collaterals of contralateral fibers, but rather from ipsilateral cortico-fugal projections originating from the ipsilateral motor and supplementary motor cortices. The functional relevance of ipsilaterally projecting motor fibers in CP patients was further investigated in subjects with intractable epilepsy who undergo hemispherectomy (HS) to disconnect the damaged hemisphere (extensively reviewed in [80]). Cortical stimulation of the undamaged hemisphere in CP patients continues to elicit bilateral responses in forearm muscles post-HS similar to pre-surgical responses indicating that these responses are independent of the damaged hemisphere [81, 82]. In a large trial of children undergoing HS, Kupper et al. demonstrated that grasping ability in the ipsilesional hand is only preserved in patients with prenatal or perinatal unilateral brain injury (or CP) and is associated with asymmetric structural connectivity of CST projections. This data suggests the reinforcement of developmentally preserved iCST fibers in these patients [83].
Although cortical re-organization involving abnormal iCST projections is infrequently seen in adult stroke, a margin of neuroplastic changes in both hemispheres may still occur and contribute to recovery [7, 13]. In fact, using functional magnetic resonance imaging (fMRI) during task-related movements of the paretic hand in chronic stroke patients, several studies have reported increased activation in the ipsilateral sensorimotor cortex, premotor cortex, SMA, and occipito-parietal cortex compared to the healthy controls [7, 27, 84]. This functional pattern was also associated with microstructural changes bilaterally in the CST of stroke patients, revealed by structural brain imaging (DTI) [85]. Longitudinal studies in patients recovering from stroke have confirmed a shift in task-related cortical activation from ipsilateral (contralesional) to contralateral (ipsilesional) activation, suggesting the importance of ipsilesional M1 activity in mediating recovery processes [7, 25,26,27,28]. Interestingly, bilateral cortical activity was also negatively correlated with the integrity of the affected CST, a finding that was not always associated with the recovery mechanisms [27, 28, 86]. Temporal analysis using electroencephalography (EEG) has also shown patterns of increased activity ipsilateral to the lesion in stroke patients during voluntary movement [5]. However, the timing of ipsilateral activation on EEG occurred after the onset of movement, discounting its role in the activity of the paretic limb [87].
Cortical functional re-organization and connectivity networks involved in stroke recovery do not appear to be uniform. Indeed, a heterogeneous pattern of cortical reorganization seen in fMRI studies reflected the heterogeneity of ischemic stroke populations [28]. It was further confirmed by positron emission tomography studies in patients with internal capsule infarct where the lesion location and the involvement of striatal structures dictated different patterns of re-organization [88]. The heterogeneity of cortical re-organization seen on brain imaging was also consistent with incongruous findings in neurophysiological experiments using TMS or transcranial direct current stimulation of motor cortices of stroke patients. TMS stimulation over the ipsilateral dorsal premotor cortex, M1, and the superior parietal lobe in patients with recovered motor performance after stroke resulted in significant interference with performance that was not observed in healthy controls [84]. However, TMS stimulation elicited ipsilateral MEPs in proximal muscles of stroke patients but more prevalent in stroke patients with poor recovery suggesting the emergence of the contralesional motor drive [89].
Collectively, studies in patients with CP and stroke have supported the role of ipsilesional primary and secondary motor cortices in rapid and better recovery after stroke. Furthermore, despite that contralateral CST projections are the major determinant for motor recovery [90], the functional contribution of iCST projections is still debatable.
Conclusions and Future Directions
Conclusive anatomical evidence that an iCST is present in adult humans is absent; however, animal studies demonstrate that iCST fibers are conserved from rodents to non-human primates supporting the existence of iCST in humans. Neurophysiological studies in humans fail to characterize an independent iCST in healthy adults. The functional relevance of iCST in healthy and stroke adult patients is still controversial, but evidence on the important contribution of iCST projections was demonstrated in pediatric patients with congenital hemiplegia. This supports the hypothesis that CST development exhibits a critical period for lateralization of projections, and that only early cortical insults may promote compensatory neurodevelopmental changes that will protect ipsilateral projections. This raises critical questions whether we can reset the developmental clock of the CST and tune up the damaged tract after brain injury in adults and poses challenges to studies that attempt to stimulate iCST fibers in stroke patients to enhance recovery. In addition, clinical studies investigating the role of undamaged motor cortical activity in stroke recovery should carefully interpret the neuroanatomical substrate of this role taking into account the possibility of involvement of inter-hemispheric commissural connections and bilateral connections to brainstem nuclei in addition to potential iCST fibers.
Moving forward, it is important that work in non-human primates replicates the approach used by Wahl et al. to use optogenetics or pharmacogenomics to specifically inhibit iCST projections in the undamaged cortex and identify the role of these fibers in sub-acute and chronic recovery after stroke. Specifically, the use of viral vectors to encode inhibitory optogenetic channels in cortical neurons with direct ipsilateral projections (not through brainstem nuclei) followed by optogenetic stimulation to specifically inhibit motor or supplementary motor neurons will allow a definite assessment of the presence and role of cortically projecting ipsilateral corticospinal connections both in normal function and after stroke. Additionally, the use of novel neuropathological approaches like CLARITY on post-mortem tissue from stroke patients as well as healthy adults allows high-resolution tracking of projecting fibers to identify whether an anatomically distinct iCST exists at pyramidal levels and to clarify the identity of neurons at the termination of these fibers. Recent proof-of-concept studies in non-human primates demonstrated feasibility of clearing spinal cord tissue with subsequent fluorescent imaging and 3D reconstruction [91]. Finally, more robust evidence on the iCST is anticipated to arise from the advancement of imaging method, like DTI, and non-invasive fiber tracking techniques, and neurophysiological techniques including virtual lesions and plasticity protocols.
References
Armand J. Topical versus diffuse organization of the corticospinal tract in the cat. Journal de physiologie. 1978;74(3):227–30.
Satomi H, Takahashi K, Kosaka I, Aoki M. Reappraisal of projection levels of the corticospinal fibers in the cat, with special reference to the fibers descending through the dorsal funiculus: a WGA-HRP study. Brain Res. 1989;492(1–2):255–60.
Lacroix S, Havton LA, McKay H, Yang H, Brant A, Roberts J, et al. Bilateral corticospinal projections arise from each motor cortex in the macaque monkey: a quantitative study. J Comp Neurol. 2004;473(2):147–61. doi:10.1002/cne.20051.
Tigges J, Nakagawa S, Tigges M. Efferents of area 4 in a south American monkey (Saimiri). I. Terminations in the spinal cord. Brain Res. 1979;171(1):1–10.
Gerloff C, Bushara K, Sailer A, Wassermann EM, Chen R, Matsuoka T, et al. Multimodal imaging of brain reorganization in motor areas of the contralesional hemisphere of well recovered patients after capsular stroke. Brain. 2006;129(Pt 3):791–808. doi:10.1093/brain/awh713.
Muller K, Kass-Iliyya F, Reitz M. Ontogeny of ipsilateral corticospinal projections: a developmental study with transcranial magnetic stimulation. Ann Neurol. 1997;42(5):705–11. doi:10.1002/ana.410420506.
Staudt M, Grodd W, Gerloff C, Erb M, Stitz J, Krageloh-Mann I. Two types of ipsilateral reorganization in congenital hemiparesis: a TMS and fMRI study. Brain. 2002;125(Pt 10):2222–37.
Lawrence DG, Kuypers HG. Pyramidal and non-pyramidal pathways in monkeys: anatomical and functional correlation. Science. 1965;148(3672):973–5.
Herbert WJ, Powell K, Buford JA. Evidence for a role of the reticulospinal system in recovery of skilled reaching after cortical stroke: initial results from a model of ischemic cortical injury. Exp Brain Res. 2015;233(11):3231–51. doi:10.1007/s00221-015-4390-x.
Buetefisch CM. Role of the Contralesional hemisphere in post-stroke recovery of upper extremity motor function. Front Neurol. 2015;6:214. doi:10.3389/fneur.2015.00214.
Dancause N, Touvykine B, Mansoori BK. Inhibition of the contralesional hemisphere after stroke: reviewing a few of the building blocks with a focus on animal models. Prog Brain Res. 2015;218:361–87. doi:10.1016/bs.pbr.2015.01.002.
Eyre JA. Development and plasticity of the corticospinal system in man. Neural Plast. 2003;10(1–2):93–106. doi:10.1155/NP.2003.93.
Eyre JA, Taylor JP, Villagra F, Smith M, Miller S. Evidence of activity-dependent withdrawal of corticospinal projections during human development. Neurology. 2001;57(9):1543–54.
Dum RP, Strick PL. Spinal cord terminations of the medial wall motor areas in macaque monkeys. J Neurosci. 1996;16(20):6513–25.
Ralston DD, Ralston HJ. The terminations of corticospinal tract axons in the macaque monkey. J Comp Neurol. 1985;242(3):325–37.
Kim SH, Pohl PS, Luchies CW, Stylianou AP, Won Y. Ipsilateral deficits of targeted movements after stroke. Arch Phys Med Rehabil. 2003;84(5):719–24.
Yarosh CA, Hoffman DS, Strick PL. Deficits in movements of the wrist ipsilateral to a stroke in hemiparetic subjects. J Neurophysiol. 2004;92(6):3276–85. doi:10.1152/jn.00549.2004.
Marchi V, Guzzetta A, Cioni G. Cerebral plasticity and functional reorganization in children with congenital brain lesions. Neonatology. 2017:1–10.
Rehme AK, Fink GR, von Cramon DY, Grefkes C. The role of the contralesional motor cortex for motor recovery in the early days after stroke assessed with longitudinal FMRI. Cereb Cortex. 2011;21(4):756–68. doi:10.1093/cercor/bhq140.
Johansen-Berg H, Rushworth MF, Bogdanovic MD, Kischka U, Wimalaratna S, Matthews PM. The role of ipsilateral premotor cortex in hand movement after stroke. Proc Natl Acad Sci U S A. 2002;99(22):14518–23. doi:10.1073/pnas.222536799.
Bestmann S, Swayne O, Blankenburg F, Ruff CC, Teo J, Weiskopf N, et al. The role of contralesional dorsal premotor cortex after stroke as studied with concurrent TMS-fMRI. J Neurosci. 2010;30(36):11926–37. doi:10.1523/JNEUROSCI.5642-09.2010.
Cauraugh JH, Summers JJ. Neural plasticity and bilateral movements: a rehabilitation approach for chronic stroke. Prog Neurobiol. 2005;75(5):309–20. doi:10.1016/j.pneurobio.2005.04.001.
Chen R, Yung D, Li JY. Organization of ipsilateral excitatory and inhibitory pathways in the human motor cortex. J Neurophysiol. 2003;89(3):1256–64. doi:10.1152/jn.00950.2002.
Soteropoulos DS, Edgley SA, Baker SN. Lack of evidence for direct corticospinal contributions to control of the ipsilateral forelimb in monkey. J Neurosci. 2011;31(31):11208–19. doi:10.1523/JNEUROSCI.0257-11.2011.
Cramer SC, Nelles G, Benson RR, Kaplan JD, Parker RA, Kwong KK, et al. A functional MRI study of subjects recovered from hemiparetic stroke. Stroke. 1997;28(12):2518–27.
Foltys H, Krings T, Meister IG, Sparing R, Boroojerdi B, Thron A, et al. Motor representation in patients rapidly recovering after stroke: a functional magnetic resonance imaging and transcranial magnetic stimulation study. Clin Neurophysiol. 2003;114(12):2404–15.
Marshall RS, Perera GM, Lazar RM, Krakauer JW, Constantine RC, DeLaPaz RL. Evolution of cortical activation during recovery from corticospinal tract infarction. Stroke. 2000;31(3):656–61.
Ward NS, Newton JM, Swayne OB, Lee L, Thompson AJ, Greenwood RJ, et al. Motor system activation after subcortical stroke depends on corticospinal system integrity. Brain. 2006;129(3):809–19.
Armand J, Kuypers HG. Organization of contralateral and bilateral projections of corticospinal tracts in cats. C R Hebd Seances Acad Sci D. 1977;285(16):1455–8.
Satomi H, Takahashi K, Aoki M, Kosaka I. Anatomical evidence for the re-crossing of lateral corticospinal fibers via the posterior gray commissure in the cat spinal cord. Neurosci Lett. 1988;88(2):157–60.
Rapisarda C, Simonelli G, Monti S. Cells of origin and topographic organization of corticospinal neurons in the guinea pig by the retrograde HRP method. Brain Res. 1985;334(1):85–96.
Armand J, Kuypers HG. Cells of origin of crossed and uncrossed corticospinal fibers in the cat: a quantitative horseradish peroxidase study. Exp Brain Res. 1980;40(1):23–34.
Brosamle C, Schwab ME. Cells of origin, course, and termination patterns of the ventral, uncrossed component of the mature rat corticospinal tract. J Comp Neurol. 1997;386(2):293–303.
Alstermark B, Ogawa J, Isa T. Lack of monosynaptic corticomotoneuronal EPSPs in rats: disynaptic EPSPs mediated via reticulospinal neurons and polysynaptic EPSPs via segmental interneurons. J Neurophysiol. 2004;91(4):1832–9.
Nielsen JB, Perez MA, Oudega M, Enriquez-Denton M, Aimonetti JM. Evaluation of transcranial magnetic stimulation for investigating transmission in descending motor tracts in the rat. Eur J Neurosci. 2007;25(3):805–14. doi:10.1111/j.1460-9568.2007.05326.x.
Brus-Ramer M, Carmel JB, Martin JH. Motor cortex bilateral motor representation depends on subcortical and interhemispheric interactions. J Neurosci. 2009;29(19):6196–206. doi:10.1523/JNEUROSCI.5852-08.2009.
Hoff E, Hoff H. Spinal terminations of the projection fibers from the motor cortex of primates. Brain. 1934.
Nakagawa S. Onuf's nucleus of the sacral cord in a south American monkey (Saimiri): its location and bilateral cortical input from area 4. Brain Res. 1980;191(2):337–44.
Rosenzweig ES, Brock JH, Culbertson MD, Lu P, Moseanko R, Edgerton VR, et al. Extensive spinal decussation and bilateral termination of cervical corticospinal projections in rhesus monkeys. J Comp Neurol. 2009;513(2):151–63.
Yoshino-Saito K, Nishimura Y, Oishi T, Isa T. Quantitative inter-segmental and inter-laminar comparison of corticospinal projections from the forelimb area of the primary motor cortex of macaque monkeys. Neuroscience. 2010;171(4):1164–79. doi:10.1016/j.neuroscience.2010.10.007.
Morecraft RJ, Ge J, Stilwell-Morecraft KS, McNeal DW, Pizzimenti MA, Darling WG. Terminal distribution of the corticospinal projection from the hand/arm region of the primary motor cortex to the cervical enlargement in rhesus monkey. J Comp Neurol. 2013;521(18):4205–35. doi:10.1002/cne.23410.
Kondo T, Yoshihara Y, Yoshino-Saito K, Sekiguchi T, Kosugi A, Miyazaki Y, et al. Histological and electrophysiological analysis of the corticospinal pathway to forelimb motoneurons in common marmosets. Neurosci Res. 2015;98:35–44. doi:10.1016/j.neures.2015.05.001.
Aizawa H, Mushiake H, Inase M, Tanji J. An output zone of the monkey primary motor cortex specialized for bilateral hand movement. Exp Brain Res. 1990;82(1):219–21.
Boudrias MH, Lee SP, Svojanovsky S, Cheney PD. Forelimb muscle representations and output properties of motor areas in the mesial wall of rhesus macaques. Cereb Cortex. 2010;20(3):704–19. doi:10.1093/cercor/bhp136.
Montgomery LR, Herbert WJ, Buford JA. Recruitment of ipsilateral and contralateral upper limb muscles following stimulation of the cortical motor areas in the monkey. Exp Brain Res. 2013;230(2):153–64. doi:10.1007/s00221-013-3639-5.
Morecraft RJ, Ge J, Stilwell-Morecraft KS, McNeal DW, Hynes SM, Pizzimenti MA, et al. Vulnerability of the medial frontal corticospinal projection accompanies combined lateral frontal and parietal cortex injury in rhesus monkey. J Comp Neurol. 2015;523(4):669–97. doi:10.1002/cne.23703.
Aisaka A, Aimi Y, Yasuhara O, Tooyama I, Kimura H, Shimada M. Two modes of corticospinal reinnervation occur close to spinal targets following unilateral lesion of the motor cortex in neonatal hamsters. Neuroscience. 1999;90(1):53–67.
Liu Z, Zhang RL, Li Y, Cui Y, Chopp M. Remodeling of the corticospinal innervation and spontaneous behavioral recovery after ischemic stroke in adult mice. Stroke. 2009;40(7):2546–51. doi:10.1161/STROKEAHA.109.547265.
Whishaw IQ, Metz GA. Absence of impairments or recovery mediated by the uncrossed pyramidal tract in the rat versus enduring deficits produced by the crossed pyramidal tract. Behav Brain Res. 2002;134(1–2):323–36.
Carmel JB, Kimura H, Berrol LJ, Martin JH. Motor cortex electrical stimulation promotes axon outgrowth to brain stem and spinal targets that control the forelimb impaired by unilateral corticospinal injury. Eur J Neurosci. 2013;37(7):1090–102.
Gonzalez CL, Gharbawie OA, Williams PT, Kleim JA, Kolb B, Whishaw IQ. Evidence for bilateral control of skilled movements: ipsilateral skilled forelimb reaching deficits and functional recovery in rats follow motor cortex and lateral frontal cortex lesions. Eur J Neurosci. 2004;20(12):3442–52. doi:10.1111/j.1460-9568.2004.03751.x.
Okabe N, Narita K, Miyamoto O. Axonal remodeling in the corticospinal tract after stroke: how does rehabilitative training modulate it? Neural Regen Res. 2017;12(2):185–92. doi:10.4103/1673-5374.200792.
Wahl AS, Omlor W, Rubio JC, Chen JL, Zheng H, Schroter A, et al. Neuronal repair. Asynchronous therapy restores motor control by rewiring of the rat corticospinal tract after stroke. Science. 2014;344(6189):1250–5. doi:10.1126/science.1253050.
Fouad K, Klusman I, Schwab ME. Regenerating corticospinal fibers in the marmoset (Callitrix jacchus) after spinal cord lesion and treatment with the anti-Nogo-a antibody IN-1. Eur J Neurosci. 2004;20(9):2479–82. doi:10.1111/j.1460-9568.2004.03716.x.
Freund P, Schmidlin E, Wannier T, Bloch J, Mir A, Schwab ME, et al. Nogo-A-specific antibody treatment enhances sprouting and functional recovery after cervical lesion in adult primates. Nat Med. 2006;12(7):790–2. doi:10.1038/nm1436.
Zaaimi B, Edgley SA, Soteropoulos DS, Baker SN. Changes in descending motor pathway connectivity after corticospinal tract lesion in macaque monkey. Brain. 2012;135(Pt 7):2277–89. doi:10.1093/brain/aws115.
Nathan PW, Smith MC, Deacon P. The corticospinal tracts in man. Course and location of fibres at different segmental levels. Brain. 1990;113(Pt 2):303–24.
Colebatch JG, Rothwell JC, Day BL, Thompson PD, Marsden CD. Cortical outflow to proximal arm muscles in man. Brain. 1990;113(Pt 6):1843–56.
Berardelli A, Priori A, Inghilleri M, Cruccu G, Mercuri B, Manfredi M. Corticobulbar and corticospinal projections to neck muscle motoneurons in man. A functional study with magnetic and electric transcranial brain stimulation. Exp Brain Res. 1991;87(2):402–6.
Carr LJ, Harrison LM, Stephens JA. Evidence for bilateral innervation of certain homologous motoneurone pools in man. J Physiol. 1994;475(2):217–27.
Strutton PH, Beith ID, Theodorou S, Catley M, McGregor AH, Davey NJ. Corticospinal activation of internal oblique muscles has a strong ipsilateral component and can be lateralised in man. Exp Brain Res. 2004;158(4):474–9. doi:10.1007/s00221-004-1939-5.
Quartarone A, MacKinnon C, Rothwell J. Ipsilateral EMG responses in pectoralis major muscle evoked by transcranial magnetic stimulation over the motor cortex. J Physiol Paris. 1999;520:74P.
Tunstill SA, Wynn-Davies AC, Nowicky AV, McGregor AH, Davey NJ. Corticospinal facilitation studied during voluntary contraction of human abdominal muscles. Exp Physiol. 2001;86(1):131–6.
Ziemann U, Ishii K, Borgheresi A, Yaseen Z, Battaglia F, Hallett M, et al. Dissociation of the pathways mediating ipsilateral and contralateral motor-evoked potentials in human hand and arm muscles. J Physiol. 1999;518(Pt 3):895–906.
Ellaway PH, Davey NJ, Maskill DW, Rawlinson SR, Lewis HS, Anissimova NP. Variability in the amplitude of skeletal muscle responses to magnetic stimulation of the motor cortex in man. Electroencephalogr Clin Neurophysiol. 1998;109(2):104–13.
Tsao H, Galea MP, Hodges PW. Concurrent excitation of the opposite motor cortex during transcranial magnetic stimulation to activate the abdominal muscles. J Neurosci Methods. 2008;171(1):132–9. doi:10.1016/j.jneumeth.2008.02.005.
Kikuchi T, Matsumoto R, Mikuni N, Yokoyama Y, Matsumoto A, Ikeda A, et al. Asymmetric bilateral effect of the supplementary motor area proper in the human motor system. Clin Neurophysiol. 2012;123(2):324–34. doi:10.1016/j.clinph.2011.06.011.
Lo YL, Dan YF, Tan YE, Fook-Chong S, Tan SB, Tan CT, et al. Intraoperative monitoring study of ipsilateral motor evoked potentials in scoliosis surgery. Eur Spine J. 2006;15(Suppl 5):656–60. doi:10.1007/s00586-006-0190-0.
Fisher KM, Zaaimi B, Baker SN. Reticular formation responses to magnetic brain stimulation of primary motor cortex. J Physiol. 2012;590(16):4045–60. doi:10.1113/jphysiol.2011.226209.
Wolpaw JR, Kaas JH. Taking sides: corticospinal tract plasticity during development. Neurology. 2001;57(9):1530–1.
Brouwer B, Smits E. Corticospinal input onto motor neurons projecting to ankle muscles in individuals with cerebral palsy. Dev Med Child Neurol. 1996;38(9):787–96.
Can L, Harrison L, Evans A, Stephens J. Patterns of central motor reorganization in hemiplegic cerebral palsy. Brain. 1993;116:1223–47.
Farmer SF, Harrison LM, Ingram DA, Stephens JA. Plasticity of central motor pathways in children with hemiplegic cerebral palsy. Neurology. 1991;41(9):1505.
Leonard CT, Goldberger ME. Consequences of damage to the sensorimotor cortex in neonatal and adult cats. II. Maintenance of exuberant projections. Dev Brain Res. 1987;32(1):15–30.
Nathan PW, Smith M, Deacon P. Vestibulospinal, reticulospinal and descending propriospinal nerve fibres in man. Brain. 1996;119(Pt 6):1809–33.
Staudt M, Gerloff C, Grodd W, Holthausen H, Niemann G, Krägeloh-Mann I. Reorganization in congenital hemiparesis acquired at different gestational ages. Ann Neurol. 2004;56(6):854–63.
Eyre JA. Corticospinal tract development and its plasticity after perinatal injury. Neurosci Biobehav Rev. 2007;31(8):1136–49. doi:10.1016/j.neubiorev.2007.05.011.
Stanfield BB. The development of the corticospinal projection. Prog Neurobiol. 1992;38(2):169–202.
Verhaart W. Hypertrophy of pes pedunculi and pyramid al result of degeneration of contralateral corticofugal fiber tracts. J Comp Neurol. 1950;92(1):1–15.
Sebastianelli L, Versace V, Taylor A, Brigo F, Nothdurfter W, Saltuari L et al. Functional reorganization after hemispherectomy in humans and animal models: what can we learn about the brain’s resilience to extensive unilateral lesions? Brain Res Bull. 2017.
Rutten GJ, Ramsey NF, van Rijen PC, Franssen H, van Veelen CW. Interhemispheric reorganization of motor hand function to the primary motor cortex predicted with functional magnetic resonance imaging and transcranial magnetic stimulation. J Child Neurol. 2002;17(4):292–7. doi:10.1177/088307380201700411.
Pilato F, Dileone M, Capone F, Profice P, Caulo M, Battaglia D, et al. Unaffected motor cortex remodeling after hemispherectomy in an epileptic cerebral palsy patient. A TMS and fMRI study. Epilepsy Res. 2009;85(2–3):243–51. doi:10.1016/j.eplepsyres.2009.03.016.
Kupper H, Kudernatsch M, Pieper T, Groeschel S, Tournier JD, Raffelt D, et al. Predicting hand function after hemidisconnection. Brain. 2016;139(Pt 9):2456–68. doi:10.1093/brain/aww170.
Lotze M, Markert J, Sauseng P, Hoppe J, Plewnia C, Gerloff C. The role of multiple contralesional motor areas for complex hand movements after internal capsular lesion. J Neurosci. 2006;26(22):6096–102. doi:10.1523/JNEUROSCI.4564-05.2006.
Schaechter JD, Fricker ZP, Perdue KL, Helmer KG, Vangel MG, Greve DN, et al. Microstructural status of ipsilesional and contralesional corticospinal tract correlates with motor skill in chronic stroke patients. Hum Brain Mapp. 2009;30(11):3461–74. doi:10.1002/hbm.20770.
Butefisch CM, Kleiser R, Korber B, Muller K, Wittsack HJ, Homberg V, et al. Recruitment of contralesional motor cortex in stroke patients with recovery of hand function. Neurology. 2005;64(6):1067–9. doi:10.1212/01.WNL.0000154603.48446.36.
Verleger R, Adam S, Rose M, Vollmer C, Wauschkuhn B, Kompf D. Control of hand movements after striatocapsular stroke: high-resolution temporal analysis of the function of ipsilateral activation. Clin Neurophysiol. 2003;114(8):1468–76.
Weiller C, Ramsay SC, Wise RJ, Friston KJ, Frackowiak RS. Individual patterns of functional reorganization in the human cerebral cortex after capsular infarction. Ann Neurol. 1993;33(2):181–9. doi:10.1002/ana.410330208.
Turton A, Wroe S, Trepte N, Fraser C, Lemon RN. Contralateral and ipsilateral EMG responses to transcranial magnetic stimulation during recovery of arm and hand function after stroke. Electroencephalogr Clin Neurophysiol. 1996;101(4):316–28.
Feng W, Wang J, Chhatbar PY, Doughty C, Landsittel D, Lioutas VA, et al. Corticospinal tract lesion load: an imaging biomarker for stroke motor outcomes. Ann Neurol. 2015; doi:10.1002/ana.24510.
Soderblom C, Lee DH, Dawood A, Carballosa M, Jimena Santamaria A, Benavides FD et al. 3D Imaging of Axons in Transparent Spinal Cords from Rodents and Nonhuman Primates. eNeuro. 2015;2(2). doi:10.1523/ENEURO.0001-15.2015.
Acknowledgments
W Feng, D Adkins, and S Kautz acknowledge grant support from National Institute of Health (P20GM109040 and HD086844).
W Feng acknowledges grant support from American Heart Association (14SDG1829003 and 15SFDRN26030003) and NIH/CTSA (UL1RR029882).
D Adkins acknowledges grant support from National Institute of Health (5R01NS065866-06).
S Tomlinson acknowledges grant support from National Institute of Health (P20GM109040) and the Department of Veterans Affairs (Merit Award 1I01RX001141 and 1BX001218).
A Alawieh acknowledges grant support from the American Heart Association (15PRE25250009).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
None.
Conflict of Interests
The authors declare that they have no conflicts of interest.
Rights and permissions
About this article
Cite this article
Alawieh, A., Tomlinson, S., Adkins, D. et al. Preclinical and Clinical Evidence on Ipsilateral Corticospinal Projections: Implication for Motor Recovery. Transl. Stroke Res. 8, 529–540 (2017). https://doi.org/10.1007/s12975-017-0551-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12975-017-0551-5