Abstract
This study aimed to investigate the contamination of drinking water sources with potentially toxic metals (PTMs) together with some hydrochemical characteristics in the highly populated industrial zone of Pakistan. For this purpose, drinking (n = 40) and surface (n = 20) water samples were collected and analyzed for PTM using graphite furnace atomic absorption spectrophotometer (GFAAS, PerkinElmer-700, USA). The metals, including cadmium (Cd), chromium (Cr), nickel (Ni), lead (Pb), and zinc (Zn), showed significantly (p = 0.05) higher concentrations than their respective limits set by the World Health Organization (WHO 2011) in drinking water. The chronic daily intake (CDI) and human hazard quotient (HQ) were also evaluated. The highest daily intake through drinking water consumption was found for Ni (4.3 μg/kg/day), while lowest for Cd (0.25 μg/kg/day). The highest hazard quotient values were found for Cd (0.33) and Ni (0.29) that could be attributed to industrial wastewater discharge. Higher CDI and HQ values of Ni and Cd may cause chronic human health problems. According to the Chadha Piper diagram, the hydrochemical facies distribution indicated that water trend in the study area followed an order such as follows: Ca–Mg–Cl < Na–Cl < Ca–HCO3 < Na–HCO3. Statistical analysis using one-way ANOVA, correlation analysis, and principal component analysis (PCA) revealed that the elevated levels of PTM were attributed to industrial wastewater discharge. This study provides baseline information for policy makers and the effective management of water in populated industrialized zone.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Safe drinking water is an absolute necessary factor for acquiring sustainable development of a society, as quality water is a high requirement for the human health and other living beings (Jehan et al. 2019; Rashid et al. 2019b). Water quality depends on the composition of recharging capacity, climate, oxidation reduction reactions, ion-exchange, lithology, reactivity of bedrock, and aquifer formation, in which potentially toxic metal (PTM) transport can be characterized by complex mechanism (Ghezzi et al. 2018; Singh et al. 2017). The assessment of hydrochemical features can enhance our understanding between water and environment which provides useful information regarding the utilization and management of groundwater resources. It has an important diagnostic chemical aspect of groundwater in the hydrological system and gives information on how the different elemental fluxes are exchanged through the aquatic and terrestrial ecosystem. Various studies worked on hydrochemical features and groundwater contamination resulted from anthropogenic intervention, such as industrial and agricultural activities (Avci et al. 2018; Raju et al. 2011; Roy et al. 2018). PTMs, such as lead (Pb), nickel (Ni), cadmium (Cd), zinc (Zn), and chromium (Cr), once enter in the ecosystem, concealed for years, rendering groundwater and posed deleterious health effects (Ahmed et al. 2019; Nedelescu et al. 2017), particularly in sensitive groups (children, pregnant women, and aged people) (Hough et al. 2004). These elements accumulate in the adipose tissues of humans (Bhuiyan et al. 2010) that badly affect the body systems, like the nervous, circulatory, and immune systems. Pb is a potential carcinogenic toxic metal which produces disorders, such as kidney dysfunctions, headache, nerve damages, and abdominal pain. Ni affects the pulmonary system and causes significant dermal problems (skin allergies) in human beings. Cd damages the skeletal system and causes mental and growth retardation among children in chronic exposure. Similarly, Cr and Zn in high concentrations induce potential health problems to plants and human health. Cr negatively affects the immune system, whereas Zn causes dermal and liver problems. Human life is greatly affected by the discharge of PTMs in the water environment; therefore, due to their devastating effects on human and ecological health, there was a focus on PTMs (Ali et al. 2019; Arain et al. 2014). PTM contamination in water is of global concern due to their hazardous, non-biodegradable, and persistent nature (El-Naggar et al. 2018; Ullah et al. 2019). Estimation of human health risk associated with PTM has been highlighted by many studies (Jones et al. 2019; Magesh et al. 2017).
Water quality has been threatened by anthropogenic and geogenic sources. Anthropogenic factors like rapid growth of industrialization, urbanization, and unwise agricultural practices are constant source of contamination that affect groundwater quality (He et al. 2019; Rashid et al. 2019a). Water contamination has been triggered by urban population, excessive industrial growth, domestic effluents, and unwise agrochemical practices during the last decades (Jadoon et al. 2019; Yetiş et al. 2019). Globally, drinking water quality deterioration is a growing problem; however, the situation is worse in developing countries (Pakistan, India, Bangladesh, and Africa) due to lack of professional expertise, management system, and financial resources (Khan et al. 2019). Pakistan is a developing country and stands 80th among the list of 122 countries for potable drinking water quality (Ahmed et al. 2015). Studies revealed that about 70% of the water resources of the country have been affected by organic, inorganic, and microbial contamination (Malik et al. 2009). Assessment of surface and groundwater is useful to trace the degree of contamination and its quality (Liao et al. 2018; Saha et al. 2017). The use of different multivariate and univariate statistical techniques, indices, and health risk assessment in different water bodies has been reported earlier in different studies for analyzing huge and complex data matrices into simple dataset (Ishtiaq et al. 2018; Mahfooz et al. 2019; Rehman et al. 2018). Therefore, the objectives of the present study were the following: (1) to evaluate the physicochemical parameters in surface and groundwater along the industrial zone; (2) to conduct non-carcinogenic health risk through CDI and HQ; (3) to confirm the hydrogeochemical features and variation trends of water; and (4) to identify sources of PTM contamination using correlation analysis (CA) and principal component analysis (PCA).
Materials and methods
Profile of the study area
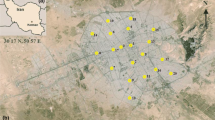
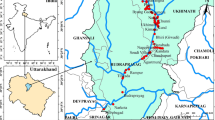
The Hattar Industrial Estate (HIE) is located in Haripur district, Khyber Pakhtunkhwa (KPK) province, Pakistan at 33°51′71 N and 72°51′8 E (Fig. 1). The study covers an area of 1063 acres, with an average altitude of 1739 ft above sea level. HIE was approved by the KPK government in 1965 (Rehman et al. 2008; Sial et al. 2006) having a large number of heavy electrical plants, iron and steel industry, food processing and chemical units, leather industry, pharmaceutical companies, and paper-manufacturing industries. The industries discharge and dump their wastewater and solid wastes into surrounding areas which finds their ways to nearby streams and natural hydrological system eventually collected in River Haro near Hassan Abdal and Wah cantonment areas, and this results in contamination of surrounding surface and groundwater (Salariya et al. 2003). According to a report published by the population census organization (PCO), 30% (tube well) of the population of Haripur district has tap water facility and the rest of the 70% generally use bore wells and dug wells for drinking purposes.
Geology and hydrogeology
The regional geology of the study area consists of different formations (Fig. 2), such as Quaternary (Q), Eocene (Eo), Mesozoic Sedimentary Rocks (Mss), Triassic (Tr), Permian (Pr), Cambrian–Ordovician (Swg), Cambrian (Cb), Paleozoic (Pz), and Peterozoic (Pt). These geological formations mostly consist of calcareous and carbonaceous phyllite, schist slate, argillite, and argillaceous meta-siltstone in the Pt settings. Mss and Pz strata are exposed to the early Cb age of Ambar formation (Nisar et al. 2018). Quaternary are composed of unconsolidated conglomerate, sandstone, siltstone, and loess. Eocene includes Jatta gypsum, Marine deposits, sandstone, and thin limestone. The Mss are mainly dolomite, sandstone, and shale. The Swg are megacrystic granite and augen gneiss, including porphyritic peraluminous granite and cordierite granite. Cb are Cambrian quartzite and schist the assemblage includes quartz, muscovite, K-feldspar, and kyanite. Pt are meta-classic and meta-carbonate sedimentary rocks (Salkhala Group, Hazar slate, Gandaf, Manki, and Sobrah formations) (Searle and Khan, 1996). The geological settings mainly control the release of PTM into the surrounding aquifers. The present research area lies in the Haripur basin that mostly consists of both alluvial and fluvial plains. Hazara slate acts as basement rocks for overlying alluvial and fluvial deposits. Haro rivers along with Nathiagali and Punjal thrusts are the main deposition agents that serve as a potential source of sediments in this area (Nisar et al. 2018).
Water sampling and analyses
Water samples (n = 60) were collected in two sets of clean and disposable polythene bottles from groundwater (dug wells, bore wells, tube wells) and surface water in August, 2016 (Fig. 1), following the standard procedures (Shah et al. 2012). One set of the bottles was acidified with a few drops of nitric acid (HNO3, 65% purity) for keeping PTM in dissolved form and inhibit their adsorption in the plastic. Before water sampling, a handheld GPS (HC Garmin) was used for the coordinate’s determination at each individual sampling point. Well samples were allowed to pump for 5 min to wash out the stagnant water and to get fresh water. Rubber bucket was used to collect water from dug well at 15–22 ft depth from the surface. The pH, electrical conductivity (EC), and total dissolved solids (TDS) were measured in situ using multi-water quality analyzer (C 6030). After sampling collection, all water samples were transported to the National Centre of Excellence in Geology (NCEG), University of Peshawar, and kept at 4 °C before further analyses.
Standard procedures were followed for the determination of physicochemical parameters in the water samples (APHA 2005). Prior to the analyses, all the water samples were filtered through Whatman No. 42 μm filter paper. The non-acidified samples were used for determination of anions, such as Cl, which were calculated via standard titration method, and NO3 and SO4, which were measured by UV–visible spectrophotometer (DR 2800, HACH Company, USA) (APHA 2005). The acid-treated water samples were analyzed for cations, i.e., calcium (Ca), magnesium (Mg), sodium (Na), and potassium (K), and PTM, such as iron (Fe), Ni, Cr, Co, Zn, Pb, and Cd, using Analyst 700 Atomic Absorption Spectrometer (Analyst Model 700 PerkinElmer, USA), having an air-acetylene flame. The limit of detection (LOD) was 1 μg/L for Cd, Ni, Pb, and Zn and 0.1 for Ca, K, Mg, and Na (Liao et al. 2018).
Reagents and standards
The purified analytical-grade (AR) chemicals were used in this research study. Double de-ionized and ultrapure water was used for the preparation of reagents and standards. The metal standard solutions were obtained from E. Merck (Germany) and used for the laboratory analysis. All the samples were run in triplicate.
Control of analytical procedure
Standard operating procedure and gravimetric analysis for each instrument have been adopted during the entire study. The atomic absorption spectrophotometer (AAS) was calibrated with standard solutions through the dilution (1000 mg/L) of the certified reference solution (Merck) of corresponding metal with deionized water, and working standards were analyzed after every 10 samples to check their precision and accuracy. A blank sample has been run for each parameter, and necessary corrections have been made to achieve accurate and precise results. All the containers and glassware used in the fields and laboratory were washed with washing detergent, rinsed with distilled water, and soaked in 10% concentrated HNO3. Percentage recoveries for the studied metals ranged from 92% (+5).
Human health risk assessment
The PTM enters to human body through ingestion, dermal (skin contact), and inhalation absorption, but the oral intake is the more critical one (ATDSR 1995). In the present study, risk assessment for PTM was made on the basis of chronic daily intake (CDI) and hazard quotient (HQ) through drinking groundwater only. CDI was calculated by the modified equation from USEPA (2011).
Where, C, DI, and BW represent the mean concentration of PTM in drinking water (μg/L), average daily intake rate (2 L/day for adult and 1 L/day for child), and body weight (72 kg for adult and 32 kg for child), respectively (Mahfooz et al. 2019). HQ was calculated by the following formula:
The RfD (reference dose) is the daily exposure of a human population to a contaminant that affects life after ingestion during a lifetime. RfD values for Cd, Cr, Ni, Pb, and Zn were 5.0 × 10−4, 1.5, 2.0 × 10−2, 3.6 × 10−2, and 3.0 × 10−1 mg/kg day, respectively (Dearfield and Moore 2005; USEPA 2000). The exposed population is assumed to be safe when HQ < 1, whereas HQ > 1 denotes a possibility of adverse health effects (Tepanosyan et al. 2017).
Statistical analysis
Descriptive statistics were conducted using Microsoft Excel 2013 and statistical package for social sciences ver. 20 (SPSS Inc., Chicago, IL, USA). The significance was defined at p < 0.05 using one-way analysis of variance (one-way ANOVA) (Aamir et al. 2016). Sigma plot (ver. 12.5, 2016 Systat Software, Inc.) was used for graphical representation. Chadha diagram and Gibbs plot were used to identify the hydrochemical feature of surface and groundwater of the area using XLSTAT software (2014 version). Geographic Information System (GIS version 10.2.2) was used to make study area and geospatial maps.
Results and discussion
Physicochemical characteristics
Statistical summary of physicochemical characteristics of surface and groundwater (bore wells, dug wells, tube wells) is summarized in Table 1. The mean value of pH was 7.2 and 6.9 in surface and groundwater, respectively. The pH was mostly acidic in groundwater, and about 64% of the samples were of acidic in nature which suggests that it has the ability to dissolve minerals and potential toxic metals which are injurious to the health in the long run (Rasool et al. 2016). The mean values of EC in surface and groundwater water were 644 and 872 μS/cm, respectively. Similarly, the TDS concentrations were found to be 315 and 366 mg/L in surface and groundwater, respectively. The presence of EC and TDS in water indicated the dissolution of ions which may be due to the gradual deposition of salts and minerals over time (Chabukdhara et al. 2017). The high mean value of EC in surface water may also be attributed to contamination with wastewater from both industrial and residential areas. TDS more than 500 mg/L in water causes uncontrolled physiological reactions affecting gastrointestinal functions and neurological setup (Shankar et al. 2008). Mean pH, EC, and TDS values were observed according to the guideline values set by WHO (2011) both in surface and groundwater. Mean concentrations of anions like Cl, HCO3, SO4, and NO3 were found lower than the recommended guideline values suggested by WHO (2011) in surface and groundwater. HCO3 was found as one of the major anions in majority of the water samples. High concentration of SO4 is associated with respiratory problems and NO3 can cause gastric cancer, goiter, methemoglobinemia, hypertension, and birth malformation (Chandrasekaran et al. 2015). Among the cations, the average concentrations of Ca, Mg, Na, and K were found within the recommended guideline values set by WHO (2011) in groundwater and surface water (Table 1).
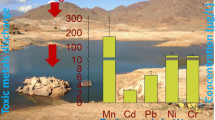
The average concentrations (μg/L) in surface water were as follows: Ni 190, Zn 120, Co 74, Cr 73., Pb 11, and Cd 5.0, and in groundwater, Ni (140.80) > Zn (76.44) > Co (51.12) > Cr (48.35) > Pb (14.31) > Cd (8.49) (Table 1). The PTM concentrations in each sampling point were summarized in Fig. 3. In both surface and groundwater, the highest concentrations were observed for Ni that surpassed the WHO drinking water guidelines (20 μg/L) for 90% of the water samples. Ni is a potential carcinogenic metal for lungs, affects the pulmonary system, and causes significant dermal problems (skin allergies) in human beings. The relative high level of Ni could be associated to the collective impact of a number of industries (e.g., textile, dying, leather, electroplating, printing, and various chemical and metallurgical industries), besides industrial input agricultural practices and Zn-based pesticides in fields that contribute to the surrounding groundwater contamination (Alves et al. 2014). The mean concentrations of Pb (11.01 μg/L) and Cd (4.96 μg/L) were observed higher in 60% and 50% of samples than the WHO (2011) guideline values of 10 and 3 μg/L, respectively. Pb is a highly carcinogenic metal and may pose several health disorders, such as kidney dysfunctions, headache, nerve damages, abdominal and pain, and cause mental and growth retardation among children in chronic exposure. Similarly, the mean concentration of Cd in surface and groundwater was higher than the recommended WHO guideline value of 3 μg/L. The accumulation of Cd in liver and kidneys can cause many pathological problems, including skeletal damages, diabetes, high blood pressure, and renal disorders (Li et al. 2018). Zn, Co, and Cr are considered as essential elements in specific amount and required for normal body functions but can cause serious health problems when taken at large doses. When Zn is taken in high dose, it can cause kidney and liver failure and affects the blood urine system. Cr in high amount affects and increases abortion and miscarriage in women (Gupta et al. 2018). The mean metal concentrations found in this study are in good agreement with the results of Zn > Cr > Pb and Cd as reported by Jabeen et al. (2014) in the groundwater of Haripur basin. The findings revealed that there were high values of PTM which are associated to industrial discharge and agricultural uses in the study area.
Health risk assessment
To obtain a more accurate risk assessment of human exposure to PTM via drinking groundwater, local inhabitants of the study area were interviewed for information regarding age, health, sex, and socioeconomic conditions. It was assessed during the interview that residents of the surrounding of HIE use water obtained from tube wells, dug wells, and bore wells for drinking and domestic purposes. The calculated CDI values for children and adults through consumption of groundwater are summarized in Fig. 4a. The highest CDI values at 4.3 and 3.91 μg/kg day for children and adults were observed for Ni through groundwater consumption, respectively. Similarly, the smallest CDI values were calculated for Cd 0.25 and 0.23 μg/kg day for children and adults through groundwater consumption, respectively. Based on the groundwater quality, the CDI values in the study area were found in a decreasing order such as follows: Ni > Zn > Cr > Pb > Cd. The CDI values calculated for toxic elements were found higher than those reported by Masood et al. (2019) in drinking water of industrial zone in Gujrat, Pakistan. The mean, minimum, and maximum concentrations of PTM, including, Cd, Cr, Cu, Pb, and Ni, conducted in the Hattar industrial estate were found higher than those in the Gujrat industrial zone and Haripur basin indicating the groundwater quality in the present study area is worse than Gujrat and Haripur basin, Pakistan. The concentrations of Cu, Pb, and Ni were found below their detection limits of 2, 10, and 20 μg/L, respectively (WHO 2011) in Gujrat and Haripur basin, Pakistan. Thus, the study of groundwater in the present study is more important.
Figure 4b summarizes the HQ values of Zn, Ni, Cr, Pb, and Cd that were 0.013, 0.293, 1.48 × 10−3, 9.36 × 10−3, and 0.304 for children and 0.011, 0.126, 1.35 × 10−3, 8.50 × 10−3, and 0.276 for adults, respectively, through groundwater consumption. Zn possesses the least non-carcinogenic risk factor due to its relatively high reference dose. The HQ index values of toxic elements in groundwater were found in the order of Cd > Ni > Pb > Zn > Cr and Cd > Ni > Zn > Pb > Cr, respectively. The risk analysis revealed that groundwater consumption is not safe for the community in the study area especially the children which are more prone to the problem. The highest HQ values of Cd and Ni could be attributed to contamination provided by industrial, agricultural, and sewage release. The HQ values for all PTM in this study were found higher than the values reported by Masood et al. (2019) and Jabeen et al. (2014).
Hydrogeochemical facies of water
Chadha (1999) presented a detailed classification of water hydrogeological data taking the difference between anions and cations (meq/L), inverse percentage relation of (Ca + Mg) and (Na + K) (Chadha 1999). The Chadha (1999) classification of groundwater is an advanced version of Piper (1944). It is considered highly useful to estimate hydrogeochemistry of aquifers based on abundance of high selected suit of anions and cations in water. Four fields of diagram containing four different types of hydrogeochemical facies have been obtained (Fig. 5). Detailed hydrogeochemical investigations have shown that the water of the study area is mostly Na-HCO3 type. In the study area, 56.6% (n = 34) of the water samples collected were Na-HCO3 type, approving that the geochemical profile of the study area showed a weakening in Ca content with increase of Na. Na is one of the extremely mobile cations that dissociates due to cation exchange processes and weathering of silicate minerals and halite. Carbonate-enriched sedimentary rocks are important natural sources of HCO3 in water (BIS 2004). Of the remaining samples, 22%, including eight groundwater (n = 8) and five surface water (n = 5), are Ca-HCO3 type, which is formed by the reactions of CO2 and CaCO3 bearing mineral in the recharge zones of rainwater area (Raj and Shaji 2017). Out of all the water samples, 19% fall in the category of Na–Cl type and 1% in Ca–Mg–Cl type. The hydro-facies distribution indicated that the trend in the water type of the study area followed an order such as follows: Ca–Mg–Cl < Na–Cl < Ca–HCO3 < Na–HCO3 (Fig. 5).
Chadha diagram showing classification of water samples of the study areas after Chadha (1999). Field 1, Ca–HCO3− type waters, reflecting recharge and weathering. Field 2, Ca2+–Mg2+–Cl− type, waters. Field 3. Na–Cl− type waters, reflecting evaporation. Field 4: Na–HCO3− type waters, reflecting base-ion exchange
Gibbs diagram
Several factors, including bedrock mineralogy, groundwater regime, and climatic conditions, control basic hydrochemistry. Water quality data were designed on Gibbs (1970) plot to infer the leading control(s) on water type and quality by plotting TDS concentration against the weight ratios of Na/(Na + Ca) for cations and the weight ratios of Cl/ (Cl + HCO3) for anions (Gibbs 1970). However, to some extent, the Gibbs diagram is unable to assess the impact of anthropogenic input on groundwater chemistry, but it does not necessarily mean that groundwater formation is totally free from human intervention (Li et al. 2016). According to Li et al. (2019), human impacts can directly alter chemical composition of groundwater or indirectly by accelerating water–rock interaction by altering hydrodynamic conditions that ultimately change groundwater intensity. Several researches have followed this type of chemical relationship to infer the water type in different parts of Pakistan (Jehan et al. 2019; Rashid et al. 2018; Rashid et al. 2019b). Three distinct types of fields are recognized by Gibbs diagram, such as rock dominance, precipitation dominance, and evaporation dominance. Figure 6 shows the chemistry of surface and groundwater which indicates that most of the water samples were dominantly controlled by rock–water interaction, with few reflections from the precipitation control rather than evaporation. This identifies that the geochemical control of the studied water is mainly from weathering of rocks (CaHCO3) and associated minerals.
(a, b) Mechanism controlling the groundwater or rock, precipitation, and evaporation dominance. Plots of (a) TDS versus (Na+/Na+ + Ca+) and (b) Cl−/Cl− + HCO3) (Gibbs 1970)
Statistical analysis
One-way analysis of variance (one-way ANOVA) was used for the statistical comparison to test the means among two or more groups, under the assumption that the sample population is normally distributed (Chen et al. 2017). A one-way ANOVA was performed to determine the variability of the water quality variables within and between the water sources (Table 2). The one-way ANOVA test revealed highly significant mean variations between Zn, Ni, Co, Fe, Cr, Pb, and Cd that behave contrarily to the average metal concentrations in groundwater sources. In addition, each metal varied differently for an individual location in the study area. The post hoc test (Tukey) indicates that surface water is significantly contaminated than groundwater. The concentrations of selected parameters were found significantly (p < 0.05) for Zn, Co, Fe, and Cd in surface and groundwater.
The inter-elemental relationship or Pearson’s correlation analysis gives us valuable and interesting information about the sources and pathways of PTM (Micó et al. 2006). Parameters significantly correlated at 0.01 and 0.05 levels are written as bold (Tables 3, 4). In the surface water collected from the study area, it showed a very significant correlation for some attributes, notably for the following: pH–Ni (r = 0.68), TDS–Pb (r = 0.60), HCO3–Na (r = 0.90), HCO3–Cl (r = 0.86), Cl–SO4 (r = 0.71), SO4–Na (r = 0.62), Zn–Ni (r = 0.60), NO3–Ca (r = 0.72), Ca–Mg (r = 0.60), Cl–Cd (r = 0.68), Na–Pb (r = 0.60), and Pb–Cd (r = 0.81), whereas some of the pairs showed negative correlation, such as pH–TDS (r = − 0.72), TDS–Ni (r = − 0.74), Na+–Ca (r = − 0.89), and Na–Mg (r = − 0.60), as shown in Table 3. The correlation analysis shown in this study is in good agreement with the study conducted by Chabukdhara et al. (2017) and Jabeen et al. (2014). The correlation in various components suggested significant contribution from multiple anthropogenic sources like improper disposal of wastes, agricultural activity, improper sanitation, discharge of industrial effluents, and organic decomposition in the study area (Howladar 2017; Kumar et al. 2009). In the case of groundwater samples, positive Pearson’s correlation matrices were observed for the following: HCO3–Cl (r = 0.50), HCO3–Na (r = 0.87), Cl–SO4 (r = 0.52), SO4–Na (r = 0.59), and NO3–Ca (r = 0.83) as given in Table 4.
The arrangement of different parameters for individual association for surface and groundwater was obtained by PCA. PCA constructs significant loading factors and variable scores. The PCA analyses of groundwater and surface water data were considered significant with eigenvalue > 1.0 and factor loading accumulative at 70%. PCA can be calculated through the following equation:
Where, Z, α, i, J, and m are the component score, component loading, original variables, component number, and number of the original variables, respectively. The loading factors established the significance of water variables.
Four principal component (PCs) were extracted from the available dataset accounting 74.05% of the total variance with eigen value greater than 1 for surface water, whereas, seven factors with probability of the total variance at 71.01% with eigen value > 1 were obtained for groundwater data (Supplementary Material, Table S1). For surface water samples, F1 exhibits 42% of the total and 74.05% with positive loading on TDS, Cl, Na, Pb, and Cd. This factor showed strong association (r = 0.78 to 0.92) of TDS, Cl, Na, Pb, and Cd for surface water. Due to high association between Na and Cl, F1 which explains high variance in the data set may be defined as hardness and salinity factor (Omonona et al. 2019). High TDS value could strongly be attributed to the presence of carbonated rocks in the study area and upon the concentration of Na and Cl while Pb and Cd were due to the recharging capacity of aquifers by contaminated streams (Jabeen et al. 2014; Omonona et al. 2019). F2 exhibits 13.24% of the total variance with positive loading on HCO3, wherein Ca and Zn indicate strong association (r = 0.63 to 0.39). F2 attributed to the geochemical processes especially influenced by anions and cation, and the contamination could be resulted from ultramafic rock erosion and steel industry effluents (Rashid et al. 2019a). F1 and F2 factor contribution via varimax rotation for surface water is shown in Fig. 7a. Infiltration of contaminated surface water creates pollution of groundwater (Guo et al. 2017). For groundwater samples, F1 exhibits 14.51% of the total variance of 70.15 with positive loadings on pH, Ca, Na, and Cd indicating strong association (r = 0.32 to 0.56). F1 indicates the strength of physicochemical parameters and suggests that these loadings are mainly from geogenic sources (Egbueri 2019). F2 shows 13.17% of the total variance with high loading on Zn and Cr. This factor suggests strong association (r = 0.67 to 0.72) of EC, Zn, and Cr for ground water. The contamination of Zn and Cr could be attributed by the weathering of mafic and ultramafic rocks, mineral dissolution, and industrial emissions that lead to the potential source of groundwater contamination in the study area (Rashid et al. 2019a). Zn is used as anti-corrosion agent and is used for coating pipeline to protect it from corrosion whereas Cr is used in the electronic manufacturing unit. F1 and F2 factors’ contribution via varimax rotation for groundwater is shown in Fig. 7b.
Conclusion
The concentrations of PTM in this study exceeded the WHO guideline values in both surface and groundwater samples. The HQ values were found (< 1) indicating no health risk to the exposed population. The outcomes of this research proved that the water of the study area was rapidly and continuously influenced by industrialization and urbanization. However, if current practices of wastewater release in the environment continued, then the exposed population will be at higher risk in the near future. The Chadha diagram indicated that hydrochemistry of the area was dominated by Na–HCO3 water type. The Gibbs diagram showed that the water samples fall in the rock–water interaction dominance field and the hydrogeochemistry of water is evolved by the continuous interaction of rocks with water. Statistical analysis, like PCA, confirmed that the contaminated surface water is strongly influencing the groundwater in the study area. Furthermore, correlation analysis showed that Zn, Ni, Pb, and Cd may originate from electroplating and metal-processing industries, agricultural practices, domestic and industrial discharge. Finally, it is concluded the both surface and groundwater in the study area posed a chronic health risk. Therefore, it is suggested that water from contaminated sites should not be used for drinking and domestic purposes. Unwise disposal methods and unplanned discharge from urban and industrial sectors should be banned to avoid overdeterioration of water sources in the study area. Proper measuring techniques, like treatment plants and immediate response team, could safeguard the drinking water sources in the study area.
References
Aamir M, Khan S, Nawab J, Qamar Z, Khan A (2016) Tissue distribution of HCH and DDT congeners and human health risk associated with consumption of fish collected from Kabul River, Pakistan. Ecotoxicol Environ Saf 125:128–134
Ahmed I, Tariq N, Al Muhery A (2019) Hydrochemical characterization of groundwater to align with sustainable development goals in the Emirate of Dubai, UAE. Environ Earth Sci 78:44
Ahmed T, Pervez A, Mehtab M, Sherwani SK (2015) Assessment of drinking water quality and its potential health impacts in academic institutions of Abbottabad (Pakistan). Desalin Water Treat 54:1819–1828
Ali L, Rashid A, Khattak SA, Zeb M, Jehan S (2019) Geochemical control of potential toxic elements (PTEs), associated risk exposure and source apportionment of agricultural soil in Southern Chitral, Pakistan. Microchem J 147:516–523
Alves RI, Sampaio CF, Nadal M, Schuhmacher M, Domingo JL, Segura-Muñoz SI (2014) Metal concentrations in surface water and sediments from Pardo River, Brazil: human health risks. Environ Res 133:149–155
APHA (2005) Standard methods for the examination of water and wastewater, vol 2. American Public Health Association
Arain MB, Ullah I, Niaz A, Shah N, Shah A, Hussain Z, Tariq M, Afridi HI, Baig JA, Kazi TG (2014) Evaluation of water quality parameters in drinking water of district Bannu, Pakistan: multivariate study. Sustainability Water Qual Ecol 3:114–123
ATDSR (1995) Agency for toxic substances and disease registry. In: Toxicological profile for polycyclic aromatic hydrocarbons (PAHs), (update) PB/95/264370. US Department of Health and Human Services, Atlanta
Avci H, Dokuz UE, Avci AS (2018) Hydrochemistry and groundwater quality in a semiarid calcareous area: an evaluation of major ion chemistry using a stoichiometric approach. Environ Monit Assess 190:641
Bhuiyan MAH, Islam MA, Dampare SB, Parvez L, Suzuki S (2010) Evaluation of hazardous metal pollution in irrigation and drinking water systems in the vicinity of a coal mine area of northwestern Bangladesh. J Hazard Mater 179:1065–1077
BIS, 2004. Drinking Water Standards IS 10500. Technical Report. Bureau of Indian Standards..
Chabukdhara M, Gupta SK, Kotecha Y, Nema AK (2017) Groundwater quality in Ghaziabad district, Uttar Pradesh, India: multivariate and health risk assessment. Chemosphere 179:167–178
Chadha D (1999) A proposed new diagram for geochemical classification of natural waters and interpretation of chemical data. Hydrogeol J 7:431–439
Chandrasekaran A, Ravisankar R, Harikrishnan N, Satapathy K, Prasad M, Kanagasabapathy K (2015) Multivariate statistical analysis of heavy metal concentration in soils of Yelagiri Hills, Tamilnadu, India—spectroscopical approach. Spectrochim Acta A Mol Biomol Spectrosc 137:589–600
Chen et al (2017) Human health risk assessment of contaminants in drinking water based on triangular fuzzy numbers approach in Yinchuan City, Northwest China. Exp Health:1–12
Dearfield KL, Moore MM (2005) Use of genetic toxicology information for risk assessment. Environ Mol Mutagen 46:236–245
Egbueri JC (2019) Evaluation and characterization of the groundwater quality and hydrogeochemistry of Ogbaru farming district in southeastern Nigeria. SN Applied Sciences 1:851
El-Naggar A, Shaheen SM, Ok YS, Rinklebe J (2018) Biochar affects the dissolved and colloidal concentrations of Cd, Cu, Ni, and Zn and their phytoavailability and potential mobility in a mining soil under dynamic redox-conditions. Sci Total Environ 624:1059–1071
Ghezzi L, D'Orazio M, Doveri M, Lelli M, Petrini R, Giannecchini R (2018) Groundwater and potentially toxic elements in a dismissed mining area: thallium contamination of drinking spring water in the Apuan Alps (Tuscany, Italy). J Geochem Explor
Gibbs RJ (1970) Mechanisms controlling world water chemistry. Science 170:1088–1090
Guo X, Zuo R, Shan D, Cao Y, Wang J, Teng Y, Fu Q, Zheng B (2017) Source apportionment of pollution in groundwater source area using factor analysis and positive matrix factorization methods. Hum Ecol Risk Assess: Int J 23:1417–1436
Gupta N, Yadav KK, Kumar V, Kumar S, Chadd RP, Kumar A (2018) Trace elements in soil-vegetables interface: translocation, bioaccumulation, toxicity and amelioration—a review. Sci Total Environ
He X, Wu J, He S (2019) Hydrochemical characteristics and quality evaluation of groundwater in terms of health risks in Luohe aquifer in Wuqi County of the Chinese Loess Plateau, northwest China. Hum Ecol Risk Assess: Int J 25:32–51
Hough RL, Breward N, Young SD, Crout NMJ, Tye AM, Moir AM, Thornton I (2004) Assessing potential risk of heavy metal exposure from consumption of home-produced vegetables by urban populations. Environ Health Perspect 112:215–221
Howladar MF (2017) An assessment of surface water chemistry with its possible sources of pollution around the Barapukuria Thermal Power Plant Impacted Area, Dinajpur, Bangladesh. Groundw Sustain Dev 5:38–48
Ishtiaq M, Jehan N, Khan SA, Muhammad S, Saddique U, Iftikhar B (2018) Potential harmful elements in coal dust and human health risk assessment near the mining areas in Cherat, Pakistan. Environ Sci Pollut Res 25:14666–14673
Jabeen et al (2014) Physico-chemical parameters of surface and ground water and their environmental impact assessment in the Haripur Basin, Pakistan. J Geochem Explor 138:1–7
Jadoon S, Hilal Z, Ali M, Muhammad S (2019) Potentially toxic elements in drinking water and associated health risk assessment in Abbottabad city, northern Pakistan. Desalin Water Treat 151:392–402
Jehan S, Khan S, Khattak SA, Muhammad S, Rashid A, Muhammad N (2019) Hydrochemical properties of drinking water and their sources apportionment of pollution in Bajaur agency, Pakistan. Measurement 139:249–257
Jones BG, Alyazichi YM, Low C, Goodfellow A, Chenhall BE, Morrison RJ (2019) Distribution and sources of trace element pollutants in the sediments of the industrialised Port Kembla Harbour, New South Wales, Australia. Environ Earth Sci 78:357
Khan SA, Abeer N, Hussain SZ, Muhammad S, Jawad F, Khan T (2019) Potentially toxic elements contamination in water and evaluation for risk assessment in the Rawalpindi, Pakistan. Desalin Water Treat 159:327–337
Kumar M, Kumari K, Singh UK, Ramanathan A (2009) Hydrogeochemical processes in the groundwater environment of Muktsar, Punjab: conventional graphical and multivariate statistical approach. Environ Geol 57:873–884
Li G, Khan S, Ibrahim M, Sun T-R, Tang J-F, Cotner JB, Xu Y-Y (2018) Biochars induced modification of dissolved organic matter (DOM) in soil and its impact on mobility and bioaccumulation of arsenic and cadmium. J Hazard Mater 348:100–108
Li P, Wu J, Qian H, Zhang Y, Yang N, Jing L, Yu P (2016) Hydrogeochemical characterization of groundwater in and around a wastewater irrigated forest in the southeastern edge of the Tengger Desert, Northwest China. Expo Health 8:331–348
Li P, Tian R, Liu R (2019) Solute geochemistry and multivariate analysis of water quality in the Guohua phosphorite mine, Guizhou Province, China. Expo Health 11:81–94
Liao F, Wang G, Shi Z, Huang X, Xu F, Xu Q, Guo L (2018) Distributions, sources, and species of heavy metals/trace elements in shallow groundwater around the Poyang Lake, East China. Expo Health 10:211–227
Magesh N, Chandrasekar N, Elango L (2017) Trace element concentrations in the groundwater of the Tamiraparani river basin, South India: insights from human health risk and multivariate statistical techniques. Chemosphere 185:468–479
Mahfooz Y, Yasar A, Sohail MT, Tabinda AB, Rasheed R, Irshad S, Yousaf B (2019) Investigating the drinking and surface water quality and associated health risks in a semi-arid multi-industrial metropolis (Faisalabad), Pakistan. Environ Sci Pollut Res:1–13
Malik AH, Khan ZM, Mahmood Q, Nasreen S, Bhatti ZA (2009) Perspectives of low cost arsenic remediation of drinking water in Pakistan and other countries. J Hazard Mater 168:1–12
Masood et al (2019) Health risk assessment of arsenic and other potentially toxic elements in drinking water from an industrial zone of Gujrat, Pakistan: a case study. Environ Monit Assess 191:95
Searle Michael P, Khan, MA (1996): Tectonic map of North Pakistan
Micó C, Recatalá L, Peris M, Sánchez J (2006) Assessing heavy metal sources in agricultural soils of an European Mediterranean area by multivariate analysis. Chemosphere 65:863–872
Nedelescu M, Baconi D, Neagoe A, Iordache V, Stan M, Constantinescu P, Ciobanu A-M, Vardavas AI, Vinceti M, Tsatsakis AM (2017) Environmental metal contamination and health impact assessment in two industrial regions of Romania. Sci Total Environ 580:984–995
Nisar UB, Khan MR, Khan S, Farooq M, Mughal MR, Ahmed KA, Razzaq SS, Niaz A (2018) Quaternary paleo-depositional environments in relation to ground water occurrence in lesser Himalayan region, Pakistan. J Himal Earth Sci 51:99–112
Omonona OV, Amah JO, Olorunju SB, Waziri SH, Ekwe AC, Olofinlade SW (2019) Hydrochemical characteristics and quality assessment of groundwater from fractured Albian carbonaceous shale aquifers around Enyigba-Ameri, southeastern Nigeria. Environ Monit Assess 191:125
Piper AM (1944) A graphic procedure in the geochemical interpretation of water-analyses. EOS Trans Am Geophys Union 25:914–928
Raj D, Shaji E (2017) Fluoride contamination in groundwater resources of Alleppey, southern India. Geosci Front 8:117–124
Raju N, Shukla UK, Ram P (2011) Hydrogeochemistry for the assessment of groundwater quality in Varanasi: a fast-urbanizing center in Uttar Pradesh, India. Environ Monit Assess 173:279–300
Rashid A, Guan D-X, Farooqi A, Khan S, Zahir S, Jehan S, Khattak SA, Khan MS, Khan R (2018) Fluoride prevalence in groundwater around a fluorite mining area in the flood plain of the River Swat, Pakistan. Sci Total Environ 635:203–215
Rashid A, Khan S, Ayub M, Sardar T, Jehan S, Zahir S, Khan MS, Muhammad J, Khan R, Ali A, Ullah H (2019a) Mapping human health risk from exposure to potential toxic metal contamination in groundwater of Lower Dir, Pakistan: application of multivariate and geographical information system. Chemosphere 225:785–795
Rashid A, Khattak SA, Ali L, Zaib M, Jehan S, Ayub M, Ullah S (2019b) Geochemical profile and source identification of surface and groundwater pollution of District Chitral, Northern Pakistan. Microchem J 145:1058–1065
Rasool A, Xiao T, Farooqi A, Shafeeque M, Masood S, Ali S, Fahad S, Nasim W (2016) Arsenic and heavy metal contaminations in the tube well water of Punjab, Pakistan and risk assessment: a case study. Ecol Eng 95:90–100
Rehman U, Khan S, Muhammad S (2018) Associations of potentially toxic elements (PTEs) in drinking water and human biomarkers: a case study from five districts of Pakistan. Environ Sci Pollut Res 25:27912–27923
Rehman W, Zeb A, Noor N, Nawaz M (2008) Heavy metal pollution assessment in various industries of Pakistan. Environ Geol 55:353–358
Roy K, Karim MR, Akter F, Islam MS, Ahmed K, Rahman M, Datta DK, Khan MSA (2018) Hydrochemistry, water quality and land use signatures in an ephemeral tidal river: implications in water management in the southwestern coastal region of Bangladesh. Appl Water Sci 8:78
Saha N, Rahman MS, Ahmed MB, Zhou JL, Ngo HH, Guo W (2017) Industrial metal pollution in water and probabilistic assessment of human health risk. J Environ Manag 185:70–78
Salariya A, Rehman Z, Ashraf M (2003) Effect of polluted water on accumulation of heavy metals in commonly consumed vegetables. J Chem Soc Pak 25:161–165
Shah M, Ara J, Muhammad S, Khan S, Tariq S (2012) Health risk assessment via surface water and sub-surface water consumption in the mafic and ultramafic terrain, Mohmand agency, northern Pakistan. J Geochem Explor 118:60–67
Shankar B, Balasubramanya N, Reddy MM (2008) Impact of industrialization on groundwater quality—a case study of Peenya industrial area, Bangalore, India. Environ Monit Assess 142:263–268
Sial R, Chaudhary M, Abbas S, Latif M, Khan A (2006) Quality of effluents from Hattar industrial estate. J Zhejiang Univ Sci B 7:974–980
Singh CK, Kumar A, Shashtri S, Kumar A, Kumar P, Mallick J (2017) Multivariate statistical analysis and geochemical modeling for geochemical assessment of groundwater of Delhi, India. J Geochem Explor 175:59–71
Tepanosyan G, Maghakyan N, Sahakyan L, Saghatelyan A (2017) Heavy metals pollution levels and children health risk assessment of Yerevan kindergartens soils. Ecotoxicol Environ Saf 142:257–265
Ullah R, Muhammad S, Jadoon IA (2019) Potentially harmful elements contamination in water and sediment: evaluation for risk assessment and provenance in the northern Sulaiman fold belt, Baluchistan, Pakistan. Microchem J 147:1155–1162
USEPA (2000) Arsenic occurrence in public drinking water supplies. US Environmental Protection Agency, Washington, D.C.
USEPA (2011) Volume I: human health evaluation manual (part E, supplemental guidance for dermal risk assessment). In: Risk assessment guidance for superfund EPA/540/R/99
WHO (2011) Guidelines for drinking-water quality. WHO chronicle 38:104–108
Yetiş R, Atasoy AD, Yetiş AD, Yeşilnacar Mİ (2019) Hydrogeochemical characteristics and quality assessment of groundwater in Balikligol Basin, Sanliurfa, Turkey. Environ Earth Sci 78:331
Acknowledgments
The authors acknowledged the financial support of project # 20–28/NRPU/R&D/HEC/10/712 received from Higher Education Commission and the technical support of the National Centre of Excellence (NCE) in Geology, University of Peshawar, Pakistan.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
All the authors declare that they have no conflict of interest.
Additional information
Responsible Editor: Philippe Garrigues
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(DOCX 20 kb)
Rights and permissions
About this article
Cite this article
Jehan, S., Khattak, S.A., Muhammad, S. et al. Human health risks by potentially toxic metals in drinking water along the Hattar Industrial Estate, Pakistan. Environ Sci Pollut Res 27, 2677–2690 (2020). https://doi.org/10.1007/s11356-019-07219-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-019-07219-y