Abstract
An eco-friendly microbial method for synthesis of silver colloid solution with antimicrobial activity is developed using a fungal strain of Penicillium purpurogenum NPMF. It is observed that increase in concentration of AgNO3 increases the formation of silver nanoparticle. At 5 mM concentration highly populated polydispersed nanoparticles form. Furthermore, change in pH of the reaction mixture leads to change in shape and size of silver nanoparticles. At lower pH two peaks are observed in the absorption spectra showing polydispersity of nanoparticles. However, highly monodispersed spherical nanoparticles of 8–10 nm size form with 1 mM AgNO3 concentration at pH 8. Antimicrobial activity of nanoparticles is demonstrated against pathogenic gram negative bacteria like Escherichia coli and Pseudomonas aeruginosa, and gram positive bacteria like Staphylococcus aureus. The antimicrobial activity of silver nanoparticles obtained at different initial pH show strong dependence on the surface area and shape of the nanoparticles.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Metal nanoparticles are intensely studied due to their unique optical, electrical, and catalytic properties. To utilize and optimize chemical or physical properties of nano-sized metal particles, a large spectrum of research has been focused to control the size and shape, which is crucial in tuning their physical, chemical, and optical properties (Alivisatos 1996; Coe et al. 2002; Bruchez et al. 1998). Various techniques, including chemical and physical means have been developed to prepare metal nanoparticles, such as chemical reduction (Yu 2007; Tan et al. 2002; Petit et al. 1993; Vorobyova et al. 1999), electrochemical reduction (Liu and Lin 2004; Sandmann et al. 2000), photochemical reduction (Mallick et al. 2005; Keki et al. 2000), heat evaporation (Bae et al. 2002; Smetana et al. 2005) and so on. Nanoparticles have a tendency to aggregate and in most cases, surface passivator reagents are needed to prevent aggregation. Unfortunately many organic passivators such as thiophenol (Ravindran et al. 1999), thiourea (Pattabi and Uchil 2000), mercaptoacetate (Lin et al. 2000), etc., are toxic enough to pollute the environment if large scale nanoparticles are produced.
The study of biosynthesis of nanomaterials offers a valuable contribution as ecofriendly technologies into materials chemistry. The ability of some microorganisms such as bacteria and fungi to control the synthesis of metallic nanoparticles should be employed in the search for new materials (Mandal et al. 2006). Among the nanoparticles, silver nanoparticles have several important applications in the field of bio-labeling, sensors, antimicrobial agents, and filters. The silver nanoparticles are capable of purifying drinking water, degrading pesticides and killing human pathogenic bacteria (Kuber and D’Souza 2006; Marambio-Jones and Hoek 2010). Recent studies of microorganisms in the synthesis of nanoparticles are a new and exciting area of research with considerable potential for development (Mandal et al. 2006; Ingle et al. 2009; Narayanan and Sakthivel 2010). Biosynthetic methods can be divided into two categories depending on the place where the nanoparticles or nanostructures are created as many microorganisms can provide inorganic materials either intra- or extra-cellularly (Mann 1996). For example, bacteria P. strutzeri isolated from silver mine materials is able to reduce Ag+ ions and accumulates silver nanoparticles, the size of such nanoparticles being in the range 16–40 nm, with the average diameter of 27 nm (Narayanan and Sakthivel 2010). Bacteria and fungus are known to produce many different types of nanomaterials like Ag, Au, ZnS, PbS, CdS, etc. (Joerger et al. 2001; Holmes et al. 1995; Juan et al. 2006; Kowshik et al. 2002). The examples also include magnetotactic bacteria which produce magnetite (Fe3O4) or greigite (Fe3S4) and diatoms which produce siliceous material (Joerger et al. 2001).
Biological methods are reported to be cost effective and ecofriendly, but still more research is needed to obtain better mono-dispersity, crystallinity, and shape control. The use of fungi in the extracellular synthesis of nanoparticles is quite exciting; fungi secrete large amounts of enzymes and it is simpler to treat the biomass (Kuber and D’Souza 2006; Kalimuthu et al. 2008; Mukherjee et al. 2002; Ahmad et al. 2003; Kathiresan et al. 2009; Shaligram et al. 2009). In case of intracellular biosynthesis, size and shape of nanoparticles is limited by the dimensions of intracellular regions which lead to monodispersity of particles; whereas obtaining monodispersity in extracellular and biological system as a whole is a challenge. This study was undertaken to study the potential of extra-cellular biosynthesis of silver nanoparticles by a fungus P. purpurogenum NPMF, isolated from Narwapahar Uranium mine of Uranium Corporation of India Ltd. (UCIL), India.
Materials and methods
Preparation of the silver nanoparticles
The fungal strain P. purpurogenum NPMF (MTCC 7356) was obtained from the culture collection center of Biominerals Department of IMMT, Bhubaneswar. All chemicals used were of analytical grade. P. purpurogenum was grown in cornmeal medium (HIMEDIA) which was composed of 20 g/L of corn meal, 10 g/L of peptic digest of animal tissue, 4 g/L of yeast extract, and 10 g/L of dextrose. The pH of the media was adjusted to 6.8 ± 0.2. For production of biomass, sterile media was inoculated with fungal spores aseptically and incubated at 35 °C under shaking condition. After growth of fungus, biomass was separated and re-suspended in distilled water. Then after incubation with water the culture filtrate (extra-cellular enzymes) so generated was added with AgNO3. The concentration of AgNO3 was maintained at 1 mM level throughout all the experiments barring few experiments. A control experiment of fungal supernatant without AgNO3 was conducted under similar conditions.
Characterization of the nanoparticles
The biosynthesis of silver nanoparticles by P. purpurogenum NPMF was supervised visually. The absorption spectra of the reaction mixture of AgNO3 and aqueous extract were analyzed by the UV–Visible spectrophotometer (CECIL) in the range of 200–800 nm. Each time 5 mL aliquots was withdrawn and the sample collection was continued at regular interval for 72 h. Transmission electron microscopy (TEM) micrographs were obtained from an FEI TECHNAI G2 TEM operating at 200 kV. In order to obtain TEM micrographs, a drop of aqueous solution containing the silver nanoparticles was placed on the carbon-coated copper grids and dried under infrared lamp. X-ray diffraction analysis was carried out by using an X-ray powder diffractometer (Philips X’pert Pro, Panalytical) having CuKα (λ = 1.54 Å) radiation and a programmable divergence slit. The voltage and current of the X-ray source were 40 kV and 20 mA, respectively.
Results and discussion
In the past few years, numerous microorganisms have been used to synthesize inorganic nanoparticles either intracellularly or extracellularly. Extracellular synthesis of nanoparticles by microbes has added advantage; the separation of biomass to get a clear supernatant containing biologically active molecules like enzymes, proteins, and other organic molecules is much easier. Fungi especially those which are filamentous are used as extracellular agents and these produce enormous amount of biologically active secretory components. The molecules of the secretary components lead to nanoparticles formation from dissolved ions by enzymatic reduction. The stability of nanoparticles is imparted by peptide capping of nanoparticles. This study is about an extracellular biosynthesis method for silver nanoparticles by a fungus—P. purpurogenum NPMF isolated locally from a local Uranium mine. The size and shape of the nanoparticle was modulated by changing the physiological condition like concentration of Ag+ ions as well as the pH of the medium. Finally, the effect of pH on antimicrobial activity was analyzed.
Effect of substrate concentration
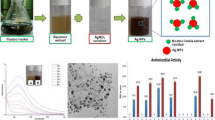
The effect of substrate concentration is a key parameter that affects the process of nanoparticle synthesis. We have found incubation of the fungal culture filtrate with silver nitrate (for 24 h) changes its color to deep brown as the concentration of the AgNO3 increases. The change in color is shown in Fig. 1, which indicates the presence of silver nanoparticles. It could have been due to the excitation of surface plasmon which is typical of the silver nanoparticles (Ahmad et al. 2003). The culture filtrate itself did not show any change in color. The increase in color intensity of culture filtrate was due to increased number of nanoparticles that forms as a result of reduction of silver ions present in the aqueous solution. Similar coloration was observed for P. fellutanum and P. brevicompactum (Kathiresan et al. 2009; Shaligram et al. 2009).
Formation of silver nanoparticle was further checked by UV–Visible spectroscopy. A broad absorption peak was observed at 420 nm. Figure 2 shows the concentration dependence of absorbance (normalized) at 420 nm. It is observed that the silver nanoparticle formation increases with increase in substrate concentration up to 1.5 mM. With further increase in substrate concentration, nanoparticle formation did not increase within 24 h. But appreciable amount of silver nanoparticle was obtained with as high as 5 mM substrate concentration. This finding is quite interesting as it contradicts the recent report of Kathiresan et al. (2009) who used P. fellutanum fungal strain. They found decrease in optical density after 1 mM substrate concentration and accordingly they proposed 1 mM substrate concentration as the optimal silver nanoparticle production condition. Hence it is worth mentioning that P. purpurogenum strain is by far the best fungal strain reported in the literature and has great potential to be considered for industrial bio-production of silver nanoparticle.
In addition to the above observations, we also noticed that the formation of silver nanoparticle was very fast. The formation of nanoparticles starts in 5 min and grows rapidly and then saturates after 24 h. The time dependent extracellular synthesis of silver nanoparticle for 1 mM AgNO3 solution is presented in Fig. 3. The observed rapid biosynthesis of silver nanoparticle is quite comparable to what has been reported by Kathiresan et al. (2009). TEM provided further insight into the morphology and size of the nanoparticles produced by changing the substrate concentration. Highly monodispersed silver nanoparticles with average size of 10 nm was found for 1 mM AgNO3 concentration as shown in Fig. 4A. Few small silver nanoparticles with 4–6 nm size were also observed. In case of 3 mM AgNO3 concentration unique star shaped aggregated silver nanoparticle was found (Fig. 4B). Polydispersed silver nanoparticles with size range between 5 and 40 nm was also observed for this concentration which is not shown in this report. Further, with 5 mM AgNO3 concentration uneven shape nanoparticle with polydispersity was observed (Fig. 4C).
Effect of pH
The culture filtrate had initial pH of 8.3 which on incubation with 1 mM AgNO3, transformed to uniform size silver nanoparticles of size around 10 nm. In order to determine the effect of initial pH on the nanoparticle formation we varied the initial pH from 4 to 9 using dilute HCl/NaOH. After 24 h of incubation with 1 mM AgNO3 at different pH culture filtrate, silver nanoparticles were obtained. We tried to correlate the formation of these nanoparticles to its morphology. First, UV–Visible spectra of fungal culture filtrate containing silver nano particles at different pH values were obtained and presented in Fig. 5. We observe that the absorption band of nanoparticles is centered at 420 nm which is the characteristic of spherical silver nanoparticles. This feature was noticed for the culture filtrate at pH 7, 8, 9 and the original culture filtrate (pH 8.3) after incubation with 1 mM AgNO3 for 24 h. The main absorption band of silver nanoparticles shifted to higher wavelengths (nearly 460 nm and even higher) for pH 4, 5, and 6, indicating that the nanoparticles are not spherical and may be of different size and shape. However, there was an unresolved absorption band corresponding to 380 nm for the nanoparticles formed at pH 4, 5, and 6. The presence of the minor absorption band at 380 nm could have been due to polydispersity in the size or shape of the nanoparticles. These results revealed that initial pH of the culture filtrate solution influence the excitation of surface plasmon vibrations of silver nanoparticles which in turn affect the morphology of nanoparticles.
The crystalline structure, phase composition, and preferential orientation, were analyzed by XRD for each product. Figure 6 shows the XRD patterns obtained for the silver nanoparticles at different initial pH using a glass substrate as support. The diffraction patterns indicate the formation of silver nanoparticles with fcc structure (JCPDS PDF Card No. 04-0783) and accordingly, the Miller indices were assigned to the observed diffraction peaks. For example, the diffraction peak located at about 38° was ascribed to the (111) of face-centered cubic metal silver structures. The average crystallite size of the samples has been determined by line broadening method using Scherer’s equation:
where D is the average crystallite size, λ is the wavelength of X ray (0.154 nm), β is the full width at half maximum (FWHM) of peak, and θ is the half of diffraction angle. Table 1 shows the crystallite sizes calculated for all the nanoparticles formed at different initial pH, by taking the major (111) peak at 2θ = 38° with d-spacing of 0.236 nm. The data in the table indicate that higher crystallite size correspond to lower pH which is consistent with the observation drawn from absorption studies. It was also observed that for higher initial pH the particles were formed with preferential orientation along (111) direction.
The role of pH is crucial in controlling the size and shape of nanoparticles. In order to understand the effect of the initial pH of the culture filtrate solution, the samples were examined by TEM. Figure 7 shows the TEM micrographs of representative silver nanoparticles synthesized by using 1 mM AgNO3 under different initial pH conditions. It is quite clear from the figure that pH strongly affects the size and shape of the silver nanoparticles. We hypothesize that the proton concentration affects conformational changes in the nitrate reducing enzymes present in the fungal culture filtrate, which may change the morphology and size of the silver nanoparticles. The nanoparticles were not uniform in size and shape. Larger particles were formed at pH 4 and 5 (Fig. 7A, B) with average particle size between 40 and 55 nm and smaller particles were formed at pH 8 and 9 (Fig. 7C, D) with average particle size between 8 and 13 nm. The nanoparticles formed at lower pH (i.e., 4 or 5) were polydispersed with many different shapes such as pyramidal, spherical, and ellipsoidal. Some anisotropic nanostructures were formed with irregular contours, which indicate that the sample was composed of a large quantity of non-uniform nanoparticles. However, nanoparticles formed at pH 8 and 9 were more uniform. The silver nanoparticles were found to be mostly spherical in shape. These results show that the pH of the culture filtrate plays an important role in determining the morphology and shape of the silver nanoparticles.
Antimicrobial activity of silver colloid solution
Antimicrobial activity of the above synthesized silver colloid solution was determined on pathogenic gram negative bacteria like E. coli and P. aeruginosa, and gram positive bacteria like S. aureus (Table 2). This test was performed on nutrient agar plates spread with microbial culture. Silver colloidal solution (40 μL) was applied on a thick paper disk of 0.4 cm diameter, dried and then applied on the surface of the media plate. The culture filtrate as such did not show any antimicrobial activity; this was doubly ensured as Penicillium species may show some antimicrobial activity of its own. We also checked for antimicrobial activity of 1 mM AgNO3 solution which did not show the visible zone. With increasing amount of nano silver solution on the disk, the zone of inhibition increased as shown for P. aeruginosa (Fig. 8A). Antimicrobial activity against all tested bacteria was visible only above 0.5 mM AgNO3 concentration. The zone of inhibition of the nanoparticles, formed at different initial pH was checked for the above mentioned bacteria. The minimum zone of inhibition was observed at pH 6. This variability in antimicrobial activity may be due to the variation in the surface area and shape of the nanoparticles. Pseudomanas aeruginosa and E. coli both gram negative, seemed to be more adversely affected by the silver nano solution compared to the tested S. aureus which is gram positive.
Conclusions
Microbial synthesis of silver nanoparticle was successfully carried out using a fungal strain of P. purpurogenum NPMF. For the first time it was possible to synthesize silver nanoparticle using 5 mM AgNO3 concentration with Penicillium strain. The method yielded higher number of particles, although the nanoparticles were not highly monodispersed. The morphology and size of the nanoparticles were largely dependent on the initial pH of the culture filtrate. Under acidic pH conditions, polydispersed nanoparticles with many different shapes such as pyramidal, spherical, and ellipsoidal structures were obtained. However, highly monodispersed spherical silver nanoparticles of average size in the range of 8–10 nm were obtained at pH 8 for 1 mM AgNO3 concentrations. Antimicrobial activity was observed against pathogenic gram negative bacteria like E. coli and P. aeruginosa, and gram positive bacteria like S. aureus. Changes in surface area and shape of the nanoparticles showed variability in antimicrobial activity.
References
Ahmad A, Mukherjee P, Senapati S, Mandal D, Khan MI, Kumar R, Sastry M (2003) Extracellular biosynthesis of silver nanoparticles using the fungus Fusarium oxysporum. Colloids Surf B 28:313–318. doi:10.1016/S0927-7765(02)00174-1
Alivisatos AP (1996) Semiconductor clusters, nanocrystals, and quantum dots. Science 271:933–937. doi:10.1126/science.271.5251.933
Bae CH, Nam SH, Park SM (2002) Formation of silver nanoparticles by laser ablation of a silver target in NaCl solution. Appl Surf Sci 197:628–634. doi:10.1016/S0169-4332(02)00430-0
Bruchez M, Moronne M, Gin P, Weiss S, Alivisatos AP (1998) Semiconductor nanocrystals as fluorescent biological labels. Science 281:2013–2016. doi:10.1126/science.281.5385.2013
Coe S, Woo WK, Bawendi M, Bulovic V (2002) Electroluminescence from single monolayer of nanocrystals in molecular organic devices. Nature 420:800–803. doi:10.1038/nature01217
Holmes JD, Smith PR, Evans-Gowing R, Richardson DJ, Russel DA, Sodeau JR (1995) Energy-dispersive-X-ray analysis of the extracellular cadmium sulfide crystallites of Klebsiella aerogenes. Arch Microbiol 163:143–147. doi:10.1007/BF00381789
Ingle A, Rai M, Gade A, Bawaskar M (2009) Fusarium solani: a novel biological agent for the extracellular synthesis of silver nanoparticles. J Nanopart Res 11:2079–2085. doi:10.1007/s11051-008-9573-y
Joerger KT, Joerger R, Olsson E, Granqvist CG (2001) Bacteria as workers in the living factory: metal-accumulating bacteria and their potential for materials science. Trends Biotechnol 19:15–20. doi:10.1016/S0167-7799(00)01514-6
Juan BH, Zhang ZM, Gong J (2006) Biological synthesis of semiconductor zinc sulfide nanoparticles by immobilized Rhodobacter sphaeroides. Biotechnol Lett 28:1135–1139. doi:10.1007/s10529-006-9063-1
Kalimuthu K, Babu RS, Venkataraman D, Bilal M, Gurunathan S (2008) Biosynthesis of silver nanocrystals by Bacillus licheniformis. Colloids Surf B 65:150–153. doi:10.1016/j.colsurfb.2008.02.018
Kathiresan K, Manivannan S, Nabeel MA, Dhivya B (2009) Studies on silver nanoparticles synthesized by a marine fungus, Penicillium fellutanum isolated from coastal mangrove sediment. Colloids Surf B Biointerfaces 71:133–137. doi:10.1016/j.colsurfb.2009.01.016
Keki S, Torok J, Deak G, Daróczi L, Zsuga M (2000) Silver nanoparticles by PAMAM-assisted photochemical reduction of Ag+. J Colloid Interface Sci 229:550–553. doi:10.1006/jcis.2000.7011
Kowshik M, Vogel W, Urban J, Kulkarni SK, Paknikar KM (2002) Microbial synthesis of semiconductor PbS nanocrystallites. Adv Mater 14:815–818. doi:10.1002/1521-4095(20020605)
Kuber CB, D’Souza SF (2006) Extracellular biosynthesis of silver nanoparticles using the fungus Aspergillus fumigatus. Colloids Surf B 47:160–164. doi:10.1016/j.colsurfb.2005.11.026
Lin SM, Lin FQ, Guo HQ, Zhang ZH, Wang ZG (2000) Surface states induced photoluminescence from Mn2+ doped CdS nanoparticles. Solid State Commun 115:615–618. doi:10.1016/S0038-1098(00)00254-4
Liu YC, Lin LH (2004) New pathway for the synthesis of ultrafine silver nanoparticles from bulk silver substrates in aqueous solutions by sonoelectrochemical methods. Electrochem Commun 6:1163–1168. doi:10.1016/j.elecom.2004.09.010
Mallick K, Witcomb MJ, Scurrell MS (2005) Self-assembly of silver nanoparticles in a polymer solvent: formation of a nanochain through nanoscale soldering. Mater Chem Phys 90:221–224. doi:10.1016/j.matchemphys.2004.10.030
Mandal D, Bolander ME, Mukhopadhyay D, Sarkar G, Mukherjee P (2006) The use of microorganisms for the formation of metal nanoparticles and their application. Appl Microbial Biotechnol 69:485–492. doi:10.1007/s00253-005-0179-3
Mann S (1996) Biomimetic materials chemistry. Wiley-VCH, New York
Marambio-Jones C, Hoek EMV (2010) A review of the antibacterial effects of silver nanomaterials and potential implications for human health and the environment. J Nanopart Res 12:1531–1551. doi:10.1007/s11051-010-9900-y
Mukherjee P, Senapati S, Mandal D, Ahmad A, Khan MI, Kumar R, Sastry M (2002) Extracellular synthesis of gold nanoparticles by the fungus Fusarium oxysporum. Chembiochem 3:461–463. doi:10.1002/1439-7633(20020503)
Narayanan KB, Sakthivel N (2010) Biological synthesis of metal nanoparticles by microbes. Adv Colloid Interface Sci 156:1–13. doi:10.1016/j.cis.2010.02.001
Pattabi M, Uchil J (2000) Synthesis of cadmium sulphide nanoparticles. Solar Energy Mater Solar Cell 63:309–314. doi:10.1016/S0927-0248(00)00050-7
Petit C, Lixon P, Pileni MP (1993) In situ synthesis of silver nanocluster in AOT reverse micelles. J Phys Chem 97:12974–12983. doi:10.1021/j100151a054
Ravindran TR, Arora AK, Balamurugan B, Mehta BR (1999) Inhomogeneous broadening in the photoluminescence spectrum of CdS nanoparticles. Nanostruct Mater 11:603–609. doi:10.1016/S0965-9773(99)00346-3
Sandmann G, Dietz H, Plieth W (2000) Preparation of silver nanoparticles on ITO surfaces by a double-pulse method. J Electroanal Chem 491:78–86. doi:10.1016/S0022-0728(00)00301-6
Shaligram NS, Bule M, Bhambure R, Singhal RS, Singh SK, Szakacs G, Pandey A (2009) Biosynthesis of silver nanoparticles using aqueous extract from the compactin producing fungal strain. Process Biochem 44:939–943. doi:10.1016/j.procbio.2009.04.009
Smetana AB, Klabunde KJ, Sorensen CM (2005) Synthesis of spherical silver nanoparticles by digestive ripening, stabilization with various agents, and their 3-D and 2-D superlattice formation. J Colloid Interface Sci 284:521–526. doi:10.1016/j.jcis.2004.10.038
Tan Y, Wang Y, Jiang L, Zhu D (2002) Thiosalicylic acid-functionalized silver nanoparticles synthesized in one-phase system. J Colloid Interface Sci 249:336–345. doi:10.1006/jcis.2001.8166
Vorobyova SA, Lesnikovich AI, Sobal NS (1999) Preparation of silver nanoparticles by interphase reduction. Colloids Surf A 152:375–379. doi:10.1016/S0927-7757(98)00861-9
Yu DG (2007) Formation of colloidal silver nanoparticles stabilized by Na+-poly (-glutamic acid) silver nitrate complex via chemical reduction process. Colloids Surf B 59:171–178. doi:10.1016/j.colsurfb.2007.05.007
Acknowledgments
Rati Ranjan Nayak would like to express his sincere thanks to Council of Scientific and Industrial Research (CSIR), India which gave an opportunity to perform research at Institute of Minerals and Materials Technology, Bhubaneswar under CSIR quick hire scheme.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Nayak, R.R., Pradhan, N., Behera, D. et al. Green synthesis of silver nanoparticle by Penicillium purpurogenum NPMF: the process and optimization. J Nanopart Res 13, 3129–3137 (2011). https://doi.org/10.1007/s11051-010-0208-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11051-010-0208-8