Abstract
Limited studies have examined the pre-counselling knowledge and attitudes of high-risk women on hereditary breast and ovarian cancer (HBOC) syndromes genetic screening in Asia Pacific regions, particularly among Chinese. After controlling cost, an intrinsic barrier to undertake such screening, comprehensive understanding of the baseline characteristics of this cohort towards HBOC genetic counselling and testing service (GT) could be sought. This study aimed at exploring the baseline knowledge, possible motivators, barriers, and decisional factors of undertaking such service. One hundred and forty-two Southern Hong Kong Chinese high-risk females (89.4% with cancer history; 10.6% were cancer-free at-risk family members) completed a questionnaire right before their pre-testing GT. Results showed that perceived benefits to self and family members with reference to cancer prevention are important decisional motivators. A sponsored cancer genetic testing service in this cohort was crucial as 71.3% would not have opted for self-financed screening. Pre-testing and post-testing counselling were essential, particularly for older and less educated high-risk individuals. More importantly, after thorough pre-counselling with Q&A session, the entire cohort in this study gave written consent to undertake GT. Moreover, those proven to be germline pathogenic variant carriers were willing to share the information with family members and successfully persuaded them to pursue GT.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Breast cancer remains one of the most common causes of cancer mortality in women worldwide (Bray et al. 2018). According to the most updated cancer statistics in Hong Kong, breast cancer accounts for 26.6% of all newly diagnosed cancers and 12.2% of all cancer deaths among females in 2016 (Hong Kong Cancer Registry, Hospital Authority 2019). Global data estimated that 5–10% of breast and ovarian cancer patients are genetically predisposed, of which majority is due to germline pathogenic variant of BRCA1/2 genes. It is the interactions of the risk predisposed by the genes with pathogenic variants, with environmental and lifestyle modifiers which result in cancer (Antoniou et al. 2003; Coyle 2009; King et al. 2003).
Asian data on hereditary breast and ovarian cancer (HBOC) has become gradually available in the past decade. Studies on Hong Kong Chinese cohort showed that around 10% of breast cancers are genetically predisposed (Kwong et al. 2016a; Kwong et al. 2016b). A most recent study on 1635 Hong Kong Chinese families with HBOC estimated that BRCA1/2-associated breast cancer risk for Chinese women is similar to that for Caucasian women, although BRCA1/2-associated ovarian cancer risks are lower for Chinese women (Zhang et al. 2018).
The demands of cancer genetic screening have increased worldwide due to the availability of new gene sequencing techniques, multi-gene panel testing, and the increasing evidence on the efficacy of targeted therapy for pathogenic variant carriers such as PARP Inhibitors (Colas et al. 2019; Domchek et al. 2016; Kaufman et al. 2015; Litton et al. 2018; Robson et al. 2017). In Hong Kong, females constitute over 98% of HBOC genetic screening enquirers and referrals (Kwong et al. 2014). Although the availability of cancer genetic screening has improved, psychosocial factors like pre-testing genetic knowledge, motivators like perceived benefits, and efficacy of test results still have decisive impact on the utilization of the test.
Unique circumstances in Asian countries
There is comparatively limited data available on these issues comparing Asian populations with their western counterparts. Asian societies have been characterized by lower availability for genetic testing services and a relatively conservative culture, which affected the utilization of genetic testing (Chin et al. 2005). Many studies listed cost and financial difficulties to be intrinsic barriers for undertaking cancer genetic screening in Asia (Chieng and Lee 2006; Chin et al. 2005; Lee et al. 2002).
Previous Asian studies showed that despite removing the cost barrier, more than half of eligible patients still declined cancer genetic testing. This suggests that other important barriers exist despite the perception that Asian cultures are generally family-oriented, and more than 50% of high-risk females who underwent genetic counselling cited “helping the family” to be a leading motivator (Chin et al. 2005; Nakagomi et al. 2016).
Contrary to cultural beliefs, a survey on the decliners in Singapore revealed strong reluctance to involve family members. The influence of being family-oriented is found to be underscored by the fact that more than 80% of respondents were put in a hypothetical situation (Chin et al. 2005). When it comes to actual genetic screening and the real possibility of involving family members for predictive testing, two fifths of the decliners mentioned difficulties in communicating the issue with family, and siblings/family members (especially cancer-free ones) were not keen to get tested. While over 80% of patients counselled were willing to share genetic information with spouses and siblings, only about 70% were willing to involve the older generation. A study in Japan echoed this finding (Nakagomi et al. 2016). After controlling cost and feasibility of getting genetic counselling, around 20% of the patients still refused testing. Older and more traditional Asian sub-urban communities might still be hesitant to discuss cancer issues within families (extended families particularly) due to “bad luck” and their concern about discrimination.
Purpose of the study
Chinese constitute the biggest population in Asia. Most studies on BRCA pathogenic variant spectrum in Chinese populations were performed in single institution or small centres. Studies exploring the psychosocial factors related to cancer genetic testing on Chinese are even less available. China does not have a centralized census system on cancer statistics which makes comprehensive estimation of cancer prevalence rate nationally difficult. In addition, the One Child Policy in Mainland China that started in the late 1970s limits the number of relatives available for genetic studies affect the estimation of BRCA1/2 prevalence. “One country, two systems” is a unique constitutional principle formulated for the reunification of China during the early 1980s. It suggests that there would be only one China, but distinct Chinese regions such as Hong Kong and Macau could retain their own capitalist economic and political systems. Under the principle, Hong Kong, which was a previous British Colony and now belongs to a Special Administrative Region of the People’s Republic of China after 1997, continues to have its own political, legal, and social system including healthcare systems.
The most updated statistics showed that Hong Kong has a population of over 7.4 million. According to the 2016 by-census, 92% of the Hong Kong population is ethnic Chinese and 8% are other ethnic groups (Nakagomi et al. 2016). It has a well-established healthcare system which consists of public hospitals managed by the Hospital Authority which is used by 85% of the entire population, and a smaller percentage of the population has access to private medical services. Hong Kong is therefore a strategic location to conduct studies of hereditary cancers on Southern Chinese populations.
The present research is the first in China region to systematically explore the psychosocial factors affecting HBOC GT utilization among at-risk females. The results of the study would provide insights on understanding Chinese patients’ baseline knowledge and attitudes towards genetic screening.
Methods
Participants
Participants eligible for HBOC GT were recruited through the Hong Kong Hereditary Breast Cancer Family Registry. They were all adult women, either with breast and/or ovarian cancer, other related cancer history themselves, or cancer-free at the time with family members who have already been proven to be BRCA1/2 germline pathogenic variant carriers. The study took place at an outpatient high-risk breast clinic of breast surgery under a research programme located in Tung Wah Hospital, an academic-affiliated public hospital, in which services are mostly sponsored. Its multidisciplinary service includes experts like genetic counsellor, geneticists, oncologists, and nurses. The team also provides long-term surveillance, clinical management, prophylactic surgeries, and psychological support for confirmed HBOC carriers. The patient recruitment criterion, procedures, and the high-risk clinic service flow has previously been reported in details by our group (Kwong et al. 2010). The present study was a nested study from a larger on-going cohort study. Its study time point marked the baseline status at participants’ first pre-testing genetic counselling consultation at the high-risk clinic (with data collection cutoff time from early 2016 to mid-2018).
Instrumentation
The present study used the form of a standardized written survey. A pilot focus group on 23 female breast cancer patients served as a blueprint to develop different questions and possible choices of answers for selected questions.
Demographics and general information
The questionnaire started with enquiries on demographic information including nationality, education level, occupation, marital status, and monthly family income. Specific questions on whether they were experiencing any financial difficulties or receiving social welfare subsidies were explored. The way participants obtain information about genetic screening and testing service of HBOC and their source of referrals were collected.
Cancer history
Participants’ cancer history and treatment received were obtained.
Attitudes and opinions on genetic testing
Participants’ attitudes and opinions on genetic testing were explored. Participants rated nine items on attitudes and opinions with a 5-point Likert scale from 1 (totally disagree) to 5 (totally agree). Examples of questions were like, to what degree “I think undergoing genetic testing for hereditary breast and ovarian cancer can help me to prevent cancer”. Three additional questions asked about the preference of sharing genetic results with family, taking the test at own costs, and consideration of making a donation to the Hereditary Breast Cancer Family Registry, a charitable organization which subsidizes genetic testing.
Knowledge on hereditary breast and ovarian cancer
Seven true-false (or I do not know) items and two additional items assessing the name of HBOC related genes and types of cancer were used assessing participants’ knowledge level on HBOC (Table 1). The total score of each participant was converted to percentage score before analyses.
Intention to share test results
Three questions were designed to explore participants’ intention and willingness to share their genetic test results, the reasons why or why not, and who they planned to share it with.
Costs as potential barrier
Questions of whether the participants were still willing to undertake such genetic testing service if they were to bear their own costs and the reasons why or why not were explored.
Procedures
Ethical approval had been obtained from the Institutional Review Board (IRB) of The University of Hong Kong before the recruitment of the subjects. One trained research assistant explained the purpose and format of the study and obtained written informed consent from all participants. They then filled in a standardized 5-page questionnaire (with assistance provided by the Research Assistant whenever necessary). After the participants finished filling in the questionnaire, they were all given an hour of face-to-face pre-testing genetic counselling in a group of 6–8 by a cancer genetic counsellor with reasonable time for clarifying misconceptions and answering questions. They were then given time to consider undertaking the genetic testing on the spot after written informed consents were obtained.
Data analysis
All statistical analyses were performed using the Statistical Package for Social Sciences 21.0. A significance level of .05 was adopted. The cancer and cancer-free groups were compared on their demographic variables using independent sample t-tests and chi-square test. Partial correlations were conducted to examine the relationship between the items in the attitudes and opinions on genetic testing. Analysis of variance (ANOVA) was utilized to compare the knowledge scores on HBOC in different age groups and education levels. Chi-square tests were employed to investigate the relationship between choices of taking genetic test, and related variables such as income and cancer history.
Results
Demographics
A total of 147 females were recruited in the present study, and 5 with uncompleted data were excluded. Descriptive statistics for the remaining 142 participants are presented in Table 2. All Probands were diagnosed with breast cancer, ovarian cancer, breast and ovarian cancers, and other cancers, while 17 (53%) were their cancer-free family members. The average age was 43.9 (SD = 10.7, ranged 18–69). The cohort was predominantly secondary/high school (46.5%) or tertiary (40.1%) educated. There was no significant difference between the cancer group and the cancer-free group in age and education levels.
The median monthly family income of the present cohort was in the range of HKD $20,000–29,999, which is slightly lower than the population in Hong Kong Census Data. Family income did not differ across the cancer and cancer-free groups.
Among the index patients (Probands), 84 (73.7%) obtained information about and referral for genetic testing from a specialist, while the remaining were recruited during educational campaigns, or self-referred by calling the service hotline of The Hong Kong Hereditary Breast Cancer Family Registry. Although family members learnt about genetic testing mostly from Probands (53.1%), some also obtained referral from specialists (37.5%).
Cancer prevention by GT and perceived family risks
Majority (80.3% and 90.8%, respectively) believed that GT allowed her and her family to prevent cancer. While 87.3% of them agreed that a negative GT result could bring relief, 84.5% worried about possible psychological distress if they were found to be a pathogenic variant carrier. Half of the participants estimated that cancer risk of their family members was high, with 88.0% believing that this risk was related to their family cancer history. After controlling the effect of cancer history, correlation analyses revealed a positive correlation between the belief that GT can prevent cancer for family members and family cancer risk perception (r = .389, p < .001), with greater confidence in the ability of GT in cancer prevention for family members associating with a higher perceived family cancer risk. Correlation analyses also revealed that the trust in GT increased with the perceived self-cancer risk based on family cancer history (r = .539, p < .001).
To share or not to share genetic test results—intention vs reality
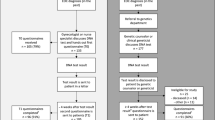
During the pre-counselling stage, 97.3% of the Probands (112 out of 142 participants were Probands) responded in a hypothetical question that they would be willing to share their GT results with their at-risk family members if they received positive results. The research team did a follow-up check after the initial data collection. All participants underwent genetic testing after thorough genetic counselling. Consistent with their stated intention, all of those who were later confirmed to be a pathogenic variant carrier or with a variant of uncertain significance (VUS) that need further investigation did communicate their genetic findings with family members. While 17 of them were found to be pathogenic variant carriers, 7 were carrying a VUS. Other than those who did not have living family members (n = 1), no biological family members (n = 1), or no family members in Hong Kong (n = 3), 16 out of 19 (84.2%) of these patients whose family members were at-risk and eligible for predictive test did successfully asked more than one family member to come for genetic counselling and predictive test (Fig. 1).
Knowledge on hereditary breast and ovarian cancer
A total score of 100 was calculated for each participant and the mean score on these items assessing basic genetic knowledge was 62.6. Only 5 participants obtained full scores. Majority scored correctly on the question regarding whether males have risk of breast cancer. 69.7% could not name any genes that were related to HBOC. Item 6 and 7 reflected that over half of the participants lacked accurate knowledge on basic principles on inheritance (Table 2). No significant difference was found between participants with and without cancer history in all items.
A correlation analysis showed that younger age was associated with overall higher scores of genetic testing knowledge (r = − .337, p < .001). An ANOVA also revealed that genetic testing knowledge was associated with education level, F(2, 141) = 10.4, p < .001. Post-hoc comparisons with Bonferroni adjustments showed significant differences between primary- and secondary school levels (p < .005), secondary school and tertiary education levels (p < .05), and primary school and tertiary education levels (p < .001) on baseline genetic knowledge at pre-counselling stage.
The questions on participants’ genetic knowledge could be divided into three categories: (1) general knowledge on breast cancer, (2) basic scientific knowledge on the inheritance of related gene, and (3) implications of the pathogenic variants towards one’s and family’s risk. Figure 2 showed the mean scores of the three categories. While participants with tertiary education levels scored highest in all three categories (with mean scores of 77.8, 71.9, and 50.9, respectively), those with primary school education levels scored lowest. An ANOVA revealed that the three groups scored significantly differently on category 2 questions, F(2, 141) = 12.0, p < .001. Post-hoc comparisons with Bonferroni adjustments showed that the significant differences lied between tertiary and primary school levels (p < .001) and tertiary and secondary school levels (p < .005).
Financial concerns
When being asked if they would consider undertaking HBOC GT at their own costs with a total service package fee of around HKD18,000 (a figure derived from comparisons of private HBOC GT with surgeon and genetic counsellor consultation at the time of data collection), 71.3% participants answered “No”. The median monthly income of our sample fell in the group “HKD20,000 – 29,999”, which was slightly lower than the median monthly income of HKD 32,000 for 3-person household (the median household size) of general public in Hong Kong (Census and Statistics Department 2018). As anticipated, 90.7% stated “expensive” or “cannot afford” as the main reasons. “Unnecessary” was the second reason though it only accounted for 5.5%. There was no significant relationship between one’s cancer history and the choice of self-financing the test (χ2 = .004, p = .947). Even though the majority would not opt for self-financing their own genetic screening, they (73.5%) would consider donating to the Hereditary Breast Cancer Family Registry—the charity that sponsored their screening.
Discussion
This study investigated the baseline knowledge level, attitudes, and receptiveness to HBOC GT. The findings indicated that this cohort, which mainly included breast cancer female patients and their female family members eligible for high-risk genetic screening, possessed a high receptive attitude towards a sponsored genetic counselling and testing programme. Only an insignificant portion of participants showed reservations to possible genetic test results at pre-counselling stage and most participants believed in the benefits of genetic testing, both to oneself and family members. The entire cohort opted for the actual testing after thorough counselling showed the crucial role professional genetic counselling service plays.
The overall performance on the HBOC knowledge test during pre-counselling stage was just average. The limited correct answers on various items revealed the lack of understanding or/and misunderstanding on the basic mechanics of cancer genetics, inheritance pattern, and the risk of HBOC. Younger and better educated participants scored higher in the knowledge test as expected. Due to the strong linear nature of the age-knowledge level relationship, it was hard to succinctly define a particular age group who did worst. Those who only received primary or elementary education scored relatively worse. Patients with poorer knowledge about breast cancer genetics would have lower interest in such service (Lipkus et al. 1999; Sussner et al. 2013; Thompson and Easton 2002). Thorough counselling with educational materials suitable to their needs is thus predominantly essential to older and less educated patients.
This cohort mainly obtained information and referral for GT from specialists. Studies also demonstrated that population relied heavily on health care providers on such information (Armstrong et al. 2015; Cragun et al. 2017). With a huge responsibility of providing accurate and timely referral of genetic screening to patients, specialists must enhance their knowledge on GT. Multilevel interventions including effective education and public promotion would help reduce the dependence on specialists.
All genetic tests of this cohort were sponsored. Financial consideration was stated as the main obstacle for this cohort if they undertake self-financed GT, especially for the relatively lower income of our sample compared to the public. Previous studies had identified this financial barrier resisting uptake of GT in various communities (Cragun et al. 2015; Hayden et al. 2008; Rajpal et al. 2017). The high rate of not considering self-financed GT also appeared in higher income groups. While there was no difference between cancer and cancer-free groups, the financial barrier was not necessarily due to medical expense for cancer management. Hong Kong has a well-established public sector; medical care is provided with a very minimal cost for general local population. Although there is increased use of the private sector and access to insurance coverage, most insurance do not cover for GT costs. These factors may also contribute to the reluctance of self-financed testing. Another reason was that most participants finished active cancer treatment by the time the specialists introduced genetic testing. They may be unable to rationalize the immediate benefits of GT on their cancer management and hence will not consider it as a priority.
Clinical implications
When considering GT, participants concerned more on the benefit to their family rather than themselves. The result is compatible with their Chinese counterparts in China with reference to cultural characteristic of interdependence in families (Ho 1974; Markus and Kitayama 1991). Previous study with Chinese subjects had demonstrated larger concern on well-being of significant others than of oneself when deciding to obtain and inform family about genetic results (Ho et al. 2003). Our follow-up works also showed that for those proven to be pathogenic variant gene carrier, 100% of them successfully invited eligible family members to attend predictive-test counselling and testing. The enthusiasm in sharing genetic results to family members enabled the extensiveness of our predictive tests utilization. Shared decision-making with an emphasis on patients and their families’ perceptions are crucial to build good clinical rapport for this cohort. Public awareness campaign marketing strategies should take the strong bonding within Hong Kong Chinese family into considerations.
Study limitations
The present cohort included high proportion of women with cancer history so it might influence the results for cancer-free relatives. However, these eligible cancer-free relatives were either from a family with an identified carrier, or strong cancer history so the effects might have been adjusted naturally. Future studies can aim for a statistically stronger comparison study. This study is not a longitudinal one and data collection was completed before PARP inhibitors, a specific targeted therapy that is beneficial to BRCA1/2 mutation carriers are made available to the public. Future research may obtain different results guided by the need for genetic testing for therapeutic purposes. In addition, this cohort mainly consisted of Chinese females in Hong Kong. Our Shenzhen clinic may contribute to evaluating the differences among females from different regions in the whole China in this area.
Conclusion
The present study showed that a sponsored cancer genetic testing service is important for this cohort. Pre-testing and post-testing genetic counselling was crucial, particularly for older and less educated high-risk individuals. Despite having inadequate knowledge in HBOC, 100% of participants gave written consent to undertake GT after thorough counselling by a professional genetic counsellor. Perceived benefits to self as well as family members with reference to cancer prevention and management are important decisional motivators. Results also showed that this cohort, if proven to be gene mutation carriers, were willing to share the information with family members and could even successfully persuade them to seek genetic counselling and testing service. Shared decision-making and emphasis on patient and family’s goals and preferences are important for Chinese cohorts. These findings showed that this Hong Kong cohort share similar decisional considerations with their China counterparts and more receptive in sharing genetic results to extended family members compared with some Southeast Asia counterparts (e.g. Singapore and Japan) mentioned in previous studies. The above insights can advise the design of client-centred cancer genetic screening services and family-oriented strategic public awareness campaign to meet the specific needs for Chinese and Asian communities.
References
Antoniou A, Pharoah PDP, Narod S, Risch HA, Eyfjord JE, Hopper JL, Loman N, Olsson H, Johannsson O, Borg Å, Pasini B, Radice P, Manoukian S, Eccles DM, Tang N, Olah E, Anton-Culver H, Warner E, Lubinski J, Gronwald J, Gorski B, Tulinius H, Thorlacius S, Eerola H, Nevanlinna H, Syrjäkoski K, Kallioniemi OP, Thompson D, Evans C, Peto J, Lalloo F, Evans DG, Easton DF (2003) Average risks of breast and ovarian cancer associated with BRCA1 or BRCA2 mutations detected in case series unselected for family history: a combined analysis of 22 studies. Am J Hum Genet 72:1117–1130. https://doi.org/10.1086/375033
Armstrong J et al (2015) Utilization and outcomes of BRCA genetic testing and counseling in a national commercially insured population: the ABOUT study. JAMA Oncol 1:1251–1260. https://doi.org/10.1001/jamaoncol.2015.3048
Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A (2018) Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 68:394–424. https://doi.org/10.3322/caac.21492
Census and Statistics Department (2018) Hong Kong in figures. Hong Kong
Chieng WS, Lee SC (2006) Establishing a cancer genetics programme in Asia - the Singapore experience. In: Hered Cancer Clin Pract 4(3):126–135. https://doi.org/10.1186/1897-4287-4-3-126
Chin TM, Tan SH, Lim SE, Iau P, Yong WP, Wong SW, Lee SC (2005) Acceptance, motivators, and barriers in attending breast cancer genetic counseling in Asians. Cancer Detect Prev 29:412–418. https://doi.org/10.1016/j.cdp.2005.06.009
Colas C, Golmard L, dePauw A, Caputo S, Stoppa-Lyonnet D (2019) “Decoding hereditary breast cancer” benefits and questions from multigene panel testing. Breast 45:29–35. https://doi.org/10.1016/j.breast.2019.01.002
Coyle YM (2009) Lifestyle, genes, and cancer. Methods Mol Biol 472:25–56. https://doi.org/10.1007/978-1-60327-492-0_2
Cragun D, Bonner D, Kim J, Akbari MR, Narod SA, Gomez-Fuego A, Garcia JD, Vadaparampil ST, Pal T (2015) Factors associated with genetic counseling and BRCA testing in a population-based sample of young Black women with breast cancer. Breast Cancer Res Treat 151:169–176. https://doi.org/10.1007/s10549-015-3374-7
Cragun D, Weidner A, Lewis C, Bonner D, Kim J, Vadaparampil ST, Pal T (2017) Racial disparities in BRCA testing and cancer risk management across a population-based sample of young breast cancer survivors. Cancer 123:2497–2505. https://doi.org/10.1002/cncr.30621
Domchek SM, Aghajanian C, Shapira-Frommer R, Schmutzler RK, Audeh MW, Friedlander M, Balmaña J, Mitchell G, Fried G, Stemmer SM, Hubert A, Rosengarten O, Loman N, Robertson JD, Mann H, Kaufman B (2016) Efficacy and safety of olaparib monotherapy in germline BRCA1/2 mutation carriers with advanced ovarian cancer and three or more lines of prior therapy. Gynecol Oncol 140:199–203. https://doi.org/10.1016/j.ygyno.2015.12.020
Hayden EP, Dougherty LR, Maloney B, Olino TM, Sheikh H, Durbin CE, Nurnberger JI Jr, Lahiri DK, Klein DN (2008) Early-emerging cognitive vulnerability to depression and the serotonin transporter promoter region polymorphism. J Affect Disord 107:227–230. https://doi.org/10.1016/j.jad.2007.07.028
Ho DYF (1974) Relational counseling: an Asian perspective on therapeutic intervention. - PsycNET. Am J Orthopsychiatry 44:620–636. https://doi.org/10.1111/j.1939-0025.1974.tb00917.x
Ho SM, Ho JW, Chan CL, Kwan K, Tsui YK (2003) Decisional consideration of hereditary colon cancer genetic test results among Hong Kong chinese adults. Cancer Epidemiol Biomark Prev 12:426–432
Hong Kong Cancer Registry, Hospital Authority (2019) Female breast cancer in 2016. Hong Kong
Kaufman B et al (2015) Olaparib monotherapy in patients with advanced cancer and a germline BRCA1/2 mutation. J Clin Oncol 33:244–250. https://doi.org/10.1200/jco.2014.56.2728
King M-C, Marks JH, Mandell JB, Group TNYBCS (2003) Breast and ovarian cancer risks due to inherited mutations in BRCA1 and BRCA2. Science 302:643–646. https://doi.org/10.1126/science.1088759
Kwong A, Wong CH, Shea C, Suen DT, Choi CL (2010) Choice of management of southern Chinese BRCA mutation carriers. World J Surg 34:1416–1426
Kwong A, Chu AT, Wu CT, Tse DM (2014) Attitudes and compliance of clinical management after genetic testing for hereditary breast and ovarian cancer among high-risk Southern Chinese females with breast cancer history. Familial Cancer 13:423–430. https://doi.org/10.1007/s10689-014-9706-7
Kwong A, Shin VY, Au CH, Law FBF, Ho DN, Ip BK, Wong ATC, Lau SS, To RMY, Choy G, Ford JM, Ma ESK, Chan TL (2016a) Detection of germline mutation in hereditary breast and/or ovarian cancers by next-generation sequencing on a four-gene panel. J Mol Diagn 18:580–594. https://doi.org/10.1016/j.jmoldx.2016.03.005
Kwong A et al (2016b) Comprehensive spectrum of BRCA1 and BRCA2 deleterious mutations in breast cancer in Asian countries. J Med Genet 53:15–23. https://doi.org/10.1136/jmedgenet-2015-103132
Lee SC, Bernhardt BA, Helzlsouer KJ (2002) Utilization of BRCA1/2 genetic testing in the clinical setting: report from a single institution. Cancer 94:1876–1885
Lipkus IM, Iden D, Terrenoire J, Feaganes JR (1999) Relationships among breast cancer concern, risk perceptions, and interest in genetic testing for breast cancer susceptibility among African-American women with and without a family history of breast cancer. Cancer Epidemiol Biomark Prev 8:533–539
Litton JK et al (2018) Talazoparib in patients with advanced breast cancer and a germline BRCA mutation New England. J Med 379:753–763. https://doi.org/10.1056/NEJMoa1802905
Markus HR, Kitayama S (1991) Culture and the self: implications for cognition, emotion, and motivation. - PsycNET. Psychol Rev 98:224–253. https://doi.org/10.1037/0033-295X.98.2.224
Nakagomi H et al (2016) Willingness of Japanese patients with breast cancer to have genetic testing of BRCA without burden of expenses. Breast Cancer 23:649–653. https://doi.org/10.1007/s12282-015-0618-7
Rajpal N, Munoz J, Peshkin BN, Graves KD (2017) Insights into BRCA1/2 genetic counseling from ethnically diverse Latina breast cancer survivors. J Genet Couns 26:1221–1237. https://doi.org/10.1007/s10897-017-0096-5
Robson M et al (2017) Olaparib for metastatic breast cancer in patients with a germline BRCA mutation N. Engl J Med 377:523–533. https://doi.org/10.1056/NEJMoa1706450
Sussner KM, Jandorf L, Thompson HS, Valdimarsdottir HB (2013) Barriers and facilitators to BRCA genetic counseling among at-risk Latinas in New York City. Psychooncology 22:1594–1604. https://doi.org/10.1002/pon.3187
Thompson D, Easton DF (2002) Breast Cancer Linkage C Cancer incidence in BRCA1 mutation carriers J Natl Cancer Inst 94 1358-1365 doi:https://doi.org/10.1093/jnci/94.18.1358
Zhang L et al (2018) Breast and ovarian cancer penetrance of BRCA1/2 mutations among Hong Kong women. In: Oncotarget 9(38):25025–25033. https://doi.org/10.18632/oncotarget.24382
Acknowledgements
The authors would like to thank Miss Alcina Kong, Miss Angela Li, and Mr Leo Ng for their contributions in literature review and data collection; Hong Kong Hereditary Breast Cancer Family Registry, The Hong Kong Sanatorium and Hospital and Dr. Ellen Li Charitable Foundation for their support in genetic testing; and the participants who took part in this study.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The current study is not a clinical trial and ethical approval (UW09-210) had been obtained from the Institutional Review Board (IRB) of The University of Hong Kong.
Conflict of interest
The authors declare no competing interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Chu, A.TW., Tse, D.MS., Suen, D.T.K. et al. Baseline knowledge and receptiveness to genetic testing for hereditary breast and ovarian cancer syndromes in Chinese high-risk females. J Community Genet 12, 431–438 (2021). https://doi.org/10.1007/s12687-021-00518-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12687-021-00518-3