Abstract
Copper nanoparticles were successfully synthesized with the help of agriculturally beneficial fungus Trichoderma harzianum through a simple green and eco-friendly route. The objectives of this study were to: (1) evaluate the application of T. harzianum and assess the effect of guar plant cultivation on heavy metal contaminated lands of copper in municipal and industrial wastewaters, and (2) develop a method to increase the antibacterial effects on the risk of two bacteria (Staphylococcus aureus and Escherichia coli). Two factors were investigated: (1) two copper (Cu) levels, Natural Hoagland Arnold solution as a control, and application of 100 μL Cu in Hoagland Arnold solution, and (2) two bio-fertilizer levels, no application, and fungus application. TEM and SEM photographs showed that synthesized Cu-NPs had spherical shapes. The formations of Cu-NPs were corroborated by FTIR and XRD analysis. Furthermore, the MIC and MBC of Cu-NPs towards bacterial growth were evaluated. The Cu-NPs and T. harzianum fungi presented antibacterial activity against Gram positive and Gram negative bacteria. The results suggested that green synthesis of nanoparticles using guar extracts can increase their antibacterial effect. The effect of Cu and fungi on biochemical properties of guar was also investigated. The results showed that the highest antioxidant enzymatic activity and proline amino acid were obtained at 100 μL Cu and T. harzianum application. Moreover, the results suggested that the use of T. harzianum can be useful in increasing the resistance to heavy metal stress in plants by increasing the activity of some antioxidant enzymes and secondary metabolites.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
In recent decades, the production of nanoparticles and their application in the various aspects of plant sciences are increasing. So, the inimitable attributes of nanoparticles have given a progress to the great research activities directed towards nanoparticle preparation and applications. In fact, nanotechnology is a science that has the wide applications today in the pharmaceuticals environmental pollutions control (Ghaedi et al. 2015), the biomedical and pharmaceutical fields as the alternative antimicrobial strategies due to the upsurge of infectious diseases and the appearance of antibiotic-resistant strains (Xu et al. 2013). Biological approaches using microorganisms and plant extracts for metallic nanoparticle synthesis have been identified as viable alternatives to the other reported chemical methods (Bhavyasree and Xavier, 2020). On the other hand, one of the production methods of nanoparticles is the green synthesis. In fact, green synthesis of nanoparticles is one of the technology branches that are rapidly developing in the field of bio-nanotechnology (Biresaw and Taneja 2021). One of the metals used in the metallic nanoparticles is copper. In fact, copper nanoparticles (Cu-NPs) have a special place in the universe. Furthermore, copper nanoparticles have the antibacterial and antifungal properties (Bhavyasree and Xavier, 2020). Cu-NPs can be made in a variety of ways, including with toxic and expensive compounds, severe reduction agents, and organic solvents. The presence of these poisonous and harmful elements on the surface of Cu-NPs makes them more toxic, and the usage and disposal of toxic solvents causes the environmental issues. However, green synthesis of NPs, utilizing various plants or gums, is an environmentally benign and cost-effective process that avoids the use of problematic chemicals (Keihan et al. 2017). Nowadays, in comparison to the other synthesis methods, green nanomaterial synthesis has become a superior choice for scientists. This is attributable to the fact that the synthesis techniques are non-toxic and ecofriendly. In the green synthesis of nanomaterials, plants have a better role compared to the microorganisms due to their availability and simplicity of the method of preparation. Also, the rate of production is quicker than in the case of microorganisms (Bhavyasree and Xavier 2020).
However, the problem of soil health is critical for the suitable functioning of earthly ecosystems. As a result, even at low concentrations, it has an effect on element emissions, lowering soil productivity, ecosystem biota, fluxes of elements and water, human and animal health, respectively (Farias et al. 2020). It is predicted that the sewage sludge will be used for the soil in the future. In this way, the valuable nutrients are returned to the soil, since the sewage sludge contains phosphorus, nitrogen, micronutrients such as copper, which can increase the crop yields and benefit the affected soil organisms. Hence, sewage sludge application to soil can create a risk of environmental contamination (Bogusz and Oleszczuk, 2020). However, urban sewage harbours a wide range of the enteric pathogens like protozoa, viruses, bacteria, parasitic worms and eggs, and its uses calls for careful management of its associated health risks. Bacteria such as Staphylococcus aureus and Escherichia coli, on the other hand, have been found in wastewater that used to irrigate the farmlands. Copper (Cu) is an essential element for humans and plants when present in shortage amount, while in excess it exerts the detrimental effects (Kumar et al. 2021). Of course, it is worth mentioning that among heavy metals, Cu is mainly found in urban sewage sludge (Reis et al. 2020). In this way, phytoremediation is a natural-based solution relying on the natural capability of plants to recover soil, sediment, water surface and groundwater contaminated with toxic metals, organic pollutants and radionuclides. In fact, plants can be used for the pollutant stabilization, extraction, degradation, or volatilization (Manjate et al. 2020).
Because of the tolerance and ability to the bioaccumulate metals, the green synthesis of nanoscaled particles based on fungi is considered an important branch. Filamentous fungi can synthesize the nanoparticles outside of the cell, making processing and biomass handling easier. They are thus preferred over bacteria and unicellular organisms (Ahluwalia et al. 2014). The use of Trichoderma harzianum is one of the available methods for reducing the harmful effects of heavy metals on plants. In fact, T. harzianum fungi can tolerate a broad range of environmental stresses such as heavy metals. Even in the presence of extreme pH, temperature, or nutrient deficiency, the fungus can uptake a variety of heavy metals such as Cu, Cd, Ag, and others from the soil and/or water. Some species of this fungus have the ability to clean the contaminated environment (Govarthanan et al. 2018). T. harzianum has been reported to increase the plant tolerance to heavy metal stress (Téllez Vargas et al. 2017). In addition, the use of fungi of the Trichoderma species has been reported to significantly promote plant growth and alleviate the oxidative stress induced by the interactions of heavy metals (Li et al. 2019).
One of the main characteristics of legumes as a resource for phytoremediation is their role in providing additional Nitrogen compounds to the soil, thus improving soil fertility and ability to support biological growth. Hence, in this study, guar is used as a legume for phytoremediation (Amin et al. 2018). Guar or cluster bean (Cyamopsis tetragonoloba L.) is a drought-resistant annual leguminous crop that is predominantly grown in India and Pakistan (Mahla et al. 2020). Guar is one of the unique beans with a large spherical endosperm that contains a significant amount of galactomannans, which are used in a variety of food and industrial uses. In fact, guar seed compound of three parts consists of 14–17% hull, 35–42% endosperm and 43–47% germ (Gresta et al. 2017).
In general, irrigation with urban sewage because of the presence of the heavy metals such as copper microbiome of E. coli and S. aureus causes plant destruction. Thus, in the present study, T. harzianum was selected with the aim of evaluating its possible application in the phytoremediation of copper-contaminated soil. Therefore, due to the rapid growth of guar, high tolerance to stress conditions, and repair potential for heavy elements, guar can be used to clean soil contaminated with heavy metals. Thus, the main objective of the present research is to examine the green synthesis of copper nanoparticles extracted from guar by T. harzianum to clean municipal sewage. In the present study, nanoparticles were characterized by using scanning electron microscopy and transmission electron microscopy. Also, FTIR, X-ray diffraction, and evaluation for antibactericidal properties using bacterial strains were performed. The present project describes the use of adsorption technology to remove new emergent pollutants using next generation nano-adsorbents. Given these facts, green synthesized Cu-NPs could be a cost-effective and efficient way to clean the urban sewage. On the other hand, because of the plant's growth conditions, which were soilless and hydroponic, it would need to be cultivated in soil conditions in future experiments. Also, the use of several pH levels for a more appropriate evaluation is one of the study's limitations.
Materials and methods
Hydrogen peroxide, Tris-chloride, Pyrogallol, Sulfosalicylic acid, Ninhydrin acid, Acetic acid, Phosphoric acid, sodium sulfate anhydrous, CuSO4 solution, Muller-Hinton Agar and Nutrient Broth (Merck, Germany) were used in this study. Also, the bacterial strains employed in this work were procured from Clinical Microbiology, Faculty of Medicine at Azad University, Ardabil, Iran. (Escherichia coli (ATCC 25922) and Staphylococcus aureus (ATCC 25923). All of the chemicals used were analytical grade.
Two Gram-positive (S. aureus) and Gram-negative (E. coli) bacteria were used. The experimental factors were two copper nanoparticles levels (Natural Hoagland Arnold solution (control) and application 100 μL Cu) and two bio-fertilizer levels (no application (control) and fungi of application). That was examined in perlite-controlled culture medium and hydroponically at pH = 6. Three replications of each treatment were used. The fungus used was T. harzianum, which was applied at the rate of 1 g per 10 kg of perlite. The biochemical effects of guar plant, on flag leaves were evaluated. The leaf tissue of each sample of copper nanoparticles (Cu–NPs) was taken from the aqueous extract by the green synthesis method for tests (FT–IR, XRD, TEM and SEM). Also, from four types of treatments (Control (first treatment), application of Cu (second treatment), application of T. harzianum (third treatment) and combined application of T. harzianum and Cu (fourth treatment)), antibacterial tests of disk diffusion (essential oil, aqueous extract and copper nanoparticles) were evaluated. Finally, minimum inhibitory concentration (MIC) and minimum bactericidal concentration (MBC) tests of Cu-NPs extracted from the four treatments were performed on two Gram-positive (S. aureus) and Gram-negative (E. coli) bacteria.
Preparation of the aqueous guar extract
About 10 g of guar dried leaves powder with 100 ml of deionized water was placed on a shaker incubator for 48 h. Then, it was centrifuged at 5000 rpm for 10 min. After 10 min of centrifugation, filtered with Whatman No. 1 filter paper to eliminate the fibrous impurities and stored at 4 °C for the further experiments.
Extraction of essential oil
Guar aerial parts after collection, cleaning and separation of impurities were crushed with an electric grinder and passed through a sieve. A Clevenger apparatus was used to extract 100 g of plant samples. Fresh distilled water was used as extraction solvent and the ratio of material to liquid was 1:10. After four hours, the essential oil was collected and dried by sodium sulfate anhydrous and then stored at 4 °C until analysis (Bai et al. 2020).
Preparation of copper nanoparticles
For the preparation of Cu-NPs, 75 ml of the aqueous guar extract was added to 100 ml of 0.01 M CuSO4 solution. It was then stirred for another 10 min to ensure the complete mixing. Afterwards, it was kept at 60 °C for one day. The solution was then centrifuged twice at 12,000 rpm for 20 min to get Cu-NPs. The nanoparticles have been prepared when the color of the solution changed from green to amber yellow. The synthesized nanoparticles were dried in the oven at 60 °C for more analysis (Mahmoudvand et al. 2020).
Antibacterial screening using the disk diffusion method
The disk diffusion method was used to evaluate the antibacterial activity and the inhibitory zone of the essential oil, aqueous extract and copper nanoparticles against the bacteria studied in the present study. 100 mL of fresh bacterial culture was gently spread on the agar surface. A 6 mm diameter filter paper disk, impregnated with a 20 mL dose of Cu-NPs with a concentration of 20 mg/mL was used for screening antibacterial activities against E. coli and S. aureus grown on culture plates. Culture plates were incubated at 37 °C for 24 h. After incubation, the inhibition zone of bacterial growth was measured in mm. Antimicrobial activity was determined by measuring the diameter of the inhibition zones around the discs against the tested bacteria. The same was done for the essential oil and “extract” extracted from guar leaves as Cu-NPs. Each disc diffusion assay in this test was repeated in triplicate (Ghaedi et al. 2015).
Minimum inhibitory concentration (MIC)
The dilution method was used to determine the MIC. MIC was assessed by dilution in a liquid medium. Accordingly, 20 ml of the liquid LB medium was transferred to a 50 ml Falcon under sterile conditions. Then 1 ml of bacterial suspension (Standardized based on McFarland turbidity) was added to the culture medium. After complete mixing with LB culture medium, 100 μL of bacterial suspension was added to each of the 96-well plates different concentrations of extract (2.5, 5, 10, 20, 40, 80, 100, and 120 mg/ml) and essential oil of guar (2.5, 5, 10, 20, 40, 80, 100, and 120 μL/ml) and Cu-NPs (0.125, 0.25, 0.5, 1, and 2 μg/ml) were added to a 96-well plate to evaluate their antibacterial properties. Then a 96-well plate containing E. coli and S. aureus bacteria were transferred to a 37 °C incubator and incubated for 24 h. The lowest concentration in which no bacterial growth was observed was determined as the MIC.
Minimum bactericidal concentration (MBC)
To determine MBC, an ounce was taken from the MIC and spread over a solid LB medium. After 24 h incubation at 37 °C, the minimum concentration with no bacterial growth was considered MBC. All tests were done in triplets. The results are presented as the average of these three replications.
Investigation of properties of metal nanoparticles
One week after the nanoparticle synthesis, the shape and size distribution of the synthesized copper nanoparticles were evaluated using TEM (model JEM-2100, JEOL, Japan) with an 200 kV voltage and a magnification of 50 nm, as well as the size and morphology using SEM (model Hitachi, Tokyo, Japan) with a voltage of 15 kV and a magnification of 300 nm. Also, for transmission electron microscopy (TEM) and scanning electron microscopy (SEM) analysis, a drop of copper nanoparticle solution was placed on a carbon-coated copper grid and completely dried prior to the TEM and SEM analysis, then a photo was taken. All of the experiments were carried out in triplets. The results are presented as the average of these three replications. However, the degree of crystallization was assessed using an XRD device. On the other hand, the percentage of compounds and functional groups of nanoparticles were evaluated by FTIR (Paulkumar et al. 2014).
Biochemical parameters measurements
Proline content in leaves was measured by the method of (Bates et al. 1973). Its absorbance was recorded at wavelength 520 nm using a spectrophotometer. The activity of catalase, peroxidase and polyphenol oxidase enzymes in flag leaves was determined by (Kar and Mishra 1976) method and by spectrophotometer which were described as OD μg Protein min−1.
Statistical analysis
All tests were performed in 3 replications and the mean comparison was based on the LSD test at 5% probability level. The statistical analysis was carried out using SAS 9.4 and Excel application software.
Results and discussions
The effect of copper (Cu) and Trichoderma harzianum fungi application on biochemical parameters of guar
Based on variance analysis, interaction effects of Cu and T. harzianum fungi were significant in enzymatic antioxidants activities (Table 1). As shown in the mean comparison table, the highest enzymatic antioxidants activities were obtained when 100 μL Cu and T. harzianum fungi were use. As, non-inoculation with T. harzianum and non-application of Cu decreased the catalase (63.59%), peroxidase (44.02%) and poly phenol oxidase (46.83%) compared to inoculation with T. harzianum and Cu application (Table 2). In fact, it can be said that copper as abiotic stress can produce more ROS in the cell and cause secondary oxidative stress to plants. On the other hand, in this paper, it is demonstrated that enzymatic antioxidant activities such as catalase, peroxidase and poly phenol oxidase were implicated in the tolerance of T. harzianum to oxidative the stress caused by exposure to Cu. So, previous studies have reported that T. harzianum inoculation and copper application have the greatest effect on enzymatic antioxidants activities (Ernesto Juniors et al. 2020).
On the other hand, the analysis of variance showed that the main effect of copper and the interaction of Cu and T. harzianum fungi significantly affected proline production (Table 1). As can be seen in Table 2, the highest content of proline was 9.35 µg/g.Fw−1 that obtained from 100 μL Cu application and inoculation with T. harzianum fungi and the lowest content of proline was in non-inoculation with T. harzianum fungi and no application of Cu (3.08 µg/g.Fw−1). In fact, the absence of fungi and Cu reduced the content of proline by approximately 67.05% when compared to the application of 100 μL Cu and T. harzianum fungi. Of many plants, the use of organic solute such as proline, mineral ions, particularly Ca and K, for osmotic regulation. In fact, when cells are under stress, increased proline content protects cell membranes, proteins, cytoplasmic enzymes, and reactive oxygen species, as well as scavenges free radicals. Plants, in fact, can withstand the stress by increasing proline, polyamine, and protein production (Hosseinzadeh et al. 2018).
Scanning electron microscopy (SEM)
Scanning electron microscopy (SEM) pictures of the synthesized Cu-NPs are shown in Fig. 1A, B. This shape is used to confirm the size of the nanoparticles. In fact, SEM provided further insight into the surface morphology of the Cu-NPs. The experimental results showed that the diameter of the prepared Cu-NPs, as measured by the SEM images and analyzed by Image J at 300 nm magnification, was approximately 15–30 nm, and the shape was found to be spherical, as shown in Fig. 1A, B. The above results are in agreement with the findings of (Hassanien et al. 2018).
Transmission electron microscopy (TEM)
Through transmission electron microscopy (TEM), it was easy to observe the shape and particle size of Cu-NPs. Therefore, for the TEM analysis and the size and shape of nanoparticles, the software Image J was used to measure the images. The TEM image of the synthesized Cu-NPs is showed in Fig. 2A, B. This figure shows the spherical-sized particles of Cu-NPs in nano-dimensions. In fact, the TEM image (Fig. 2A, B), also confirms the spherical shape of Cu-NPs which the average particles are in the size range of 10–30 nm. The above results are in agreement with the findings of (Ismail 2019).
X-ray diffraction
XRD analysis is a very useful tool for identifying the structure of metal nanoparticles. Therefore, the XRD analysis was used to evaluate the crystallinity of green synthesized Cu-NPs, type and crystal phase. The XRD pattern of Cu-NPs as shown in Fig. 3 that demonstrates the diffraction peaks of Cu-NPs exhibiting three peaks of \(2\theta \) at 43.6 (111), 50.80 (200) and 74.4 (220). The peaks match with the literature values of metallic copper (File No. 04-0836) (Rajesh et al. 2018), which further proves the formation of crystals of the Cu-NPs. In fact, it can be said that the material in question was the Cu-NPs and the sharpness indicates the crystalline nature of the as-prepared Cu nanostructure. The peaks observed in the XRD spectrum of Cu-NPs synthesized in this study are consistent with the above (Hasheminya et al. 2018). Moreover, the average crystallite size of Cu-NPs was analyzed using Scherrer’s formula.
where k = 0.94, the Scherrer ‘s constant, D is the mean crystallite size, λ is the wavelength of the copper target, β is the full width half maximum value (FWHM) of the diffraction peaks and θ is the diffraction angle. Thus, XRD is commonly used to determine the chemical composition and crystal structure, type and crystal phase of a material. (Caroling et al. 2015).
Evaluation of factor groups by FT–IR
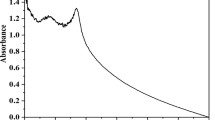
The FT–IR technique was used to confirm the synthesis of nanoparticles as well as to investigate the interactions between the different species and changes in chemical compositions of the mixtures during bio-synthesis. The FT–IR spectra of the synthesized Cu-NPs are shown in Fig. 4. As can be seen in the Figure, the peaks observed in the range of 3410 and 2920 are associated with the O–H and H-bonded functional groups in the copper nanoparticles, respectively. Also, the band at the 2848 was ascribed to C–H stretching vibrations. Furthermore, the carbonyl group, C–OH stretching vibrations, and C–O stretching were represented by the peaks at 1620, 1504, 1224, and 1320, respectively. This result also confirms that water soluble compound such as saponins which are present in the aqueous extract of guar leaf that have the ability to perform the stabilization of Cu-NPs. A similar observation has been reported by the several works (Gopalakrishnan and Muniraj 2019).
Antibacterial properties of copper nanoparticles, aqueous extract and essential oil extracted from the guar plant
The results of the MIC and MBC analysis of the effect of extracted nanoparticles, aqueous extract and essential oil (Control (first treatment), application of Cu (second treatment), application of T. harzianum (third treatment) and combined application of T. harzianum and Cu (fourth treatment)) on bacteria E. coli and S. aureus treated guar showed that, the essential oils treated with T. harzianum and copper at a concentration of 80 μL/ml inhibited the growth of E. coli and S. aureus. Also, the results of the MBC study showed that the concentration of 100 μL/ml in this treatment is effective in killing these two bacteria. E. coli and S. aureus bacteria were both inhibited by extracts treated with T. harzianum and copper at concentrations of 80 and 40 mg/ml, respectively. The concentration of 100 mg/ml in this treatment is effective in killing these two bacteria. The other factors investigated in this study included the MIC and MBC of copper nanoparticles extracted from guar.
According to the findings of this study, the extracted Cu-NPs of plant inhibited the growth of E. coli and S. aureus bacterial in all of the treatments. Also, according to MBC results, the Cu-NPs at a concentration of 0.25 mg/ml can kill this bacterium. Besides that, the extracted nanoparticles in the fourth treatment inhibit the growth of S. aureus bacteria at a concentration of 0.5 mg/ml, and a concentration of 0.25 mg/ml is sufficient to kill this bacterium. Generally, by comparing MIC and MBC obtained from nanoparticles, the aqueous extract and essential oils of the studied treatments it can be concluded that, the Cu-NPs and aqueous extract of the fourth treatment have better antibacterial properties compared to the essential oils of this plant. So, the essential oil of this plant in concentrations of 120 μL/ml inhibited the growth of E. coli and S. aureus. Sources revealed that no research on the synthesis of Cu-NPs with aqueous extract of guar plant has been published to date, and this is the first. However, the similar studies on other plants have been conducted (Hasheminya et al. 2018; Dlamini et al. 2020; Amer and Awwad 2021). The results of the MIC and MBC of Cu-NPs on the aforementioned bacteria are presented in Fig. 5 and Table 3.
Investigation of antibacterial properties using disk diffusion test
The results of studying the diameter and area of non-growth halo by image J software on the antibacterial properties of guar aqueous extract, essential oil and Cu-NPs on E. coli and S. aureus by disk diffusion test showed that the nanoparticles extracted from the plant have the antibacterial properties on these two bacteria. In fact, by applying a concentration of 100 μL of copper with T. harzianum, each of these three extracted compounds (extract, essential oil and nanoparticles) increased the diameter and area of non-growth halo in E. coli and S. aureus bacteria.
Copper nanoparticles extracted from guar in all of the four treatments showed the high antibacterial properties on E. coli and S. aureus. So that, in the concentration of 1 mg of copper nanoparticles, the highest diameter and area of non-growth halo (3.268 mm) was observed in S. aureus bacteria and in the fourth treatment. Furthermore, a 50 μL aqueous extracts concentration significantly increased the diameter and area of the non-growth halo in E. coli bacteria. So that, the fourth treatment had the largest diameter and area of non-growth halo in S. aureus (2.382 mm), while the control treatment had the smallest diameter and area of non-growth halo (0.931 mm). In S. aureus bacteria, therefore, a concentration of 50 μL of guar essential oil increased the diameter and area of non-growth halo. The results show that in comparison with the essential oil of this plant, the nanoparticles and aqueous extract from the fourth treatment had stronger antibacterial properties. In summary, most of the diameter and area of non-growth halo in the two studied bacteria in Cu-NPs and aqueous extract was related to the fourth treatment. The above results are in agreement with the findings of (Amer and Awwad 2021) (Fig. 6).
Conclusions
Plants try to maintain the optimal conditions when they are stressed. As a result, many plant metabolites undergo the quantitative and qualitative changes. What was observed in this study also confirms this fact. The current study showed that the presence of T. harzianum fungi had a significant role in increasing antioxidant enzymes in guar under heavy metal stress conditions. This study founded that the copper nanoparticles, aqueous extract, and essential oils extracted from the fourth treatment (combined application of fungus and copper) plant increased the inhibition and killing of E. coli and S. aureus bacteria. Cu-NPs and aqueous extracts from the plant, in fact, had more inhibitory and lethal effects on Gram-positive and Gram-negative bacteria than the plant essential oil. However, because of the thicker walls, Gram-negative bacteria were more resistant than Gram-positive bacteria, according to the findings of this study. Therefore, in order to minimize the risks of irrigated products with municipal wastewater in agricultural lands, plants with high growth rates should be used. Hence, due to the rapid growth of guar, high tolerance to stress conditions and repair potential to heavy elements, guar can be used to clean the soils contaminated with heavy metals. Because of its high growth volume and antibacterial properties, using this plant as a cover plant is desirable. In general, the guar plant is considered to have a high economic and ecological value. As a result, this plant has the potential to clean heavy metal-contaminated areas while also producing valuable biomass that can generate income for landowners, that is considered the plant's economic value. In reality, the harvested biomass could be incinerated and disposed of, or the accumulated metal could be recovered and reused as biofuel.
References
Ahluwalia V, Kumar J, Sisodia R et al (2014) Green synthesis of silver nanoparticles by Trichoderma harzianum and their bio-efficacy evaluation against Staphylococcus aureus and Klebsiella pneumonia. Ind Crops Prod 55:202–206. https://doi.org/10.1016/j.indcrop.2014.01.026
Amer MW, Awwad AM (2021) Green synthesis of copper nanoparticles by Citrus limon fruits extract, characterization and antibacterial activity. Chem Int 7:1–8. https://doi.org/10.5281/zenodo.4017993
Amin H, Arain BA, Jahangir TM et al (2018) Accumulation and distribution of lead (Pb) in plant tissues of guar (Cyamopsis tetragonoloba L.) and sesame (Sesamum indicum L.): profitable phytoremediation with biofuel crops. Geol Ecol Landsc 2:51–60. https://doi.org/10.1080/24749508.2018.1452464
Bai X, Aimila A, Aidarhan N et al (2020) Chemical constituents and biological activities of essential oil from Mentha longifolia: effects of different extraction methods. Int J Food Prop 23:1951–1960. https://doi.org/10.1080/10942912.2020.1833035
Bates LS, Waldren RP, Teare ID (1973) Rapid determination of free proline for water-stress studies. Plant Soil 39:205–207
Bhavyasree PG, Xavier TS (2020) Green synthesis of Copper Oxide/Carbon nanocomposites using the leaf extract of Adhatoda vasica Nees, their characterization and antimicrobial activity. Heliyon 6:e03323. https://doi.org/10.1016/j.heliyon.2020.e03323
Biresaw SS, Taneja P (2021) Copper nanoparticles green synthesis and characterization as anticancer potential in breast cancer cells (MCF7) derived from Prunus nepalensis phytochemicals. Mater Today Proc. https://doi.org/10.1016/j.matpr.2021.07.149
Bogusz A, Oleszczuk P (2020) Effect of biochar addition to sewage sludge on cadmium, copper and lead speciation in sewage sludge-amended soil. Chemosphere 239:124719. https://doi.org/10.1016/j.chemosphere.2019.124719
Caroling G, Vinodhini E, Mercy Ranjitham A, Shanthi P (2015) Biosynthesis of copper nanoparticles using aqueous Phyllanthus emblica (Gooseberry) extract-characterisation and study of antimicrobial effects. Int J Nano Chem Int 1:53–63
Dlamini NG, Basson AK, Simonis J et al (2020) Biosynthesis of bioflocculant passivated copper nanoparticles, characterization and application. Phys Chem Earth. https://doi.org/10.1016/j.pce.2020.102898
Ernesto Juniors PT, Valeria CL, Santiago PO et al (2020) Tolerance to oxidative stress caused by copper (Cu) in Trichoderma asperellum To. Biocatal Agric Biotechnol 29:2–8. https://doi.org/10.1016/j.bcab.2020.101783
Farias CP, Alves GS, Oliveira DC et al (2020) A consortium of fungal isolates and biochar improved the phytoremediation potential of Jacaranda mimosifolia D. Don and reduced copper, manganese, and zinc leaching. J Soils Sediments 20:260–271. https://doi.org/10.1007/s11368-019-02414-3
Ghaedi M, Yousefinejad M, Safarpoor M et al (2015) Rosmarinus officinalis leaf extract mediated green synthesis of silver nanoparticles and investigation of its antimicrobial properties. J Ind Eng Chem 31:167–172. https://doi.org/10.1016/j.jiec.2015.06.020
Gopalakrishnan V, Muniraj S (2019) Neem flower extract assisted green synthesis of copper nanoparticles—optimisation, characterisation and anti-bacterial study. Mater Today Proc 36:832–836. https://doi.org/10.1016/j.matpr.2020.07.013
Govarthanan M, Mythili R, Selvankumar T et al (2018) Myco-phytoremediation of arsenic- and lead-contaminated soils by Helianthus annuus and wood rot fungi, Trichoderma sp. isolated from decayed wood. Ecotoxicol Environ Saf 151:279–284. https://doi.org/10.1016/j.ecoenv.2018.01.020
Gresta F, Ceravolo G, Lo Presti V, D’Agata A, Rao R, Chiofalo B (2017) Seed yield, galactomannan content and quality traits of different guar (Cyamopsis tetragonoloba L.) genotypes. Ind Crops Prod 107:122–129. https://doi.org/10.1016/j.indcrop.2017.05.037
Hasheminya S-M, Rezaei Mokarram R, Ghanbarzadeh B et al (2018) Physicochemical, mechanical, optical, microstructural and antimicrobial properties of novel kefiran-carboxymethyl cellulose biocomposite films as influenced by copper oxide nanoparticles (CuONPs). Food Packag Shelf Life 17:196–204. https://doi.org/10.1016/j.fpsl.2018.07.003
Hassanien R, Husein DZ, Al-Hakkani MF (2018) Biosynthesis of copper nanoparticles using aqueous Tilia extract: antimicrobial and anticancer activities. Heliyon 4:e01077. https://doi.org/10.1016/j.heliyon.2018.e01077
Hosseinzadeh SR, Amiri H, Ismaili A (2018) Evaluation of photosynthesis, physiological, and biochemical responses of chickpea (Cicer arietinum L. cv. Pirouz) under water deficit stress and use of vermicompost fertilizer. J Integr Agric 17:2426–2437
Ismail MIM (2019) Green synthesis and characterizations of copper nanoparticles. Mater Chem Phys. https://doi.org/10.1016/j.matchemphys.2019.122283
Kar M, Mishra D (1976) Catalase, peroxidase, and polyphenoloxidase activities during rice leaf senescence. Plant Physiol 57:315–319
Keihan AH, Veisi H, Veasi H (2017) Green synthesis and characterization of spherical copper nanoparticles as organometallic antibacterial agent. Appl Organomet Chem 31(7):e3642. https://doi.org/10.1002/aoc.3642
Kumar V, Pandita S, Singh Sidhu GP et al (2021) Copper bioavailability, uptake, toxicity and tolerance in plants: a comprehensive review. Chemosphere 262:127810. https://doi.org/10.1016/j.chemosphere.2020.127810
Li X, Zhang X, Wang X et al (2019) Bioaugmentation-assisted phytoremediation of lead and salinity co-contaminated soil by Suaeda salsa and Trichoderma asperellum. Chemosphere 224:716–725. https://doi.org/10.1016/j.chemosphere.2019.02.184
Mahla HR, Sharma R, Kumar S, Gaikwad K (2020) Independent segregation of qualitative traits and estimation of genetic parameters and gene action for some quantitative traits in guar (Cyamopsis tetragonoloba L. Taub.). Indian J Genet Plant Breed 80:186–193. https://doi.org/10.31742/IJGPB.80.2.9
Mahmoudvand H, Khaksarian M, Ebrahimi K et al (2020) Antinociceptive effects of green synthesized copper nanoparticles alone or in combination with morphine. Ann Med Surg 51:31–36. https://doi.org/10.1016/j.amsu.2019.12.006
Manjate E, Ramos S, Almeida CMR (2020) Potential interferences of microplastics in the phytoremediation of Cd and Cu by the salt marsh plant Phragmites Australis. J Environ Chem Eng 8:103658. https://doi.org/10.1016/j.jece.2020.103658
Paulkumar K, Gnanajobitha G, Vanaja M et al (2014) Piper nigrum leaf and stem assisted green synthesis of silver nanoparticles and evaluation of its antibacterial activity against agricultural plant pathogens. Sci World J 2014:829894. https://doi.org/10.1155/2014/829894
Rajesh KM, Ajitha B, Reddy YAK et al (2018) Assisted green synthesis of copper nanoparticles using Syzygium aromaticum bud extract: Physical, optical and antimicrobial properties. Optik 154:593–600
Reis IMS, Alves SCN, de Melo WJ et al (2020) Cadmium, copper, and chromium levels in maize plants and soil fertilized with sewage sludge. Aust J Crop Sci 14:244–249. https://doi.org/10.21475/ajcs.20.14.02.p2006
Téllez Vargas J, Rodríguez-Monroy M, López Meyer M et al (2017) Trichoderma asperellum ameliorates phytotoxic effects of copper in onion (Allium cepa L.). Environ Exp Bot 136:85–93. https://doi.org/10.1016/j.envexpbot.2017.01.009
Xu T, Li C, Li H et al (2013) Synthesis of well-dispersed copper nanoparticles in electrospun polyacrylonitrile nanofibres. Micro Nano Lett 8:849–852. https://doi.org/10.1049/mnl.2013.0570
Acknowledgements
The author would like to thank Professor Seyed Ataollah Siadat, of the Agricultural Sciences and Natural Resources University of Khuzestan, Iran, Professor Mohammad Taghi Alebrahim, of the University of Mohaghegh Ardabili Iran and Dr. Faina Kraverskaja of the AWO (Arbeiterwohlfahrt Kreisverband Gütersloh e.V.); for inspire us and helpful advice on various technical issues in this manuscript.
Funding
This study has been supported by the Research Grant of Tabriz University of Medical Sciences (Tabriz, Iran) and University of Mohaghegh Ardabili (Ardabil, Iran).
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of a Topical Collection in Environmental Earth Sciences on Earth Surface Processes and Environment in a Changing World: Sustainability, Climate Change and Society, guest edited by Alberto Gomes, Horácio García, Alejandro Gomez, Helder I. Chaminé.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Ahmadi-Nouraldinvand, F., Afrouz, M., Elias, S.G. et al. Green synthesis of copper nanoparticles extracted from guar seedling under Cu heavy-metal stress by Trichoderma harzianum and their bio-efficacy evaluation against Staphylococcus aureus and Escherichia coli. Environ Earth Sci 81, 54 (2022). https://doi.org/10.1007/s12665-022-10184-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12665-022-10184-4