Abstract
Nanocrystals of PbS were synthesized by using Aspergillus sp. isolated from the rhizosphere of Chickpea. For this, PbS-tolerant fungi were isolated from the rhizosphere soil by serial dilution method and plated on potato dextrose agar supplemented with 1 mM lead acetate and 0.1 % sodium sulfide. PbS-tolerant fungus was identified as Aspergillus sp. by fluorescence microscopy. The PbS nanocrystals were characterized by UV-visible absorption spectroscopy, x-ray diffraction (XRD), particle size analyzer (PSA), and transmission electron microscope (TEM). UV-visible absorption scan revealed a peak at 300 nm, a characteristic of the nanosize range. Characteristic peaks at around 26° and 30° were observed in XRD analysis of samples of fungal fabricated quantum dots. Crystallite size as determined from transmission electron microscopy was found to be in the range of 10–15 nm.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
The study of biosynthesis of nanomaterials offers a valuable contribution into materials science. The ability of some microorganisms such as bacteria and fungi to control the synthesis of metallic nanoparticles can be employed in the search for new materials e.g., bio-reduction of selenite by Pantoea agglomerans strain UC-32 [1], gold nanoparticles by the fungus Penicillium sp. [2], silver nanoparticle by Penicillium purpurogenum NPMF, Aspergillus tamarii, Penicillium diversum, and Bacillus sp. [3–6]. Ingle et al. (2011) reported extracellular biosynthesis of silver nanoparticles by Fusarium solani (USM-3799), a phytopathogen causing disease in onion [7]. Srivastava et al. (2012) synthesized multiple nanoparticles e.g., Ag, Pd, Fe, Rh, Ni, Ru, Pt, Co, and Li by Pseudomonas aeruginosa [8].
For large-scale biogenesis of nanoparticles, fungi possess unique advantages over other microorganisms. Most fungi have a very high wall-binding capacity as well as intracellular metal uptake capacities [9]. Fungi produce large amount of enzymes per unit biomass. The use of specific enzymes such as reductase secreted by fungi opens up exciting possibilities of designing a rational biosynthesis strategy for metal nanoparticles of different chemical compositions. A number of different genera of fungi have been investigated for biotechnological process research, and it has been shown that fungi are extremely good candidates in the synthesis of nanoparticles [10, 11].
Synthesis of quantum dots using microorganism is a relatively new and unexplored area that could be cost effective, less toxic, and a reproducible method that could be carried out at ambient temperatures and pressure. Bai et al. (2009) synthesized cadmium sulfide nanoparticles by photosynthetic bacteria Rhodopseudomonas palustris [12]. Intracellular synthesis of nanoscale PbS crystallites by Torulopsis species when exposed to aqueous Pb+2 ions was reported by Kowshik et al. (2002) [13]. Seshadri et al. (2011) synthesized lead sulfide nanoparticles by using the lead resistant marine yeast, Rhodosporidium diobovatum [14]. The synthesis of highly luminescent water soluble QDs has become a priority, because of application of quantum dots in various field e.g., cell labeling, cell tracking, in vivo imaging, and DNA detection [15–18]. In view of the potential advantages of their synthesis through biological route, research in this area merits significance.
In the present work, biosynthesis of PbS nanocrystals by rhizospheric fungi is reported for the first time. The PbS nanocrystals were obtained by using Aspergillus fungi isolated from the rhizosphere soil of chickpea and subsequently characterized using various techniques available. Rhizospheric fungus Aspergillus sp. when challenged with aqueous lead acetate and sodium sulfide (Na2S) at room temperature for 96 h under shaking conditions on a rotary shaker (200 rpm) resulted in the formation of highly stable PbS nanoparticles possessing the following characteristics: ≥15–20 nm, polydisperse, and highly stable.
2 Experimental Details
2.1 Materials
All chemicals were of analytical grade and procured from S. D. Fine Chem. Ltd. and Sigma Aldrich (India). All culture media were purchased from Hi Media and Sisco Research Laboratory (India).
2.2 Organism and Cultivation
A lead tolerant fungus was isolated from rhizospheric soil of chickpea using spread plate method [19]. One gram of the soil sample was serially diluted in sterilized distilled water and spread on potato dextrose agar (PDA) media amended with lead acetate. The plates were incubated at 25 °C for 4 days. Fungal strains showing growth on the above media and hence tolerance to lead acetate were picked up and subcultured on PDA media slants (potato 20 % w/v, dextrose 2 % w/v, and agar 2 % w/v) at 25 °C. Fungal biomass to be used for biosynthetic experiments was grown aerobically in potato dextrose broth.
Identification of the fungal isolate was carried out by morphological and microscopic observations (such as color, texture of the mycelia, spore formation pattern) with the help of identification scheme of Barnett and Hunter [20]. Lead-tolerant fungus isolated from the rhizosphere of chickpea was observed by fluorescence microscope (Leica DFC425C). For this, 72 hold specimens were mounted on glass slides in several drops of the stain solution. The stain solution was prepared at least 4 h prior to use as 0.05 % aniline blue dye in 0.067 M potassium phosphate dibasic (K2HPO4 ). Stain solution was stored at room temperature in brown glass bottles for not more than 6 months [21].
2.3 Synthesis of PbS Nanocrystals
For fabrication of PbS nanocrystals, fungal strain was inoculated in potato dextrose broth (PDB) and incubated at 25 °C for 72 h on a rotary shaker at 150 rpm in the dark. Thereafter, lead acetate (1 mM) and 0.1 % sodium sulfide were added to the culture, which was further incubated under the same conditions and observed for change in color of PDB [22]. After 4 days of incubation, change in color of PDB was observed and nanoparticles were recovered from the cultures. For this, first, the culture was centrifuged at 5,000 rpm for 30 min to sediment the fungal mass and, then, the supernatant containing the nanoparticles was centrifuged at 15,000 rpm (4 °C/30 min). Controls containing PDB, with fungal strain but without lead acetate and Na2S as positive control, and with lead acetate and Na2S but without cell biomass as negative control, were also run simultaneously along with the experimental flasks in three replicates. The obtained pellet was washed with distilled water and lyophilized. The nanocrystals so fabricated were characterized by UV–vis spectrophotometer, zeta potential, particle size analyzer, TEM, and XRD.
2.4 Characterization of PbS Nanocrystals
Samples of 1 cm3 were withdrawn from the 4-day-old fungal cultures, for measuring the absorbance at the resolution of 1 nm using a UV-visible spectrophotometer (UV-2450, Shimadzu).
Size and zeta potential of sample was measured by using particle size analyzer (Zetasizer Nanoseries ZS-90, Malvern). The mean particle size of nanoparticles was analyzed at 25 °C using photon correlation spectroscopy. The lyophilized samples were suspended in distilled water and dispersed by sonication. One milliliter of the nanosuspension was scanned in disposable cuvette with an equilibrium time of 120 s in particle size analyzer.
Zeta potential of samples was measured using disposable zetasizer cuvette with the help of syringe. The RI viscosity and dielectric constant were kept constant for all determinations. One milliliter sample was taken from each formulation. The cuvette was placed in the instrument for zeta potential. Zeta potential was measured after intervals of 1, 12, 24, 48, and 72 h and after 1 month. All experiments were conducted in triplicates.
For transmission electron microscope (TEM) measurements, a drop of cell-free filtrate-containing PbS nanocrystals was placed on the carbon-coated copper grids and kept under vacuum desiccation overnight before loading them onto a specimen holder. TEM micrographs were taken by analyzing the prepared grids on Morgagni 268D (Fei Electron Optics) TEM.
Chemical analysis of lyophilized nanoparticles obtained above was done using x-ray diffraction (XRD). XRD patterns were recorded on a RigaKuC/max-2500 diffractometer using graphite filtered CuKα radiation at 30 kV and 100 mA with a scanning rate of 4° min−1 from 2θ = 10° to 70°.
3 Results and Discussion
3.1 Identification of Fungal Isolate
Lead-tolerant fungus isolated from the rhizosphere of chickpea was observed by fluorescence microscopy. On the basis of observation of asexual fruiting body with conidiophores, septate hyphae, vesicles, phialides, and conidiospores in chain, the fungus was identified to be Aspergillus sp. according to Barnett and Hunter (Fig. 1) [20].
3.2 Synthesis and Characterization of PbS Nanocrystals
The UV–vis spectra recorded for the reaction mixture after observing visible change in color of PDB on the fourth day of incubation of fungal strain on media with lead acetate and sodium sulfide is shown in Fig. 2. Strong surface plasmon resonance centered at approximately 300 nm is clearly visible, indicating the formation of PbS nanocrystals. In a previous study [23] with alkanethiolate-capped PbS nanoparticles synthesized in a bicontinuous cubic lipid matrix, it was reported that a broad absorption band was also observed at ca. 300 nm. Jain et al. [24] reported that peak ranges of 200–280 nm were due to the presence of proteins and other biomolecules of plant extract responsible for fabrication of nanoparticles. In Fig. 2, absorption band of nearly 270–280 nm is also visible which is contributed by proteins present in the extracellular broth, suggesting a possible reducing enzyme-based process for the synthesis of PbS nanoparticles.
The zeta potential of PbS nanocrystals was 51.3 mV (Fig. 3). This result demonstrated that the PbS nanocrystals obtained were extremely stable, with no evidence of flocculation of the particles even after a month of preparation. The long-term stability of the PbS nanoparticle solution could be due to the presence of the capping protein/peptide in the solution that binds to the surface of the nanoparticles and prevent its flocculation [25]. Greenwood et al. [26] described that a value of zeta potential between 40–60 mV indicates good stability of colloids.
Particle size analysis for sample solutions gives the average particle diameter as 788.4 nm, and first peak observation shows that the size of the smallest particle comes out to be 90.1 nm (Fig. 4).
A representative TEM image of the PbS nanoparticles obtained using lead acetate as the metal source is shown in Fig. 5. The particles are essentially spherical and appear to be reasonably monodisperse, and the average particle size is 10–15 nm. The present report was correlated with the report of Ahmad et al. [27] where they have synthesized spherical shaped CdS nanoparticles by using fungus Fusarium oxysporum.
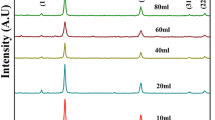
Usually, size observed in TEM is quite different from that obtained through particle size analyzer. Firstly, particle size analyzer is an assorted technique because of its low measuring range thus it is quite difficult to determine the presence of microparticles in nanosuspension whereas, TEM shows the particle as well as its size so it is easy to differentiate between microparticles and nanoparticles in nanosuspension [28]. Secondly, particle size analyzer measures the hydrodynamic diameter which not only depends on the particle core but also on surface structure, concentration, and type of ions in the medium while, in TEM the particle is removed from its native environment [29]. The XRD pattern of the synthesized PbS nanocrystals biosynthesised with Aspergillus sp. is shown in Fig. 6. It is obvious that all of the XRD peaks of the sample are consistent with the values in the standard card (JCPDS No.5–592). Its main diffraction peaks are indexed as (111), (200), (220), (311), (222), (400), (420), and (422) planes of the cubic crystalline structure of PbS. The characteristic peak for QD’s comes at 26.057 and 30.173. Gong et al. (2007) also found similar results [22].
4 Conclusion
A protocol for biosynthesis of PbS nanoparticles using Aspergillus sp. has been demonstrated. The formation of PbS nanoparticles in intercellular as well as extracellular secretion of fungal strain in potato dextrose medium at room temperature can be easily handled during downstream processing. Since the nanoparticles formed by fungi can be directly used in various biomedical applications [30, 31], understanding the fungi-nanoparticle interactions during the synthesis mechanism will lead to the possibility of using the system reported here as future “nano-factory”. The simple and energy-conserving nature of fungal-based processes for PbS nanoparticles synthesis in comparison with chemical synthesis cannot be overemphasized. The possibility of rational use of this fungal strain isolated from metal-rich soils, in synthesis of other important metal nanoparticles, is an exciting area and holds enormous future possibility.
References
Torres, S. K., Campos, V. L., León, C. G., Rodríguez-Llamazares, S. M., Rojas, S. M., González, M., et al. (2012). Biosynthesis of selenium nanoparticles by Pantoea agglomerans and their antioxidant activity. Journal of Nanoparticle Research, 14, 1236. doi:10.1007/s11051-012-1236-3.
Du, L., Xian, L., Feng, J. X. (2011). Rapid extra-/intracellular biosynthesis of gold nanoparticles by the fungus Penicillium sp. Journal of Nanoparticle Research, 13, 921–930. doi:10.1007/s11051-010-0165-2.
Nayak, R. R., Pradhan, N., Behera, D., Pradhan, K. M., Mishra, S., Sukla, L. B., et al. (2011). Green synthesis of silver nanoparticle by Penicillium purpurogenum NPMF: the process and optimization. Journal of Nanoparticle Research, 13, 3129–3137. doi:10.1007/s11051-010-0208-8.
Kumar, R. R., Priyadharsani, K. P., Thamaraiselvi, K. (2012). Mycogenic synthesis of silver nanoparticles by the Japanese environmental isolate Aspergillus tamarii. Journal of Nanoparticle Research, 14, 860. doi:10.1007/s11051-012-0860-2.
Ganachari, S. V., Bhat, R., Deshpande, R., Venkataraman, A. (2012). Extracellular biosynthesis of silver nanoparticles using fungi penicillium diversum and their antimicrobial activity studies. BioNanoScience, 2, 316–321. doi:10.1007/s12668-012-0046-5.
Pugazhenthiran, N., Anandan, S., Kathiravan, G., Udaya Prakash, N. K., Crawford, S., Ashokkumar, M. (2009). Microbial synthesis of silver nanoparticles by Bacillus sp. Journal of Nanoparticle Research, 11, 1811–1815. doi:10.1007/s11051-009-9621-2.
Ingle, A., Rai, M., Gade, A., Bawaskar, M. (2009). Fusarium solani: a novel biological agent for the extracellular synthesis of silver nanoparticles. Journal of Nanoparticle Research, 11, 2079–2085. doi:10.1007/s11051-008-9573-y.
Srivastava, S. K., & Constanti, M. (2012). Room temperature biogenic synthesis of multiple nanoparticles (Ag, Pd, Fe, Rh, Ni, Ru, Pt, Co, and Li) by Pseudomonas aeruginosa SM1. Journal of Nanoparticle Research, 14, 831. doi:10.1007/s11051-012-0831-7.
Mohanpuria, P., Rana, N. K., Yadav, S. K. (2008). Biosynthesis of nanoparticles: technological concepts and future applications. Journal of Nanoparticle Research, 10, 507–517. doi:10.1007/s11051-007-9275-x.
Kalishwaralal, K., Deepak, V., Pandian, S. R., Kottaisamy, M., Kanth, S. M., Kartikeyan, B., et al. (2010). Biosynthesis of silver and gold nanoparticles using Brevibacterium casei. Colloids Surf B: Biointerfaces, 77, 257–262. doi:10.1016/j.colsurfb.2010.02.007.
Sadhasivam, S., Shanmugam, P., Yun, K. S. (2010). Biosynthesis of silver nanoparticles by Streptomyces hygroscopicus and antimicrobial activity against medically important pathogenic microorganisms. Colloids Surf B: Biointerfaces, 81, 358–362. doi:10.1016/j.colsurfb.2010.07.036.
Bai, H. J., Zhang, Z. M., Guo, Y., Yang, G. E. (2009). Biosynthesis of cadmium sulfide nanoparticles by photosynthetic bacteria Rhodopseudomonas palustris. Colloids Surf B: Biointerfaces, 70, 142–146. doi:10.1016/j.colsurfb.2008.12.025.
Kowshik, M., Vogel, W., Urban, J., Kulkarni, S. K., Paknikar, K. M. (2002). Microbial synthesis of semiconductor PbS nanocrystallites. Advanced Materials, 14, 815–818. doi:10.1002/1521-4095(20020605).
Seshadri, S., Saranya, K., Kowshik, M. (2011). Green synthesis of lead sulfide nanoparticles by the lead resistant marine yeast. Rhodosporidium diobovatum. Biotechnology Progress, 27, 1464–1469. doi:10.1002/btpr.651.
Wong, K. K. W., & Mann, S. (1996). Biomimetic synthesis of cadmium sulfide-ferritin nanocomposites. Advanced Materials, 8, 928–932. doi:10.1002/adma.19960081114.
Hennequin, B., Turyanska, L., Ben, T., Beltran, A. M., Molina, S. I., Li, M., et al. (2008). Aqueous near infrared fluorescent composites based on apoferritin-encapsulated PbS quantum dots. Advanced Materials, 20, 3592–3596. doi:10.1002/adma.200800530.
Chan, W. C., & Nie, S. (1998). Quantum dot bioconjugates for ultrasensitive nonisotopic detection. Science, 281, 2016–2018. PMID: 9748158.
Bruchez, M. J., Moronne, M., Gin, P., Weiss, S., Alivisatos, A. P. (1998). Semiconductor nanocrystals as fluorescent biological labels. Science, 281, 2013–2016. PMID: 9748157.
Salle, A. J. (1973). Laboratory manual of fundamental principles of bacteriology (p. 201). New York: McGraw-Hill.
Barnett, H. L., & Hunter, B. B. (1972). Illustrated general of imperfect fungi (3rd ed.). Minnesota: Burgess Publishing Co. 241.
Hood, M. E., & Shew, H. D. (1996). Application of KOH-aniline blue fluorescence in the study of plants-fungal interactions. Phytopathology, 86, 704–708.
Gong, J., Zang, Z. M., Bai, H. J., Yang, G. E. (2007). Microbiological synthesis of nanophase PbS by Desulfotomaculum sp. Science China Series E-Technology Science, 50, 302–307. doi:10.1007/s11431-007-0045-x.
Eflink, M. R., & Ghiron, C. A. (1981). Fluorescence quenching studies with proteins. Analytical Biochemistry, 114, 199–227. doi:10.1016/0003-2697(81)90474-7.
Jain, P., & Pradeep, T. (2005). Potential of silver nanoparticles-coated polyurethane foam as an antibacterial water filter. Biotechnology Bioengineering, 90, 59–63. doi:10.1002/bit.20368.
Henglein, A. (1989). Small-particle research: physicochemical properties of extremely small colloidal metal and semiconductor particles. Chemical Reviews, 89, 1861–1873. doi:10.1021/Cr00098a010.
Greenwood, R., & Kendall, K. J. (1999). Selection of suitable dispersants for aqueous suspensions of zirconia and titania powders using acoustophoresis. European Ceramics Society, 19, 479–488. doi:10.1016/S0955-2219(98)00208-8.
Ahmad, A., Mukherjee, P., Mandal, D., Senapati, S., Khan, M. I., Kumar, R., et al. (2002). Enzyme mediated extracellular synthesis of CdS nanoparticles by the fungus, Fusarium oxysporum. Journal of the American Chemical Society, 124, 12108–12109. doi:10.1021/ja027296o.
Patravale, V. B., Date, A. A., Kulkarni, R. M. (2004). Nanosuspensions: a promising drug delivery strategy. Journal of Pharmacy and Pharmacology, 56(2004), 827–840. doi:10.1211/0022357023691.
http://www.malvern.com/LabEng/technology/dynamic light scattering/dynamic light scattering.htm (accessed 10.08.11).
Yaghini, E., Seifalian, A. M., MacRobert, A. J. (2009). Quantum dots and their potential biomedical applications in photosensitization for photodynamic therapy. Nanomedicine (London, England), 4, 353–363. doi:10.2217/nnm.09.9.
Shao, L., Gao, Y., Yan, F. (2011). Semiconductor quantum dots for biomedicial applications. Sensors, 11, 11736–11751. doi:10.3390/s111211736.
Acknowledgments
The authors gratefully acknowledge Sophisticated Advanced Instrument Facility, All India Institute of Medical Sciences, New Delhi for TEM characterization, and the Department of Science and Technology, New Delhi for providing the financial support for carrying out the present work.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kaur, P., Jain, P., Kumar, A. et al. Biogenesis of PbS Nanocrystals by Using Rhizosphere Fungus i.e., Aspergillus sp. Isolated from the Rhizosphere of Chickpea. BioNanoSci. 4, 189–194 (2014). https://doi.org/10.1007/s12668-014-0135-8
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12668-014-0135-8