Abstract
Groundwater quality monitoring is of great importance in Iran’s arid and semi-arid regions where water scarcity exists. This study assessed background information on groundwater quality and heavy metals concentration in the spring water of the Beheshtabad Basin, located in Chaharmahal and Bakhtiari Province, Iran, to examine the suitability of the groundwater for drinking. Groundwater samples were collected from five springs in the basin during the time frame of February 2014 and September 2015 and analyzed in terms of physicochemical characteristics such as pH, electrical conductivity (EC), total dissolved solids (TDS), cations, anions, and heavy metal concentration. These parameters were used to determine the groundwater’s suitability for domestic purposes by comparing their measured values to the maximum permissible limits according to recommendations of the World Health Organization. The results revealed that most groundwater samples are suitable for drinking. During the rainy season, however, spring waters are bacteriologically contaminated and unsuitable for human consumption. As important parameters for determining drinking water quality, water quality index (WQI) values in the present study indicated very poor quality water for some groundwater samples in the area dominated by weathering of rocks and dissolution of salts from the bedrock into the water resources, which can be a serious threat to the ecological habitat.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Groundwater resources are considered valuable water sources around the globe and they are an increasingly important water supply source in regions with frequent water stress (Richey et al. 2015; Yaghobi et al. 2017). Investigating groundwater quality, as one of the most important and most vulnerable water supply sources, is of high priority (Prasanth et al. 2012). With increasing population and water demand for various purposes including agriculture, drinking, and industry, the need for investment in the water sector is inevitable. These developments have put a great deal of pressure on Iran’s groundwater resources. One of the important responsibilities of water decision makers is to assess water quality parameters. Iran, as a vast country with extensive agricultural land, is always faced with water shortages because of high water demand, low rainfall, high evaporation, and uneven rainfall distribution (Khosravi et al. 2017; Abbasnia et al. 2018). At present, agriculture plays a vital role in the national economy and food production in Iran and consumes more than 90% of the available water. In addition to reducing crop yields and creating problems for irrigation systems, poor groundwater quality in agriculture degrades the soil’s physical properties and consequently results in land degradation. Therefore, it is necessary to consider the qualitative aspects of water and heavy metal pollution in groundwater (Krishna et al. 2009). In general, groundwater movement along underground pathways increases the concentration of chemical compounds in the water. Groundwater contains varying amounts of nutrients, such as carbonate (CO32−), bicarbonate (HCO3−), calcium (Ca2+), magnesium (Mg+2), and sodium (Na+), which affect the suitability of groundwater for human consumption, irrigation, and other uses (Bear and Cheng 2010). Water quality assessment for drinking-water purposes involves the determination of the groundwater’s chemical composition and remedial measures for restoration of the water quality (Annapoorna and Janardhana 2015; Neisi et al. 2018). Several tools such as water quality indices are implemented (Lermontov et al. 2009) to determine water quality conditions. Water quality index (WQI) is a practical and comparatively simple approach for evaluating the composite influence of the overall quality. It also reflects the composite influence of the different water quality parameters (Singh et al. 2016).
To date, many research studies and projects have been conducted on surface water, and groundwater quality measurements for the domestic, irrigation, and industrial activities in different regions of the world. Some of these studies include the reports in Spain of Valenzuela et al. (2006); in Portugal, by Stigter et al. (2006a, b); in India, by Edmunds and Shand (2008), Hakim et al. (2009), Vasanthavigar et al. (2010), Gurunadha et al. (2011), Nag (2014), Mahendra and Patode (2014), and Ravikumar and Somashekar (2015); in Ghana, by Yidana et al. (2011); in Malaysia, by Prasanna et al. (2012); in Ghana, by Ewusi et al. (2013); in Libya, by Oişte (2014); in Romania, by Abd El-Aziz (2017); in Nigeria, by Majolagbe et al. (2017); in the UAE, by Zhang et al. (2017); in Egypt, by Masoud et al. (2017); in China, by Zhang et al. (2017); in the USA, by Law et al. (2017); and in Iran, by Khosravi et al. (2017).
Given the importance of groundwater quality monitoring, the present study characterized groundwater quality by testing spring water samples in Iran and comparing them with the guidelines stated by the World Health Organization (WHO). To this aim, the quality of spring water from aquifers in the Beheshtabad Basin, located in Chaharmahal and Bakhtiari Province, was evaluated to assess the groundwater suitability for the purpose of drinking. The present achievements can provide decision makers with useful information.
Material and methods
Study area and sampling location
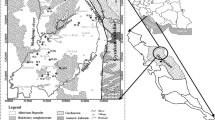
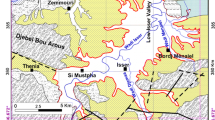
The Beheshtabad Basin, with a total area of 3822 square meters, is in northeastern Chaharmahal and Bakhtiari Province. This watershed is situated between latitudes of 31° 28′ N, 32° 56′ N, longitudes of 50° 36′ E and 51° 45′ E. The basin accounts for about 12.9% of the central Zagros Mountains area, where nearly 44% of the area is mountainous and 56% is plain. It is characterized as a rural setting in which most of the people work in agriculture.
The average annual precipitation is around 419 mm and its climate is essentially semi-arid. The springs are recharged by direct precipitation infiltration, as the main source of groundwater recharge. The average annual temperature is 10.25 °C. Figure 1 depicts the location of the study area together with its five sampling stations.
The sampling method along with the physicochemical and elemental analyses
Water samples from Beheshtabad Basin springs were collected in the months of February (rainy season) and September (dry season) 2014. Groundwater samples from five springs in the study area were collected in triplicate in new, pre-cleaned polypropylene bottles (1 l capacity). After the sample collection, the samples were held at 4 °C in a laboratory refrigerator to avoid microbial degradation. All samples pertinent to the physiochemical parameters were analyzed within 24 h. The parameters of pH and electrical conductivity (EC) were measured with a Hach HQ40d portable meter (USA). Other physiochemical parameters such as soluble cations (Ca2+, Mg2+, Na+ and K+) and soluble anions (CO32−, SO42−, Cl−, HCO3− and NO3−) were analyzed within 24 h after transferring the water samples to the laboratory, according to methods described in the American Public Health Association manual (APHA 2012). Ca2+, Mg2+, CO32−, Cl−, and HCO3− were analyzed by volumetric titration methods. Na+ and K+ were measured using a flame photometer, and SO42− and NO3− were determined with the spectrophotometric technique.
To analyze the heavy metals (Ag, As, B, Ba, Cd, Co, Cr, Cs, Cu, In, Mn, Ni, Pb, Rb, Se, Sr, U, and Zn), water samples were preserved with ultrapure nitric acid and then transferred to the laboratory. Water samples were analyzed using an ICP mass spectrometer (Agilent 7500, USA). The bacteriological component as total coliform was measured using the most probable number (MPN) method (APHA 2012).
Analytical process precision was evaluated by the relative standard deviation (RSD). To this aim, one sample was analyzed in five replicates. RSD values were obtained for heavy metals during the rainy and dry seasons.
Water quality assessment
In the first step, the proportion of groundwater for domestic purposes was assessed by comparing the values of various water quality parameters to those of the WHO guidelines for drinking water (WHO 2004).
In general, the suitability of water sources for human consumption has been described in terms of WQI, which is one of the most effective ways for describing water quality. The unique feature of WQI is the use of several key parameters of groundwater chemistry for investigating the influence of natural and anthropogenic activities. This index has been widely used by various scientists (Zhang et al. 2017; Law et al. 2017; Khosravi et al. 2017) and is defined as:
where
Qi is the quality rating scale for each parameter, and
Wi is the unit weight for each water quality parameter.
The quality rating scale for each parameter is determined as follows:
in which Pi stands for the estimated concentration of the ith parameter in the analyzed water and P0 is the ideal value of this parameter in the pure water. Si is the recommended standard value of the ith parameter and Wi is calculated using the following formula:
Iran’s groundwater resource quality index (IRWQI) creates a score to evaluate the general water quality of Iran’s water resources for conventional and toxic pollutants, via combining ten water quality variables into a single number (Hashemi et al. 2011). The parameters covered in this method for conventional pollutants include electrical conductivity (EC), dissolved oxygen (DO), chemical oxygen demand (COD), biochemical oxygen demand (BOD), pH, sodium adsorption ratio (SAR), nitrate-nitrogen, total phosphorus, total dissolved solids (TDS), and fecal coliform bacteria.
Those covered in this method for the toxic heavy metal pollutants are arsenic (As), mercury (Hg), cadmium (Cd), lead (Pb), chromium (Cr), cyanide (CN), iron (Fe), manganese (Mn), phenol, and detergent. Table 1 gives the water quality rating according to this WQI for conventional and toxic pollutants.
Results and discussion
Physicochemical characteristics of the investigated groundwater samples
The five examined groundwater samples from springs in February and September 2014 samplings indicated that EC is significantly higher in spring No. 1 compared to the other four ones (more than 100 times higher), according to the springs’ physical and chemical parameters. Therefore, this salt spring was eliminated from Table 2 calculation of the mean and standard analysis.
pH
pH is an acidic or basic indicator associated with water and is an important indicator of water quality in the present study. According to WHO guidelines, the appropriate pH range for drinking water purposes is 6.5–8.5 (Table 2) and the potability of drinking water is significantly impaired by pH less than 6.5 or greater than 8.5. pH values of the samples were in the range of 7.95–8.38 and 8.14–8.52 for dry and rainy seasons, respectively. Therefore, the pH values associated with all water samples from the springs, for both periods of sampling in the study area are within the permissible limits prescribed by the WHO.
EC
The EC value of water defines the amount of soluble salts (concentration of ionized substances) in the water samples. The maximum permissible limit of EC is 1500 µs/cm for drinking water. In this basin, the EC values of the samples, except that of spring No. 1, were in the range of 331–841 µs/cm, and 312–1000 µs/cm for dry and rainy seasons, respectively, indicating suitable values for the drinking water. However, the EC values associated with spring No. 1 in the northeastern part of the basin were estimated at 104,700 and 130,800 µs/cm for dry and rainy seasons, respectively, which indicate very high EC values beyond the prescribed limit for drinking water. The most important reason for the high EC in spring No. 1 is salt water intrusion from the parent rock, characterized as a fine grained, micaceous, sandy shale and salt (Table 3).
Total dissolved solid (TDS)
According to the WHO (2004), total dissolved solids (TDS) are a measure of all dissolved substances in water (such as calcium, magnesium, potassium, sodium, bicarbonate, chloride, and sulfate) and the small amounts of organic matter dissolved in it. High concentrations of TDS could have adverse effects on taste. As suggested by the WHO, TDS > 1500 mg/l (Table 2) significantly impairs water potability and it is considered to be the maximum permissible limit for drinking water. TDS values of the samples were in the range of 185.4–471 mg/l and 158–560 mg/l for dry and rainy seasons, respectively. Generally, in the study area, the TDS values of samples were below 560 mg/l and the low TDS values of these groundwaters clearly indicate their suitability for drinking with regard to this parameter. Only the TDS value for spring No. 1, for both periods of sampling, exceeded the maximum permissible limit, indicating that it is not suitable for drinking.
Cations and anions
Calcium was the major cation in the groundwater during the rainy season (Table 1). It contributes to water hardness. Higher Ca2+ concentrations cause abdominal ailments, are undesirable for domestic uses, and cause encrustation and scaling. Calcium sources in groundwater are calcite, aragonite, gypsum, and anhydrite minerals. The maximum permissible limit of calcium concentration for drinking water is reported as 200 mg/l and the desirable limit for this cation is 75 mg/l (WHO 2004). No investigated water samples except that of spring No. 1, for both periods of sampling, exceeded the permissible limit of Ca2+. According to the WHO (2004), the maximum permissible limit of Mg2+ concentration in drinking water is 150 mg/l. Once again, only the water samples from spring No. 1, for both periods of sampling, exceeded this limit.
The maximum permissible limits of Na+ and K+ in drinking water are reported as 200 mg/l and 12 mg/l, respectively. In the studied basin, both Na+ and K+ values of the water samples (except for spring No. 1) were in the standard range prescribed by the WHO. Na+ and K+ values of the samples from spring No. 1 for the dry season were 28,000 and 15 mg/l, respectively, and for the rainy season were 23,300 mg/l for Na+ and 18 mg/l for K+. Water samples from spring No. 1 indicated higher Na+ and K+ values than the standards outlined by the WHO. The intake of high levels of Na can cause increased blood pressure, arteriosclerosis, and hyperosmolarity. Potassium concentrations in all groundwater samples were lower compared to Na+, which could be due to the fact that potassium minerals are more resistant to the weathering in the study area.
Overall, the cations and anions of the spring water (except for HCO3−and Cl−) were higher during the rainy season and lower in the dry season, as shown on Fig. 2.
The average concentrations of electrical conductivity (EC), pH, total dissolved solids (TDS), and sum of cations and anions in groundwater samples in Beheshtabad Basin in the rainy season (dark columns) and the dry season (grey columns). Error bars indicated standard deviation. Spring number indicated; 2 (Sardab Spring), 3 (Bagh-e-Rostom), 4 (Spring 19), and 5 (Khadrzaneh). Spring number 1 was eliminated from the analysis for reasons explained in the text
Chloride in groundwater is likely from a variety of sources such as the climate, saturation of sedimentary rocks and soils, salt water influx, household waste, industrial waste, and urban sewage. Obviously, Cl affects the taste of water. Sulfate is found in water as sulfate (SO42−) presence in drinking water can cause a bitter taste at concentrations greater than 200 mg/l. Soluble anions were dominated by Cl− and SO42− and no groundwater samples (except that of spring No. 1), for both periods of sampling, exceeded the maximum permissible limit for drinking water as recommended by the WHO (2004). The high SO4−2 concentration in spring No. 1 is most likely, from gypsum dissolution. The maximum allowable limits of Cl−, SO4−2, HCO3−, and NO3− in drinking water are 600, 400, 240, and 100 mg/l, respectively. During the dry season, the concentrations of HCO3− in springs 1, 3, and 4 exceeded the maximum permissible limit, which is 240 mg/l for drinking water.
Nitrate is likely to enter groundwater from fertilizer, food preservatives, and human and animal waste. It is highly soluble in water and easily transported to drinking water through soil. All samples showed a NO3− concentration below the maximum allowable concentration of 100 mg/l (WHO 2004).
Bacterial content
In general, the bacterial content of drinking water is one of the most important aspects of water quality. Drinking bacterial contamination in water is one of the most common and widespread health hazards, caused either directly or indirectly by human or animal excrement. In this study, all four groundwater samples collected in the rainy season were found to be contaminated. The permissible limit of bacterial fecal coliform in drinking water is 4/100 ml (MPN/100 ml). The obtained results indicate that the groundwater from springs is bacterially contaminated and, therefore, unsuitable for human consumption.
Iran water quality index calculation (IRWQI)
The results of IRWQI classification (Table 3) indicate that out of the five spring samples in the rainy season, only one sample was in the second category (very poor quality) and the others were in the fourth category (good water). There was a significant difference in WQI among the four springs, Nos. 2, 3, 4 and 5, but all were found to be in the fourth category and were desirable for drinking.
IRWQI values associated with spring No. 1 were 17.2 ± 0.01 and 20.1 ± 0.01 for rainy and dry seasons, respectively, and were significantly (p < 0.05) lower compared to the other four springs (very poor water). The very poor water quality in spring No. 1 for both sampling periods can be attributed to the process of rocks weathering and dissolution of salts from bedrock into the water. The bedrock or mother rock material in this region is fine grained, micaceous, sandy shale and salt.
Moreover, two sampled springs, Nos. 3 and 5, in the dry season were found to be in the third category (poor water) compared to the excellent quality in spring No. 2. The significantly (p < 0.05) low water quality in these two springs compared to the excellent quality in spring No. 2 and the good quality in spring No. 4 could result from some high values of physicochemical characteristics of the groundwater samples associated with these springs (Table 2). This is also attributable to the low rainfall infiltration into the groundwater of these regions, leading to a decline in groundwater quality.
In general, the results indicate a significant decrease in WQI in the dry season compared to the rainy season; however, for spring Nos. 2 and 4, a significant increase and insignificant increase in WQI, respectively was observed compared to the rainy season.
Table 4 compares the available groundwater quality data with the drinking water of selected areas around the world.
Saeedi et al. (2010) developed a simple process of determining the WQI in Iran’s Qazvin plateau and reported that the groundwater quality in this region is closely related to mineral water quality. Jamshidzadeh and Mirbagheri (2011) estimated the groundwater quality in most parts of the Kashan basin in central Iran. They found that the groundwater was undesirable for drinking and could be harmful to human health. However, these authors pointed out that the groundwater quality in this basin is affected by the intrusion of Salt Lake, in Kashan Province’s Dasht-e Kavir-Salt Desert National Park.
A few years later, considering the newly published data, Khosravi et al. (2017) indicated that the WQI for the groundwater quality in Birjand, Iran, was not in the range recommended by the WHO and thus the water was unsuitable for drinking.
Abdelaziz (2017) reported that the groundwater samples in northwestern Libya were unsuitable for drinking and household uses and illustrated that the examined parameter levels exceeded the WHO’s permissible limits. The geochemical characteristics of the groundwater and drinking water quality for Chennai, Tamil Nadu, India were reported to be of excellent and good categories and suitable for drinking water purposes (Krishna et al. 2015).
According to a report by Majolagbe et al. (2017), the groundwater vulnerability to pollution from the Solus dumpsite in Lagos, Nigeria, ranged between moderate and high groundwater pollution potential. Zhang et al. (2017) evaluated the groundwater chemistry in the Hutuo River in China to be of poor quality, which could be the result of anthropogenic activities as well as the river’s vulnerability to contamination.
Compared to the published groundwater quality data for other basin regions in the world (Table 4), the calculated groundwater quality in the Beheshtabad Basin with regard to the permissible limits of the WHO is between low and moderate groundwater pollution potential.
As mentioned earlier, the very poor groundwater quality of spring No. 1, for both periods of sampling in the northeastern of the study area can be attributed to the process of rock weathering and dissolution of salts from the bedrock into the water resources.
As shown in Table 2, the total viable bacteria in each of the four water samples during the rainy season were too numerous to count. Excessively high colony numbers of total viable bacteria indicate that the water is highly contaminated with microorganisms and unsuitable for drinking because its consumption could lead to waterborne diseases such as nausea, vomiting, diarrhea, and gastroenteritis. High colony numbers of total viable bacteria in rainy season compared to dry season, are mostly likely from greater infiltration of rainwater into the groundwater, pointing to contamination from agriculture and wastewater runoff in these regions, resulting in a decline of groundwater quality.
Metal concentrations of the investigated groundwater samples
The results for the concentrations of trace metals in the groundwater samples collected from the Beheshtabad Basin are listed in Table 5. RSD values were obtained for heavy metals during the rainy and dry seasons, ranging from 0.0 to 7.0% and 2.0 to 9.8%, respectively (Table 5).
The heavy metal indices (IRWQI) are shown in Table 6. As can be observed from Table 5, almost all measured metal concentrations of spring No. 1, for both periods of sampling, were higher than in the other four.
Table 6 illustrates that all heavy metal indices except those of spring No. 1 in the rainy season were below the detectable values. Therefore, for both periods of sampling, all springs except spring No.1 were in the categories of very good water, very excellent water, and suitable water for drinking purposes categories in regard to dissolved metals (Table 5).
All toxic metals (Ag, As, B, Ba, Cd, Co, Cr, Cs, Cu, In, Mn, Ni, Pb, Rb, Se, Sr, U, and Zn) were below their respective WHO limits except that of spring No. 1 for rainy season. The five examined groundwater samples from springs in February indicated that As and B are significantly higher in spring No. 1 compared to the other four (more than ten times higher). The spatial variations revealed that only samples collected from spring No. 1 in the rainy season had poor water quality and would require measures for mitigation. The other data revealed that IRWQI values for all investigated metals were within safe limits. Therefore, these groundwater springs can be used for drinking without any health risk with regard to dissolved metals.
Given the mean concentrations of trace metals reported in Table 5, the Beheshtabad Basin’s groundwater from springs was comparable to and even lower than these elements’ concentrations as reported by Shuhaimi-Othman et al. (2008) for Chini Lake, Malaysia; by Kazi et al. (2009) for Manchar Lake, Pakistan; by Singanan et al. (2008) for Wenchi Crater Lake, Ethiopia; by Masresha Alemayehu et al. (2011) for Lake Awassa, Ethiopia; and by Prasanna et al. (2012) for Curtin Lake, Malaysia (Table 7).
Table 7 gives the concentrations of Se and Pb, which were significantly lower in this study compared to other waters worldwide. Thus, the studied basin was in the normal range in terms of its heavy metal concentrations.
Conclusion
The present work provides background information for the groundwater quality of springs in the Beheshtabad Basin to be utilized in future research. The research presents initial results for the identification of groundwater quality and heavy metal concentrations, provides baseline data for future studies, identifies possible sources, and determines the degree of metals pollution in the spring waters from the Beheshtabad Basin.
The electrical conductivity, total dissolved solids, cations, and anions of the water samples did not exceed the maximum permissible limits of the WHO. However, some of the water samples were not bacteriologically suitable for human consumption.
The water quality analysis clearly shows that trace elements have not been released from natural hydrogeochemical processes and do not have high potential to contaminate the groundwaters. Finally, based on the WHO classification, the spring waters of the Beheshtabad Basin generally are suitable for drinking purposes, although the weathering of rocks and dissolution of salts from bedrock into spring No. 1 poses a severe threat to this habitat and requires serious attention.
References
Abbasnia A, Yousefi N, Mahvi AH, Nabizadeh R, Radfard M, Yousefi M, Alimohammadi M (2018) Evaluation of groundwater quality using water quality index and its suitability for assessing water for drinking and irrigation purposes: case study of Sistan and Baluchistan province (Iran). Hum Ecol Risk Assess. https://doi.org/10.1080/10807039.2018.1458596
Abd El-Aziz SH (2017) Evaluation of groundwater quality for drinking and irrigation purposes in the north-western area of Libya (Aligeelat). Environ Earth Sci. https://doi.org/10.1007/s12665-017-6421-3
Annapoorna H, Janardhana MR (2015) Assessment of groundwater quality for drinking purpose in rural areas surrounding a defunct copper mine. Aquatic Procedia 4:685–692
APHA (2012) Standard methods for the examination of water and waste waters. APHA, Washington
Bear J, Cheng AHD (2010) Modeling groundwater flow and contaminant transport. Springer, Berlin
Edmunds WM, Shand P (2008) Natural groundwater quality. Wiley, New York
Ewusi A, Obiri-yeboah S, Voig H, Asabere SB, Bempah CK (2013) Groundwater quality assessment for drinking and irrigation purposes in Obuasi municipality of Ghana, a preliminary study. Environ Earth Sci 5:6–17
Gurunadha VVSR, Tamma GR, Surinaidu L, Rajesh R, Mahesh J (2011) Geophysical and geochemical approach for seawater intrusion assessment in the Godavari Delta Basin, A.P India. Water Air Soil Pollut 217:503–514
Hakim MA, Juraimi AS, Begum M, Hasanuzzaman M, Uddin MK, Islam MM (2009) Suitability evaluation of groundwater for irrigation, drinking and industrial purposes. Am J Environ Sci 5:413–419
Hashemi SH, Pourasghar F, Nasrabadi T, Ramezani S, Khoshrou Gh (2011) Guideline for calculation of Iran Water Resources Quality Index (IRWQI). Shahid Beheshti University, Iran Department of Environment, Tehran
Jamshidzadeh Z, Mirbagheri SA (2011) Evaluation of groundwater quantity and quality in the Kashan Basin, Central Iran. Desalination 270:23–30
Kazi TG, Arain MB, Jamali MK, Jalbani N, Afridi HI, Sarfraz RA, Baig JA, Abdu S (2009) Assessment of water quality of polluted lake using multivariate statistical techniques: a case study. Ecotoxicol Environ Saf 72:301–309
Khosravi R, Eslamia H, Almodaresib SA, Heidaric M, Fallahzadeha RA, Taghavid M, Khodadadie M, Peirovi R (2017) Use of geographic information system and water quality index to assess groundwater quality for drinking purpose in Birjand City Iran. Desalin Water Treat 67:413–419. https://doi.org/10.5004/dwt.2017.204585
Krishna AK, Satyanarayanan M, Govil PK (2009) Assessment of heavy metal pollution in water using multivariate statistical techniques in an industrial area: a case study from Patancheru, Medak District, Andhra Pradesh, India. J Hazard Mater 167:366–373
Law RK, Murphy MW, Choudhary E (2017) Private well groundwater quality in West Virginia, USA–2010. Sci Total Environ. https://doi.org/10.1016/j.scitotenv.2017.02.018
Lermontov A, Yokoyama L, Lermontov M, Machado MAS (2009) River quality analysis using fuzzy water quality index: Ribeira do Iguape river watershed, Brazil. Ecol Indic 9:1188–1197. https://doi.org/10.1016/j.ecolind.2009.02.006
Mahendra GT, Patode HS (2014) Evaluation of groundwater quality and its suitability for drinking and agricultural use in and around Hingoli region, Maharashtra, India. Int J Humanit Arts Soc Sci 6:256–263
Majolagbe AO, Adeyi AA, Osibanjo O, Adams AO, Ojuri OO (2017) Pollution vulnerability and health risk assessment of groundwater around an engineering Landfill in Lagos, Nigeria. Chem Int 3:58–68
Masoud AA, El Bouraie MM, El-Nashar W, Mashaly H (2017) Spatiotemporal evaluation of the groundwater quality in Gharbiya Governorate, Egypt. Environ Sci Pollut Res Int 24(9):8256–8278
Masresha Alemayehu E, Skipperud L, Rosseland BO, Zinabu GM, Meland S, Teien HC, Salbu B (2011) Speciation of selected trace elements in three Ethiopian Rift Valley lakes (Koka, Ziway, and Awassa) and their major inflows. Sci Total Environ 409:3955–3970
Nag SK (2014) Evaluation of hydrochemical parameters and quality assessment of the groundwater in Gangajalghati Block, Bankura District, West Bengal, India. Arab J Sci Eng 39:5715–5727
Neisi A, Mirzabeygi M, Zeyduni G, Hamzezadeh A, Jalili D, Abbasnia A, Yousefi M, Khodadadi R (2018) Data on fluoride concentration levels in cold and warm season in City area of Sistan and Baluchistan Province Iran. Data Brief 18:713–718. https://doi.org/10.1016/j.dib.2018.03.060
Oişte AM (2014) Groundwater quality assessment in urban environment. Int J Environ Sci Technol 11:2095. https://doi.org/10.1007/s13762-013-0477-8
Prasanna MV, Praveena SM, Chidambaram S, Nagarajan R, Elayaraja A (2012) Evaluation of water quality pollution indices for heavy metal contamination monitoring: a case study from Curtin Lake, Miri City, East Malaysia. Environ Earth Sci 67:1987–2001
Prasanth SSV, Magesh NS, Jitheshlal KV, Chandrasekar N, Gangadhar K (2012) Evaluation of groundwater quality and its suitability for drinking and agricultural use in the coastal stretch of Alappuzha District, Kerala India. Appl Water Sci. https://doi.org/10.1007/s13201-012-0042-5
Ravikumar R, Somashekar RK (2017) Principal component analysis and hydrochemical facies characterization to evaluate groundwater quality in Varahi river basin, Karnataka state, India. Appl Water Sci 7:745–755
Richey AS, Thomas BF, Lo MH, Reager JT, Famiglietti JS, Voss K, Rodell SS (2015) Quantifying renewable groundwater stress with GRACE. Water Resour Res 51:5217–5238
Saeedi M, Abessi O, Sharifi F, Meraji H (2010) Development of groundwater quality index. Environ Monit Assess 163:327–335
Shuhaimi-Othman M, Ahmad A, Mushrifah I, Lim EC (2008) Seasonal influence on water quality and heavy metals concentration in Tasik Chini, Peninsular Malaysia. In: Sengupta M and Dalwani R (eds). The 12th World Lake Conference, pp: 300–303
Singanan M, Wondimu L, Tesso M (2008) Water quality of Wenchi Crater Lake in Ethiopia, Maejo. Int J Food Sci Tech 02:361–373
Singh S, Hussian A, Dubey S (2016) Water quality index development for groundwater quality assessment of Greater Noida sub-basin, Uttar Pradesh India. Cogent Eng. https://doi.org/10.1080/23311916.2016.1177155
Stigter TY, Ribeiro L, Carvalho Dill AMM (2006a) Application of a groundwater quality index as an assessment and communication tool in agroenvironmental policies–two Portuguese case studies. J Hydrol 327:578–591
Stigter TY, Ribeiro L, Carvalho Dill AMM (2006b) Evaluation of an intrinsic and a specific vulnerability assessment method in comparison with groundwater salinisation and nitrate contamination levels in two agricultural regions in the south of Portugal. J Hydro 14:79–99
Valenzuela JC, Quilez D, Lafarga R (2006) Groundwater quality in CR-V irrigation district (Bardenas I, Spain): alternative scenarios to reduce off-site salt and nitrate contamination. Agr Water Manag 84:281–289
Vasanthavigar M, Srinivasamoorthy K, Vijayaragavan K, Ganthi RR, Chidambaram S, Sarama VS, Anandhan P, Manivannan R, Vasudevan S (2010) Application of water quality index for groundwater quality assessment: Thirumanimuttar sub-basin, Tamilnadu, India. Environ Monit Assess 171:595–609
WHO (2004) Hardness in drinking-water: background document for development of WHO Guidelines for drinking-water quality. World Health Organization, Geneva
Yaghobi S, Faramarzi M, Karimi H et al (2017) Simulation of land-use changes in relation to changes of groundwater level in arid rangeland in western Iran. Int J Environ Sci Technol. https://doi.org/10.1007/s13762-017-1610-x
Yidana SM, Sakyi PA, Stamp G (2011) Analysis of the suitability of surface water for irrigation purposes: the southwestern and Coastal River Systems in Ghana. J Water Resource Prot 3:695–710
Zhang O, Wang H, Wang Y, Yang M, Zhu L (2017) Groundwater quality assessment and pollution source apportionment in an intensely exploited region of northern China. Environ Sci Pollut Res. https://doi.org/10.1007/s11356-017-9114-2
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Gharahi, N., Zamani-Ahmadmahmoodi, R. Evaluation of groundwater quality for drinking purposes: a case study from the Beheshtabad Basin, Chaharmahal and Bakhtiari Province, Iran. Environ Earth Sci 79, 82 (2020). https://doi.org/10.1007/s12665-020-8816-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12665-020-8816-9