Abstract
This investigation has been conducted to evaluate the concentrations of heavy metals, including lead, cadmium, and copper in the drinking water in Kerman city, Iran. In this descriptive cross-sectional research, there have been 160 samples of tap water and 64 samples of bottled water brands collected to achieve comparable results. The atomic absorption spectrophotometer has been used to measure the concentrations of lead, cadmium and copper and the United States Environmental Protection Agency (USEPA) indexes have been used to evaluate the human health risk. The results showed that the mean concentrations of lead metal in tap water have been higher than the recommended quantity based on the standards of the World Health Organization (WHO) and USEPA. The mean concentrations of cadmium and copper in urban tap water have been in the acceptable ranges defined by Iranian National Standards, WHO and USEPA. Likewise, the target heavy metals concentrations in the bottled water have been conformed to these standard limits. Although there is no potential risk of carcinogenic detrimental health effect in children and adult groups, the children group consuming the drinking water are at the risk of non-carcinogenic adverse health effect. It is recommended that the heavy metals concentrations in drinking water are periodically monitored to minimize the environmental pollutions and health risks in consumers.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Nowadays, environmental pollutions, including water pollution, have become a global issue and human life depends on the healthy drinking water intake required to prevent any risks to human health (WHO 2004). Although the surface and groundwater are the main sources of drinking water in most parts of the world, the desalinated seawater, bottled water as well as spring water are regularly used in other areas especially the regions encountering the water shortage (Kim et al. 2015; Sullivan and Leavey 2011). Several human activities have influences on the capacity and availability of heavy metals in ecosystems, and these metals can enter the body following the emission in water (Sardar et al. 2013). Although some metals such as iron, cobalt, copper, zinc, chromium, vanadium, selenium, and molybdenum act as a catalyst in the activities of the human bodies′ enzymes and are considered as the essential elements for the growth and reproduction, their accumulation in the human body causes toxicity (Karamanis et al. 2007; Ghaderpoori et al. 2009). The non-essential metals such as lead and cadmium having no role in the metabolic activities lead to the toxic effects on the body tissues (Bruins et al. 2000).
The concentrations of heavy metals higher than the standard levels defined by USEPA in the drinking water cause systematic effects on human health (USEPA 2007). The water pollution and its effects are increasing, and this pollution has become the global issue (Volety 2008; Shanbehzadeh et al. 2014; Liu et al. 2009; Mandour and Azab 2011; Montuori et al. 2013; Sekabira et al. 2010). The toxicities of lead and cadmium metals lead to the dangerous effects such as abortion and the increase in the birth of premature infants, pair injury, birth weight loss, adverse effects on the kidney system, and high blood pressure (Jarup 2003; Neeti and Prakash 2013). Similarly, copper accumulation in drinking water causes Alzheimer’s disease (Kaplan et al. 2011).
The revival of heavy metals under the sediments of the water columns acts as a source of heavy metals (Wang et al. 2015; Wu et al. 2014; Das et al. 2008; Davidson et al. 2004). The sedimentation of the heavy metals by corroding the water pipes and the washing-up through the water distribution system (WDS) pollutes the drinking water. The used materials in the household piping are transferred to the drinking water through the contacting corrosive water with pipes, fittings, valves, municipal and domestic distribution networks. This event creates a great amount of heavy metals in the drinking water (Al-Saleh and Al-Doush 1998; Alabdula’aly and Khan 2009; Craun and Calderon 2001). The transferred metals to the drinking water include lead, cadmium, copper, zinc, and manganese (Savari et al. 2008).
Healthy drinking water has a high priority in the water supply all over the world (Salvato et al. 2003). The use of bottled water has risen in many countries since the last 30 years (Karamanis et al. 2007; Jakus et al. 2009).
The water kept in the room temperature for a long time increases the risk of heavy metal sedimentation from the bottle walls into water (Keresztes et al. 2009). Many studies have investigated the human health risk assessment by the heavy metals through the bottled water intake (Hadiani et al. 2015; Kolawole and Obueh 2015). The lead concentration in the urban water by the metallic piping system is very high, and the dissolved lead in the piping system depends on water chlorine, dissolved oxygen, pH, temperature, water hardness, and retention time in the water pipe (FiketI et al. 2007). The consumption of water containing a certain amount of heavy metals may lead to health problems such as breath shortness and multiple types of cancer in humans (Kavcar et al. 2009). Stegavik (1975) examined the heavy metal pollution in the drinking water distribution network of Trondheim city in Norway and the results showed that the concentrations of lead, cadmium, copper, and zinc in the drinking water of this city have been less than the standard level, and there is no concern for the public health. Water pollution of Ginzoo River by cadmium from Kamioka zinc mine in Japan has caused kidney disorders among the people (Yoshida et al. 1999).
Due to the importance of this issue, several studies have been conducted to investigate the chronic health effects of exposure to heavy metals from drinking water consumption worldwide (Jaishankar et al. 2014; Zhang et al. 2014; Colak et al. 2015; Avino et al. 2011; Shah et al. 2012).
Although evaluating the qualitative parameters of drinking water is a direct method to compare these parameters with the standard limits, it cannot provide a comprehensive description of the drinking water quality. On the other hand, there is no documentation about the detrimental effects of the heavy metals exposure on the human body since the exposure levels lower than the recommended standards for heavy metals do not lead to recognizable clinical symptoms (Manassaram et al. 2010). Accordingly, one of the most important methods to evaluate the potential adverse effects of human exposure to hazardous pollutants is health risk assessment (Karim 2011; Sun et al. 2007). The information obtained from the risk assessment is used as one of the crucial tools to help the decision-makers in environmental and health management (Falk-Filipsson et al. 2007). Based on the importance of heavy metals in water resources, this study has been conducted to assess the carcinogenic and non-carcinogenic risks by consumption of drinking water network, and bottled water in Kerman city located in the South-East of Iran.
Materials and methods
Study area
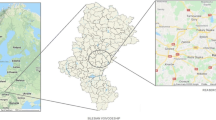
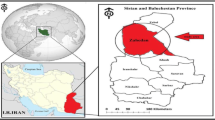
Kerman city is the capital and the biggest city of Kerman province. The geographical coordinates of Kerman are 50°–57° east and 17°–30° north. This city has hot and dry summers and cold winters (Nazarialamabadi 2008). Based on the census of the statistical center of Iran, the population of the city has reached 537,718 people, including 272,715 men and 265,003 women (Iran 2016). The map of the study area and sampling sites are presented in Fig. 1.
Chemicals and reagents
All standard acids, as well as the solutions of heavy metals (lead, cadmium, and copper) used in this investigation, have been bought from Merck Co. in Germany. A variety of heavy metal concentrations were prepared by diluting with the standard solutions and deionized water.
Sample collection
Polyethylene bottles previously sterilized without any pollutions have been used to randomly collect the samples of drinking waters from 20 public taps in Kerman city. There have been eight samples with 1000 ml of water collected from each tap. Also, eight samples from eight bottled water brands have been bought from supermarkets and stores. The collected samples have been kept in a cold box and transferred to the laboratory. The samples have been stored in the refrigerator of the laboratory until the experiment time.
Sample preparation and digestion
The heavy metals in the water samples have been prepared using the acidic digestion method adopted from other researches (Hseu 2004; Momodu and Anyakora 2010). The amount of 5 ml concentrated nitric acid has been added to the 5 ml water and heated to reach 3 ml for the acidic digestion. Also, the residue has been passed through the filter to reach 25 ml volume.
Sample analysis
Atomic Absorption Spectrophotometer, model AA240, Varian Company, made in Australia equipped with a GTA120 graphite tube atomizer, a PSD120 autosampler and a Varian hallow cathode lamp has been used to measure the existed heavy metals in the water samples. The detection limit (LOD) for lead, cadmium and copper have been found to be 0.036 ng/mL, 0.01 ng/mL and 1 ng/mL, respectively. The optimal instrumental parameters for VARIAN AA240 determination of target heavy metals are shown in Table 1. All concentrations have been measured (mg/L) and compared with the standard limit of the heavy metals in drinking water based on USEPA and WHO (Reynolds et al. 2008; Shotyk and Krachler 2007b). The results have been analysed using SPSS v.25 (IBM spss) to achieve the mean value, standard deviation, maximum and minimum values. The office and Excel software 2016 have been used to plot all tables.
Human health risk assessment
Exposure assessment
One step of human health risk assessment is exposure assessment (Means 1989; EPA 2001). According to a precise definition, the pollutants intake by humans refers to an effective dose of the pollutant that enters the body through various exposure pathways such as ingestion, inhalation or skin contact, and reach blood circulation and affect the body tissues and other organs. The daily chronic intake of heavy metals given the conservative measures and limitations of this study, the chronic daily intakes of drinking water (CDI) in children and adult groups have been calculated based on the chronic daily intake of toxic dangerous materials during the contact period (mg/kg/day) (Yu et al. 2014). The CDI of heavy metals is obtained based on the following formula:
where CDI: chronic daily intake of heavy metal by water ingestion (mg/kg/day), C: pollutant concentration in tap water or bottled water (mg/L), IR: ingestion rate per unit time (IR) (1 l/day for children and 2 l/day for adults), ED: exposure period to metals (6 years for children and 30 years for adults), EF: represents the annual exposure frequency to metal (365 day/year), BW: average body weight (15 kg for children and 70 kg for adults), AT: average exposure time (for carcinogenicity AT = 365 × 70 = 25,550 days for children and adults for non-carcinogenicity AT = ED × 365, which is 2190 days for children and 10,950 days for adults) (EPA 2001).
Non-carcinogenic risk assessment
The probability of non-carcinogenic risk is assessed by the hazard quotient (HQ) factor. This factor is non-carcinogenic CDI based on the oral reference dose (RFD) calculated according to the following equation:
where HQ: the non-cancer hazard quotient, CDI: non-carcinogenic chronic daily intake (mg/kg/day), RFD: RFD is an estimation of the daily human contact surface of a population and includes a sensitive population that does not have a harmful effect on their health throughout their lifetime (Yu et al. 2014; Bamuwamye et al. 2015).
Non-carcinogenic potential risk to human health through higher than one heavy metal was assessed by hazard index (HI) index, which is the sum of all HQ calculated for individual heavy metal (Liu et al. 2013). The HI is used to estimate the total non-carcinogenic risk effects of the exposures to a multiple of heavy metals in the drinking water and calculated as follows: (Huang et al. 2008; Bamuwamye et al. 2015):
Therefore, if the value of HQ or HI > 1, there is a possibility of adverse effects on human health so that the closer to 1, the greater the non-carcinogenic risk (Wei et al. 2015).
Carcinogenic risk assessment
The carcinogenic risk potential in heavy metals through the drinking water is estimated using incremental lifetime cancer risk (ILCR) (Liu et al. 2013).
CDI in the following equation is the chronic daily intake of carcinogenic chemical materials (mg/kg/day). This index shows the mean daily dose of exposure to the carcinogenic materials in a lifetime:
Based on USEPA standard, ILCR is obtained by cancer slope factor (CSF) that is the risk by one dose of 1 mg/kg in total body weight for the certain pollutant in all lifetime.
The cumulative cancer risk as a result of exposure to multiple carcinogenic heavy metals due to the consumption of water was assumed to be the sum of the individual heavy metal increment risk and calculated using the following equation (Liu et al. 2013):
In this equation, n is the individual carcinogenic of each heavy metal in the bottled water or tap water. The level of acceptable cancer risk (ILCR) or for regulatory purposes is considered between 10−4 and 10−6 (Li and Zhang 2010).
Results
Heavy metals concentration
Heavy metals concentration in tap water
Based on the results in Table 2, it is observed that the amount of lead in tap water is between 0.0001 and 0.21 mg/L. The concentration of copper has been determined as the significant level (ND–0.03 mg/L), while the concentration of cadmium has been considered negligible (ND–0.002 mg/L). The concentrations of the target heavy metals in tap water have been compared with the provided standard limit by USEPA and WHO (Table 9). In this regard, the lead concentration in the samples of the 8th site has been higher than the Iranian National Standards (INS). Also, the lead concentrations in the samples of four sites (1, 4, 7, and 11) have been higher than the acceptable limits defined in WHO and USEPA standards. The concentrations of cadmium and copper in all samples have been lower than the permitted levels by (INS), WHO, and USEPA standards. In general, the mean concentration of lead in the tap water has been lower than that recommended by the Iranian standard (INS). However, it has been higher than the guidelines in WHO and USEPA.
The concentration of heavy metals in bottled water
The concentration of lead in the bottled water has been between ND and 0.0009 mg/L. Based on the values obtained for this metal, although the concentration of lead in brand four has been higher than the levels in INS, its concentrations in other brands have been less than the acceptable limits in INS, WHO and USEPA as represented in Table 3. The mean concentrations of cadmium and copper in all brands of the bottled water have been lower than the recommended levels in the INS, WHO and USEPA. Generally, the mean concentration of lead in the bottled water has been lower than the recommendations in INS. However, it has been higher than the guidelines in WHO and USEPA.
Human health risk assessment
The carcinogenic and non-carcinogenic risks have been determined based on the mean concentration of the heavy metals using the formulations of ILCR and HQ (Liu et al. 2013). RFD and CSF values of carcinogenic risk are shown in Table 4 (USEPA 2016).
Non-carcinogenic risk
Table 5 represents the chronic daily intakes of non-carcinogenic through the target heavy metals in tap and bottled water. The results show that the chronic daily intake of non-carcinogenic of heavy metals has been higher in children as compared with that in adults. The chronic daily intake of non-carcinogenic reduction procedure in both children and adults are similar as follows: Pb > Cu > Cd.
The non-carcinogenic risk of the target heavy metals in the drinking water was calculated by the HQ, and the results are shown in Table 6. Based on the guideline of USEPA mentioned before, If the HQ level of any of the heavy metals or HI \( \left[ {\left( {HI = \sum\nolimits_{k = 1}^{n} H Q} \right) \,{\text{in}}\,{\text{the}}\,{\text{drinking}}\,{\text{water}}} \right] \) is less than one, it means that there is no considerable risk regarding the detrimental health effects. The ratios above one for HQ and HI can indicate the potential non-carcinogenic effect are likely to occur and is in the unacceptable range. The results showed that HQ levels of lead metal in tap water are more than one in the children group placed in the range of unacceptable non-carcinogenic risk. The potential risk of non-carcinogenic detrimental effect due to exposure to copper metal in all water samples of the bottled and tap water in both groups of children and adults is minimal (HQ < 0.009), indicating that cu does not lead to the significant risk of systemic toxicity.
The HQ of lead metal for the bottled water in both children and adults and the tap water in adults have been lower than one. Likewise, the risk quotient of cadmium and copper for all water samples in all studied groups have been less than one. Therefore, there is not any potential non-carcinogenic effect are unlikely to occur for the children exposed to lead metal through the bottled water which is the same for the adults exposed to lead metal through the tap water and the bottled water. Furthermore, there has not been any probability of non-carcinogenic effect through the exposure to Cd and Cu metals by consuming the drinking water (bottled water and tap water) in the studied population. The decreasing trend of HQ in both groups of children and adults through the tap water and the bottled water has been the same as follows: HQPb > HQCd > HQCu.
The multiple non-carcinogenic HI of heavy metals (HI) for the tap water in the children and adults groups have been 2.78 and 0.126, respectively, and that for the bottled water have been 0.311 and 0.131, respectively. Based on the USEPA guidelines, the HI above one (HI > 1) for the tap water in the children group is within the range of unacceptable non-carcinogenic risk, and there are the detrimental effects of the target heavy metals causing the adverse health effect in children consuming the tap water. It should be noted that the HI level in children has been greater than that in adults, indicating that children are more at risk of non-carcinogenic risks of heavy metals in the drinking water.
Carcinogenic risk
The carcinogenic risk and chronic daily intake due to lead and cadmium are, respectively shown in Tables 7 and 8 for the tap and bottled water in both children and adult groups.
Carcinogenic chronic daily intake in both children and adult groups by consuming the tap and bottled water has reduction procedure as CDIPb > CDICu > CDICd.
Incremental lifetime carcinogenic risk (ILCR) of cadmium has been more than in the lead for both types of tap and bottled water. In general, ILCR of cadmium and lead in tap water has been monitored and controlled in the standard and permitted level for adults group (1 × 10−4–1 × 10−6), while this amount in children group for lead has been lower than the standard limit of USEPA (lower than 1 × 10−6) and is under monitored and controlled about the standard level for cadmium. ILCR for lead in the bottled water has been negligible for both adult and children groups (lower than 1 × 10−6), but regarding cadmium, it has been under monitored and controlled in the standard level for the bottled water (1 × 10−4–1 × 10−6). Generally, the ILCR of lead and cadmium have been higher in adults as compared with children group. Furthermore, the consumption of tap water has led to higher carcinogenic risks of lead and cadmium than that of bottled water.
Overall, the cumulative cancer risk (\( \sum ILCR) \) by drinking water in Kerman city is at the standard level for both adult and children groups (1 × 10−4–1 × 10−6). Thus, there is no potential to the carcinogenic diseases in both groups.
Discussion
The results showed that the mean concentration of lead in the tap water exceeds the acceptable limits in INS, WHO, and USEP as represented in Table 9.
The maximum concentration of lead in groundwater in Kerman city is 0.045 mg/L (Hassanzadeh et al. 2011). Thus, an increase in the lead concentration of tap water from the standard limits indicates a high concentration of this metal in the main resource and inefficiency of the water treatment system in Kerman. Alternatively, the corrosion of lead pipes, as one of the used piping material in water and indoor plumbing systems of buildings, transfers lead from the walls of the pipes to the urban water systems. The absorbed cadmium in the tap drinking water depends on several factors including lead piping in the plumping system, the number of fittings exposed to water, and soldering of pipes (Quevauviller and Thompson 2005). The results of this research agree well with the investigation of Nahid and Moslehi (2008), about the drinking water of various areas in Tehran city, Iran which has indicated that the measured contact concentrations of heavy metals were in acceptable standard limits by WHO and USEPA except for lead. The mean concentrations of cadmium and copper in tap water in this research have been lower than the defined standard limits by INS, WHO, and USEPA. The low values can be for optimal coagulation or settling process in the process of drinking water purification. In spite of the difference in concentrations of the mentioned elements in various sampling parts, their concentration have not been higher than the compared standards. The difference in the concentrations of copper and cadmium has been due to the erosion of sediment or corrosion of pipes and fittings used in the urban plumbing system, corrosion of galvanized pipes, and brass valves to the domestic networks of urban houses (Shahriari et al. 2010).
The results are also in agreement with the similar researches conducted by SJ et al. (1976) in Taiwan, Hashem (1993) in Saudi Arabia, and Nouri et al. (2006) in Iran, indicating the lower copper concentration than standard limits. The lower concentrations of copper as compared with the standard level in these studies are due to the flows of draining water into the aquifers. Another research by Abbasnezhad and Khajehpour (2009), on the concentration of heavy metal in groundwaters of Rafsanjan city, Iran showed that the concentrations of lead and arsenic have been higher than standard levels since the main source of groundwater is under the volcanic mountains which are in agreement with the results of this research, also Khajehpour (2010), showed that the concentrations of cadmium, copper, and zinc have been lower than standard levels in Iran which is in agreement with the results of this research.
The mean concentration of lead in the bottled water in this study has been lower than INS, WHO, and USEPA. Nonetheless, the difference in the lead concentrations in various bottled water samples has been from the difference in bottled water resource such as spring, well, as well as the difference in the treatment process or their storage containers. The results of studies by Pip (2000) and Shotyk and Krachler (2007b) showed that lead concentration in bottled containers increases by retention time. Moreover, glass and polyethylene terephthalate (PTE) have been used as the container material to protect the bottled water. There is a probability of sedimentation of these materials based on the studies carried out in other countries (Shotyk and Krachler 2007a; Shotyk et al. 2006; Westerhoff et al. 2008). The results of Pip (2000) and Dabeka et al. (2002) showed that the lead concentration in bottled water has been lower than the standard limits which are in agreement with the result of this research.
Pip (2000) carried out a research on 40 brands of bottled water in Canada showed that the mean concentration of lead in the examined mineral water brands is 5 μg/L which has been higher than the lead concentration of the studied samples of this research. However, it has been lower than the maximum standard limits in WHO and USEPA. The mean concentrations of cadmium and copper in the bottled water have been lower than the compared standards and they agree well with the results of obtained for the bottled water in Azerbaijan (Forouzan et al. 2008). Another research on the bottled water in Tehran city, Iran, by Ghaderpoori et al. (2009), indicated that the concentrations of metals in bottled water have been at the desirable level. The studies in Croatia reported that the range of the heavy metals and elements in all water samples have been within the standard limits (FiketI et al. 2007).
In this study, HI for the consumption of tap water in children has been higher than one showing that it is likely to have detrimental health effects on children. Although this index has been lower than one in both children and adults groups consuming the tap water and bottled water, it has been less than one only for the adults group using the tap water. Therefore, it is unlikely to have an adverse health effect on the two groups. In general, the HI in children group has been higher than the adult group, which shows the potential of carcinogenic risk has been higher in children than adults (Akkus and Ozdenerol 2014). The previous studies have confirmed this observation (Guerra et al. 2012). It indicates that the HI of target heavy metals (cadmium and zinc) in aqueous flows for drinking water around mine area in Korea country has been acceptable (Lim et al. 2008).
In periodic studies adopting HI of health risk assessment of heavy metals in the drinking water of the mountainous area in northern Pakistan, it was found that there is no risk threatening the human health (Muhammad et al. 2011). Adamu et al. (2015) conducted a research on State River in the vicinity of the barite mine in the southeastern of Nigeria and showed that multiple non-carcinogenic risk (HI) of heavy metals has been in the unacceptable level. The heavy metal assessment of cumulative carcinogenic risk for target metals (\( \sum ILCR \)) through the tap and bottled water in both children and adult groups has been in the permitted limit of the monitored and controlled level (1 × 10−4–1 × 10−6).
Cumulative cancer in both children and adult groups for the tap water has been higher as compared with the bottled water. Generally, \( \sum ILCR \) the adult group has been greater for children through the tap and bottled water. The research carried out with the aim of heavy metals health risk assessment in the middle Russian area showed that the risk of the carcinogenic disease had been 3.9 × 10−3 (Momot and Synzynys 2005). Health risk assessment in this research for the consumers of drinking water resources through both the tap and bottled water has been within the limit of 1 × 10−4–1 × 10−6 indicating lower carcinogenic risk potential in the studied area as compared with that in Russia. The results of the heavy metals risk assessment conducted by Rajaei and Hesari (2012), in Aliabad Plain, Iran showed that the potential of carcinogenic risk had been 2.23 × 10−4, and the potential of non-carcinogenic risk has been 2.53 × 10−4. It showed a lower potential of carcinogenic risk and higher potential of non-carcinogenic risk.
Conclusions
In this investigation, the concentrations of target heavy metals (lead, cadmium, and copper) in the tap water and the bottled water have been measured, and then the carcinogenic and non-carcinogenic risks have been calculated in both children and adults in Kerman city, Iran. The results are summarized as follows:
- 1.
The highest and lowest mean concentrations of heavy metals in the samples of tap water and bottled water have been related to lead and cadmium, respectively. The lead concentrations in the tap and bottled water have been lower than the recommended levels by Iranian standards, but higher than the International organizations guidelines (WHO and EPA). The mean concentrations of Cd and Cu in the studied water samples have been lower than all considered standards.
- 2.
In the assessment of non-carcinogenic risk, only the HQ values of lead metal for the tap water in the children group have been greater than one, which is considered at an unacceptable range.
- 3.
The multiple non-carcinogenic HI of heavy metals for the tap water in children has been higher than one (HI = 2.78), indicating the unacceptable level of the non-carcinogenic risk for the target metals in the tap water and their detrimental impacts on children’s’ health.
- 4.
The cumulative cancer risk of the target heavy metals for the children and adults using the tap water and bottled water are within the acceptable monitored and controlled levels (1 × 10−4–1 × 10−6).
- 5.
It is recommended that the relevant health authorities periodically monitor heavy metals to prevent health risk to the consumers.
References
Abbasnezhad A, Khajehpour S (2009) Survey of heavy metal concentration in Rafsanjan underground aquifer. In: Third specialized conference on environmental engineering (in Persian). https://www.civilica.com/Paper-CEE03-CEE03_109.html
Adamu C, Nganje T, Edet A (2015) Heavy metal contamination and health risk assessment associated with abandoned barite mines in Cross River State, southeastern Nigeria. Environ Nanotechnol Monitor Manag 3:10–21. https://doi.org/10.1016/j.enmm.2014.11.001
Akkus C, Ozdenerol E (2014) Exploring childhood lead exposure through GIS: a review of the recent literature. Int J Environ Res Public Health 11:6314–6334. https://doi.org/10.3390/ijerph110606314
Alabdula’aly AI, Khan MA (2009) Heavy metals in cooler waters in Riyadh, Saudi Arabia. Environ Monit Assess 157(1–4):23–28. https://doi.org/10.1007/s10661-008-0511-3
Al-saleh I, AL-doush I (1998) Survey of trace elements in household and bottled drinking water samples collected in Riyadh, Saudi Arabia. Sci Total Environ 216:181–192. https://doi.org/10.1016/s0048-9697(98)00137-5
Avino P, Capannesi G, Rosada A (2011) Ultra-trace nutritional and toxicological elements in Rome and Florence drinking waters determined by instrumental neutron activation analysis. Microchem J 97(2):144–153. https://doi.org/10.1016/j.microc.2010.08.007
Bamuwamye M, Ogwok P, Tumuhairwe V (2015) Cancer and non-cancer risks associated with heavy metal exposures from street foods: evaluation of roasted meats in an urban setting. J Environ Pollut Hum Health 3:24–30. https://doi.org/10.12691/jephh-3-2
Bruins MR, Kapil S, Oehme FW (2000) Microbial resistance to metals in the environment. Ecotoxicol Environ Saf 45:198–207. https://doi.org/10.1006/eesa.1999.1860
Colak EH, Yomralioglu T, Nisanci R, YildirimV Duran C (2015) Geostatistical analysis of the relationship between heavy metals in drinking water and cancer incidence in residential areas in the Black Sea region of Turkey. J Environ Health 77(6):86–93
Craun GF, Calderon RL (2001) Waterborne disease outbreaks caused by distribution system deficiencies. J Am Water Works Assoc 93:64–75. https://doi.org/10.1002/j.1551-8833.2001.tb09287.x
Dabeka R, Conacher H, Lawrence J, Newsome W, Mckenzie A, Wagner H, Chadha R, Pepper K (2002) Survey of bottled drinking waters sold in Canada for chlorate, bromide, bromate, lead, cadmium and other trace elements. Food Addit Contam 19:721–732. https://doi.org/10.1080/02652030210140905
Das B, Nayak B, Pal A, Ahamed S, Hossain MA, Sengupta MK, Rahman MM, Maity S, Saha KC, Chakraborti D, Mukherjee SC (2008) Groundwater arsenic contamination and its health effects in the Ganga-Meghna-Brahmaputra plain. In: Groundwater for sustainable development. Taylor & Francis, London, UK, pp 257-296
Davidson C, Peters N, Britton A, Brady L, Gardiner P, Lewis B (2004) Surface analysis and depth profiling of corrosion products formed in lead pipes used to supply low alkalinity drinking water. Water Sci Technol 49:49–54. https://doi.org/10.2166/wst.2004.0086
Falk-Filipsson A, Hanberg A, Victorin K, Warholm M, Wallén M (2007) Assessment factors—applications in health risk assessment of chemicals. Environ Res 104(1):108–127. https://doi.org/10.1016/j.envres.2006.10.004
FiketI Ž, Roje V, Mikac N, Kniewald G (2007) Determination of arsenic and other trace elements in bottled waters by high resolution inductively coupled plasma mass spectrometry. Croatica Chemica Acta 80:91–100
Forouzan SH, Bani I, Rahimi A (2008) Survey on nitrite, nitrate, and heavy metal concentrations in bottled waters in Azarbaijan_Gharbi supermarkets. In: 18th congress on food industry, Mashhad, Iran, Oct 14–17 (In Persian). https://www.google.com/url?sa=t&rct=j&q=&esrc=s&source=web&cd=12&cad=rja&uact=8&ved=2ahUKEwjwtop9vzjAhUnuaQKHYC7A1MQFjALegQIABAC&url=http%3A%2F%2Fconfnews.um.ac.ir%2Fimages%2F41%2Fconferences%2Ffoodcongress%2Fpdfs%2Fpp_27.pdf&usg=AOvVaw3uFjrYJjlcnlB5nNfFvrx9. Accessed 4 Feb 2019
Ghaderpoori M, Khaniki G, Nazmara S (2009) Determination of trace elements in bottled water in Tehran. In: 12th national congress on environmental health, Shahid Beheshti University of Medical Sciences, Tehran, Iran (In Persian). https://www.civilica.com/Paper-NCEH12-NCEH12_072.html. Accessed 4 Feb 2019
Guerra F, Trevizam AR, Muraoka T, Marcante NC, Canniatti-Brazaca SG (2012) Heavy metals in vegetables and potential risk for human health. Scientia Agricola 69:54–60. https://doi.org/10.1590/S0103-90162012000100008
Hadiani MR, Dezfooli-Manesh S, Shoeibi S, Ziarati P, Mousavi Khaneghah A (2015) Trace elements and heavy metals in mineral and bottled drinking waters on the Iranian market. Food Addit Contam Part B 8:18–24. https://doi.org/10.1080/19393210.2014.947526
Hashem A (1993) Heavy metals analysis of water and soils from Saudi Arabia. J King Saud Univ 5:39–45
Hassanzadeh R, Abbasnejad A, Hamzeh AM (2011) Assessment of groundwater pollution in Kerman urban areas. Environ Stud J 56:101–110 (in Persian)
Hseu Z-Y (2004) Evaluating heavy metal contents in nine composts using four digestion methods. Biores Technol 95:53–59. https://doi.org/10.1016/j.biortech.2004.02.008
Huang M, Zhou S, Sun B, Zhao Q (2008) Heavy metals in wheat grain: assessment of potential health risk for inhabitants in Kunshan, China. Sci Total Environ 405(1–3):54–61. https://doi.org/10.1016/j.scitotenv.2008.07.004
Iran SCO (2016) National portal of statistics: population census and housing (in Persian). https://www.amar.org.ir. Accessed 25 Dec 2018
Jaishankar M, Tseten T, Anbalagan N, Mathew BB, Beeregowda KN (2014) Toxicity, mechanism and health effects of some heavy metals. Interdiscip Toxicol 7(2):60–72. https://doi.org/10.2478/intox-2014-0009
Jakus PM, Shaw WD, Nguyen TN, Walker M (2009) Risk perceptions of arsenic in tap water and consumption of bottled water. Water Resour Res 45(5):1–8. https://doi.org/10.1029/2008WR007427
Jarup L (2003) Hazards of heavy metal contamination. Br Med Bull 68:167–182. https://doi.org/10.1093/bmbldg032
Kaplan O, Yildirim NC, Yildirim N, Tayhan N (2011) Assessment of some heavy metals in drinking water samples of Tunceli, Turkey. J Chem 8(1):276–280. https://doi.org/10.1155/2011/370545
Karamanis D, Stamoulis K, Ioannides K (2007) Natural radionuclides and heavy metals in bottled water in Greece. Desalination 213:90–97. https://doi.org/10.1016/j.desal02006.03.604
Karim Z (2011) Risk assessment of dissolved trace metals in drinking water of Karachi. Pakistan. Bull Environ Contam Toxicol 86(6):676–678. https://doi.org/10.1007/s00128-011-0261-8
Kavcar P, Sofuoglu A, Sofuoglu SC (2009) A health risk assessment for exposure to trace metals via drinking water ingestion pathway. Int J Hyg Environ Health 212(2):216–227. https://doi.org/10.1016/j.ijheh.2008.05.002
Keresztes S, Tatar E, Mihucz VG, ViragI I, Majdik C, Zaray G (2009) Leaching of antimony from polyethylene terephthalate (PET) bottles into mineral water. Sci Total Environ 407:4731–4735. https://doi.org/10.1016/j.scitotenv.2009.04.025
Khajehpour S (2010) Evaluation of heavy metal concentrations in the groundwater table in Rafsanjan. In: Third symposium on environmental engineering (in Persian). https://scholar.google.com/scholar?hl=en&as_sdt=0,5&q=Khajehpour+S,+Abbasnejad+A.+Evaluation+of+heavy+metal+concentrations+in+the+groundwater+table+in+Rafsanjan.+In+Third+Symposium+on+Environmental+Engineering.+2010+. Accessed 4 Feb 2019
Kim D, Amy GL, Karanfil T (2015) Disinfection by-product formation during seawater desalination: a review. Water Res 81:343–355. https://doi.org/10.1016/j.watres.2015.05.040
Kolawole SE, Obueh HO (2015) Evaluation of the minerals, heavy metals and microbial compositions of drinking water from different sourcesin Utagba-Uno, Nigeria. ISABB J Health Environ Sci 2(2):6–10. https://doi.org/10.5897/ISAAB-JHE2015.0017
Li S, Zhang Q (2010) Risk assessment and seasonal variations of dissolved trace elements and heavy metals in the Upper Han River, China. J Hazard Mater 181(1–3):1051–1058. https://doi.org/10.1016/j.jhazmat.2010.05.120
Lim H-S, Lee J-S, Chon H-T, Sager M (2008) Heavy metal contamination and health risk assessment in the vicinity of the abandoned Songcheon Au–Ag mine in Korea. J Geochem Explor 96:223–230. https://doi.org/10.1016/j.gexplo.2007.04.008
Liu J, Li Y, Zhang B, Cao J, Cao Z, Domagalski J (2009) Ecological risk of heavy metals in sediments of the Luan River source water. Ecotoxicology 18:748–758. https://doi.org/10.1007/s10646-009-0345-y
Liu X, Song Q, Tang Y, Li W, Xu J, Wu J, Wang F, Brookes PC (2013) Human health risk assessment of heavy metals in soil–vegetable system: a multi-medium analysis. Sci Total Environ 463:530–540. https://doi.org/10.1016/j.scitotenv.2013.06.064
Manassaram DM, Backer LC, Messing R, Fleming LE, Luke B, Monteilh CP (2010) Nitrates in drinking water and methemoglobin levels in pregnancy: a longitudinal study. Environ Health 9(1):1–12. https://doi.org/10.1186/1476-069X-9-60
Mandour R, Azab Y (2011) The prospective toxic effects of some heavy metals overload in surface drinking water of Dakahlia Governorate, Egypt. Int J Occup Environ Med (The IJOEM) 2. http://theijoem.com/ijoem/index.php/ijoem/article/view/80. Accessed 4 Feb 2019
Means B (1989) Risk-assessment guidance for superfund. Volume 1. Human health evaluation manual. Part A. Interim report (Final). Environmental Protection Agency, Washington DC, USA. Office of Solid Waste. https://www.osti.gov/biblio/7037757. Accessed 4 Feb 2019
Momodu M, Anyakora C (2010) Heavy metal contamination of ground water: the Surulere case study. Res J Environ Earth Sci 2:39–43
Momot O, Synzynys B (2005) Toxic aluminium and heavy metals in groundwater of middle Russia: health risk assessment. Int J Environ Res Public Health 2:214–218. https://doi.org/10.3390/ijerph005020003
Montuori P, Lama P, Aurino S, Naviglio D, Triassi M (2013) Metals loads into the Mediterranean Sea: estimate of Sarno River inputs and ecological risk. Ecotoxicology 22:295–307. https://doi.org/10.1007/s10646-012-1026-9
Muhammad S, Shah MT, Khan S (2011) Health risk assessment of heavy metals and their source apportionment in drinking water of Kohistan region, northern Pakistan. Microchem J 98:334–343. https://doi.org/10.1016/j.microc.2011.03.003
Nahid P, Moslehi MP (2008) Heavy metals concentrations on drinking water in different areas of Tehran and methods of removal them. J Food Sci Technol 5(1):29–35 (in Persian)
Nazarialamabadi M (2008) Investigation of part of Kerman city (residential neighborhood design). Dissertation, Islamic Azad University of Kerman (Persian language)
Neeti K, Prakash T (2013) Effects of heavy metal poisoning during pregnancy. Int Res J Environ Sci 2:88–92
Nouri J, Mahvi A, Babaei A, Jahed G, Ahmadpor HE (2006) Investigation of heavy metals in groundwater. Pak J Biol Sci 9:377–384
Pip E (2000) Survey of bottled drinking water available in Manitoba, Canada. Environ Health Perspect 108(9):863–866. https://doi.org/10.1289/ehp.00108863
Quevauviller P, Thompson KC (2005) Analytical methods for drinking water: advances in sampling and analysis. Wiley, New York
Rajaei GH, Hesari S (2012) health risk assessment of heavy metals in groundwater AliAbad Katool. J North Khorasan Univ Med Sci 4(2):155–162 (Persian language)
Reynolds KA, Mena KD, Gerba CP (2008) Risk of waterborne illness via drinking water in the United States. Reviews of environmental contamination and toxicology. Springer, Berlin, pp 117–158. https://doi.org/10.1007/978-0-387-71724-1_4
Salvato JA, Nemerow NL, Agardy FJ (2003) Environmental engineering. Wiley, New York
Sardar K, Ali S, Hameed S, Afzal S, Fatima S, Shakoor MB, Bharwana SA, Tauqeer HM (2013) Heavy metals contamination and what are the impacts on living organisms. Greener J Environ Manag Public Saf 2(4):172–179. https://doi.org/10.15580/gjemps.2013.4.060413652
Savari J, Jaafarzadeh N, Hassani AH, Shams Khoramabadi G (2008) Heavy metals leakage and corrosion potential in Ahvaz drinking water distribution network. Water Wastew J 18:16–24
Sekabira K, Origa HO, Basamba T, Mutumba G, Kakudidi E (2010) Heavy metal assessment and water quality values in urban stream and rain water. Int J Environ Sci Technol 7:759–770. https://doi.org/10.1007/BF03326185
Shah MT, Ara J, Muhammad S, Khan S, Tariq S (2012) Health risk assessment via surface water and sub-surface water consumption in the mafic and ultramafic terrain, Mohmand agency, northern Pakistan. J Geochem Explor 118:60–67. https://doi.org/10.1016/j.gexplo.2012.04.008
Shahriari T, Khodadadi M, Azizi M (2010) The survey of chromium and copper concentration in Birjand city’s drinking water resources and water supply network. In: 13th environ health conference, Kerman, Iran. https://www.sid.ir/fa/journal/ViewPaper.aspx?id=147540. Accessed 4 Feb 2019 (Persian language)
Shanbehzadeh S, Vahid Dastjerdi M, Hassanzadeh A, Kiyanizadeh T (2014) Heavy metals in water and sediment: a case study of Tembi River. J Environ Public Health. https://doi.org/10.1155/2014/858720
Shotyk W, Krachler M (2007a) Contamination of bottled waters with antimony leaching from polyethylene terephthalate (PET) increases upon storage. Environ Sci Technol 41:1560–1563. https://doi.org/10.1039/B517844B
Shotyk W, Krachler M (2007b) Lead in bottled waters: contamination from glass and comparison with pristine groundwater. Environ Sci Technol 41(10):3508–3513. https://doi.org/10.1021/es062964h
Shotyk W, Krachler M, Chen B (2006) Contamination of Canadian and European bottled waters with antimony from PET containers. J Environ Monit 8(2):288–292. https://doi.org/10.1039/B517844B
Sj Y, Py C, Tanaka S (1976) Heavy metals in drinking water in Taiwan and their possible bearing on an endemic disease. Geochem J 10:211–214. https://doi.org/10.2343/geochemj.10211
Stegavik K (1975) An investigation of heavy metal contamination of drinking water in the city of Trondheim, Norway. Bull Environ Contam Toxicol 14:57–60. https://doi.org/10.1007/BF01685599
Sullivan MJ, Leavey S (2011) Heavy metals in bottled natural spring water. J Environ Health 73(10):8–13
Sun F, Chen J, Tong Q, Zeng S (2007) Integrated risk assessment and screening analysis of drinking water safety of a conventional water supply system. Water Sci Technol 56(6):47–56. https://doi.org/10.2166/wst.2007.583
USEPA (2001) Quality assurance guidance document-model quality assurance project plan for the PM ambient air, 2. United States Environmental Protection Agency. https://www3.epa.gov/ttnamti1/files/ambient/pm25/qa/m212.pdf. Accessed 4 Feb 2019
USEPA (2007) Dermal exposure assessment: a summary of EPA approaches. National Center for Environmental Assessment. 20460. United States Environmental Protection Agency. https://www.google.com/search?client=firefox-b-d&q=USEPA.+Dermal+Exposure+Assessment%3A+A+Summary+of+EPA+Approaches.+National+Center+for+Environmental+Assessment.+2007%3A20460. Accessed 4 Feb 2019
USEPA (2016) United States Environmental Protection Agency (EPA). Retrieved from IRIS chemical assessment quick list. https://www.google.com/url?sa=t&rct=j&q=&esrc=s&source=web&cd=10&cad=rja&uact=8&ved=2ahUKEwiNjpjT-_zjAhXEKQKHRY2BjgQFjAJegQIAhAB&url=https%3A%2F%2Fcfpub.epa.gov%2Fncea%2Firis%2Fsearch%2Findex.cfm&usg=AOvVaw1GKehbdVZTUm5UbWGtlVxt. Accessed 4 Feb 2019
Volety AK (2008) Effects of salinity, heavy metals and pesticides on health and physiology of oysters in the Caloosahatchee Estuary, Florida. Ecotoxicology 17:579–590. https://doi.org/10.1007/s10646-008-0242-9
Wang G, Yinglan A, Jiang H, Fu Q, Zheng B (2015) Modeling the source contribution of heavy metals in surficial sediment and analysis of their historical changes in the vertical sediments of a drinking water reservoir. J Hydrol 520:37–51. https://doi.org/10.1016/j.jhydrol.2014.11.034
Wei H, Le Z, Shuxian L, Dan W, Xiaojun L, Lan J, XipingI M (2015) Health risk assessment of heavy metals and polycyclic aromatic hydrocarbons in soil at coke oven gas plants. Environ Eng Manag J 14(2):487–496
Westerhoff P, Prapaipong P, Shock E, HillaireauI A (2008) Antimony leaching from polyethylene terephthalate (PET) plastic used for bottled drinking water. Water Res 42:551–556. https://doi.org/10.1016/j.waters.2007.07.048
WHO (2004) Guidelines for drinking-water quality, vol 1. World Health Organization. https://books.google.com/books?hl=en&lr=&id=SJ76COTmQC&oi=fnd&pg=PR15&dq=ORGANIZATION,+W.+H.+2004.+Guidelines+for+drinkwater+quality,+World+Health+Organization.+PIP,+E.+2000.+Survey+of+bottled+drinking+water+available+in+Manoba,+Canada.+Environmental+health+perspectives,+108,+863866.&ots=V8uZqaN53a&sig=sbarXwzIYxRx-6K3RAScSj6MSJg. Accessed 1 Aug 2019
Wu B, Wang G, Wu J, Fu Q, Liu C (2014) Sources of heavy metals in surface sediments and an ecological risk assessment from two adjacent plateau reservoirs. PLoS One 9:101–124. https://doi.org/10.1371/journal.pone.0102101
Yoshida F, Hata A, Tonegawa H (1999) Itai–Itai disease and the countermeasures against cadmium pollution by the Kamioka mine. Environ Econ Policy Stud 2:215–229. https://doi.org/10.1007/BF03353912
Yu B, Wang Y, Zhou Q (2014) Human health risk assessment based on toxicity characteristic leaching procedure and simple bioaccessibility extraction test of toxic metals in urban street dust of Tianjin, China. PLoS One 9:924–959. https://doi.org/10.1371/journal.pone.0092459
Zhang N, Zang S, Sun Q (2014) Health risk assessment of heavy metals in the water environment of Zhalong Wetland. China. Ecotoxicology 23(4):518–526. https://doi.org/10.1007/s10646-014-1183-0
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Abedi Sarvestani, R., Aghasi, M. Health risk assessment of heavy metals exposure (lead, cadmium, and copper) through drinking water consumption in Kerman city, Iran. Environ Earth Sci 78, 714 (2019). https://doi.org/10.1007/s12665-019-8723-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12665-019-8723-0