Abstract
We have reported that neuron-specific conventional protein kinase C (cPKC)γ is involved in the development of cerebral hypoxic preconditioning (HPC) and the neuroprotection against ischemic injuries, but its molecular mechanism is unclear. In this study, the adult and postnatal 24 h C57BL/6J wild-type (cPKCγ+/+) and cPKCγ knockout (cPKCγ−/−) mice were respectively used to establish the models of middle cerebral artery occlusion (MCAO)-induced ischemic stroke in vivo and oxygen-glucose deprivation (OGD)-treated primarily cultured cortical neurons as cell ischemia in vitro. The results showed that cPKCγ knockout could increase the infarct volume and neuronal cell loss in the peri-infarct region, and enhance the neurological deficits, the impaired coordination, and the reduced muscle strength of mice following 1 h MCAO/1–7 days reperfusion. Meanwhile, cPKCγ knockout significantly increased the conversion of LC3-I to LC3-II and beclin-1 protein expression, and resulted in more reductions in P-Akt, P-mTOR, and P-S6 phosphorylation levels in the peri-infarct region of mice with ischemic stroke. The autophagy inhibitor BafA1 could enhance or reduce neuronal cell loss in the peri-infarct region of cPKCγ+/+ and cPKCγ−/− mice after ischemic stroke. In addition, cPKCγ knockout and restoration could aggravate or alleviate OGD-induced neuronal ischemic injury in vitro through Akt-mTOR pathway-mediated autophagy. These results suggested that cPKCγ-modulated neuron-specific autophagy improves the neurological outcome of mice following ischemic stroke through the Akt-mTOR pathway, providing a potential therapeutic target for ischemic stroke.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
As a kind of acute brain blood circulation disorder, ischemic stroke has high incidence, morbidity, and mortality [1, 2]. Recently, some progress has been made, such as intravenous recombinant tissue plasminogen activator (rt-PA) treatment [3, 4] and recombinant T cell receptor ligand combined with rt-PA treatment [5], but the majority of clinical cases still use rt-PA thrombolytic therapy as the main method. Due to the narrow therapeutic window of 4.5 h, the therapeutic strategy for ischemic stroke is still unsatisfactory. Thus, a true breakthrough calls for the full understanding of the molecular mechanism underlying endogenous neuroprotection against cerebral ischemic injury.
Autophagy is an important route for the degradation of proteins and damaged organelles for maintaining the cellular hemostasis [6]. At present, three types of autophagy have been discovered thus far, which are macroautophagy (the most common type), microautophagy, and chaperone-mediated autophagy [7, 8]. The microtubule-associated protein 1 light chain 3 (LC3-I) is diffusely distributed in the cytoplasm, while another protein Beclin 1, which was firstly described in a yeast two-hybrid screen as a Bcl-2-interacting protein, is essential for the initiation of autophagy [9, 10]. When autophagy is activated, the cytoplasmic LC3-I can be hydrolyzed to LC3-II, which is attached to the autophagosome membrane during autophagy process. The ratio of LC3-II/LC3-I is related to the level of autophagosome formation [11]. Maintaining autophagy at a certain level can degrade misfolded proteins and damaged organelles to maintain homeostasis of cells. However, an insufficient or excessive autophagy may degrade the proteins and organelles incorrectly to induce cell damage. Cerebral ischemia/reperfusion (I/R) injury is a complex pathophysiological process, in which the autophagy is activated and plays a double-edged sword for neuronal survival in the progression of cerebral I/R injury [12].
Conventional protein kinase C (cPKC)γ, an important isoform of the PKC family, is located only in neurons of the central nervous system. By using hypoxic preconditioning (HPC) and middle cerebral artery occlusion (MCAO)-induced ischemic-stroked mouse models, we have demonstrated that cPKCγ activation was involved in the development of cerebral HPC and the neuroprotection against ischemic injuries [13]. Recent studies also demonstrated that phosphoinositide 3-kinase (PI3K)/Akt or PKC could modulate mammalian target of rapamycin (mTOR)-mediated autophagy in various cell types [14]; palmitic acid (PA) can induce activation of mTOR-mediated autophagy via PKC [15]. However, whether cPKCγ can modulate neuronal autophagy through the mTOR pathway remains unclear in the brain of mice with ischemic stroke. In this study, we found that cPKCγ-modulated autophagy could alleviate ischemic injuries in vitro and in vivo through the Akt-mTOR pathway.
Experimental Procedure
Except for some antibodies and agents that were individually indicated in the text, all general chemicals were purchased from Sigma-Aldrich (St. Louis, MO 63103, USA). The C57BL/6J wild-type (WT, cPKCγ+/+) and cPKCγ knockout (KO, cPKCγ−/−) mice were purchased from the Jackson Laboratory (Bar Harbor, Maine 04609, USA). The mice with free access to food and water were maintained and genotyped in the Experimental Animal Center of Capital Medical University, People’s Republic of China. Adult male (at the age of 8–10 weeks old, weighing 22–25 g) and postnatal 24-h C57BL/6J mice were respectively used to establish the models of MCAO-induced ischemic stroke in vivo and oxygen-glucose deprivation (OGD)-treated primarily cultured cortical neurons as cell ischemia in vitro. All procedures were performed in strict accordance with the recommendations in the Guide for the Care and Use of Laboratory Animals of the National Institutes of Health and approved by the experimental animal ethics committee of Capital Medical University.
MCAO-Induced Ischemic Stroke Mouse Model
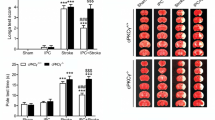
The MCAO-induced ischemic stroke mouse model was prepared as described before [13, 16, 17]. Briefly, the mice were anesthetized using pentobarbital sodium (60 mg/kg i.p.), and then, an incision in the ventral midline neck was made to expose the left common and external carotid arteries. Next, the left common and left external carotid arteries were ligated, and the carefully exposed internal carotid artery was clipped by using microvascular aneurysm clips. A 6-0 surgical nylon monofilament (0.23 mm in diameter) was inserted into the internal carotid artery to occlude the middle cerebral artery (a point approximately 12 mm distal to the carotid bifurcation). Laser Doppler flowmetry (Perimed PeriFlux system 5000, Jarfalla, Stockholm, Sweden) was used to ensure that the blood circulation was occluded successfully and to inspect the cerebral blood flow (CBF). Regional CBF decreased by 80 % in mice after MCAO, and CBF recovered completely after the occlusion was removed 1 h later (Fig. 1a).
Effect of cPKCγ knockout on regional CBF, infarct volume, and neuronal cell loss of mice with MCAO-induced ischemic stroke. a The regional CBF decreased by about 80 % after MCAO and recovered completely after the occlusion was removed 1 h later both in cPKCγ+/+ and cPKCγ−/− mice. b The typical TTC staining results showed the effect of cPKCγ knockout on the infarct area of mice after 1 h MCAO/1–7 days reperfusion. The quantitative analysis demonstrated that the percentage of infract volume increased in the brain of cPKCγ−/− mice when compared with that of cPKCγ+/+ mice after 1 h MCAO/1–7 days reperfusion (n = 5 per group). c Typical images and quantitative analysis results of Nissl staining showed that neuronal cell loss in the peri-infarct region significantly increased in cPKCγ−/− mice when compared with that of cPKCγ+/+ mice after 1 h MCAO/3 days reperfusion (scale bar = 100 μm, n = 5 per group). Three asterisks P < 0.001 versus sham; two number signs P < 0.01 and three number signs P < 0.001 versus corresponding cPKCγ+/+ mice with the same treatment
Sham-operated mice received the same procedure, without inserting the nylon monofilament to occlude the middle cerebral artery. The autophagy inhibitor BafA1 (0.00, 0.25, 2.5, and 25.0 mg/kg) [18] was dissolved in dimethyl sulfoxide (DMSO) and injected into the intracerebroventricle (ICV) of mice 15 min before the onset of MCAO [19]. In brief, the anesthetized mice (pentobarbital sodium, 60 mg/kg, i.p.) were placed upon a stereotaxic frame, and the cannula (28 gauge, inner diameter 0.18 mm, outer diameter 0.36 mm) was lowered into the left cerebral ventricle according to the following coordinates: 0.5 mm posterior and 1.0 mm lateral to the bregma and 3.5 mm below the skull surface [20]. In this study, the reason for delivering BafA1 via ICV injection is the lack of information on BafA1 crossing the blood-brain barrier.
OGD-Induced Ischemic Injury In Vitro in Primarily Cultured Cortical Neurons and Lentiviral Transduction
The primarily cultured cortical neurons were prepared from postnatal 24-h C57BL/6J (cPKCγ+/+ and cPKCγ−/−) mice. The cortical neurons were dissociated carefully and seeded onto plates at a density of 5 × 105 cells/cm2. The neurons were grown in neurobasal medium with 2 % B27 supplement (Gibco Inc, Carlsbad, CA 92008, USA), and the culture medium was replaced half of fresh medium every 3 days. The cPKCγ−/− neurons were transduced with lentiviral vectors containing cPKCγ gene and their controls (Genepharma, Suzhou, People’s Republic of China) according to the manufacturer’s instruction. After 48 h lentiviral transduction and 7 days culture, the neurons were subjected to 1 h OGD/24 h reoxygenation to mimic ischemic injury in vitro. For OGD treatment, the neurons were replaced with glucose-free DMEM and placed in the hypoxia incubator (Thermo Scientific, Marietta, OH 45750, USA) under the condition of 5 % CO2/1 % O2/94 % N2. At the end of 1 h OGD exposure, the cells were maintained in regular neurobasal medium containing 2 % B27 under normoxic conditions (5 % CO2/21 % O2/74 % N2) for 24 h reoxygenation. The cell survival rate was determined by using thiazolyl blue tetrazolium bromide (MTT, 0.5 mg/ml; Applichem Inc., Omaha, NE 68135, USA), and cell death was detected via the LDH release rate using the CytoTox 96 Non-Radioactive Cytotoxicity Assay (Promega Cor, Madison, WI 53711, USA) following the manufacturer’s instructions.
TTC Staining for Assessment of Cerebral Infarct Volume
After 1 h MCAO/1–7 days reperfusion, the animals were sacrificed and perfused with ice-cold phosphate-buffered saline (PBS). The brains were removed and sectioned coronally to 2-mm-thick slices. The slices were stained with 0.5 % triphenyltetrazolium chloride (TTC) solution (Sigma-Aldrich, St. Louis, MO 63103, USA) at 37 °C for 15 min. Normal brain tissue was stained brightly, whereas the infarcted areas were pale white. The cerebral infarct area was measured by using Image-Pro Plus 5.0 image processing software (Media Cybernetics, Rockville, MD 20852, USA). The infarct volume is counted according to our previous reports as follows [13;16;17]. The cerebral edema rate (S) = (∑LT − ∑RT)/(∑LT + ∑RT) × 100 %, where ∑LT and ∑RT represent the volume of the left (ischemic) and right (non-ischemic) hemisphere, respectively. Background (B) = volume of TTC unstained white matter in sham group/total brain volume of sham group × 100 %. Considering the effects of edema and background, the infarct volume was represented as the percentage of total brain through the following equation: I = ∑SIN(1 − S)/(∑LT + ∑RT)(1 − B) × 100 %, where ∑SIN(1 − S) represents the total infarct volume after deducting the edema rate.
Measurement of Neurological Deficit
At 1, 3, and 7 days after 1 h MCAO, the mice were tested for neurobehavioral score according to the neurological disability status scale reported by Rodriguez et al. [21]. Briefly, at each time period after MCAO surgery, the animals were graded on a scale of 0 to 10 according to the severity of their deficits, with the detailed criteria as follows: 0, no neurological dysfunction; 2, slight dysfunction in mobility and the presence of passivity; 4, moderate neurological dysfunction; 6, corresponding to more handicapped animals but still able to walk, with more marked hypomobility, circling, tremor, jerks and/or convulsions, forelimb flexion, and moderate motor incoordination; 8, corresponding to respiratory distress and total incapacity to move/coordinate. Status 10 refers to death due to 1 h MCAO/1–7 days reperfusion. If the level of deficits did not meet the precise criteria, the nearest appropriate number 1, 3, 5, 7, and 9 was used.
At 1, 3, and 7 days after 1 h MCAO, the neurological function of mice was assessed as follows. The time it takes for a mouse to finish an upside-down 180° turn (T turn) and time to the ground (T total) were recorded and used as an indicator of limb coordination in the pole test [22]; the wire hanging test criteria were reflected by latency to fall [23]; the cylinder test records the number of contacts for each forelimb, and the ratio for contralateral limb usage is calculated using the formula (L − R)/(L + R + both) × 100 % [24]; results for the handedness test criteria are shown as the laterality index [25]; and the foot fault test criteria are indicated as the number of missteps mice took while walking on a wire mesh [26]. The mice were trained three days before MCAO surgery; meanwhile, all trainings and tests were performed in triplicates each day.
Nissl Staining
The mice were perfused transcardially with 4 % paraformaldehyde in PBS after 1 h MCAO/3 days reperfusion, and then, the brains were removed and post-fixed in 4 % paraformaldehyde. After dehydration by successive immersion in 20 and 30 % sucrose solution, the brains were cut into 20-μm-thick sections to stain with 0.04 % cresyl violet dissolved in acetate buffer for 1 h. Five sections per brain were used for cell counting in the peri-infarct region of MCAO mice from three views under the light microscope (Nikon, 50i, Japan).
Immunofluorescent Staining
Immunofluorescent staining with NeuN was used to detect neuronal cells according to the manufacturer’s instructions. The 20-μm-thick sections of mouse brain were washed in phosphate-buffered saline (PBS) and permeabilized with 0.3 % Triton X-100 (pH 7.5) for 1 h. Next, they were blocked with 3 % goat serum for 1 h. The blocked slices were incubated with primary mouse monoclonal antibodies against NeuN (ab104224, Abcam, Boston, MA 02754, USA) at 1:100 for 12 h at 4 °C. Then, the slices were incubated with Alexa Fluor 488 goat anti-mouse IgG (Green color, A11029, Molecular Probes, Thermo Fisher Scientific, Eugene, OR 97401, USA) at a 1:200 dilution for 2 h at 37 °C. Finally, the slices were washed and sealed with medium containing DAPI. The images (three images per section) were acquired by using the fluorescent microscope system (Nikon, 50i, Japan).
Western Blot Analysis
In this study, the antibodies were purchased from the following indicated companies: rabbit anti-Beclin 1 monoclonal antibody (1:1000; catalogue number #3495), rabbit anti-LC3 polyclonal antibody (1:1000; catalogue number #2775), rabbit anti-phospho-Akt (Ser473) polyclonal antibody (1:1000; catalogue number #9271), rabbit anti-Akt polyclonal antibody (1:1000; catalogue number #9272), rabbit anti-phospho-mTOR (Ser2448) polyclonal antibody (1:1000; catalogue number #2971), rabbit anti-mTOR polyclonal antibody (1:1000; catalogue number #2972), rabbit anti-phospho-S6 (Ser235/236) polyclonal antibody (1:1000; catalogue number #2211), and mouse anti-S6 monoclonal antibody (1:1000; catalogue number #2317) from Cell Signaling Technology (Danvers, MA 01923, USA); mouse anti-β-actin monoclonal antibody (1:10,000) from Sigma-Aldrich Corp., St. Louis, MO 63103, USA; rabbit anti-cPKCγ polyclonal antibody (1:1000; catalogue number SC-98952) from Santa Cruz Biotechnology, Inc., Santa Cruz, CA 95060, USA; and horseradish peroxidase-conjugated goat anti-rabbit or anti-mouse IgG as secondary antibody (1:4000) from Stressgen Biotechnologies Corporation, Victoria, BC, Canada.
Total protein was extracted from the brain-specific regions and primarily cultured cortical neurons as our previous reports [27, 28]. Thirty micrograms of total protein was loaded in 12 % SDS-PAGE. After electrophoresis, proteins were transferred onto polyvinylidene difluoride membrane (GE Healthcare, UK). The transferred membrane was blocked with 10 % non-fat milk in Tween/Tris-buffered salt solution (TTBS; 20 mM Tris–Cl, pH 7.5, 0.15 M NaCl, and 0.05 % Tween-20) for 1 h. After the incubations of primary and secondary antibodies, the Enhanced Chemiluminescence kit (GE Healthcare, UK) was used to detect the signals. The amount of proteins was quantified by densitometry and normalized to β-actin, an internal standard.
Statistical Analysis
The GelDoc-2000 Imagine System (Bio-Rad, Hercules, CA 94547, USA) was used to perform the quantitative analysis of Western blot. The protein expression levels of Beclin 1 and cPKCγ (band density of target protein/band density of β-actin); the phosphorylation levels of P-mTOR, P-Akt, and P-S6 (the band densities of phosphorylated form/total protein); the proteolysis levels of caspases (cleaved caspase/total caspase); and LC3 (band density of LC3-II/band density of LC3-I and II/) were calculated as 100 % in the control or sham group, and then, the other groups were expressed as percentage of that of the control or sham group. Statistical analysis was performed by using one-way analysis of variance (ANOVA) followed by all pairwise multiple comparison procedures using the Bonferroni test. The values were presented as mean ± SEM, and P < 0.05 was considered as statistically significant.
Results
Effect of cPKCγ Knockout on the Outcome of Mice with Ischemic Stroke
We have reported that HPC-induced cPKCγ activation is involved in the endogenous neuroprotection against cerebral ischemic injuries [13], but how cPKCγ affects the outcome of mice with ischemic stroke is unclear. To investigate the role of cPKCγ in ischemic stroke, the regional CBF, infarct volumes, neuronal cell loss, and neurological deficits were assessed in cPKCγ−/− mice after 1 h MCAO/1–7 days reperfusion. As shown in Fig. 1a, the regional CBF decreased by 80 % after MCAO and recovered completely after the occlusion was removed 1 h later both in cPKCγ+/+ and cPKCγ−/− mice, suggesting that cPKCγ did not affect CBF levels during the procedure of 1 h MCAO/reperfusion [Fig. 1a, F(19, 99) = 1588.573, n = 5 per group]. However, the infarct volume increased significantly in cPKCγ−/− mice after 1 h MCAO [Fig. 1b, F(9, 49) = 81.6, n = 5 per group] at 1 day (P < 0.001), 3 days (P < 0.001), and 7 days (P = 0.004) reperfusion when compared with that of cPKCγ+/+ mice. Meanwhile, neuronal cell loss in the peri-infarct region significantly increased in cPKCγ−/− mice [Fig. 1c, F(5, 29) = 152.41, P < 0.001, n = 5 per group] when compared with that of cPKCγ+/+ mice after 1 h MCAO/3 days reperfusion. Similarly, compared with cPKCγ+/+ mice following 1 h MCAO/1–7 days reperfusion, results on the neurological score (Fig. 2a), pole test (Fig. 2b), wire hanging test (Fig. 2c), cylinder test (Fig. 2d), handedness test (Fig. 2e), and foot fault test (Fig. 2f) showed that cPKCγ knockout could aggravate the neurological deficits [Fig. 2a, F(9, 59) = 93.1, P < 0.001], the impaired coordination, and reduced muscle strength [Fig. 2b, time turn: F(9, 59) = 78.117, P < 0.001; time total: F(9, 59) = 97.528, P < 0.001; Fig. 2c, F(9, 59) = 327.267, P < 0.001; Fig. 2d, F(9, 59) = 511.265, P < 0.001; Fig. 2e, F(9, 59) = 516.222, P < 0.001; and Fig. 2f, F(9, 59) = 71.023, P < 0.001].
Effect of cPKCγ knockout on the neurological outcome of mice with ischemic stroke. a Results of the neurological score indicated that cPKCγ knockout could enhance the neurological deficit of mice after 1 h MCAO/1–7 days reperfusion (n = 6 per group). In addition, the results of the pole test (b), wire hanging test (c), cylinder test (d), handedness test (e), and foot fault test (f) showed that cPKCγ knockout could enhance the impaired coordination and reduced muscle strength (n = 6 per group). Three asterisks P < 0.001 versus sham; two number signs P < 0.01 and three number signs P < 0.001 versus corresponding cPKCγ+/+ mice with the same treatment
cPKCγ Affects MCAO-Induced Autophagy In Vivo Through Akt-mTOR Pathway
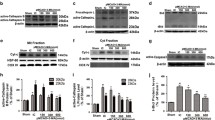
The previous studies have reported that autophagy might be a potential therapeutic target for stroke [29]. To further validate the role of cPKCγ in cerebral ischemia-induced autophagy in vivo, the autophagy-related protein expressions were determined in the peri-infarct region of mice after 1 h MCAO/1–7 days reperfusion. LC3-II is the most widely used marker for revealing the presence of autophagosomes, while Beclin 1 belongs to the class 3 phosphoinositide 3-kinase (PI3K) complex and is involved in the early stages of autophagosome formation [30]. As shown in Fig. 3a, c, the conversion of LC3-I to LC3-II increased evidently in cPKCγ−/− mice with 1 h MCAO after 1 day (P < 0.001), 3 days (P < 0.001), and 7 days (P = 0.005) reperfusion compared with that of the same treated cPKCγ+/+ mice [F(9, 69) = 294.152, n = 7 per group]. Similarly, cPKCγ knockout also significantly increased the expressions of Beclin 1 in the peri-infarct region of mice with 1 h MCAO after 1 day (P < 0.001), 3 days (P < 0.001), and 7 days (P < 0.001) reperfusion (Fig. 3a, b, F(9, 69) = 106.476, n = 7 per group). Moreover, to further explore whether cPKCγ affects MCAO-induced autophagy through the Akt-mTOR pathway, the phosphorylation levels of Akt, mTOR, and p70 ribosomal S6 (S6) were examined by using Western blot analysis (Fig. 3d). The results showed that cPKCγ−/− could cause more reduction in P-Akt [Fig. 3f, F(5, 29) = 269.81, P < 0.001], P-mTOR [Fig. 3e, F(5, 29) = 226.98, P < 0.001], and P-S6 [Fig. 3g, F(5, 29) = 99.567, P < 0.001] phosphorylation levels in the peri-infarct region of cPKCγ+/+ mice with 1 h MCAO/3 days reperfusion (n = 5 per group). These results indicated that cPKCγ knockout may strengthen MCAO-induced autophagy by affecting Akt, mTOR, and S6 phosphorylation levels.
Effect of cPKCγ knockout on autophagy and phosphorylation levels of the Akt-mTOR pathway in the peri-infarct region of mice with ischemic stroke. a Typical results of Western blot showed the changes of cPKCγ and Beclin1 protein expression levels and the ratio of LC3-II/LC3-I in the peri-infarct region of cPKCγ+/+ and cPKCγ−/− mice after 1 h MCAO/1–7 days reperfusion. The quantitative analysis results demonstrated that cPKCγ knockout significantly increased the expressions of Beclin 1 (b) and the conversion of LC3-I to LC3-II (c) in the peri-infarct region of mice with 1 h MCAO after 1–7 days reperfusion (n = 7 per group). The representative (d) and quantitative analysis results of Western blot showed that P-mTOR (e), P-Akt (f), and P-S6 (g) phosphorylation levels in the peri-infarct region of cPKCγ−/− mice decreased more significantly than those of cPKCγ+/+ mice with 1 h MCAO/3 days reperfusion (n = 5 per group). Two asterisks P < 0.01 and three asterisks P < 0.001 versus sham; two number signs P < 0.01 and three number signs P < 0.001 versus corresponding cPKCγ+/+ mice with the same treatment
cPKCγ-Mediated Neuroprotection Against Ischemic Injury Acts Through Modulating Autophagy In Vivo
cPKCγ could modulate the level of MCAO-induced autophagy in vivo; then, whether cPKCγ-mediated neuroprotection acted through modulating autophagy was determined by using intracerebralventricular (i.c.v.) injection of the autophagy inhibitor BafA1 before the onset of MCAO. As BafA1 inhibits the autophagosome docking and fusion with the lysosome to stop the process of autophagy, the level of autophagy marker LC3-II accumulation could be recognized as the indicator of BafA1 pharmacological actions. As shown in Fig. 4a, b, BafA1 at 2.5 and 25.0 mg/kg could significantly enhance the LC3-II accumulation in the peri-infarct region of both cPKCγ+/+ and cPKCγ−/− mice following 1 h MCAO/3 days reperfusion [F(13, 69) = 163.323, P < 0.001, n = 5 per group], confirming the inhibition of BafA1 on MCAO-induced autophagy in vivo. Interestingly, the results of Nissl (Fig. 4c) and immunofluorescent staining with NeuN (Fig. 5a) demonstrated that BafA1 could aggravate MCAO-induced neuronal cell loss in cPKCγ+/+ mice but reduced the neuronal cell loss in the peri-infarct region of cPKCγ−/− mice after 1 h MCAO/3 days reperfusion [Fig. 4d, F(9, 49) = 183.401, P < 0.001; Fig. 5b, F(9, 49) = 369.43, P < 0.001, n = 5 per group], which implicated that cPKCγ-mediated neuroprotection acts through the modulation of MCAO-induced autophagy.
Effect of BafA1 on the ratio of LC3-II/LC3-I and neuronal cell loss in the peri-infarct region of cPKCγ+/+ and cPKCγ−/− mice with ischemic stroke. a Typical results of Western blot showed the effect of autophagy inhibitor BafA1 on the ratio of LC3-II/LC3-I in the peri-infarct region of cPKCγ+/+ and cPKCγ−/− mice after 1 h MCAO/3 days reperfusion. b The quantitative analysis results demonstrated that BafA1 at 2.5 and 25.0 mg/kg could significantly enhance the LC3-II accumulation in the peri-infarct region of both cPKCγ+/+ and cPKCγ−/− mice with ischemic stroke (n = 5 per group), confirming the inhibition of BafA1 on MCAO-induced autophagy in vivo. c Typical images of Nissl staining showed the effect of BafA1 on neuronal cell loss in the peri-infarct region of both cPKCγ+/+ and cPKCγ−/− mice after 1 h MCAO/3 days reperfusion (scale bar = 100 μm). d The quantitative analysis results demonstrated that BafA1 could aggravate neuronal cell loss in cPKCγ+/+ mice, but attenuate neuronal cell loss in the peri-infarct region of cPKCγ−/− mice with ischemic stroke (n = 5 per group). Three asterisks P < 0.001 versus sham; three number signs P < 0.001 versus 0.00 (DMSO) group
Effect of BafA1 on neuronal cell loss in the peri-infarct region of cPKCγ+/+ and cPKCγ−/− mice with ischemic stroke. a Typical images of immunofluorescent staining with neuron-specific maker NeuN showed the effect of BafA1 on NeuN-positive cell loss in the peri-infarct region of both cPKCγ+/+ and cPKCγ−/− mice after 1 h MCAO/3 days reperfusion (scale bar = 100 μm). b The quantitative analysis results demonstrated that BafA1 could aggravate NeuN-positive cell loss in cPKCγ+/+ mice, but attenuate NeuN-positive cell loss in the peri-infarct region of cPKCγ−/− mice with ischemic stroke (n = 5 per group). Three asterisks P < 0.001 versus sham; three number signs P < 0.001 versus 0.00 (DMSO) group
cPKCγ Protects Neurons Against OGD-Induced Ischemic Injury in Primarily Cultured Cortical Neurons In Vitro Through Akt-mTOR Pathway-Mediated Autophagy
To further investigate the role of cPKCγ in neuronal ischemic injury in vitro, MTT and LDH assays were used to detect OGD-induced cell death in both primarily cultured cortical cPKCγ+/+ and cPKCγ−/− neurons. It is found that the cell survival rate is decreased [Fig. 6h, F(3, 23) = 207.195, P < 0.001], while the cell death rate is significantly increased [Fig. 6i, F(3, 23) = 321.388, P < 0.001] in cPKCγ−/− neurons when compared with that of cPKCγ+/+ neurons after 1 h OGD/24 h reoxygenation. Similarly, the effect of cPKCγ knockout on the ratio of LC3-II to LC3-I and Beclin 1 expression levels was determined in 1 h OGD/24 h reoxygenation-treated neurons (Fig. 6a). The results showed that cPKCγ knockout could increase the protein expressions of Beclin 1 [Fig. 6b, F(3, 23) = 275.142, P < 0.001] and the ratio of LC3-II/LC3-I [Fig. 6c, F(3, 23) = 1363.085, P < 0.001] in primarily cultured cortical neurons after 1 h OGD/24 h reoxygenation. For the affection of cPKCγ on Akt-mTOR pathway-mediated autophagy, the phosphorylation levels of Akt, mTOR, and S6 (Fig. 6d) were determined by using Western blot analysis. The results showed that cPKCγ knockout resulted in more reduction in P-Akt (Fig. 6f, F(3, 23) = 77.157, P < 0.001), P-mTOR (Fig. 6e, F(3, 23) = 338.694, P < 0.001), and P-S6 (Fig. 6g, F(3, 23) = 280.594, P < 0.001) in primarily cultured cortical neurons after 1 h OGD/24 h reoxygenation. Similar with the results of autophagy inhibitor BafA1 at the onset of MCAO in vivo, the Western blot analysis showed that BafA1 at 100 nM could significantly enhance the LC3-II accumulation in cultured cortical neurons from both cPKCγ+/+ and cPKCγ−/− mice after 1 h OGD/24 h reoxygenation [Fig. 7a, b, F(7, 47) = 328.128, P < 0.001]. The results of MTT (Fig. 7c) and LDH (Fig. 7d) assays also showed that BafA1 could further enhance or alleviate OGD-induced ischemic injuries in cPKCγ+/+ and cPKCγ−/− neurons after 1 h OGD/24 h reperfusion [Fig. 7c, F(7, 47) = 207.195, P < 0.001; Fig. 7d, F(7,47) = 321.388, P < 0.001], respectively.
Effect of cPKCγ knockout on the ratio of LC3-II/LC3-I, Beclin 1 protein levels, and ischemic injury in primarily cultured cortical neurons after 1 h OGD/24 h reoxygenation. a Typical results of Western blot showed the effect of cPKCγ knockout on the ratio of LC3-II/LC3-I and Beclin 1 expression levels in 1 h OGD/24 h reoxygenation-treated neurons. The quantitative analysis results showed that cPKCγ knockout could increase the protein expressions of Beclin 1 (b) and the ratio of LC3-II/LC3-I (c) in primarily cultured cortical neurons after 1 h OGD/24 h reoxygenation (n = 6 per group). d Typical results of Western blot showed the effect of cPKCγ knockout on the phosphorylation levels of the AKt-mTOR pathway in 1-h OGD/24-h reoxygenation-treated neurons. The quantitative analysis results demonstrated that cPKCγ knockout could enhance the OGD-induced decrease of P-mTOR (e), P-Akt (f), and P-S6 (g) in primary cultured cortical neurons after 1 h OGD/24 h reoxygenation (n = 6 per group). The results of MTT assays (h) and LDH assay (i) indicated that the cell survival rate is decreased, while the cell death rate is significantly increased in cPKCγ−/− neurons when compared with that of cPKCγ+/+ neurons after 1 h OGD/24 h reoxygenation (n = 6 per group). Three asterisks P < 0.001 versus normoxia; three number signs P < 0.001 versus cPKCγ+/+ neurons after 1 h OGD/24 h R
Effects of BafA1 and cPKCγ restoration on the ratio of LC3-II/LC3-I, Beclin 1 protein levels, and ischemic injury in cPKCγ+/+ and cPKCγ−/− neurons after 1 h OGD/24 h reoxygenation. The typical (a) and quantitative analysis (b) results of Western blot showed that BafA1 at 100 nM could significantly induce LC3-II accumulation in cultured cortical neurons of both cPKCγ+/+ and cPKCγ−/− after 1 h OGD/24 h reoxygenation (n = 6 per group). The results of MTT (c) and LDH (d) assays also showed that BafA1 could further enhance or alleviate OGD-induced ischemic injuries in cPKCγ+/+ and cPKCγ−/− neurons, respectively (n = 6 per group). The cPKCγ−/− cortical neurons were transduced with lentivirus coding cPKCγ, and the successfully expressed cPKCγ was confirmed by using Western blot (e). The results of typical Western blot (f) and quantitative analysis showed that cPKCγ restoration could significantly decrease both the ratio of LC3-II/LC3-I (g) and Beclin 1 expression levels (h) when compared with those of the LV-cPKCγ (−) group of cPKCγ−/− neurons after 1 h OGD/24 h reoxygenation (n = 6 per group). In addition, the results of MTT (i) and LDH (j) assays also confirmed that cPKCγ restoration (LV-cPKCγ) could improve OGD-induced neuronal ischemic injuries compared with those of the LV-cPKCγ (−) group of cPKCγ−/− neurons after 1 h OGD/24 h reoxygenation (n = 6 per group). Three asterisks P < 0.001 versus normoxia; three number signs P < 0.001 versus OGD-treated cPKCγ+/+/cPKCγ−/− neurons with 0.0 nM BafA1 or versus OGD-treated LV-cPKCγ (−) group of cPKCγ−/− neurons
To confirm the role of cPKCγ in OGD-induced neuronal ischemic injuries and autophagy, the cPKCγ−/− cortical neurons were transduced with lentivirus coding cPKCγ (Fig. 7e). The results of typical Western blot (Fig. 7f) and quantitative analysis showed that cPKCγ restoration could significantly decrease both the ratio of LC3-II/LC3-I [Fig. 7g, F(3, 23) = 297.229, P < 0.001] and the expression of Beclin 1 [Fig. 7h, F(3, 23) = 320.330, P < 0.001] when compared with that of LV-cPKCγ (−) group of cPKCγ−/− neurons after 1 h OGD/24 h reoxygenation. In addition, cPKCγ restoration (LV-cPKCγ) could also improve OGD-induced neuronal ischemic injuries [Fig. 7i, F(3, 23) = 394.544, P < 0.001; Fig. 7j, F(3, 23) = 503.668, P < 0.001, n = 6 per group] compared with LV-cPKCγ (−) group of cPKCγ−/− neurons after 1 h OGD/24 h reoxygenation. The results demonstrated that cPKCγ restoration could effectively decrease OGD-induced neuronal ischemic injuries through modulating autophagy in vitro.
Discussion
Autophagy is a highly conserved pathway for degradation, by which cytosolic long-lived proteins and organelles are broken down and re-used to maintain cellular metabolic turnover and homeostasis [31–33]. Accumulating reports have demonstrated that autophagy increases in cerebral ischemic injury, including hypoxia-ischemia (HI) [6, 34–38], global [39–41], and focal ischemia [18, 42–44]. In neurons, moderate autophagy is important for preserving cellular homeostasis under normal conditions [45], but inadequate autophagy promotes neuronal cell death [46–48]. In this study, we found that cPKCγ could affect the neurological outcome of mice with ischemic stroke through modulating the Akt-mTOR pathway-mediated autophagy.
A group of autophagy-related genes (ATG) controls autophagy by regulating several sequential steps of the autophagic process, including induction, autophagosome nucleation and elongation, autophagosome docking and fusion with the lysosome, and finally autophagic body breakdown and release of the contents back into the cytosol. The serine/threonine-protein kinase mTOR has been identified as a key negative regulatory protein involved in the induction of autophagy upstream of ATG proteins [49]. mTOR belongs to the family of PI3K-like kinases [50] and can masterly regulate cell growth and metabolism via predominantly controlling the phosphorylation of p70 ribosomal S6 kinase (p70S6K) [51]. The autophagy marker LC3-II is a main hydrolysis product of LC3-I (known as ATG8). In mammalian cells, the initiation process of autophagy depends on a complex consisting of ULK1 (ATG1 homologue), ATG13, and FIP2000 proteins. BafA1, a downstream inhibitor of autophagy, could inhibit autophagosome fusing with lysosome to induce the accumulation of LC3-II. In this study, we found that cPKCγ could modulate the level of MCAO-induced autophagy, and the effective blockage of autophagy by BafA1 could significantly cause the LC3-II accumulation in the peri-infarct region of both cPKCγ+/+ and cPKCγ−/− mice with ischemic stroke. For cPKCγ+/+ mice with ischemic stroke, the autophagy at moderate level is neuroprotective, as BafA1 inhibiting autophagy to a lower level could cause more loss of neuronal cells in the peri-infarct region. However, the excessive autophagy is detrimental, as BafA1-induced inhibition of autophagy attenuated the neuronal cell loss in the peri-infarct region of cPKCγ−/− mice with ischemic stroke. In addition, cPKCγ−/− causes more reduction in P-Akt, P-mTOR, and P-S6 phosphorylation levels in the peri-infarct region of mice with 1 h MCAO/3 days reperfusion. These results indicated that cPKCγ-modulated autophagy alleviates cerebral ischemic injury of mice with ischemic stroke through the Akt-mTOR pathway.
To further determine the neuron-specific cPKCγ-modulated autophagy and its possible molecular mechanism, both primarily cultured cortical cPKCγ+/+ and cPKCγ−/− neurons were subjected to 1 h OGD/24 h reoxygenation to mimic ischemic injury in vitro. We found that cPKCγ knockout could increase the protein expressions of Beclin 1 and the ratio of LC3-II/LC3-I as well as augmented the OGD-induced decrease in P-Akt, P-mTOR, and P-S6 phosphorylation levels in primarily cultured cortical neurons; the autophagy inhibitor BafA1 could further aggravate or alleviate OGD-induced ischemic injuries in cPKCγ+/+ and cPKCγ−/− neurons, respectively. In addition, we found that cPKCγ restoration could significantly decrease both the ratio of LC3-II/LC3-I and Beclin 1 expressions and improve ischemic injuries in primarily cultured cortical cPKCγ−/− neurons after OGD treatment. These results demonstrated that cPKCγ-modulated autophagy is neuron-specific through regulating the Akt-mTOR pathway, but how cPKCγ modulates the Akt-mTOR pathway is unclear.
According to recent reports, the Akt phosphorylation might be modulated by cPKCγ in neurons. In response to various stimulations, activated PI3K promotes the production of phosphatidylinositol 3,4,5-trisphosphate (PIP3), leading to phosphorylate Akt at Ser473 by PDK2, βII isoform of cPKC [52]. cPKCβII can physically associate Akt and regulate Akt activity by directly phosphorylating Ser473 in vitro and in IgE/antigen-stimulated mast cells [53, 54]. Bacopa monnieri (BM) plays a neuroprotective role via significantly activating PKC and elevated the level of p-Akt (S473) on OGD-induced ischemic injury in hippocampal cells [55]. We also reported that cPKCγ membrane translocation (activation) significantly increased in the development of cerebral HPC to play neuroprotection. These reports indicated that cPKCγ may play an important role in maintaining the Akt phosphorylation status, but the detailed molecular mechanism is needed to be explored in the future experiment.
In conclusion, cPKCγ could alleviate ischemic injury and improve the neurological outcome of mice with ischemic stroke through the modulation of Akt-mTOR pathway-mediated autophagy. cPKCγ-modulated autophagy is neuron-specific and cPKCγ may play a key role in maintaining the Akt phosphorylation status, but the detailed molecular mechanism requires further exploration. In addition, the autophagy must be moderate to allow for neuroprotection, since either inhibiting or stimulating this process could be detrimental to neurons. However, the translation of this knowledge into medicine and clinical use still requires further investigation, particularly in finding the right window, be it time period or a specific medical reagent, to mediate a moderate level of autophagy that could induce neuroprotection.
References
Liu M, Wu B, Wang WZ, Lee LM, Zhang SH, Kong LZ. Stroke in China: epidemiology, prevention, and management strategies. Lancet Neurol. 2007;6(5):456–64.
Sidney S, Rosamond WD, Howard VJ, Luepker RV. The “Heart Disease and Stroke Statistics–2013 Update” and the need for a national cardiovascular surveillance system. Circulation. 2013;127(1):21–3.
Bas DF, Ozdemir AO, Colak E, Kebapci N. Higher insulin resistance level is associated with worse clinical response in acute ischemic stroke patients treated with intravenous thrombolysis. Transl Stroke Res. 2016;6(6):167–71.
Mandava P, Shah SD, Sarma AK, Kent TA. An outcome model for intravenous rt-PA in acute ischemic stroke. Transl Stroke Res. 2015;7(3):451–7.
Zhu W, Libal NL, Casper A, Bodhankar S, Offner H, Alkayed NJ. Recombinant T cell receptor ligand treatment improves neurological outcome in the presence of tissue plasminogen activator in experimental ischemic stroke. Transl Stroke Res. 2014;5(5):612–7.
Carloni S, Buonocore G, Balduini W. Protective role of autophagy in neonatal hypoxia-ischemia induced brain injury. Neurobiol Dis. 2008;32(3):329–39.
Levine B, Kroemer G. Autophagy in the pathogenesis of disease. Cell. 2008;132(1):27–42.
Rubinsztein DC, Difiglia M, Heintz N, Nixon RA, Qin ZH, Ravikumar B, et al. Autophagy and its possible roles in nervous system diseases, damage and repair. Autophagy. 2005;1(1):11–22.
Liang XH, Jackson S, Seaman M, Brown K, Kempkes B, Hibshoosh H, et al. Induction of autophagy and inhibition of tumorigenesis by beclin 1. Nature. 1999;402(6762):672–6.
Pattingre S, Tassa A, Qu X, Garuti R, Liang XH, Mizushima N, et al. Bcl-2 antiapoptotic proteins inhibit Beclin 1-dependent autophagy. Cell. 2005;122(6):927–39.
Kabeya Y, Mizushima N, Ueno T, Yamamoto A, Kirisako T, Noda T, et al. LC3, a mammalian homologue of yeast Apg8p, is localized in autophagosome membranes after processing. EMBO J. 2000;19(21):5720–8.
Chen W, Sun Y, Liu K, Sun X. Autophagy: a double-edged sword for neuronal survival after cerebral ischemia. Neural Regen Res. 2014;9(12):1210–6.
Zhang N, Yin Y, Han S, Jiang J, Yang W, Bu X, et al. Hypoxic preconditioning induced neuroprotection against cerebral ischemic injuries and its cPKCgamma-mediated molecular mechanism. Neurochem Int. 2011;58(6):684–92.
Choi YH, Jin GY, Li LC, Yan GH. Inhibition of protein kinase C delta attenuates allergic airway inflammation through suppression of PI3K/Akt/mTOR/HIF-1 alpha/VEGF pathway. PLoS One. 2013;8(11):e81773.
Wang X, Yu W, Nawaz A, Guan F, Sun S, Wang C. Palmitate induced insulin resistance by PKCtheta-dependent activation of mTOR/S6K pathway in C2C12 myotubes. Exp Clin Endocrinol Diabetes. 2011;118(9):657–61.
Bu X, Zhang N, Yang X, Liu Y, Du J, Liang J, et al. Proteomic analysis of cPKCbetaII-interacting proteins involved in HPC-induced neuroprotection against cerebral ischemia of mice. J Neurochem. 2011;117(2):346–56.
Feng S, Li D, Li Y, Yang X, Han S, Li J. Insight into hypoxic preconditioning and ischemic injury through determination of nPKCepsilon-interacting proteins in mouse brain. Neurochem Int. 2013;63(2):69–79.
Wen YD, Sheng R, Zhang LS, Han R, Zhang X, Zhang XD, et al. Neuronal injury in rat model of permanent focal cerebral ischemia is associated with activation of autophagic and lysosomal pathways. Autophagy. 2008;4(6):762–9.
Tian Z, Wang C, Hu C, Tian Y, Liu J, Wang X. Autophagic-lysosomal inhibition compromises ubiquitin-proteasome system performance in a p62 dependent manner in cardiomyocytes. PLoS One. 2014;9(6):e100715.
Munoz A, Nakazaki M, Goodman JC, Barrios R, Onetti CG, Bryan J, et al. Ischemic preconditioning in the hippocampus of a knockout mouse lacking SUR1-based K(ATP) channels. Stroke. 2003;34(1):164–70.
Rodriguez R, Santiago-Mejia J, Gomez C, San-Juan ER. A simplified procedure for the quantitative measurement of neurological deficits after forebrain ischemia in mice. J Neurosci Methods. 2005;147(1):22–8.
Balkaya M, Krober J, Gertz K, Peruzzaro S, Endres M. Characterization of long-term functional outcome in a murine model of mild brain ischemia. J Neurosci Methods. 2013;213(2):179–87.
Broughton BR, Brait VH, Guida E, Lee S, Arumugam TV, Gardiner-Mann CV, et al. Stroke increases g protein-coupled estrogen receptor expression in the brain of male but not female mice. Neurosignals. 2013;21(3–4):229–39.
Tennant KA, Jones TA. Sensorimotor behavioral effects of endothelin-1 induced small cortical infarcts in C57BL/6 mice. J Neurosci Methods. 2009;181(1):18–26.
Lubjuhn J, Gastens A, Von WG, Bargiotas P, Herrmann O, Murikinati S, et al. Functional testing in a mouse stroke model induced by occlusion of the distal middle cerebral artery. J Neurosci Methods. 2009;184(1):95–103.
Chen J, Zhang C, Jiang H, Li Y, Zhang L, Robin A, et al. Atorvastatin induction of VEGF and BDNF promotes brain plasticity after stroke in mice. J Cereb Blood Flow Metab. 2005;25(2):281–90.
Yang X, Zhang X, Li Y, Han S, Howells DW, Li S, et al. Conventional protein kinase Cbeta-mediated phosphorylation inhibits collapsin response-mediated protein 2 proteolysis and alleviates ischemic injury in cultured cortical neurons and ischemic stroke-induced mice. J Neurochem. 2016;137(3):446–59.
Wang P, Liang J, Li Y, Li J, Yang X, Zhang X, et al. Down-regulation of miRNA-30a alleviates cerebral ischemic injury through enhancing beclin 1-mediated autophagy. Neurochem Res. 2014;39(7):1279–91.
Wei K, Wang P, Miao CY. A double-edged sword with therapeutic potential: an updated role of autophagy in ischemic cerebral injury. CNS Neurosci Ther. 2012;18(11):879–86.
Xingyong C, Xicui S, Huanxing S, Jingsong O, Yi H, Xu Z, et al. Upregulation of myeloid cell leukemia-1 potentially modulates beclin-1-dependent autophagy in ischemic stroke in rats. BMC Neurosci. 2013;14:56.
Levine B, Klionsky DJ. Development by self-digestion: molecular mechanisms and biological functions of autophagy. Dev Cell. 2004;6(4):463–77.
Maiuri MC, Zalckvar E, Kimchi A, Kroemer G. Self-eating and self-killing: crosstalk between autophagy and apoptosis. Nat Rev Mol Cell Biol. 2007;8(9):741–52.
Klionsky DJ, Emr SD. Autophagy as a regulated pathway of cellular degradation. Science. 2000;290(5497):1717–21.
Zhu C, Xu F, Wang X, Shibata M, Uchiyama Y, Blomgren K, et al. Different apoptotic mechanisms are activated in male and female brains after neonatal hypoxia-ischaemia. J Neurochem. 2006;96(4):1016–27.
Zhu C, Wang X, Xu F, Bahr BA, Shibata M, Uchiyama Y, et al. The influence of age on apoptotic and other mechanisms of cell death after cerebral hypoxia-ischemia. Cell Death Differ. 2005;12(2):162–76.
Adhami F, Liao G, Morozov YM, Schloemer A, Schmithorst VJ, Lorenz JN, et al. Cerebral ischemia-hypoxia induces intravascular coagulation and autophagy. Am J Pathol. 2006;169(2):566–83.
Koike M, Shibata M, Tadakoshi M, Gotoh K, Komatsu M, Waguri S, et al. Inhibition of autophagy prevents hippocampal pyramidal neuron death after hypoxic-ischemic injury. Am J Pathol. 2008;172(2):454–69.
Ginet V, Puyal J, Clarke PG, Truttmann AC. Enhancement of autophagic flux after neonatal cerebral hypoxia-ischemia and its region-specific relationship to apoptotic mechanisms. Am J Pathol. 2009;175(5):1962–74.
Liu C, Gao Y, Barrett J, Hu B. Autophagy and protein aggregation after brain ischemia. J Neurochem. 2010;115(1):68–78.
Nitatori T, Sato N, Waguri S, Karasawa Y, Araki H, Shibanai K, et al. Delayed neuronal death in the CA1 pyramidal cell layer of the gerbil hippocampus following transient ischemia is apoptosis. J Neurosci. 1995;15(2):1001–11.
Wang JY, Xia Q, Chu KT, Pan J, Sun LN, Zeng B, et al. Severe global cerebral ischemia-induced programmed necrosis of hippocampal CA1 neurons in rat is prevented by 3-methyladenine: a widely used inhibitor of autophagy. J Neuropathol Exp Neurol. 2011;70(4):314–22.
Puyal J, Vaslin A, Mottier V, Clarke PG. Postischemic treatment of neonatal cerebral ischemia should target autophagy. Ann Neurol. 2009;66(3):378–89.
Rami A, Langhagen A, Steiger S. Focal cerebral ischemia induces upregulation of Beclin 1 and autophagy-like cell death. Neurobiol Dis. 2008;29(1):132–41.
Zheng YQ, Liu JX, Li XZ, Xu L, Xu YG. RNA interference-mediated downregulation of Beclin1 attenuates cerebral ischemic injury in rats. Acta Pharmacol Sin. 2009;30(7):919–27.
Wong E, Cuervo AM. Autophagy gone awry in neurodegenerative diseases. Nat Neurosci. 2010;13(7):805–11.
Banerjee R, Beal MF, Thomas B. Autophagy in neurodegenerative disorders: pathogenic roles and therapeutic implications. Trends Neurosci. 2010;33(12):541–9.
Nixon RA. Autophagy in neurodegenerative disease: friend, foe or turncoat? Trends Neurosci. 2006;29(9):528–35.
Marino G, Madeo F, Kroemer G. Autophagy for tissue homeostasis and neuroprotection. Curr Opin Cell Biol. 2011;23(2):198–206.
He C, Klionsky DJ. Regulation mechanisms and signaling pathways of autophagy. Annu Rev Genet. 2009;43:67–93.
Keith CT, Schreiber SL. PIK-related kinases: DNA repair, recombination, and cell cycle checkpoints. Science. 1995;270(5233):50–1.
Raught B, Gingras AC, Sonenberg N. The target of rapamycin (TOR) proteins. Proc Natl Acad Sci U S A. 2001;98(13):7037–44.
Mora A, Komander D, van Aalten DM, Alessi DR. PDK1, the master regulator of AGC kinase signal transduction. Semin Cell Dev Biol. 2004;15(2):161–70.
Kawakami Y, Nishimoto H, Kitaura J, Maeda-Yamamoto M, Kato RM, Littman DR, et al. Protein kinase C betaII regulates Akt phosphorylation on Ser-473 in a cell type- and stimulus-specific fashion. J Biol Chem. 2004;279(46):47720–5.
Stephens L, Anderson K, Stokoe D, Erdjument-Bromage H, Painter GF, Holmes AB, et al. Protein kinase B kinases that mediate phosphatidylinositol 3,4,5-trisphosphate-dependent activation of protein kinase B. Science. 1998;279(5351):710–4.
Le XT, Nguyet Pham HT, Van NT, Minh NK, Tanaka K, Fujiwara H, et al. Protective effects of Bacopa monnieri on ischemia-induced cognitive deficits in mice: the possible contribution of bacopaside I and underlying mechanism. J Ethnopharmacol. 2015;164:37–45.
Acknowledgments
This work was supported by grants from the Seed Grant of International Alliance of Translational Neuroscience (PXM2014-014226-000006), Beijing Municipal Program for Hundred-Thousand-Ten Thousand Excellent Talents of New Century (Li J), Beijing Natural Science Foundation (7144188, 7132070, and 7141001), and National Natural Science Foundation of China (81301015, 81400948, and 31471142).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Ethical Approval
All animal procedures were performed in strict accordance with the recommendations in the Guide for the Care and Use of Laboratory Animals of the National Institutes of Health and approved by the Capital Medical University.
Conflict of Interest
The authors declare that they have no conflicts of interest.
Rights and permissions
About this article
Cite this article
Wei, H., Li, Y., Han, S. et al. cPKCγ-Modulated Autophagy in Neurons Alleviates Ischemic Injury in Brain of Mice with Ischemic Stroke Through Akt-mTOR Pathway. Transl. Stroke Res. 7, 497–511 (2016). https://doi.org/10.1007/s12975-016-0484-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12975-016-0484-4