Abstract
Polycyclic aromatic hydrocarbons (PAHs) are ubiquitous organic pollutants which can be accumulated in river sediment, posing potential threats to ecosystem and human health. A total of 20 surface sediment samples were collected in Wei River, and 16 priority PAHs were analyzed for pollution features as well as ecological and human health risk. The results showed that the sum of the 16 PAHs (Σ16PAHs) in the sediment of Wei River ranged from 60.5 to 10,241.1 ng g−1 dw, with an average of 2250.4 ng g−1 dw. The total of seven carcinogenic PAHs (ΣCPAHs) was in the range of 5.8–7232.7 ng g−1 dw, with a mean of 1276.5 ng g−1 dw, accounting for 56.7% of the Σ16PAHs and presenting a high carcinogenic potential. Elevated Σ16PAHs and ΣCPAHs were observed in the lower reach of Wei River. PAHs in the sediment were dominated by 3- and 5-ring PAHs, and mainly related to various combustion processes. The ecological risk of individual PAHs (except for Chy) in the sediment was not to be neglected, with the highest risk of Acy and Flu. The total ecological risk of PAHs in the sediment was a moderate to high level. The non-carcinogenic risk of exposure to PAHs for children, adolescences and adults was insignificant. The carcinogenic risk of exposure to PAHs was in the range of 10–6—10–4 for children, adolescences and adults, indicating an acceptable carcinogenic risk.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Polycyclic aromatic hydrocarbons (PAHs) are a group of organic compounds containing two or more fused benzene rings, with the characteristics of high toxicity, long-distance migration, long-term persistence, degradation-resistant, and bio-accumulation (Liu et al. 2009; IARC 2010; Lei and Wania 2011; Kim et al. 2013; Bi et al. 2018). Some PAHs have carcinogenic, teratogenic, and mutagenic effects. As a result, the United States Environmental protection Agency (US EPA) has listed sixteen PAHs as priority pollutants and seven of them are considered to be probable carcinogens. PAHs in the environment are derived from natural and anthropocentric processes. Volcanic eruptions, forest fires, and diagenesis are natural sources of PAHs; the incomplete combustion of fossil fuels (coal, petroleum, and natural gas) and biomass (wood and grass), traffic exhausts, and solid waste incineration are main anthropocentric sources of PAHs (Zhu et al. 2009; Pietzsch et al. 2010; Wang et al. 2015). With rapid urbanization and industrialization, PAHs are largely released into the environment and widely detected in water bodies, atmospheric gas/particulates, soil, and sediment (Readman et al. 1986; Chen et al. 2015; Pongpiachan et al. 2013; Wang et al. 2018).
River sediment is an ecosystem matrix composed of solid particles in the bottom of river, directly influencing water quality and living environment of aquatic organisms, and being considered as an important sink and source of pollutants. Characterized by high octanol–water partition coefficient (logKow) as well as lipophilic and hydrophobic properties, PAHs in aquatic environment are prone to accumulate in sedimentary phase, which leads to a high concentration level of PAHs and long-term impact on aquatic organisms, posing potential threats to aquatic ecosystem (Sun et al. 2009; Manariotis et al. 2011; Dudhagara et al. 2016). In addition, the transportation of PAHs from sediment to benthic organisms is of great health concern because PAHs can be transferred into human bodies through the intake of aquatic products contaminated by PAHs, posing potential threats to human health (Li et al. 2016; Yang et al. 2014). Therefore, the quality and chemical composition of river sediment can be important indicators of environmental pollution and risk (Liu et al. 2016).
The pollution and risk associated with PAHs in rivers are of global concern. Many studies on the concentration, distribution, source, and risk of PAHs have been conducted in the sediment from Guan River and Luan River of China (He et al. 2014; Zhang et al. 2016), Delaware River of USA (Kim et al. 2018), Poxim River of Brazil (Souza et al. 2018), and Brisbane River of Australia (Duodu et al. 2017). However, limited data on PAHs in Wei River of Northwest China are available. Wei River is the largest tributary of the Yellow River, flowing through some semi-arid cities, such as Tianshui, Baoji, Xianyang, Xi’an, and Weinan, some of which are important industrial cities in Northwest China. The section of Shaanxi of Wei River is also called as Weihe/Guanzhong Plain/Basin, which is an important production base of grain and cotton for Shaanxi Province. Wei River and its tributaries make a great contribution to the development of local industry and agriculture. With the rapid development of social economy, a large quantity of pollutants have been discharged into Wei River, posing potential threats to aquatic ecosystem and human health (Chen et al. 2015). Therefore, the objectives of this study were to analyze the pollution characteristics of PAHs in sediment of Wei River, assess the ecological risk of PAHs in sediment of Wei River; and evaluate the health risk of human exposure to PAHs by the intake of fishes living in Wei River.
Materials and methods
Description of study area
Wei River originates from the Niaoshu mountain in the southwest part of Weiyuan county of Gansu Province, flows through the Loess Plateau of the east part of Gansu, Tianshui basin and Baoji valley, enters into Guanzhong/Weihe plain, and merges into the Yellow River in Tongguan. Wei River is the largest tributary of the Yellow River. The total length of Wei River is 818 km, with an annual average runoff of 7.57 billion m3 and a runoff area of 62.4 × 103 km2. The origin and south part of Wei River are surrounded by the Qinling mountain composed by granite gneiss, granite and biotite granite. The north part of Wei River is Loess Plateau consisting of coal and horizontal sand shale covered by loess. Weihe plain is a graben type tectonic plain due to the alluviation of Wei River and its tributaries, enriched by sandstone, sharpening rock and mudstone. There are many tributaries in the drainage area of Wei River. The tributaries in north bank originate from Loess Plateau, with the high content of silt; the ones in south bank come from the Qinling mountain, with the features of short headwater, steep slope and fast flow. The two important tributaries of Wei River are Jing River and Luo River, originating from Loess Plateau. Jing River is the largest tributary of Wei River, with a length of 455.1 km, an annual average runoff of 2.07 billion m3, and a runoff area of 45,400 km2. Luo River is the longest tributary of Wei River, with a length of 680 km and a runoff area of 26,900 km2. Large refineries and factories are distributed in the basin of Luo River. Approximate 40 species of fishes have been found in Wei River basin, and Cyprinidae and Cobitidae constitute the major structure of fish communities (Shen et al. 2019; Wu et al. 2014). In the headwater and upstream tributaries of Wei River, Triplophysa shaanxiensis and Triplophys are dominant species among fishes; In the upstream of the Luo River and Jing River, the species of fishes are dominated by Triplophysa dalaica, Triplophysa stoliczkae dorsonotata and Abbottina rivularis; It is dominated by Misgurnus anguillicaudatus, Opsariichthys bidens, Pseudorasbora parva, Abbottina rivularis and Carassius auratu in Mid-tail of Wei River, Luo River and Jing River (Wu et al. 2014).
Sample collection and pre-treatment
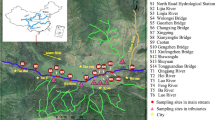
Twenty river sediment samples were collected from Wei River (Fig. 1). Among them, thirteen samples (S1–S13) were from Wei River, three samples (S14–S16) from Jing River, and four samples (S17–S20) from Luo River. These sampling sites were respectively located in the national hydrological monitoring section of Wei River. At each sampling site, a sediment sample of about 2 kg was collected by using a grab sludge sampler in Oct of 2017, and stored in an aluminum box. The longitude, latitude, and surrounding conditions of each sampling site were recorded. The river sediment samples were kept in dry ice on the way of transport back to laboratory. All the collected sediment samples were freeze-dried in laboratory, passed through 1 mm stainless steel sieve to remove gravel, detritus and small stones, transferred into brown glass bottles, and stored at 4 °C before analysis (Wang et al. 2016).
PAHs analysis
A total of 8 g sediment sample was weighted and placed into a 50 mL glass centrifuge tube with a lid, and then 30 mL mixed solution of n-hexane and acetone (v:v = 1:1) was added. They were extracted for 30 min by ultrasonic extraction method, and centrifuged for 10 min at 3000 r min−1. The supernatant was transferred into a 250 mL evaporation flask. This process was repeated three times and the supernatant was merged. After the extracts was concentrated to 1–2 mL by a rotary evaporator, 20 mL n-hexane was added and concentrated to 1–2 mL again. PAHs in the extracts were cleaned up by a silica gel-alumina oxide glass purification column, which was described in our previous work (Wang et al. 2016, 2018a). Sixteen priority PAHs including naphthalene (NaP), acenaphthylene (Acy), acenaphthene (Ace), fluorene (Flu), phenanthrene (Phe), anthracene (Ant), fluoranthene (Fla), pyrene (Pyr), benzo [a] anthracene (BaA), chrysene (Chy), benzo [b] fluoranthene (BbF), benzo [k] fluoranthene (BkF), benzo [a] pyrene (BaP), diphenyl [a, h] anthracene (DBA), indene [1, 2, 3-cd] pyrene (InP) and benzo [g, h, i] perylene (BghiP) were separated and analyzed by a AT-5 capillary chromatography column (30 m × 0.25 mm × 0.25 mm) on Agilent 7890A gas chromatography equipped with a hydrogen flame ionization detector. The detailed instrument conditions were provided in our previous work (Wang et al. 2016).
Plasticware was prohibited during the whole experiment. All glassware was first soaked for 24 h in K2CrO4-H2SO4 solution, and then rinsed by tap water, distilled water, and deionized water, respectively, finally baked for 4 h at a 450 °C muffle furnace and rinsed with the corresponding organic reagents before use. Anhydrous sodium sulfate, silica gel, and aluminum oxide used in purification were soxhlet-extracted for 24 h by 150 mL methylene chloride, and activated for 2, 6, and 4 h at 450, 180, and 250 °C, respectively. The method detection limit (MDL) was calculated by adopting the ratio of three times signal to noise, and the MDL for NaP, Acy, Ace, Flu, Phe, Ant, Fla, Pyr, BaA, Chy, BbF, BkF, BaP, InP, DBA and BghiP was 0.75, 0.375, 0.375, 0.375, 0.375, 0.375, 0.625, 0.75, 1.5, 1.5, 1.5, 1.5, 1.875, 2.5, 2.875 and 2.125 ng g−1, respectively. The recovery of decafluorobiphenyl as surrogate standard ranged from 79 to 113%, with an average of 102%. The recovery of matrix addition standard varied between 67 and 119%. Ten percent of sediment samples were duplicated and the relative standard deviation was below 10%.
Ecological risk assessment methods
PAHs in river sediment may be released into water bodies, posing potential ecological risks (Dudhagara et al. 2016). Risk quotient (RQ) is a valid method for evaluating the ecological risk of PAHs in river sediment (Kalf et al. 1997; Cao et al. 2010). The RQ was calculated by Eq. 1.
where, CPAHs is the concentration of PAHs in sediment; CQV is the corresponding evaluation standard of PAHs and generally adopts negligible concentrations (NCs) and maximum acceptable concentrations (MPCs). NCs are concentrations in the environment below which the risk of adverse effects is considered to be negligible, and MPCs are the concentrations in the environment above which the risk of adverse effects is considered unacceptable to ecosystems (Crommentuijn et al. 2000). The corresponding RQNCs and RQMPCs were calculated as follows.
where, CQV(NCs) is the NCs of PAHs in sediment; CQV(MPCs) is the MPCs of PAHs in sediment. RQΣPAHs, RQΣPAHs (NCs) and RQΣPAHs (MPCs) were calculated as follows.
For individual PAHs, RQNCs < 1.0, indicating that the ecological risk is negligible; RQNCs ≥ 1.0 and RQMPCs < 1.0, moderate risk; RQ(MPCs) ≥ 1, high risk. For ΣPAHs, RQΣPAHs(NCs) = 0, implying no potential ecological risk; 1 ≤ RQΣPAHs(NCs) < 800 and RQΣPAHs(MPCs) = 0, low risk; RQΣPAHs(NCs) ≥ 800 and RQΣPAHs(MPCs) < 1, moderate risk; RQΣPAHs(NCs) ≥ 800 and RQΣPAHs(MPCs) ≥ 1, high ecological risk (Kalf et al. 1997; Cao et al. 2010).
Health risk assessment methods
Biota-sediment accumulation model
Based on the principle of equilibrium partitioning, biota-sediment accumulation model can predict the migration and accumulation of PAHs from sediment to fish, which was described by Eq. 7 (Baumard et al. 1998; US EPA 1998).
where, Ci biota is the predicted concentration of the ith PAH in fish; CSi is the concentration of corresponding PAH in sediment; flipid is the lipid part of fish (0.07); OCsediment is the part of biochar in sediment (0.04) (US EPA 1998); and BSAF is the bio-sediment accumulation factor and 0.29 for PAHs (Tracey and Hansen 1996).
Carcinogenic risk assessment model
Among 16 priority PAHs, BaA, Chy, BbF, BkF, BaP, DBA, and InP are carcinogens (Qiao et al. 2006). The carcinogenic risk (CR) of human exposure to PAHs in river sediment through the intake of fish was calculated by Eq. 8 (Yang et al. 2014; Li et al. 2016).
where, CSFi is carcinogenic slope factors (Table 1), IR is the daily intake rate of fish, EF is the exposure frequency, ED is the exposure duration, BW is the body weight, AT is the average exposure time, and CF is a conversion factor (10–6). The parameter values used in health risk assessment are shown in Table 2. If the CR value is below 10–6, the carcinogenic risk is low or negligible; the CR value is between 10–6 and 10–4, the carcinogenic risk is within an acceptable range; and the CR value is above 10–4, the carcinogenic risk is significant (Wang et al. 2011; Kamal et al. 2014; US EPA 2019).
Non-carcinogenic risk assessment model
For non-carcinogenic PAHs, the non-carcinogenic risk is assessed based on harm quotient (HQ). The HQ was calculated by Eq. 9 (Chen et al. 2012; Yang et al. 2014).
where, RfDo is reference doses (Table 1). The HQ less than one indicates no significant non-carcinogenic risk, while HQ greater than one indicates a significant non-carcinogenic risk (Pongpiachan et al. 2013).
Results and discussion
Concentrations, distribution, composition and sources of PAHs in sediment
As shown in Table 3, the individual PAH concentrations ranged from undetected (ND) to 5388.3 ng g−1 dw (BkF). The total concentrations of the 16 PAHs (Σ16PAHs) ranged from 60.5 to 10,243.1 ng g−1 dw, with an average of 2250.4 ng g−1 dw. The total concentrations of seven carcinogenic PAHs (ΣCPAHs) were in the range of 5.8–7232.7 ng g−1 dw, with a mean of 1276.5 ng g−1 dw, accounting for 7.8–92.1% of the Σ16PAHs (average 56.7%), indicating a relatively high carcinogenic potential. The sum of specific combustion compounds (ΣCOMB) was from 10.4 to 7505.3 ng g−1 dw, with an average of 1483.0 ng g−1 dw, responsible for 17.2–95.5% of the Σ16PAHs (average 54.2%), implying that PAHs in the sediment were mainly associated with various incomplete combustion processes. The average concentration of HMWPAHs was 2.36 times higher than that of LMWPAHs, indicating that PAHs in sediment mainly originated from combustion and pyrolytic processes. In addition, the coefficient of variation (CV) of individual PAHs was greater than 0.6, which suggested a large spatial difference of PAHs in sediment of Wei River (Karim Nezhad et al. 2015).
It is a common practice to compare the current level with other rivers around the world. As shown in Table 4, the Σ16PAHs in the sediment of Wei River were higher than that in Luan River Estuary of China (Zhang et al. 2016), Guan River Estuary of China (He et al. 2014), Poxim River of Brazil (Souza et al. 2018), Amazon River Estuary of Brazil (Dos Santos et al. 2018), and Brisbane River of Australia (Duodu et al. 2017). However, the Σ16PAHs was lower than that in Delaware river Estuary of USA (Kim et al. 2018) and Erjen River of Taiwan (Wang et al. 2015). Meanwhile, the Σ16PAHs was lower than the result of previous studies (Chen et al. 2015). The concentration comparisons indicated that the current Σ16PAHs in the sediment of Wei River was at a relatively high level. In addition, (Baumard et al. 1998) proposed that the contamination of PAHs in river sediment can be classified as low level (0–100 ng g−1 dw), moderate level (100–1000 ng g−1 dw), high level (1000–5000 ng g−1 dw), and very high level (> 5000 ng g−1 dw) based on the Σ16PAHs. In the present study, the contamination level of PAHs was low in S14, moderate in S1, S3, S4, S6, S11, S12, S14, S19 and S20, high in S2, S5, S7–S9, and S15–18, and very high in S10 and S13. The overall contamination level of PAHs in the sediment of Wei River was high.
As shown in Fig. 1, the Σ16PAHs and ΣCPAHs in the sediment of Wei River (main stream) were higher than those of Jing River and Luo River (tributaries). Wei River is the main wastewater discharge channel compared with Jing River and Luo River. Elevated Σ16PAHs and ΣCPAHs were found in the lower reach of Wei River. For Jing River, the Σ16PAHs and ΣCPAHs presented upper reach higher than lower reach. For Luo River, S18 being close to a refinery had larger Σ16PAHs. The relative high Σ16PAHs were observed in S8-10 and S13, which were 4845.3, 4438.8, 10,243.1 and 7856.9 ng g−1 dw, respectively. These samples were in lower reach of Wei River and might be heavily influenced by the anthropogenic activities of nearby cities, including Xianyang, Xi’an, and Weinan. The relative low Σ16PAHs was found in S14 (60.5 ng g−1 dw), which was far from cities and received slight disturbance from anthropogenic activities.
The composition characteristics of PAHs in the environment are able to reflect their potential sources. Individual PAHs, including Acy, Ace, Flu, Phe, Ant, Fla, Pyr, and BkF, were detected in all the sediment samples and the detection rates of others PAHs also exceeded 70%. The percentage of individual PAH concentration to the Σ16PAHs decreased in the order of BkF (26.27%) > InP (12.34%) > BaA (9.68%) > Acy (9.21%) > Flu (7.98%) > Pyr (5.29%) > BghiP (4.38%) > Phe (4.11%) > Fla (3.89%) > Ant (3.79%) > Ane (3.01%) > BbF (2.95%) > DBA (2.42%) > NaP (1.63%) > Chy (1.57%) > BaP (1.50%), indicating that PAHs in the river sediments were mainly dominated by BkF, followed by InP, BaA, and Acy. As shown in Fig. 2, 2-ring PAHs accounted for 0–16.30% (1.63%) of the Σ16PAHs, 3-ring PAHs 4.23–79.76% (28.10%), 4-ring PAHs 5.76–50.41% (20.42%), 5-ring PAHs 5.47–73.70% (33.14%), and 6-ring PAHs 0–50.10% (16.72%), indicating that PAHs in the river sediment were dominated by 3- and 5-ring PAHs, followed by 4- and 6-ring PAHs. PAHs in S1, S4-S6, S11, S14, S18, and S20 were mainly low molecular weight PAHs (2–3 ring PAHs, more than 50%), implying petroleum leakage source; while PAHs in S2, S3, S7–S10, S12, S13, S15-S17, and S19 mainly consisted of high molecular weight PAHs (4–6 ring PAHs), suggesting combustion sources.
The isomer ratio of PAHs was commonly used to identify the possible sources of PAHs in the environment due to simplicity and validity (Yunker et al. 2002; Tobiszewski and Namieśnik et al. 2012). In this study, Ant/(Ant + Phe), BaA/(BaA + Chy) and InP/(InP + BghiP) were used to identify the possible sources of PAHs in the sediment of Wei River. A ratio of Ant/(Ant + Phe) > 0.1 refers to pyrolytic origin, while < 0.1 suggests petrogenic source (Hu et al. 2019). A ratio of BaA/(BaA + Chr) < 0.20 indicates petrogenic sources, 0.20—0.35 implies mixed sources, and > 0.35 suggests pyrolytic origin (Tobiszewski and Namieśnik et al. 2012; Baniemam et al. 2017). A ratio of InP/(InP + BghiP) < 0.2, 0.2–0.5, and > 0.5 indicates petrogenic origin, petrogenic combustion (e.g., liquid fossil fuel, vehicle and crude oil), and burning biomass (i.e., coal, wood and grass), respectively (Martins et al. 2011). As shown in Fig. 3, the ratio of Ant/(Ant + Phe) exceeded 0.1 in all the sediment samples, corresponding to pyrolytic source. The ratio of BaA/(BaA + Chr) indicated that PAHs were from petrogenic source in S3 and S6, mixed source only in S8, and pyrolytic origin in the rest of sediment samples. The ratio of InP/(InP + BghiP) suggested that PAHs were related to petrogenic origin in S1, S7 and S17, petroleum combustion in S2, S3, S11, S16, S18 and S20, and the combustion of coal, wood and grass in S4, S5, S8-S10, S12-S15 and S19. In summary, it was demonstrated that PAHs in the sediment of Wei River were mainly associated with the combustion of coal, wood and grass as well as petroleum (e.g., gasoline, diesel and crude oil), except for S1, S3, S6, S7 and S17 in which PAHs were petrogenic origin, being presumably due to the leakage of natural oil by oil-producing industries.
Ecological risk of PAHs in sediment
As shown in Table 5, the mean of RQ(NCs) for Chy was less than 1, indicating a low ecological risk. The mean of RQ(MNCs) for Acy and Flu exceeded 1, implying a high ecological risk. Other PAHs presented RQ(NCs) ≥ 1 and RQ(MPCs) < 1, indicating a moderate ecological risk. Meanwhile, the RQ(MPCs) of NaP, Ace, Ant, Pyr, BaA, BbF, and BkF in several samples exceeded 1, suggesting a high ecological risk. As shown in Fig. 4, the Σ16PAHs in S5 (Baoji), S8 (Xiangyang), S9 and S10 (Xi’an), S13 (Weinan), and S18 (refinery) showed RQΣPAHs(NCs) ≥ 800 and RQΣPAHs(MPCs) ≥ 1, illustrating a high ecological risk. S18 was located in Luo River, probably influenced by the nearby refinery. Other sites were located around the cities as well as the lower reach of Wei River, mainly affected by the anthropogenic activities of nearby cities. However, the Σ16PAHs in S6, S12, S14, S17, and S19 showed 1 ≤ RQΣPAHs(NCs) ≤ 800 and RQΣPAHs(MPCs) = 0, indicating a low ecological risk. These sites were away from cities or factories, slightly affected by anthropogenic activities (Dudhagara et al. 2016). The Σ16PAHs in other sites were a moderate ecological risk. The ecological risk of PAHs in Wei River were higher than that in Jing River and Luo River, with Luo River higher than Jing River. The relative high ecological risk of PAHs were observed in lower reach of Wei River close to cities, being consistent with the concentration distributions.
Non-carcinogenic and carcinogenic health risks
Table 6 shows the carcinogenic and non-carcinogenic risk of human exposure to PAHs by fish intake. The 95% upper confidence limit (95%UCL) was adopted to replace the maximal values in health risk assessment (Li et al. 2016). The CR of children and adolescences ranged from 4.99 × 10–10 to 6.08 × 10–6, with a 95%UCL of 2.01 × 10–6, and from 5.30 × 10–10 to 6.46 × 10–6, with a 95%UCL of 2.14 × 10–6. The CR of adults was in the range of 3.62 × 10–9 to 4.41 × 10–5, with a 95%UCL of 1.46 × 10–5. From the 95%UCL, the CR of Children, adolescences and adults was between 10–6 and 10–4, indicating an acceptable carcinogenic risk. The highest CR was found in S10 (Xi’an), followed by S8, S9, S13, and S15 (Xi’an and Weinan), indicating a relatively high health risk in the section of Xi’an and Weinan of Wei River, which was consistent with the distribution results of concentration and ecological risk of PAHs. In addition, the CR showed an decreasing order of adults > adolescence > children.
The HQ of children and adolescence ranged from 4.18 × 10–5 to 6.19 × 10–2, with a UCL95% of 2.05 × 10–2, and from 8.88 × 10–5 to 1.31 × 10–1, with a 95%UCL of 4.34 × 10–2. The HQ of adults was from 7.00 × 10–5 to 1.04 × 10–1, with a 95%UCL of 3.43 × 10–2. All the values of HQ were less than 1, indicating a low or negligible non-carcinogenic risk. The non-carcinogenic risk presented adolescences > adults > children.
Conclusions
The pollution characteristic, ecological and human health risks of PAHs in the sediment of Wei River were investigated in present study. The results showed that the Σ16PAHs ranged from 60.5 to 10,243.1 ng g−1 dw, with an average of 2250.4 ng g−1 dw, presenting a high contamination level. The Σ16PAHs in the sediment of Wei River (main stream) was higher than that in Jing River and Luo River (tributaries), and elevated Σ16PAHs and ΣCPAHs were mainly distributed in the lower reach of Wei River. PAHs in the sediment were dominated by 3- and 5-ring PAHs. PAHs in the sediment of Wei River were mainly associated with the combustion of fossil fuels and biomass. The ecological risk of individual PAHs (except for Chy) was assignable, with the highest risk of Acy and Flu. The total ecological risk of PAHs in the sediment was a moderate to high level. The CR of human exposure to PAHs ranged from 10–6 to 10–5, and the HQ of human exposure to PAHs was 10–2, less than 1. There were acceptable cancer risk and no significant non-carcinogenic risk of human exposure to PAHs in sediment of Wei River.
References
Baniemam M, Moradi AM, Bakhtiari AR, Fatemi MR, Khanghah KE (2017) Seasonal variation of polycyclic aromatic hydrocarbons in the surface sediments of the southern Caspian Sea. Mar Pollut Bull 117:478–485
Baumard P, Budzinski H, Garrigues P (1998) Polycyclic aromatic hydrocarbons in sediments and mussels of the Western Mediterranean Sea. Environ Toxicol Chem 17:765–776
Bi CJ, Wang XP, Jia JP, Chen ZL (2018) Spatial variation and sources of polycyclic aromatic hydrocarbons influenced by intensive land use in an urbanized river network of East China. Sci Total Environ 627:671–680
Cao ZG, Liu JL, Luan Y, Li YL, Ma MY, Xu J, Han SL (2010) Distribution and ecosystem risk assessment of polycyclic aromatic hydrocarbons in the Luan River, China. Ecotoxicology 19:827–837
Chen JW, Wang SL, Hsieh DPH, Yang HH, Lee HL (2012) Carcinogenic potencies of polycyclic aromatic hydrocarbons for back-door neighbors of restaurants with cooking emissions. Sci Total Environ 417:68–75
Chen YY, Jia R, Yang SK (2015) Distribution and source of Polycyclic Aromatic Hydrocarbons (PAHs) in water dissolved phase, suspended particulate matter and sediment from Weihe River in Northwest China. Int J Environ Res Public Health 12:14148–14163
Crommentuijn T, Sijm D, De Bruijn J, Van Leeuwen K, Van De Plassche E (2000) Maximum permissible and negligible concentrations for some organic substances and pesticides. Environ Manage 58:297–312
Dos Santos RCC, Santos LGGV, Santos E, Damasceno FC, Corrêa JAM (2018) Polycyclic aromatic hydrocarbons in sediments of the Amazon River Estuary (Amapá, Northern Brazil): Distribution, sources and potential ecological risk. Mar Pollut Bull 135:769–775
Dudhagara DR, Rajpara RK, Bhatt JK, Gosai HB, Sachaniya BK, Dave BP (2016) Distribution, sources and ecological risk assessment of PAHs in historically contaminated surface sediments at Bhavnagar coast, Gujarat, India. Environ Pollut 213:338–346
Duodu GO, Ogogo KN, Mummullage S, Goonetilleke FA, Ayoko GA (2017) Source apportionment and risk assessment of PAHs in Brisbane River sediment, Australia. Ecol Ind 173:784–799
He XR, Pang Y, Song XJ, Chen BL, Feng ZH, Ma YQ (2014) Distribution, sources and ecological risk assessment of PAHs in surface sediments from Guan River Estuary, China. Mar Pollut Bull 80:52–58
Hu H, Tian M, Zhang L, Yang F, Peng C, Chen Y, Shi G, Yao X, Jiang C, Wang J (2019) Sources and gas-particle partitioning of atmospheric parent, oxygenated, and nitrated polycyclic aromatic hydrocarbons in a humid city in southwest China. Atmos Environ 206:1–10
IARC (International Agency for Research on Cancer) (2010) Monographs on the evaluation of carcinogenic risks to humans. Some non-heterocyclic polycyclic aromatic hydrocarbons and some related exposures. IARC, France, p 92
Jiang YF, Hu XF, Yves UJ, Zhan HY, Wu YQ (2014) Status, source and health risk assessment of polycyclic aromatic hydrocarbons in street dust of an industrial city, NW China. Ecotoxicol Environ Saf 106:11–18
Kalf DF, Crommentuijn T, Van De Plassche EJ (1997) Environmental quality objectives for 10 polycyclic aromatic hydrocarbons (PAHs). Ecotoxicol Environ Saf 36:89–97
Kamal A, Malik RN, Martellini T, Cincinelli A (2014) Cancer risk evaluation of brick kiln workers exposed to dust bound PAHs in Punjab province (Pakistan). Sci Total Environ 493:562–570
Karim Nezhad MT, Tabatabaii SM, Gholami A (2015) Geochemical assessment of steel smelter-impacted urban soils, Ahvaz. Iran J Geochem Exp 152:91–109
Khuman SN, Chakraborty P, Cincinelli A, Snow D, Kumar B (2018) Polycyclic aromatic hydrocarbons in surface waters and riverine sediments of the Hooghly and Brahmaputra Rivers in the Eastern and Northeastern India. Sci Total Environ 636:751–760
Kim KH, Jahan SA, Kabir E, Brown RJC (2013) A review of airborne polycyclic aromatic hydrocarbons (PAHs) and their human health effects. Environ Int 60:71–80
Kim AW, Vane CH, Moss-Hayes V, Engelhart SE, Kemp AC (2018) PAH, PCB, TPH and mercury in surface sediments of the Delaware River Estuary and Delmarva Peninsula, USA. Mar Pollut Bull 129:835–845
Lei YD, Wania F (2011) Transport of polycyclic aromatic hydrocarbons and pesticides during snowmelt within an urban watershed. Water Res 45:1147–1156
Li J, Dong H, Li X (2016) Quantitatively assessing the health risk of exposure to PAHs from intake of smoked meats. Ecotoxicol Environ Saf 124:91–95
Liu AX, Lang YH, Xue LD, Liu J (2009) Ecological risk analysis of polycyclic aromatic hydrocarbons (PAHs) in surface sediments from Laizhou Bay. Environ Monit Assess 159:429–436
Liu S, Liu XR, Liu M, Yang B, Cheng L, Li Y, Qadeer A (2016) Levels, sources and risk assessment of PAHs in multi-phases from urbanized river network system in Shanghai. Environ Pollut 219:555–567
Manariotis ID, Karapanagioti HK, Chrysikopoulos CV (2011) Degradation of PAHs by high frequency ultrasound. Water Res 45:2587–2594
Martins C, Bicego MC, De Mahiques MM, Figueira RCL, Tessler MG, Montone RC (2011) Polycyclic aromatic hydrocarbons (PAHs) in a large South American industrial coastal area (Santos estuary, Southeastern Brazil): sources and depositional history. Mar Pollut Bull 63:452–458
Norris GA, Henry RC (2019) Unmix optimum analysis of PAH sediment sources. Sci Total Environ 631:831–838
Pietzsch R, Patchineelam SR, Torres JPM (2010) Polycyclic aromatic hydrocarbons in recent sediments from a subtropical estuary in Brazil. Mar Chem 118:56–66
Pongpiachan S, Tipmanee D, Deelaman W, Muprasit J, Feldens P, Schwarzer K (2013) Risk assessment of the presence of polycyclic aromatic hydrocarbons (PAHs) in coastal areas of Thailand affected by the 2004 tsunami. Mar Pollut Bull 76:370–378
Qiao M, Wang CX, Huang SB, Wang DH, Wang ZJ (2006) Composition, sources, and potential toxicological significance of PAHs in the surface sediments of the Meiliang Bay, Taihu Lake, China. Environ Int 32:28–33
Readman JW, Preston MR, Mantoura RFC (1986) An integrated technique to quantify sewage, oil and PAH pollution in estuarine and coastal environments. Mar Pollut Bull 17:298–308
Santos LO, Santos AG, De Andrade JB (2018) Methodology to examine polycyclic aromatic hydrocarbons (PAHs) nitrated PAHs and oxygenated PAHs in sediments of the Paraguaçu River (Bahia, Brazil). Mar Pollut Bull 136:248–256
Shen HB, Li RJ, Lv BB, Zhang J, Yin XuW, Wang Yi C, Wang ZQ, Wang F (2019) Characterisyics of fish community structure in the Weihe River of shaanxi section. Acta Hydrobiol Sin 43:1311–1320 ((In Chinese))
Shi Z, Tao S, Pan B, Fan W, He XC, Zuo Q, Wu SP, Li BG, Cao J, Liu WX, Xu FL, Wang XJ, Shen WR, Wong PK (2005) Contamination of rivers in Tianjin, China by polycyclic aromatic hydrocarbons. Environ Pollut 134:97–111
Souza MRR, Santos E, Suzarte JS, Frena CLOM, DamascenoAlexandre FCMR (2018) Concentration, distribution and source apportionment of polycyclic aromatic hydrocarbons (PAH) in Poxim River sediments, Brazil. Mar Pollut Bull 127:478–483
Sun JH, Wang GL, Chai Y, Zhang G, Li J, Feng JL (2009) Distribution of polycyclic aromatic hydrocarbons (PAHs) in Henan Reach of the Yellow River, Middle China. Ecotoxicol Environ Saf 72:1614–1624
Tobiszewski M, Namieśnik J (2012) PAH diagnostic ratios for the identification of pollution emission sources. Environ Pollut 162:110–119
Tracey GA, Hansen DJ (1996) Use of biota-sediment accumulation factors to assess similarity of nonionic organic chemical exposure to benthically-coupled organisms of differing trophic mode. Arch Environ Contam Toxicol 30:467–475
Tu YT, Ou JH, Tsang DCW, Dong CD, Chen CW, Kao CM (2018) Source identification and ecological impact evaluation of PAHs in urban river sediments: a case study in Taiwan. Chemosphere 194:666–674
Urban JD, Tachovsky JA, Haws LC, Wikoff Staskal D, Harris MA (2009) Assessment of human health risks posed by consumption of fish from the Lower Passaic River, New Jersey. Sci Total Environ 408:209–224
US EPA (United States Environmental Protection Agency) (1998) Methodology for Assessing Health Risks Associated with Multiple Pathways of Exposure to Combustor Emissions. Environmental Protection Agency, National Center for Environmental Assessment, Cincinnati, OH, US EPA-600/R-98–137.
US EPA (United States Environmental Protection Agency) (2001) Supplemental Guidance for Developing Soil Screening Level for Superfund Sites. OSWER 9355: 4–24. Office of Solid Waste and Emergency Response; U.S. Environmental Protection Agency: Washington, DC, USA.
US EPA (United States Environmental Protection Agency) (2019) Regional Screening Levels (RSLs), Generic Tables.
Wang W, Huang MJ, Kang Y, Wang HS, Anna OW, Leung AOW, Cheung KC, Wong MH (2011) Polycyclic aromatic hydrocarbons (PAHs) in urban surface dust of Guangzhou, China: status, sources and human health risk assessment. Sci Total Environ 409:4519–4527
Wang W, Huang MJ, Kang Y, Wang HS, Anna OW, Leung AOW, Cheung KC, Wong MH (2011) Polycyclic aromatic hydrocarbons (PAHs) in urban surface dust of Guangzhou, China: status, sources and human health risk assessment. Sci Total Environ 409:4519–4527
Wang YB, Chen W, Liu CW, Kao YH, Jang CS (2014) Characterization and risk assessment of PAH-contaminated river sediment by using advanced multivariate methods. Sci Total Environ 524–525:63–73
Wang CH, Wu SH, Zhou SL, Wang H, Li BJ, Chen H, Yu YN, Shi YX (2015a) Polycyclic aromatic hydrocarbons in soils from urban to rural areas in Nanjing: concentration, source, spatial distribution, and potential human health risk. Sci Total Environ 527:375–383
Wang YB, Chen W, Liu CW, Kao YH, Jang CS (2015b) Characterization and risk assessment of PAH-contaminated river sediment by using advanced multivariate methods. Sci Total Environ 524–525:63–73
Wang LJ, Wang L, Tao WD, Smardon RC, Shi XM, Lu XW (2016) Characteristics, sources, and health risk of polycyclic aromatic hydrocarbons in urban surface dust: a case study of the city of Xi’an in Northwest China. Environ Sci Pollut Res 23:13389–13402
Wang LJ, Zhang SW, Wang L, Zhang WJ, Shi XM, Lu XW, Li XP, Li XY (2018) Concentration and risk evaluation of polycyclic aromatic hydrocarbons in urban soil in the typical semi-Arid City of Xi’an in Northwest China. Int Environ Res Public Health 15:607
Wu W, Xu ZX, Yin XW (2014) Fish community structure and the effect of environmental factors in the Wei River basin. Acta Sci Circum 34:1298–1308 ((In Chinese))
Yang W, Lang YH, Li GL (2014) Cancer risk of polycyclic aromatic hydrocarbons (PAHs) in the soils from Jiaozhou Bay wetland. Chemosphere 112:289–295
Yunker MB, Macdonald RW, Vingarzan R, Mitchell RH, Goyette D, Sylvestre S (2002) PAHs in the Fraser River basin: a critical appraisal of PAH ratios as indicators of PAH source and composition. Org Geochem 33:489–515
Zhang Y, Guo CS, Xu J, Tian YZ, Shi GL, Feng YC (2012) Potential source contributions and risk assessment of PAHs in sediments from Taihu Lake, China: comparison of three receptor models. Water Res 46:3065–3073
Zhang DL, Liu JQ, Jiang XJ, Cao K, Yin P, Zhang XH (2016) Distribution, sources and ecological risk assessment of PAHs in surface sediments from the Luan River Estuary, China. Mar Pollut Bull 102:223–229
Zhu LZ, Lu H, Chen SG, Amagai T (2009) Pollution level, phase distribution and source analysis of polycyclic aromatic hydrocarbons in residential air in Hangzhou, China. J Hazard Mater 162:1165–1170
Acknowledgements
This research was supported by the National Natural Science Foundation of China through Grants 41877516 and 41471420, and the Fundamental Research Funds for the Central Universities through Grants GK201701010 and GK201601009.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Pang, L., Zhang, S., Wang, L. et al. Pollution characteristics and risk assessment of polycyclic aromatic hydrocarbons in the sediment of Wei River. Environ Earth Sci 80, 203 (2021). https://doi.org/10.1007/s12665-021-09483-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12665-021-09483-z