Abstract
The primary challenge for farmers at present is providing for an ever-increasing population while having little available land that is severely polluted. Zinc oxide (ZnO) nanoparticles (NPs) exhibit interesting properties and potential for applications in various disciplines, especially as nanomaterials in agriculture. In this study, to improve the quality of green gram (Vigna radiata) seeds and the first-time cultivation of green gram pods, ZnO NPs were synthesized using seaweed (Codium decorticatum) extract. Several investigations show that the synthesis procedure of ZnO NPs determines the physicochemical properties of ZnO NPs. The antimicrobial efficacy of plant pathogenic organisms such as Xanthomonas phaseoli, Colletotrichum lindemuthianum, Cercospora canescens, Rhizoctonia bataticola, and Ascochyta phaseolorum was examined using the agar well technique. X-ray diffraction, UV spectrum, and field emission scanning electron microscopy analyses were used to investigate the structural, optical, and morphological characteristics of the thus-synthesized NPs, respectively. These analyses demonstrated the crystal structure and the spherical shape of the NPs and showed that they ranged in size from 25 to 35 nm. The purity of the NPs and the functional moieties contributing to their efficient manufacturing and stability were investigated using energy-dispersive X-ray analysis and Fourier transform infrared spectroscopy, respectively. Green gram seeds were subjected to foliar treatments of various concentrations of the synthesized ZnO nano-fertilizer. Among these concentrations, the 20 mg/L ZnO nano-fertilizer resulted in the highest level of biochemical content and improvements in different growth metrics in plants. These results show that the biosynthesis of ZnO NPs was safe, effective, non-toxic, and environmentally friendly. Furthermore, the thus-synthesized ZnO NPs showed strong antibacterial activity in plants. In addition, they were found to be efficient in improving the cultivation and production of green grams. Hence, these ZnO NPs show outstanding antibacterial activities and could be recommended as nano-fertilizers.
Graphical Abstract

Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Statement of novelty
This is the first time, we have used Codium decorticatum seaweed extract for the treatment of nano-fertilizer (Vigna radiata).
Introduction
Nanoparticles (NPs) play a vital role in various disciplines, such as medicine, cosmetics, agriculture, and food industries. Recent research has shown that using plants and microorganisms to synthesize metal NPs is an effective and environmentally friendly approach [1]. The application of nanotechnology in agriculture can improve product quality, minimize the adverse effects on the environment and human health due to pesticide usage, increase crop productivity, and improve food security, especially in terms of climate change and population growth. Using NP-based fertilizers can promote plant development and enhance product quality as well as ensure soil quality [2]. Furthermore, by using carriers and chemicals based on NPs, the need for fertilizers and pesticides is decreased without affecting production.
The agriculture sector significantly benefits from nanotechnology. One of the goals of nanotechnology is to use NPs to synthesize novel, high-quality with potential for applications in wide-ranging fields [3]. Nanotechnology and biotechnology have significantly contributed to the advances in the agriculture industry [4]. Today, the majority of researchers agree that nanomaterials (NMs) are a key component of modern science and technology. Since NMs can enter the environment via various routes, the relationship between NMs and ecosystems has become a contentious issue worldwide [5]. As they show immense potential in many disciplines, NMs with at least one structural dimension at the nanoscale level receive more scientific interest [6].
Zinc (Zn) is a micronutrient that is crucial for plant development as it plays a key role in auxin production, and growth hormone necessary for tryptophan synthesis, a precursor of indole-3-acetic acid [7]. Furthermore, Zn contains a component that is necessary for the activity of various enzymes and proteins [8, 9]. Moreover, Zn is a cofactor for many enzymes, such as carbonic anhydrase, carboxypeptidase, and superoxide dismutase. Zn deficiency negatively affects plants by limiting their development, reducing tiller count, inducing chlorosis, reducing the leaf size, increasing the crop maturity period, and resulting in sterility of spikelet’s and poor quality end products [10]. Plant enzymes triggered by Zn are involved in various processes such as regulation of auxin production, preservation of cellular membrane integrity, protein synthesis, pollen development, and carbohydrate metabolism [11]. ZnO nano-fertilizers provide beneficial solutions to many environmental issues, such as fertilizer overuse, chemical leaching, and pollution. Thus, the synthesis of NPs has been increasing worldwide.
Seaweeds find applications in several disciplines, such as the production of food products, feed, fodder, fertilizers, industrial goods, and various chemicals, as they are inexpensive and eco-friendly due to the presence of fiber, probiotics, antioxidants, vitamins, and minerals [12]. In addition, the presence of auxin, cytokinins, betanins, macronutrients, and micronutrients, which promote seed germination, crop production, and resistance to frost, insect, and fungal assaults, has made seaweeds more prevalent in contemporary agriculture practices in the last decade [13]. Hence, chemical fertilizers can be substituted by seaweed-based nano-fertilizers.
The findings of this study suggest, for the first time, the seed quality of green gram and the production of green gram pods as important parameters in ZnO NPs synthesis using seaweed extract. The synthesized ZnO NPs were characterized using several physicochemical characteristics. In addition, their antimicrobial activities were also investigated against the microorganisms that cause diseases in plants.
Materials and Methods
Chemicals, Sample Collection, and Seaweed Extract Preparation
All chemicals and solvents used were bought from Sigma-Aldrich Chemicals, India. The seaweed Codium decorticatum, a member of the Chlorophyceae family, was procured between July and August 2023 from Devipattinam, Tamil Nadu, India (Lat, 9° 47 N, Long, 78° 89E) (Fig. 1). To remove undesirable contaminants and epiphytes from the samples, they were carefully washed in seawater upon collection. The samples were then separated from the seawater and packed in new polythene bags before being transferred to the laboratory.
The seaweed samples were cleaned and sliced into pieces. The samples were then grilled in distilled water for 10 min at 95 °C. To prepare the seaweed extract, 100 g of seaweed was crushed in 200 mL of distilled water, which was then filtered using a muslin cloth. The filtrate was then treated with equal volumes of Millipore water, filtered through Whatman No. 1 paper, and kept at 4 °C until further analysis.
ZnO NPs Synthesis
With ∼ 400 mL of 0.1 M zinc acetate, 80 mL of seaweed extract was mixed using a magnetic mixer. To the mixture was then added 400 mL of 0.2 M NaOH, which was then vigorously stirred at 60 °C. After 3 h of stirring, a pale yellow precipitate appeared, which showed that ZnO NPs were successfully synthesized. The precipitate was collected, centrifuged, and oven-dried. The dried powder was then heated in a muffle furnace for 4 h at 400 °C.
Properties of ZnO NPs
Optical absorption of the ZnO NPs ranged between 200 and 600 nm, which was determined using a UV–Visible spectroscope (Shimadzu UV-1800). To determine particle size and crystallized nature, powder diffraction measurements (BRUKER AXS, D8 Discover, etc.) were carried out in the temperature range of 20° to 80° with CuKα radiation (λ = 0.15 nm). NPs’ size, shape, and surface morphology were determined using field emission scanning electron microscopy (FE-SEM) with energy-dispersive X-ray (EDX) analysis (Supra 55-Carl Zeiss, Germany). In addition, the functional compounds present in the NPs were studied using a IRAffinity-1 S Fourier transform infrared spectroscopy (FT-IR) spectrophotometer operated at a range of 3500–500 cm−1.
Antimicrobial Efficiencies
Disease-causing plant pathogens such as Xanthomonas phaseoli, Colletotrichum lindemuthianum, Cercospora canescens, Rhizoctonia bataticola, and Ascochyta phaseolorum were purchased from MTCC, Chandigarh, India, which investigated using the agar well diffusion method. Bacterial and fungal strains were subcultured in MHA and SDA media at 35 °C and 30 °C, respectively. A 6-mm hole was dug on MHA and SDA plates, and a 20 µL sample was injected into each well using a micropipette. Gentamycin (50 µg) was used as the bacterial and fungal positive control, and MilliQ water as the negative control. The bacterial and fungal cultures were grown for 24 and 72 h, respectively, at 30 ± 2 °C and 37 ± 2 °C. Experiments were carried out in triplicate, and the outcomes were expressed in millimeters.
Preparation of ZnO Solution and Green Gram Seeds
The following ZnO concentrations were refined using ultrasonication and deionized water dispersion for 30 min: 5, 10, 20, 40, 80, and 160 mg/L. An all-deionized water priming control was used. Green gram seeds (Vigna radiata) purchased from the Seed Centre at the Tamil Nadu Agricultural University (TNAU), Coimbatore, Tamil Nadu, India, were utilized in all analyses. Seed priming was conducted by mixing with 3% H2O2 and then cleaning with deionized water. The seeds were then soaked in ZnO solutions, vented for 12 h, and air-dried at room temperature until further analysis.
Influence of ZnO Nano-priming on the Sprouting of Seeds and Juvenile Indices
The ISTA (International Seed Testing Association) tissue paper technique was used to examine the germination of green gram seeds using both nanoprimed and unprimed ZnO (control). About 15 seeds were placed in sprouting plates, which were then moistened using distilled water for every experiment. Then, the sheets were gently rolled in a butter knife, fastened using an elastic band, and stored at 25 °C and 90% RH in a dark sprouting room. The seed sprouting proportion was evaluated on day three. This procedure was repeated four times, and the findings were expressed as means.
The Impact of Foliar Spraying of ZnO NPs on Pot Culture
The efficiency of the NPs was examined in a cultivated field in Ayyampet, Thanjavur District, Tamil Nadu, India. After germinating the sprouts for three days in cement pots, they were kept in a garden. Young plants were transferred to containing a 1:1 mixture of agricultural soil and sand. Then, for the first 30 days, all plants were provided a 100% water level of the field potential, and either tap water was used to water the plants or various concentrations of the nano-fertilizer were sprayed on them before and after blossoming. All experiments were conducted in triplicate. Before spraying, the NPs were soured for 30 min using an ultrasonicator.
Determination of Biochemical Constituents
The biochemical constituents determined in 30-day-old plants were as follows: chlorophylls a and b, entirety chlorophyll, entirety carbohydrates, and entirety proteins [12].
Statistical Analysis
All tests were conducted in triplicate for each sample. The findings were analyzed using one-way analysis of variance and expressed as means and standard deviations.
Results and Discussion
Ultraviolet–Visible Spectroscopy Analysis
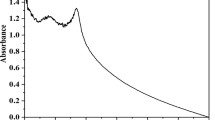
The NP formation was further examined using UV–Visible spectrophotometry. The UV peaks detected by the spectrophotometer are presented in Fig. 2. The highest peak for seaweed-based ZnO NPs was observed at 298 nm. In general, all oxides show broad band gaps but shorter wavelengths, which indicates the conventional ZnO absorption pattern. Furthermore, nanoscale materials tend to have even shorter wavelengths. This observation is in line with the findings of this study [14].
XRD Analysis
Figure 3 shows the X-ray diffraction (XRD) pattern the ZnO NPs, which clearly demonstrates the widening lines of the XRD peaks, thus showing the nanoscale range of the thus-generated particles. The Miller index planes for the XRD peaks at 31.6, 33.8, 35.3, 47.4, 56.2, 63.5, 66.3, 68.2, 69.4, 73.2, and 78.6° were indexed as follows: (100), (002), (101), (102), (110), (103), (200), (112), (201), (004), and (202). These findings show that the peaks observed in the XRD pattern spectrum (JPCDS card number 36-1451) were consistent with the previous reports, indicating the hexagonal crystal structure of the NPs [15, 16]. All distinctive peaks of the NPs were identified as being unique, which shows that there were no contaminants in them. The Debye–Scherrer formula was used to calculate the diameter of the crystallites, which was found to be around 26 nm, and based on Bragg’s diffraction angle and full width at half-maximum of stronger peaks corresponding to 101 planes present at position 35.3°, the average size of crystallites was found to be 38.24 nm. The indexation supports the findings of prior reports showing the typical hexagonal wurtzite structure of ZnO NPs (JCPDF file no. 00-036-1451) [17].
FT-IR Spectroscopy
The FT-IR analysis of the NPs showed peaks at 3621, 3122, 1712, 1256, 998, and 524 cm1 (Fig. 4). The wide peak at 3621 cm−1 was attributable to O–H bending [18]. The peak at 3122 cm−1 was attributable to C–H alkene stretching [19], whereas the peak at 1712 cm−1 was attributable to C=O vibration stretching [20]. The bands at 1256 cm−1 showed the presence of the C–O–C group [21]. In addition, the bands at 998 cm−1 were attributable to C–C stretching vibration [22]. The peak at 524 cm−1 may be attributable to ZnO stretching vibrations due to the presence of metal-oxygen [23].
FE-SEM with EDX
The morphology of the NPs was analyzed using FE-SEM. As presented in Fig. 5a, the NPs showed a semispherical shape and highly agglomerated state. This shows the homogenous state of the NPs, which is crucial to the various functions they perform. The NPs ranged in size from 29 to 41 nm. The increase in NP size was attributable to their overlapping with one another. Spherical to hexagonal NPs were observed, with a grain size of 34.5 nm. A plot of the particle distribution of the NPs is presented in Fig. 5c. EDX analysis was used to analyze the composition and purity of the NPs. Only the presence of Zn and O was observed, as shown in Fig. 5b. A comparison of the particle sizes observed in XRD patterns confirmed NP synthesis. These findings are consistent with previous publications [17, 24].
Determination of Antimicrobial Properties
The antimicrobial activity of the NPs was evaluated against various pathogens such as Xanthomonas phaseoli, Colletotrichum lindemuthianum, Cercospora canescens, Rhizoctonia bataticola, and Ascochyta phaseolorum, of the synthesized ZnO NPs were assessed. In contrast to plant extract, zinc acetate, and gentamycin – all suppressed the growth of the tested bacterial strains—ZnO NPs showed a broad spectrum of antibacterial activity (Fig. 6). Inhibition zones against X. phaseoli were most prominent (24 mm), followed by those against C. lindemuthianum (21 mm), C. canescens (20 mm), A. phaseolorum (18 mm), and R. bataticola (16 mm).
Antibiotic resistance is a significant challenge that affects both developing and developed countries, in turn affecting a significant proportion of the global environment. The increasing spread of diseases with multiple drug resistance has had a significant impact on current herbicide therapy. The search for novel sources of antimicrobials has been expanded to include seaweed-mediated NMs, which have a wide range of bioactive chemicals with well-established curative potential [25, 26]. Environmentally friendly techniques for synthesizing metal NPs have shown significant progress of late. While seaweed extracts serve as both capping and reducing agents in NP synthesis, seaweed extract phytochemicals have also attracted major interest in NP synthesis.
Abdelhakim et al. [27] proposed that the antimicrobial activity of NPs is usually attributable to the high effectiveness of NPs in restricting cell growth due to an increase in the formation of reactive oxygen species (ROS). ROS are responsible for ZnO NPs’ ability to limit bacterial and fungal growth, as reported in a recent study by Keerthana et al. [24]. They reported that ZnO NPs may form ROS in aqueous suspensions. NPs can interact effectively with both the cell membrane and cell wall, resulting in the leakage proteins, genetic material, and minerals, ultimately leading to cell death [28, 29].
Potted Planting Examinations
A previous study on potted planting in green gram plants using various concentrations of nano-fertilizers reported that all biochemical components in plants had grown at the 30-day stage (Fig. 7). Plants subjected to 20 mg/L NPs showed the highest biochemical contents. At the 60-day stage, the 20 mg/L concentration showed the highest level of seed sprouting, root diameter, length of shoots, fresh shoot weight, whole dry mass, quantity of twigs, number of pods, and area of leaves. In addition, the lowest values for the above parameters were observed for the 160 mg/L concentration. In line with these findings, analysis of ladies’ finger and cluster bean plants given a starting fertilizer showed that increasing Zn concentration (20 mg) in seeds significantly enhanced the Zn content in roots and shoot elongation [24, 28]. In addition, Zn priming effectively enhanced seed germination and seedling development.
Various parameters such as shoot length, root length, plant height, total dry weight, the number of branches, and the number of pods are presented in Table 1. Significant improvements were observed in the growth metrics of seeds primed using ZnO NPs, such as seed germination (97%), root height (9 cm), total plant height (38 cm), shoot height (23 cm), total fresh weight (28 gm), root fresh weight (5 gm), shoot fresh weight (19 gm), total dry weight (5.60 gm), root dry weight (0.98 gm), shoot dry weight (6.71 gm), leaf area (8.12 cm), number of pods (47), length of pods (20 cm), weight of pods (42 gm), and number of branches (14), (Table 1; Fig. 8). Keerthana et al. [24] and Rexlin et al. [28] reported similar findings, who observed that ladies’ finger and cluster bean seedlings treated with a low concentration (20 mg/L) of ZnO NPs-based nano-fertilizers promoted plant growth. However, tomato seedlings exposed to different concentrations of ZnO NPs showed a substantial increase in seed germination rate, root weight, and shoot weight [29].
Due to the low absorption efficiency of traditional fertilizers, they should be administered in considerable quantities. The two primary constraints with fertilizers based on phosphorus and nitrogen are their low efficiency in absorbing nutrients and rapid transformation into forms that plants cannot use [30]. These aspects are harmful to the land and the ecosystem as they increase the production of hazardous greenhouse gases and eutrophication. In nano-fertilizers, nutrients are released gradually, which is beneficial in improving the efficiency of nutrient usage without any adverse effects. These nano-fertilizers are designed to slowly release nutrients and to significantly reduce nutrient loss, hence ensuring environmental safety [31]. Not only are traditional fertilizers expensive but they also have adverse effects on people and the environment; however, nano-fertilizers preserve soil health and increase agricultural output [32]. Nano-fertilizers promote plant development through both direct and foliar application techniques (Fig. 9). They are the most efficient solution for preventing eutrophication and microbial infections and improving nutrient usage efficiency.
Conclusion
These results showed that ZnO NPs can be synthesized from seaweed extract in a straightforward, affordable, and environmentally responsible manner. The NPs thus synthesized were investigated for their optical, structural, elemental, and morphological parameters using XRD, UV–Visible spectroscopy, FE-SEM with EDX, and FT-IR. XRD showed tetragonal-shaped wurtzite ZnO NPs and indicated that the seaweed extract comprised various biological elements that decreased the number of ZnO NPs and rendered them more stable. Along with the EDX data, the findings of FE-SEM revealed that the NPs were spherical and pure, ranging in size from 25 to 35 nm. In conclusion, ZnO NPs with high disease resistance and promising potential as nano-fertilizers can be used in the cultivation of green grams.
Data Availability
On behalf of all the authors, the corresponding author states that our data are available upon reasonable request.
References
Howra, B., Saghi, N., Nahid, J., Hossein, T., Vasighe, S.M., Andrew, J.E., Gholamreza, A.: Green synthesis of metal nanoparticles using microorganisms and their application in the agri-food sector. J. Nanobiotechnol. 19, 86 (2021). https://doi.org/10.1186/s12951-021-00834-3
Duhan, J.S., Kumar, R., Kumar, N., Kaur, P., Nehra, K., Duhan, S.: Nanotechnology: the new perspective in precision agriculture. Biotechnol. Rep. (Amst.) 15, 11–23 (2017)
Govarthanan, M., Srinivasan, P., Selvankumar, T., Janaki, V., Fahad, A.A., Hamed, A.E., Woong, K., Kamala-Kannan, S.: Utilization of funnel-shaped ivory flowers of Candelabra cactus for zinc oxide nanoparticles synthesis and their in-vitro anti-cancer and antibacterial activity. Mater. Lett. 273, 127951 (2020)
Pooja, G., Jyoti, M., Nidhi, S.: Silica nanoparticles as novel sustainable approach for plant growth and crop protection. Heliyon 8, e09908 (2022). https://doi.org/10.1016/j.heliyon.2022.e09908
Haleema, S., Syed, Z.: Sustainable use of nanomaterials in textiles and their environmental impact. Materials 13(22), 5134 (2020). https://doi.org/10.3390/ma13225134
Sivasubramanian, M., Radhakrishnan, Y.K., Sundaram, V., Ramasamy, S., Woong, K., Govarthanan, M., Natchimuthu, K.: Emerging nanotechnology in renewable biogas production from biowastes: impact and optimization strategies—a review. Renew. Sustain. Energy Rev. 181, 113345 (2023)
Ahmad, R., Ishaque, W., Khan, M., Ashraf, U., Riaz, M.A., Ghulam, S., Ahmad, A., Rizwan, M., Ali, S., Alkahtani, S.: Relief role of lysine chelated zinc (zn) on 6-week-old maize plants under tannery wastewater irrigation stress. Int. J. Environ. Res. Public Health 17, 5161 (2020). https://doi.org/10.3390/ijerph17145161
Zaheer, I.E., Ali, S., Rizwan, M., Abbas, Z., Bukhari, S.A.H., Wijaya, L., Alyemeni, M.N., Ahmad, P.: Zinc-lysine prevents chromium-induced morphological, photosynthetic, and oxidative alterations in spinach irrigated with tannery wastewater. Environ. Sci. Pollut. Res. 26, 28951–28961 (2019). https://doi.org/10.1007/s11356-019-06084-z
Zaheer, I.E., Ali, S., Saleem, M.H., Yousaf, H.S., Malik, A., Abbas, Z., Rizwan, M., Abualreesh, M.H., Alatawi, A., Wang, X.: Combined application of zinc and iron-lysine and its effects on morpho-physiological traits antioxidant capacity and chromium uptake in rapeseed (Brassica napus L.). PLoS One (2022). https://doi.org/10.1371/journal.pone.0262140
Rizwan, M., Ali, S., Rehman, M.Z., Maqbool, A.: A critical review on the effects of zinc at toxic levels of cadmium in plants. Environ. Sci. Pollut. Res. 26, 6279–6289 (2019). https://doi.org/10.1007/s11356-019-04174-6
Bhantana, P., Rana, M.S., Sun, X., Moussa, M.G., Saleem, M.H., Syaifudin, M., Shah, A., Poudel, A., Pun, A.B., Bhat, M.A.: Arbuscular mycorrhizal fungi and its major role in plant growth, zinc nutrition, phosphorous regulation and phytoremediation. Symbiosis 84, 19–37 (2021). https://doi.org/10.1007/s13199-021-00756-6
Vijayakumar, S., Durgadevi, S., Arulmozhi, P., Rajalakshmi, S., Gopalakrishnan, T.: Effect of seaweed liquid fertilizer on yield and quality of Capsicum annum L. Acta Ecol. Sinca 39, 406–410 (2019). https://doi.org/10.1016/j.chnaes.2018.10.001
Zafar, A., Ali, I., Rahayu, F.: Marine seaweeds (biofertilizer) significance in sustainable agricultural activities: a review. Earth Environ. Sci. 974, 012080 (2022). https://doi.org/10.1088/1755-1315/974/1/012080
Naseer, M., Aslam, U., Khalid, B., Chen, B.: Green route to synthesize zinc oxide nanoparticles using leaf extracts of Cassia fistula and Melia Azadarach and their antibacterial potential. Sci. Rep. 10(1), 9055 (2020). https://doi.org/10.1038/s41598-020-65949-3
Shah, F., Hasnain, J., Sajjad, A.S., Sumaira, S., Adnan, K., Muhammad, T.A., Muhammad, R., Faheem, J., Wajidullah, Noreen, A., Aishma, K., Suliman, S.: Green synthesis of zinc oxide (ZnO) nanoparticles using aqueous fruit extracts of Myristica fragrans: their characterizations and biological and environmental applications. ACS Omega 6(14), 9709–9722 (2021). https://doi.org/10.1021/acsomega.1c00310
Hamidian, K., Sarani, M., Sheikhi, E., Khatami, M.: Cytotoxicity evaluation of green synthesized ZnO and Ag-doped ZnO nanoparticles on brain glioblastoma cells. J. Mol. Struct. 1251, 131962 (2022). https://doi.org/10.1016/j.molstruc.2021.131962
Tamil Elakkiya, M., Vijayakumar, S., Sangeetha, R., Vidhya, E., Prathipkumar, S., Sekar, V.: Bio-mediated zinc oxide nanoparticles through tea residue: ecosynthesis, characterizations, and biological efficiencies. Sustainability 14, 15572 (2022). https://doi.org/10.3390/su142315572
Bodiul Islam, M., Jahidul Haque, M., Shehab, N.M., Saifur Rahman, M.: Synthesis and characterization (optical and antibacterial) of silver doped zinc oxide nanoparticles. Open Ceram. 14, 100370 (2023). https://doi.org/10.1016/j.oceram.2023.100370
Pillai, A.M., Sivasankarapillai, V.S., Rahdar, A., Joseph, J., Sadeghfar, F., Anuf, A.R., Rajesh, K., Kyzas, G.Z.: Green synthesis and characterization of zinc oxide nanoparticles with antibacterial and antifungal activity. J. Mol. Struct. (2020). https://doi.org/10.1016/j.molstruc.2020.128107
Rui, S., Ying, L., Linghao, H.: Synthesis and characterization of mercaptoacetic acid-modified ZnO nanoparticles. Solid State Sci. 10(11), 1563–1567 (2008). https://doi.org/10.1016/j.solidstatesciences.2008.02.006
Sorna Gowri, V., Kumar, R., Sunil Kumar, S., Avanish Kumar, S.: New epoxy poly(dimethyl acrylamide) polymer for the dispersion of ZnO nanoparticles. Adv. Sci. Eng. Med. 12, 1–5 (2020). https://doi.org/10.1166/asem.2020.2683
Yousefi, F., Seyed, B.M., Saeed, Z.H., Samin, N.H.: UV-shielding properties of a cost-effective hybrid PMMA-based thin film coatings using TiO2 and ZnO nanoparticles: a comprehensive evaluation. Sci. Rep. 13, 7116 (2023)
Rajan, A., Elsa, C., Baskar, G.: Biosynthesis of zinc oxide nanoparticles using Aspergillus fumigatus JCF and its antibacterial activity. Int. J. Mod. Sci. Technol. 1(2), 52–57 (2016)
Keerthana, P., Vijayakumar, S., Vidhya, E., Punitha, V.N., Nilavukkarasi, M., Praseetha, P.K.: Biogenesis of ZnO nanoparticles for revolutionizing agriculture: a step towards anti-infection and growth promotion in plants. Ind. Crops Prod. 170, 113762 (2021). https://doi.org/10.1016/j.indcrop.2021.113762
Prerna, I.D., Govindaraju, K., Tamilselvan, S., Kannan, M., Raja, K., Subramanian, K.S.: Seaweed-based biogenic ZnO nanoparticles for improving agro-morphological characteristics of Rice (Oryza sativa L.). J. Plant Growth Regul. (2019). https://doi.org/10.1007/s00344-019-10012-3
Raja, K., Sowmya, R., Sudhagar, R., Sathya, M.P., Govindaraju, K., Subramanian, K.S.: Biogenic ZnO and Cu nanoparticles to improve seed germination quality in blackgram (Vignamungo). Mater. Lett. 235, 164–167 (2019)
Abdelhakim, H.K., El-Sayed, E.R., Rashidi, F.B.: Biosynthesis of zinc oxide nanoparticles with antimicrobial, anticancer, antioxidant and photocatalytic activities by the endophytic Alternaria tenuissima. J. Appl. Microbiol. (2020). https://doi.org/10.1111/jam.14581
Rexlin, J., Vijayakumar, S., Nilavukkarasi, M., Vidhya, E., Nahed, S., Alharthi, M., Sajjad, Punitha, V.N., Praseetha, P.K.: Bioengineered ZnO nanoparticles as a nano priming agent in Cyamopsis tetragonoloba (L).Taub. To improve yield and disease resistance. Appl. Nanosci. (2022). https://doi.org/10.1007/s13204-022-02526-2
Punitha, V.N., Vijayakumar, S., Alsalhi, S.M., Sandhanasamy, D., Nilavukkarasi, M., Vidhya, E., Prathipkumar, S., Woong, K.: Biofabricated ZnO nanoparticles as vital components for agriculture revolutionization—a green approach. Appl. Nanosci. (2023). https://doi.org/10.1007/s13204-023-02765-x
Raliya, R., Saharan, V., Dimkpa, C., Biswas, P.: Nanofertilizer for precision and sustainable agriculture: current state and future perspectives. J. Agric. Food Chem. 66, 6487–6503 (2018). https://doi.org/10.1021/acs.jafc.7b02178
Nongbet, A., Mishra, A.K., Mohanta, Y.K., Mahanta, S., Ray, M.K., Khan, M., Baek, K.H., Chakrabartty, I.: Nano-fertilizers: a smart and sustainable attribute to modern agriculture. Plants. 11, 2587 (2022). https://doi.org/10.3390/plants11192587
Jiang, M., Song, Y., Kanwar, M.K., Ahammed, G.J., Shao, S., Zhou, J.: Phytonanotechnology applications in modern agriculture. J. Nanobiotechnol. 19, 430 (2021)
Funding
The authors are grateful to the DBT-STAR (HRD-11011/18/2022-HRD-DBT), DST-FIST (SR/FST/College-222/2014). The authors express their sincere appreciation to the Researchers Supporting Project Number (RSP2023R398) King Saud University, Riyadh, Saudi Arabia.
Author information
Authors and Affiliations
Contributions
DD: has monitored the nano-fertilizer treatment. SV: has designed the work, and monitored the overall setup including writing the paper, All authors have been helped to finish the paper. EV, and SD, has a collection of plant materials and extract preparations. SP, MSA and WK has analyzed the characterizations of nanoparticles. AA, and RM have analyzed the antimicrobial activity.
Corresponding author
Ethics declarations
Conflict of interest
The authors have declared no conflict of Interest.
Additional information
Publisher’s Note
Springer nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Devadharshini, D., Vijayakumar, S., Vidhya, E. et al. Eco-synthesized ZnO Nanoparticles Pertaining to Agricultural Revolution: An Infection Curative and Plant Growth Promoter for Green Gram. Waste Biomass Valor 15, 1869–1879 (2024). https://doi.org/10.1007/s12649-023-02346-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12649-023-02346-7