Abstract
To evaluate the 2-year results obtained with self-expandable stent for chronic total occlusion (CTO) of the iliac artery, a retrospective study was performed of patients who underwent endovascular therapy (EVT) for chronic iliac artery CTO who presented from April 2007 to September 2012. 82 patients with 86 occluded iliac arteries underwent successful recanalization and stenting with a self-expandable stent. The primary equivalence end point was a composite of restenosis, mortality, target vessel revascularization, and limb salvage rates. Patients were followed up with the presence of a palpable femoral artery pulse, resolution of symptoms, and noninvasive vascular laboratory testing reviewed at 1, 3, and 6 months after EVT and then were evaluated at 6-month intervals. In patients who gave consent, repeat angiography was done in sixty-one of 86 lesions (70.1 %) for follow-up. The mean follow-up was at 27.6 ± 17.8 months (range 3–60 months). All stents were placed in the true lumen under intravascular ultrasound (IVUS) guidance. There were no cases of peripheral embolization or iliac artery rupture after the procedure. The ankle-brachial index increased significantly from 0.55 ± 0.19 to 0.88 ± 0.17 (P < 0.001). The primary patency rate was 96.5 % at 2 years. The MLD immediately after the procedure was 5.10 ± 0.26 mm and increased significantly to 5.40 ± 0.28 mm at the period of follow-up angiography. The 2-year outcome of endovascular therapy with self-expandable stents for CTO of the iliac artery had an acceptable result.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
The Transatlantic Inter-Society Consensus II (TASC II) was revised in 2007, with endovascular therapy for the iliac artery being established as a minimally invasive treatment option [1]. Stenting of chronic iliac occlusion is a safe and durable alternative to surgical treatment, especially in comparison with aortobifemoral bypass, and is also considered to be the primary modality for TASC A, B, and C lesions [2]. Several authors have reported suboptimal results when treating chronic total occlusion (CTO) of the iliac artery with stents because of difficulty in crossing heavily calcified lesions, as well as a higher incidence of major complications such as distal embolization and iliac artery rupture [3]. However, the outcome of primary stenting for iliac disease has continued to improve since Rees et al. [4] reported on this modality in 1989.
Endovascular treatment (EVT) for iliac artery disease is generally recognized as the first-line procedure among less invasive therapeutic options, even for chronic iliac occlusion [4–13]. Balloon-expandable stents are often used for the common iliac artery, but such stents are susceptible to extrinsic compression and deformation [9–14]. Fracture and restenosis of first-generation balloon-expandable stents placed in the external iliac artery has been reported [15]. However, there have been few reports regarding the results obtained with self-expanding stents for CTO of the iliac artery. Accordingly, the purpose of this study was to evaluate the 2-year results obtained with self-expandable stents for CTO of the iliac artery.
Methods
Subjects
Between April 2007 and September 2012, a retrospective study was carried out on consecutively presenting limbs, including CTO of the iliac artery that underwent primary stent placement at Saiseikai Yokohama city Eastern Hospital. Patients who could not have stent deployed were not eligible for this study. Only patients with chronic iliac artery occlusions and stable clinical symptoms of at least 3 months duration were included.
Eligible patients presented with lifestyle-limiting claudication. Patients who had undergone prior major amputation and those who presented with severe ischemia were not eligible for this study. The necessary clinical, perioperative, and demographic data were obtained through review of hospital and physician records. This study was approved by the hospital ethics committee and all patients gave informed consent.
Symptoms affecting the lower limbs were described according to the Fontaine classification. Preoperative evaluation included duplex scanning of the lower limb arteries, as well as computed tomography or arteriography. Vascular lesions were classified according to TASC II (2007).
Angiographic analysis
Preoperative and postoperative stenosis of the reference lumen was determined by digital angiography (Phillips Xper FD10, Royal Philips Electronics, Amsterdam, Netherlands) with a standard external reference device placed at the level of the target lesion for calibration. The minimum lumen diameter (MLD), percent diameter stenosis, and reference vessel diameter were measured before and after the procedure and at follow-up on a single matched image showing the smallest lumen diameter. Late loss was defined as the difference between the MLD immediately after the procedure and that at follow-up angiography.
Stenting procedure
All procedures were performed under local anesthesia in an angiography suite. Access was obtained via the ipsilateral, contralateral femoral artery, or a brachial artery through an introducer sheath ranging in size from 5 to7 F. A bilateral approach was used frequently. Patients were given 5,000 IU of heparin sodium before the procedure, and a bolus of 1,000 was added hourly during extended procedures. The retrograde approach was attempted from the ipsilateral femoral artery using a 0.018-in. spring guide wire with contralateral injection from the femoral artery or brachial artery. If the guide wire failed to cross the lesion from the retrograde approach, another approach (antegrade approach) was made from the contralateral femoral artery or brachial artery with 6-Fr Mach1IMA Catheter (Boston Scientific, Natick, MA). The guide wire was not stiff enough to penetrate the lesion and a stiffer wire was employed. If the wire crossed the lesion antegradely, this wire was pulled through from the ipsilateral sheath. After successfully crossing the guide wire to the lesion, we confirmed that the guide wire was in the true lumen in all cases with IVUS manually. Then, the lesion was predilated with an undersized balloon (4 or 5 mm). If the guide wire was not placed in the true lumen, we tried to get the true lumen with a 2nd wire by IVUS guidance. Extensive thrombus was detected in the occluded vessel by IVUS; we routinely compressed the popliteal artery manually for distal protection and performed thrombectomy at the end of the procedure.
All successful EVT cases were implanted with stent using self-expandable SMART stents (Cordis, Johnson & Johnson, Warren, NJ) with a diameter 1 or 2 mm larger than the reference vessel diameter at the lesion. Stents were placed retrogradely through a 6- or 7-Fr sheath in the ipsilateral femoral artery. We routinely performed balloon dilatation after stenting, 1 or 2 mm smaller than the reference vessel diameter. For cases with proximal lesions of the common iliac artery, we release the stent at about 1 cm and hold it in the aorta the first time. Then, we take off the system with contralateral injection to decide the landing position.
Preoperatively, all patients were started on 100 mg of aspirin and 200 mg of cilostazol or 200 mg of ticlopidine daily, and this treatment was continued indefinitely.
Follow-up and definition
Follow-up evaluations was performed before hospital discharge, at 1, 3, and 6 months and then at every 6-month intervals. The patients were followed up for the presence of a palpable femoral artery pulse, resolution of symptoms according to the criteria of Fontaine, and noninvasive vascular laboratory testing. Such testing included ankle-brachial index (ABI) and duplex ultrasound. Patients complaining of symptom recurrence or suspicion of restenosis on routine clinical follow-up were referred for repeat angiography. In patients who gave consent but without any symptoms, repeat angiography was also done at 12 months after EVT for follow-up.
Primary and secondary patency and limb salvage were determined in concordance with the Society for Vascular Surgery guidelines [16]. Loss of patency was determined by the loss of previously palpable pulses, recurrent symptoms, a decrease in ABI greater than 0.15, or a peak systolic velocity ratio greater than 2.5 times from Doppler ultrasound findings [16]. In-stent restenosis can be defined angiographically. Binary angiographic restenosis was defined as >50 % diameter stenosis in the stented segment. Indications for repeat EVT included recurrent symptoms, accompanied by loss of patency.
For IVUS analysis, heavy calcification was defined as more than 270 degrees of calcification at the occluded segment.
Statistical analysis
The results are expressed as average ± standard deviation. Discrete variables were evaluated using the Fisher exact test where applicable. Comparisons between pre- and post-MLD were computed by paired t test. The Kaplan–Meier method was used to determine patency and limb salvage and survival in the follow-up period. SPSS v.16.0 (SPSS Inc, Chicago, IL, USA) was used for all statistical calculations. Individual differences were considered to be statistically significant for P < 0.05.
Results
Patient and lesion characteristics
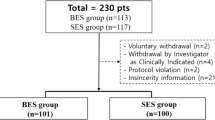
Figure 1 shows the participants’ flowchart of this study. During the study period, 309 patients (348 limbs) had an iliac artery lesion and 92 (97 limbs) of them had an iliac CTO lesion. Successful EVT cases of 82 patients (86 limbs) were eligible for this study. The patient characteristics of 82 patients are summarized in Table 1. The population of patients with diabetes mellitus was 50 (61.0 %). The mean ABI was 0.56 ± 0.21 at presentation.
Lesion characteristics are summarized in Table 2. According to the TASC II classification, 34 limbs (39.5 %) were classified as type B, 22 (25.6 %) as type C, and 30 (34.9 %) as type D.
Procedural results
Fifteen lesions (17.4 %) were treated by the retrograde approach via the ipsilateral femoral artery with contralateral injection from the femoral artery or brachial artery. Seventy-one lesions (72.6 %) required multiple access sites, bifemoral in 47 lesions (54.7 %), femoral/brachial in 20 lesions (23.3 %), and femoral/popliteal in 4 lesions (4.7 %).
One hundred and twenty-one stents were successfully deployed in the 86 lesions: 39 in the external iliac artery, 60 in the common iliac artery, and 22 across both arteries. Every stent was deployed in the true lumen. Twenty-eight lesions required more than one stent due to the length of the obstruction. In these patients, the overlap was approximately 10 mm. Fourteen stents were placed at the ostium of the common iliac artery. The diameter of the stents ranged from 6 to 10 mm (median 8.5 ± 1.4 mm), and the length ranged from 40 to 100 mm (median 64.2 ± 23.4 mm) (Table 3).
During the study period, the ABI and symptoms based on the Fontaine criteria were significantly increased after the procedure (Table 4). After stent implantation, ABI was significantly increased from 0.51 ± 0.23 to 0.89 ± 0.18 (P < 0.001) (Fig. 2a). Fontaine grade was also significantly decreased from 2.68 ± 0.84 to 1.11 ± 0.31 (P < 0.001). (Fig. 2b).
Complications
There were no cases of peripheral embolization or iliac artery rupture after the procedure. There were also few puncture site complications, perhaps owing to the use of a closure device (Angio-Seal STS Plus, SJM, St. Paul, MI). Two hematomas occurred, but these could be treated conservatively and no patient required surgery. Thus, the complication rate was 2.4 %.
Follow-up
In the period of 2-year follow-up, ten patients (12.1 %) died at a median of 8.4 month after stent implantation (range 1–18) from unrelated causes. The survival rate was 89.5 %. In 57 patients of angiographic outcome, 4 patients had in-stent restenosis. Three of these patients required an additional procedure within the first year of follow-up. Thus, the primary patency rate was 96.5 % (Fig. 3). The target lesion of these 3 cases were common iliac artery. One case of reocclusion and two cases of restenosis were treated with endovascular treatment by using balloon and/or additional stent implantation. These 3 cases were successfully revascularized and the secondary patency rate was 100 %. The predictor of primary patency was not detected from this study.
Amputation was not required for any of the limbs.
Angiographic outcome
Every patient had angiographic review. The length of occlusion revealed that it was 0–50 mm in 36 limbs, 50–100 mm in 33 limbs, and >100 mm in 17 limbs. The mean length of the occlusion was 65.0 ± 26.9 mm. The mean reference diameter was 6.8 ± 1.9 mm and the mean MLD after the procedure was 5.10 ± 0.26 mm.
Follow-up angiography was also done after the procedure in 57 patients (61 limbs 70.9 %) 85 stents (70.2 %) who gave consent at a mean of 14.5 ± 9.5 months after the procedure. At the time of angiographic review, the proximal reference diameter was 8.4 ± 1.4, the distal reference diameter was 6.0 ± 1.2, and the mean reference diameter was 7.0 ± 1.6 mm. The MLD was significantly increased from 5.10 ± 0.26 to 5.40 ± 0.28 mm (P < 0.05) (Fig. 4). The angiography of a typical case is shown in Fig. 5. Late loss was −0.53 ± 2.50 mm. There were no cases of stent fracture from follow-up radiography.
Discussion
The importance of percutaneous interventional procedures has been reinforced by the Transatlantic Inter-Society Consensus II (TASC II) Report [1]. Many investigators have reported excellent results after primary stenting of aorto-iliac denovo lesions. Retrospective studies have shown that the primary patency after stenting of these lesions is 86.3 % at 2 years and 73.1 % at 6 years, and secondary patency is 98.9 % at 2 years and 98.5 % at 6 years. [17] A randomized controlled trial (the Dutch Iliac Stent Trial) also showed that the 2-year/primary patency rate was 71 % for selective stenting and 70 % for primary stenting [18]. However, these studies also included patients with iliac artery stenosis and those with iliac CTO were treated by subintimal angioplasty. In this study, we evaluate the procedural and 2-year results of primary treatment for iliac artery CTO with self-expandable stents placed in the true lumen.
Initial success and primary patency rates
Initial success rate for iliac CTO of 92 patients was 89.1 % (82/92 cases). Seven of 10 failed cases were successfully wire crossing, but any device could not cross the lesion. The other 3 cases of the lesions could not cross the wire. Those 3 cases referred to bypass surgery. The other 7 cases received medical therapy.
Several authors reported on the outcome after treatment of more complex iliac occlusions (TASC-C and TASC-D). Review of the data published since 1995 shows that the primary patency rate ranges from 69 to 76 % at 2 years, with the secondary patency rate being 85 to 95 % at that time [6, 9, 11]. Overall complication rates have also been low in recent series (1.4–4.8 %), probably because of improvements in the techniques and devices employed. In a series of 212 patients with chronic iliac occlusion, successful recanalization was achieved for nearly 90 % and marked clinical improvement in the vast majority of patients [8]. Also, the primary patency rate at 4 year was 75.7 %.
In this series, the primary patency rate was 95.9 % and secondary patency rate was 100 % at 2 years. These results were better than previous report. Most of the other reports were based on subintimal stenting [19]. Muramatsu et al. [20] reported that IVUS-guided intervention should be useful for indicating the re-entry site for the retrograde guide wire in many patients. IVUS may help the wire into the true lumen even in the case of retrograde the approach. We placed the stent after confirming by IVUS that the wire was in the true lumen by IVUS. Our results suggest that employing IVUS guidance for EVT may increase the stent patency rate. In femoropopliteal artery disease, Soga et al. [21] reported that stent diameter/vessel diameter (S/V) ratio was an independent predictor of in-stent restenosis after stenting, and it was also associated with the clinical outcome. In this series, we selected the 1- or 2-mm larger stent than the reference vessel diameter at the lesion from IVUS findings. However, the S/V ratio did not predict restenosis and clinical outcome.
Higashiura et al. [22] reported that chronic occlusion of the iliac artery is a risk factor for stent fracture. However, fracture of stents placed in this artery rarely affects patency. Stents for iliac CTO placed subintimally can suffer from eccentric compression by plaque and thrombus in the true lumen and adopt an elliptical shape that could alter their rigidity. Then, extrinsic or intrinsic forces acting on the stent might cause fracture. In contrast, we did not experience stent fracture after follow-up for an average of 27.6 months, suggesting that stent implantation in the true lumen decreases the fracture rate.
Complications
It has been reported that iliac artery rupture may occur due to subintimal passage of the guide wire or overdilation of the vessel by the balloon catheter. This is a rare, but potentially devastating complication [8, 9, 11, 12].
In the present series, we chose the diameter of the balloon and stent based on IVUS findings and used self-expandable stents. If a large amount of thrombus was seen in the occluded vessel on IVUS, we routinely compressed the common femoral artery manually for distal protection and then performed thrombectomy via the sheath inserted into the ipsilateral femoral artery. Consequently, we had no cases of peripheral embolization or iliac artery rupture.
Angiographic findings
In 56 patients (59 limbs, 68.7 %), follow-up angiography was done after a mean of 14.4 ± 9.4 months. The MLD immediately after the procedure was 5.10 mm and was increased significantly to 5.40 mm at the period of follow-up angiography. This result suggests that self-expandable stents when placed in the true lumen continue to expand to the optimum diameter over time after insertion.
Study limitations
Although the data were collected prospectively, this was a retrospective single-center analysis. Also, this study was limited to patients who underwent EVT and provides no data on the outcome for management of CTO with medical therapy or surgical revascularization. Moreover, the small sample size may have influenced the results of the statistical analysis.
Conclusion
In conclusion, this study showed that an acceptable 2-year outcome of EVT for iliac CTO was achieved by implantation of self-expandable stents in the true lumen.
References
Norgren L, Hiatt WR, Dormandy JA, Nehler MR, Harris KA, Fowkes FG. Inter-Society Consensus for the Management of Peripheral Arterial Disease (TASC II). J Vasc Surg. 2007;45(Suppl):S5–67.
Motarjeme A, Keifer JW, Zuska AJ. Percutaneous transluminal angioplasty of the iliac arteries: 66 experiences. AJR. 1980;135:934–44.
Ring EJ, Freiman DB, McLean GK, Schwarz W. Percutaneous recanalization of common iliac artery occlusions: unacceptable complication rate? AJR. 1982;139:587–9.
Rees CR, Palmaz JC, Garcia O, Roeren T, Richter GM, Gardiner G Jr, et al. Angioplasty and stenting of completely occluded iliac arteries. Radiology. 1989;172:953–9.
Vorwerk D, Gunther RW, Schurmann K, Wendt G, Peters I. Primary stent placement for chronic iliac artery occlusions: follow-up in 103 patients. Radiology. 1995;194:745–9.
Dyet JF, Gaines PA, Nicholson AA, Cleveland T, Cook AM, Wilkinson AR, et al. Treatment of chronic iliac artery occlusions by means of percutaneous endovascular stent placement. J Vasc Interv Radiol. 1997;8:349–53.
Reyes R, Maynar M, Lopera J, Ferral H, Gorriz E, Carreira J, et al. Treatment of chronic iliac artery occlusions with guide wire recanalization and primary stent placement. J Vasc Interv Radiol. 1997;8:1049–55.
Scheinert D, Schröder M, Ludwig J, Bräunlich S, Möckel M, Flachskampf FA, et al. Stent-supported recanalization of chronic iliac artery occlusions. Am J Med. 2001;110:708–15.
Uher Petr, Nyman Ulf, Lindh Mats, et al. Long-term results of stenting for chronic iliac artery occlusion. J Endovasc Ther. 2002;9:67–75.
Funovics MA, Lackner B, Cejna M, Peloschek P, Sailer J, Philipp MO, et al. Predictors of long-term results after treatment of iliac artery obliteration by transluminal angioplasty and stent deployment. Cardiovasc Interv Radiol. 2002;25:397–402.
Carnevale FC, De Blas M, Merino S, Egana JM, Caldas JGMP. Percutaneous endovascular treatment of chronic iliac artery occlusion. Cardiovasc Interv Radiol. 2004;27:447–52.
Gandini R, Fabiano S, Chiocchi M, Chippa R, Simonetti G. Percutaneous treatment in iliac artery occlusion: long-term results. Cardiovasc Interv Radiol. 2008;31:1069–76.
Greiner A, Dessl A, Klein-Weigel P, Neuhauser B, Perkmann R, Waldenberger P, et al. Kissing stents for treatment of complex aorto-iliac disease. Eur J Vasc Endovasc Surg. 2003;26:161–5.
Kondo Y, Alan D, Muto A, et al. Primary stent placement for iliac artery chronic total occlusions. Surg Today. 2010;40:433–9.
Sacks BA, Miller A, Gottlieb M. Fracture of an iliac artery Palmaz stent. J Vasc Interv Radiol. 1996;7:53–5.
Rutherford RB, Baker JD, Ernst C, et al. Recommended standards for reports dealing with lower extremity ischemia: revised version. J Vasc Surg. 1993;17:1103–7.
Soga Y, Iida O, Kawasaki D, et al. REAL-AI investigators. Contemporary outcomes after endovascular treatment for aorto-iliac artery disease. Circ J. 2012;76:2697–704.
Klein WM, van der Graaf Y, Seeger J, Spithoven JH, Buskens E, van Baal JG, et al. Dutch iliac stent trial: long-term results in patients randomized for primary and selective stent placement. Radiology. 2006;238:734–44.
Jacobs DL, Motaganahalli RL, Cox DE, Wittgen CM, Peterson GJ. True lumen re-entry devices facilitate subintimal angioplasty and stenting of total chronic occlusions: initial report. J Vasc Surg. 2006;43:1291–6.
Muramatsu T, Tsukahara R, Ito Y, et al. A novel intravascular ultrasound-guided percutaneous coronary angioplasty technique via the retrograde approach for chronic total occlusion. Cardiovasc Interv Ther. 2011;26:45–51.
Soga Y, Yokoi H, Urakawa T, Iwabuchi M, et al. Clinical impact of self-expandable stent diameter after femoropopliteal stenting. Cardiovasc Interv Ther. 2011;26:38–44.
Higashiura W, Kubota Y, Sakaguchi S, Kurumatani N, et al. Prevalence, factors, and clinical impact of self-expanding stent fractures following iliac artery stenting. J Vasc Surg. 2009;49:645–52.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Araki, M., Hirano, K., Nakano, M. et al. Two-year outcome of the self-expandable stent for chronic total occlusion of the iliac artery. Cardiovasc Interv and Ther 29, 40–46 (2014). https://doi.org/10.1007/s12928-013-0210-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12928-013-0210-z