Abstract
Research on the hydrochemical characteristics of groundwater can help to elucidate its evolution and distribution, as well as other hydrogeological conditions. The present study investigated the current status of groundwater hydrochemistry and nitrogen contamination in Yinchuan area, Yinchuan basin of northwest China. Piper and Gibbs diagrams were constructed and multivariate statistical methods were used to explore the groundwater hydrochemical characteristics and its controlling factors, as well as nitrogen contamination of groundwater and its sources. The results demonstrated that phreatic water was moderately salty with a large variation of TDS and major ion concentrations, while the confined water was mainly fresh water with TDS less than 1000 mg/L. Phreatic water was mainly HCO3·Cl–Na·Mg and HCO3·SO4–Na·Mg types, while the confined water was HCO3·Cl–Na·Ca and HCO3·SO4–Na·Ca types. The major ion chemistry of the confined water is governed by water–rock interactions, while evaporation plays a key role in phreatic water chemistry formation. Furthermore, the concentration of NO3− in phreatic water is significantly higher than that of confined water, and the opposite was observed for NH4+. The concentrations of NO3− and NH4+ exhibited an increasing tendency in groundwater in the last 26 years. The nitrogen sources in phreatic water involved human activities like nitrogen fertilizer application in agriculture, and industrial and domestic sewage. In confined aquifer, the higher NH4+ concentration is comprehensively affected by hydrogeological conditions, leakage of phreatic water and an anaerobic environment. Overall, the results of the present study are helpful for the groundwater protection in Yinchuan area, and will contribute to the effective management and reasonable development of groundwater resources in Yinchuan basin.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Groundwater is a vital natural resource for daily domestic demands and socio-economic development, and has supported extensively the sustainable living of humans, the development of agricultures and industries (Chen et al. 2017; Gao et al. 2012; Li and Qian 2018a, b; Li et al. 2018a, b; Zabala et al. 2016; Zhang et al. 2018). The groundwater is particularly important in arid and semiarid large river basins where population is usually concentrated and agricultures and industries are more rapidly developed. Shortages in water resources and the contamination of shallow groundwater seriously restrict economic development in many river basins such as in Yinchuna basin, northwest China (Adimalla et al. 2019; Chen et al. 2017; Vega et al. 1998). With the rapid population increase and economic expansion, the gap between water supply and demand for fresh groundwater is widening (Qian and Li 2011; Zhang et al. 2014), while groundwater pollution worsens this situation (Heaton et al. 2012; Li et al. 2017a, b). Therefore, in the past few decades, studies on the hydrochemical characteristics and contamination of groundwater have received extensive attention from scholars (He and Wu 2019; He et al. 2019; Li et al. 2014a, b; Wu et al. 2015, 2017).
Many studies on groundwater quality and chemical characteristics have been carried out (Kumar et al. 2006; Li et al. 2016a, b, 2018c, d; Zhang et al. 2018). Lithology, geomorphology, hydrogeological conditions and human activities are all known factors affecting the hydrogeochemical characteristics of groundwater. Interaction with surface water, mineral dissolution, redox reaction and ion exchange are the important hydrogeochemical processes that control groundwater quality (Djorfi et al. 2017; Qian and Li 2011; Kumar et al. 2006; Li et al. 2018c). The presence of nitrogen pollutants, including NO3−, NO2− and NH4+, in groundwater is one of most frequently occurring types of pollution in northern China. Statistical data indicate that the nitrogen concentrations are positively correlated with anthropogenic activities and regional socio-economic indices, such as the GDP, population density, use of fertilizers and industrial input. The nitrogen pollutants (NO3−, NO2− and NH4+) can interconvert with each other in soil and groundwater systems, and this conversion is mainly controlled by the chemical characteristics of groundwater. Many studies have verified that elevated NO2− concentration can induce methemoglobinemia, result in the formation of the carcinogenic and mutagenic nitrosamines, and consequently cause digestive system cancer (Gao et al. 2012; Hu et al. 2005; Li et al. 2019a). Long-term drinking of groundwater with excessive nitrate concentration is detrimental to the human health, affecting the sustainable living of local residents (Li and Wu 2019a, b). At the same time, the concentrations of the nitrogen pollutants are greatly affected by the chemical characteristics of groundwater as well as the hydrogeological conditions (Han et al. 2015; Li et al. 2014b; Qian et al. 2012). Therefore, the presence of the nitrogen pollutants in groundwater is inevitably related to its hydrogeochemistry.
Yinchuan is the capital city of Ningxia Hui Autonomous Region, which is a densely populated and traditional agricultural area, situated in the continental-arid climate region of northern temperature zone (Qian et al. 2014; Sun et al. 2007). The local population relies heavily on confined water for the daily domestic demands, irrigation and municipal water supply, owing to its good water quality. (Chen et al. 2018; Qian and Li 2011; Li et al. 2018a). With long-term withdrawals, a cone of depression has been formed in the confined aquifer. Previous studies by Qian et al. (2012, 2013, 2014) examined the recharge, discharge, and the hydrochemical and isotopic characteristics of the phreatic water and the first confined water in Yinchuan plain. The results of these studies are helpful for defining the groundwater flow systems and water cycle in the area, and are meaningful for further studies. In addition, Chen et al. (2015, 2016, 2018) investigated the presence of the nitrogen pollutants in groundwater in Yinchuan plain and concluded that anthropogenic sources are the major contributors to the nitrogen contamination. They suggested that the cone of depression of groundwater in Yinchuan area exhibited a growing trend from 1991 to 2010, its expansion and the increases of groundwater head between phreatic and confined aquifers may deteriorate the water quality in the confined aquifer. Although several studies regarding nitrogen pollution in the soil and groundwater have been carried out in Yinchuan plain, the hydrochemical characteristics of nitrogen polluted groundwater based on long-term monitoring in Yinchuan area have rarely been studied. Previous analytical results revealed that the concentration of NH4+ in the confined water is higher than that in the phreatic water (Qian et al. 2014; Sun et al. 2007). All these make it more difficult to take effective measures against nitrogen contamination.
The purposes of the present paper were to assess the hydrochemical characteristics of groundwater in Yinchuan area, and its controlling factors. Based on this, the presence of the nitrogen pollutants in groundwater in the study area was discussed. The causes and mechanisms of the pollution characteristics of nitrogen in the phreatic and confined aquifers were also analyzed.
Study area
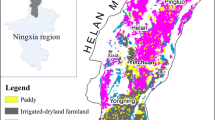
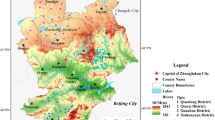
The study area is located in the middle part of Yincuan plain, which is situated in the northern area of Ningxia Hui Autonomous Region in northwest China, covering an area of 555 km2, situated between 106°02′ and 106°20′E, 38°23′–38°37′N. The study area covers Xixia, Jinfeng and Xingqing Districts of Yinchuan City (Fig. 1), and its elevation ranges at 1100–1200 m above mean sea level. The landform slopes from a leaning pluvial plain at the foot of Helan Mountain in the west, the pluvial–alluvial plain of the Yellow River in the middle, and the alluvial–lacustrine plain and alluvial flat in the east with a zonal distribution. The hydraulic gradient is between 1 and 3‰ (Fang 2009; Zhang et al. 2014). Traditionally, most of the study area consists of farmland and has been gradually urbanized in the last few decades. Many industrial pollution sources exist in north of the study area, with Fe and Mn levels in groundwater exceeding the national standard, indicating a reducing environment (Zhang 2014, 2015).
The study area is characterized as an arid climate with intense evaporation and little precipitation. The annual average precipitation is 186.7 mm and is mainly concentrated from June to September, while the potential evaporation is 1834.44 mm, which is approximately ten times the precipitation (Qian et al. 2013, 2014).
The aquifers are found within a depth of 260 m, and can be divided into the following three groups: phreatic aquifer, first confined aquifer and second confined aquifer, respectively. These aquifers are separated by aquitards, which are normally continuous and have a thickness of 3–10 m. The phreatic aquifer is composed of mountain pluvial deposits, and alluvial sands and gravel form the Yellow River, while the confined aquifers are covered by lacustrine and alluvial deposits (Fig. 2).
The hydrogeological section of A–A (a) and B–B (b). (See Fig. 1 for location)
In most regions, the phreatic water table depth is 1–3 m. The phreatic water movement is greatly affected by topography, lithology, distribution of channels and ditches, and groundwater exploitation. As observed in the contour map of the phreatic water table (Fig. 3a), this water flows mainly from west to east with a hydraulic gradient of 0.8–6.7‰, and the flow directions and conditions vary in different regions. In the southwestern area, the phreatic water flows to the northeast with a relatively larger hydraulic gradient. In the middle and eastern area, the hydraulic gradient is significantly decreased to 0.8–1.2‰, indicating that the movement of phreatic water slows down in this area.
As shown in Fig. 3b, c, the hydraulic gradient of the confined aquifer is between 1.5 and 3.8‰ in the western parts of the study area, while the flow direction is toward the center of the cone of depression. Due to long-term extraction, the water head of the phreatic aquifer is higher than that of the confined aquifer, leading to the leakage of phreatic water into the confined aquifer. The maximum hydraulic head difference between the two aquifers reaches up to 25 m.
Groundwater is mainly recharged by channel leakage and the irrigation infiltration, which account for 90% of the total groundwater recharge (Fang 2009; Qian et al. 2012). Furthermore, the confined aquifer is recharged by the phreatic water leakage and the lateral flow, accounting for 54.03% and 45.97% of the total recharge, respectively (Zhang 2014). The groundwater discharge includes evapotranspiration, discharge to drainage ditches and artificial exploitation, accounting for 62.44%, 33.49% and 4.06% of the total groundwater discharge, respectively.
Materials and methods
Sample description and preparation
Water samples from the phreatic and confined aquifers were collected from 47 wells in the study area from 1991 to 2016 (Fig. 1), including 21 phreatic and 26 confined water samples. The water quality was analyzed twice a year, before spring and autumn irrigation period. All samples were collected from private, irrigation, and monitoring wells in the region. Each of the groundwater samples was analyzed for 18 parameters including water temperature(T), pH, COD, TDS, Tfe, TH, HCO3−, Cl−, SO2−4, PO3−4, Ca2+, Mg2+, Na+, K+, Mn2+ and nitrogen compounds (NO3−, NO2− and NH4+). Water temperature and pH were measured in the field with a portable Hanna pH meter.
Analysis result samples were checked for accuracy by calculating the percent charge balance errors (%CBE) with the following equation:
In the calculation, all cations and anions are expressed as milliequivalents per liter. The relative error (%CBE) for all samples was less than ± 5%, confirmed the reliability of the analysis results. Therefore, all the 47 samples were used for analysis. A total of 17 groundwater quality indicators except temperature were used for multivariate statistical analysis. The original data were standardized to avoid misclassification due to wide differences in the dimensions of data (Hossain et al. 2013; Everitt 1980; Peters 1987). The entire multivariate statistical process was performed using the statistical analysis software SPSS (version19.0).
Analytical procedures
In the present study, groundwater hydrochemical characteristics were investigated using statistical analysis, and Piper and Gibbs diagrams were constructed based on data collected in the study area in 2016. These diagrams are widely used to study the main mechanisms governing water evolution. Piper diagrams are commonly used to reveal the groundwater types, while Gibbs diagrams are used to quantitatively analyze groundwater evolution, which is indicated by three main mechanisms: evaporation dominance, rock dominance, and precipitation dominance (Qian and Li 2011; Gibbs 1970; Li et al. 2016b; Wu and Sun 2016). According to long-term monitoring chemistry data obtained in the study area from 1991 to 2016, the nitrogen pollution of groundwater was analyzed based on multivariate statistical methods, including correlation analysis, principal component analysis (PCA) and cluster analysis.
The PCA combines and reduces many original variables into a small number of independent new variables and summarizes the information contained in multidimensional variables with fewer representative factors; this method is widely used in water resources and water environment research (Peters 1987; Sebzalli and Wang, 2001; Lee et al. 2006; Li et al. 2019b, c; Wu et al. 2019). Cluster analysis, a new branch of multivariate statistical analysis, is an analytical method that classifies variables (or indicators) according to their similarity (Everitt 1980). According to the characteristics of the research object (sample or variable), the similarly high sample (variable) is classified into one class by calculating the cluster statistic between the samples (variables) (Willett 1987). In cluster analysis, the research objects can be divided into Q-type clustering, which serves to classify the research objects themselves, and R-type clustering, which serves to classify the variables and indicators that characterize the research objects (Everitt 1980; Zhang et al. 2014).
Results and discussion
Hydrochemical characteristics
The major groundwater components of the present study are summarized in Table 1. It was observed that groundwater is mildly alkaline, with pH values ranging at 7.7–8.19. Phreatic water was reported to be usually moderately salty, with TDS ranging from 432.23 to 3087.49 mg/L (mean value, 1277.52 mg/L). The confined aquifer was consisted of fresh water with TDS less than 1,000 mg/L. TDS level (Fig. 1) shows that TDS values in groundwater around Jinfeng district is high, above 1050 mg/L, indicating groundwater in these area are unsuitable for drinking. Based on the mean concentrations (expressed as milliequivalents per liter), the order of cations in all samples of the phreatic aquifer was found to be Na+>Mg2+>Ca2+>K+, while the order of anions was HCO3− > SO2−4 > Cl−. In confined aquifer, the order of cations was Na+> Ca2+> Mg2+>K+, and the anions was HCO3− > Cl– > SO2−4. Compared with the confined water, the concentration of major ions is higher and has large variations in phreatic water.
Figure 4 shows that the chemical types of the phreatic water were mainly HCO3·SO4–Na·Mg and HCO3·Cl–Na·Mg, and the chemical types of the confined water were mainly HCO3·Cl–Na·Ca and HCO3·SO4–Na·Ca. Along the flow direction from west to east, the chemical types of the phreatic water varied in the following sequences: HCO3− → HCO3− + SO2−4 → SO2−4 + Cl– → Cl− + SO2−4 The TDS, and the concentrations of Na+ and Cl− in phreatic water generally increase along the flow direction. The chemical type of confined water showed a similar distribution to phreatic water.
As shown in Fig. 5, most of the confined water samples are found in the middle part of the diagrams, indicating that water–rock interactions are the leading factors in water chemistry evolution in the confined aquifer. A few confined water samples and most phreatic water samples are plotted in the evaporation dominant zone. The phreatic samples that fall into the evaporation domain are obtained from an area where the groundwater level is shallow (1–3 m). With the rise in groundwater levels caused by irrigation and leakage of channels, groundwater evaporation becomes more intense. Owing to intense evaporation, the water escapes and ions remain in the soil and accumulate in phreatic water which deteriorates the groundwater quality. Furthermore, some of the samples are plotted outside the Gibbs diagrams, indicating that the ion concentration in groundwater is also affected by other factors, such as cation exchange and absorption.
The chemical components and formation of groundwater are controlled by the geological environment, which is the result of long-term geological evolution processes. Hydrochemical component relationships can be distinguished by the correlations among groundwater quality parameters (Li et al. 2016b, c, 2018b, c, d; Wu et al. 2014; Zhang et al. 2014; Montety et al. 2008). The correlation coefficients are shown in Table 2 and Fig. 6. From Fig. 6, with the increase in TDS, all ion concentrations increased in different degrees. Ca2+ and Mg2+ provide larger contribution to cations than that of Na+, while HCO3− provided an important contribution to anions. From the relationship between the major ions and TDS, it is preliminarily concluded that the dissolution of minerals containing Ca2+, Mg2+, Na+, and HCO3− constitutes a major part of the water–rock interaction. These minerals may be dolomite, calcite, albite. Cl− and SO2−4 may be from gypsum and halite dissolution.
From Table 2, TH was strongly correlated with Ca2+ and Mg2+, and weakly correlated with HCO3− (r = 0.453), indicating that TH is essentially a permanent hardness. It also means hardness is an approximate measure of Ca2+ and Mg2+. The correlation of Na+ and Cl− was found to be 0.844, indicating that halite dissolution is the reaction influencing the concentrations of Na+ and Cl−. The correlation of SO2−4 with Ca2+ is strong, indicating its main source is the dissolution and precipitation of gypsum. With dissolution of calcite and dolomite, concentrations of Ca2+ and Mg2+ increase meanwhile HCO3− decreases, resulting in higher TH and lower TDS.
Nitrogen contamination
Current status
The groundwater quality data collected from 1991 to 2016 were analyzed based on the Class III of “Standard for groundwater quality” (General Administration of Quality Supervision, Inspection and Quarantine of China and Standardization Administration of China 1993; Sebzalli and Wang 2001) (NO3− < 20 mg/L; NO2− < 0.02 mg/L; NH4+ < 0.2 mg/L). The nitrogen concentration of the phreatic and confined aquifers is shown in Fig. 7. The concentration of NH4+ in confined water was found to be significantly higher than that in the phreatic water; however, the opposite was observed for NO3− concentration. After 1995, the concentration of NO3− in the confined water continued to be low, while it continuously increased in phreatic water. Furthermore, NO2− concentration in the confined aquifer was low, and fluctuated greatly in phreatic water, while NH4+ concentration was generally increasing in the phreatic and confined water.
Figure 7 indicates that from 1991 to 2016, the concentration of NO3− in the phreatic and confined water was within the standard value. However, its detection rate was relatively high, ranging at 35–98.88% in phreatic water and 6.06–84% in the confined water. The standard exceeding rate of NH4+ was found to be 4.7–35.9% in phreatic water and 3.3–90.3%in the confined water. The highest detection values (2006) of NH4+, NO2− and NO3− in phreatic water were 14 mg/L, 6.78 mg/L, and 143.43 mg/L, respectively, which exceeded the permissible limit by 70, 7 and 7.2 times, respectively. The highest detection value (2006) of NH4+ in confined aquifer was 2.02 mg/L, exceeding the standard value by 10.1 times, while the highest NO2− concentration was 0.62 mg/L.
Nitrogen sources
The results of PCA are listed in Table 3. The first five main factors account for 72% of the total original information extracted from the analysis objects, which can generally reflect the changes of each variable (Ghahremanzadeh et al. 2017; Sebzalli and Wang 2001). The first three principal components (PCs) explained most of the variance, at 33.524%, 11.14% and 10.197%, respectively, while PC4 and PC5 explained 9.924% and 7.377%, respectively. The results of correlation analysis are listed in Table 2.
The main indicators highly related to PC1 were Cl−, TH, Mg2+, SO2−4, Ca2+ and Na+. The interrelationship between SO2−4, Ca2+ and Mg2+ in Table 2 indicated that these ions come from the dissolution of minerals. SO2−4 is significantly and positively correlated with Cl− (r = 0.808), indicating that the groundwater environment faces a number of problems, such as sewage discharge, gypsum fertilizer and irrigation infiltration. It can infer that PC1 reflected the dissolution of minerals and sewage discharge.
The results of PCA also demonstrated that PC2 was greatly influenced by NO3− and K+, PC3 was strongly related with pH and COD, and PC5 was intensely affected by NH4+ and PO3−4 concentrations. NO3− and K+ are related to the use of fertilizers in agriculture, while the main sources of COD and PO3−4 are industrial and domestic sewage. Therefore, we can infer that these three PCs reflected the intensity of human activities, including agricultural activities, and discharge of human and animal sewage. Furthermore, PC4 was positively influenced by HCO3− with a loading of 0.698. HCO3− is produced by the interaction of groundwater with aquifer sediments that are rich in carbonate and organic matter. Thus, PC4 is related to water–rock interaction.
Cluster analysis was also carried out to further interpret the nitrogen sources. The classified results of Q-type cluster analysis are presented in Fig. 8. The 47 samples can be divided into three categories with a distance of 10: A, B and C, listed in Table 4. The mean concentrations of the main ions for each category are shown in Table 5, combined with Fig. 8 and Table 5, which clarify that the three categories are geochemically independent groups. Among them, there are 35 samples in Category A, which are divided into 4 sub-categories, including A1, A2, A3 and A4. In addition, Category B includes 11 samples that are divided into 2 sub-categories, namely B1 and B2, while Category C includes 1 sample.
Each category has a distinct difference in water quality. Taking TDS as an example, Category A is characterized as fresh water and includes four sub-categories with an average TDS of 877.98 mg/L, ranging from 402.7 to 1454.3 mg/L. Category B is characterized as moderately salty water influenced by evaporation and contamination, and includes two sub-categories with an average TDS of 1655.81 mg/L that ranges from 460.02 to 3087.49 mg/L. Category C is characterized as salty water and contains only one sample (P009), which has TDS of 2212.77 mg/L. TDS, TH and most major ions increased significantly from Category A to Category C.
Compared with the other two categories, the chemical composition of these water samples in Category A had the lowest mean concentration of major ions, but the pH values are the highest among the three categories. Samples of Category A are mainly distributed in the west and southeast of the study area, where groundwater quality is better, since the groundwater flow is relatively quick and the depth down to the groundwater table is greater.
The samples of Category B have higher concentration of ions, indicating that evaporation and human activities affect their hydrochemical compositions more intensely than in Category A. The samples in Category B are located in areas that are more susceptible to a shallow groundwater level (1–3 m), mainly distributed in the east of the study area, where scattered agricultural and chemical industrial areas are found.
The sample included in Category C has the highest mean concentrations of major ions, while its pH is the lowest. The single sample is located in the east part of the study area, in which the population is relatively concentrated, but there is no industrial activity. The main sources of pollution include untreated domestic sewage and agricultural irrigation infiltration to phreatic water, which affects groundwater quality. The depth of the groundwater table is less than 2.4 m and thus evaporation is intense.
The R-type cluster analysis was used to classify 17 indicators of groundwater samples (Fig. 9).
In this analysis, the 17 indicators can be divided into three categories: Cluster 1, cluster 2 and cluster 3. There are ten parameters in cluster 1, including TDS, pH, COD, NO3−, NO2−, NH4+, PO3−4, Fe, K+ and Cl−, which can be summarized as indicators of human activity impact, include agricultural fertilizers, domestic sewage and organic pollution. There are six parameters in cluster 2, including TH, SO2−4, Mg2+, Ca 2+, Na+, and Mn, which can be summarized as indicators of mineral dissolution, along with the comprehensive indicator TH. Cluster 3 only includes HCO3−, which is related to water–rock interaction.
NH4 + contamination in confined aquifer
The analysis of current nitrogen contamination status revealed that the concentration of NH4+ in the confined water is higher than that in phreatic water. The reasons behind this difference in contamination were investigated.
Hydrogeological conditions In the irrigated area, the excessive application of nitrogen fertilizers elevated the nitrogen concentration in groundwater, since these compounds are continuously absorbed in the soil (Heatwole and McCray 2007). Yinchaun is the typical irrigation area. The self-purification ability of vadose zone is weak owing to its thickness of 1–3 m and extremely good vertical permeability of approximately 0.25 m/d (Fang 2009; Zhang 2010). Fertilizers are nitrified in the vadose zone and cannot be fully utilized due to excessive application, it will enter the phreatic aquifer with precipitation and irrigation water and cause pollution.
Groundwater evaporation The evaporation in the study area is intense, the water escapes and the ions remain in soil, leading the ions in groundwater tend to accumulate. Due to the irrigation infiltration recharge, the phreatic water evaporation is more intense with the rising water table. The evapotranspiration discharge accounting for 62.44% of the total groundwater discharge. Therefore, it can be concluded that evaporation is another important reason for the increase in nitrogen concentration in phreatic water.
Leakage recharge of phreatic water An increasing tendency in the cone of depression was observed from 1991 to 2010 (Chen et al. 2015). The confined water level is greatly reduced by exploitation, leading to enlargement of the cone of depression and to a higher phreatic water level than the confined water level. The increasing vertical hydraulic gradient results in leakage recharge of the phreatic to confined aquifer. In addition, in some areas, the aquitard between the phreatic and confined aquifer is thin and has “skylights”. A larger head difference and heavier phreatic water contamination would result in a higher concentration of nitrogen pollutants in the confined aquifer. This is another reason of the leakage recharge of phreatic water to the confined aquifer. Wu et al. (2015) have reported the leakage in the northern part of the Yinchuan Basin, which has induced confined groundwater quality deterioration.
Redox conditions The use of the Yellow River water for irrigation has a long history in the Yinchuan area, resulting in long-term flooding of the soil and lack of oxygen. Furthermore, the high concentrations of Fe and Mn, and the long residence time of groundwater result in anaerobic environment in the confined aquifer, which is not conducive to nitrification in the soil. Mn and Fe are reducing agents, and NO3− is an oxidizing agent; these undergo redox reactions in mildly alkaline solutions according to the following reaction formula:
From the correlation of NH4+ and NO3− with Fe and Mn in Table 2, it was also observed that NO3− is negatively correlated with Fe and Mn, indicating that anaerobic environment is not conducive to NO3− accumulation, but benefits to NH4+ accumulation. Once NO3− enters the confined aquifer, it will transform into NH4+ under anaerobic environment, which will accumulated in the aquifer.
Conclusions
-
1.
Groundwater is one of the important sources for domestic, agricultural and industrial activities in Yinchuan area. Phreatic water in Yinchuan area is usually moderately salty with TDS ranging from 432.23 to 3087.49 mg/L, while the confined water mainly consisted of fresh with TDS less than 1000 mg/L. The chemical types of the phreatic water are mainly HCO3·SO4 –Na·Mg and HCO3·Cl–Na·Mg, and the chemical types of the confined water are mainly HCO3·Cl–Na·Ca and HCO3·SO4–Na·Ca. Along the flow direction from west to east, the chemical types of phreatic water in the study area varied in the following sequences: HCO3− → HCO3− + SO2−4 → SO2−4 + Cl−→Cl− + SO2−4. Water–rock interactions are the leading factors in the water chemistry evolution in the confined aquifer, and evaporation is the leading factor controlling phreatic water chemistry.
-
2.
The concentration of NO3− in the phreatic and confined aquifers is within the standard value for drinking, but its detection rate is relatively high. The concentration of NO3− in the phreatic aquifer is significantly higher than that in confined aquifer, and the opposite was observed for NH4+. The concentrations of NO3− and NH4+ exhibited an increasing tendency in groundwater since 1995. According to the results of multivariate statistical analysis, the main nitrogen sources in phreatic water in the study region are human activities, including nitrogen fertilizer application in agriculture, and industrial and domestic sewage.
-
3.
Because of the comprehensive influences of hydrogeological conditions, leakage of phreatic water and redox conditions, the concentration of NH4+ in the confined water is higher than that of phreatic water. The formation of the regional cone of depression favors the leakage of phreatic water to confined aquifer, which is the most important factor affecting concentration of pollutants in the confined water, because the poor quality of phreatic water. The anaerobic environment in confined aquifer is not conducive to NO3− accumulation, but benefits to NH4+ accumulation. Once NO3− enters the confined aquifer, it will transform into NH4+ under the anaerobic environment.
In view of above problems, we recommended balancing fertilization in agricultural areas, controlling of irrigation quotas and combined application of organic and inorganic fertilizers to prevent groundwater pollution. Further enhancement of the monitoring and research of environmental hydrogeology is necessary.
References
Adimalla N, Li P, Qian H (2019) Evaluation of groundwater contamination for fluoride and nitrate in semi-arid region of Nirmal Province, South India: a special emphasis on human health risk assessment (HHRA). Hum Ecol Risk Assess 25(5):1107–1124. https://doi.org/10.1080/10807039.2018.1460579
Chen J, Wu H, Qian H, Liu Q (2015) Analysis of evolvement for confined water cone of depression and its influence on groundwater resource sustainability in Yinchuan area. Adv Mater Res 1073–1076:1656–1659. https://doi.org/10.4028/www.scientific.net/AMR.1073-1076.1656
Chen J, Wu H, Qian H (2016) Groundwater nitrate contamination and associated health risk for the rural communities in an agricultural area of Ningxia, Northwest China. Expo Health 8(3):349–359. https://doi.org/10.1007/s12403-016-0208-8
Chen J, Qian H, Wu H (2017) Nitrogen contamination in groundwater in an agricultural region along the New Silk Road, northwest China: distribution and factors controlling its fate. Environ Sci Pollut Res 24:13154–13167. https://doi.org/10.1007/s11356-017-8881-0
Chen J, Wu H, Qian H, Li X (2018) Challenges and prospects of sustainable groundwater management in an agricultural plain along the Silk Road Economic Belt, north-west China. Int J Water Resour Dev 34(3):354–368. https://doi.org/10.1080/07900627.2016.1238348
Djorfi S, Beloulou L, Djidel M, Guechi S (2017) Hydrochemical evolution of groundwater in the Tamlouka Plain, influence of lithology, geomorphology and anthropogenic actions. Springer, Cham, pp 685–687. https://doi.org/10.1007/978-3-319-70548-4_203
Everitt B (1980) Cluster analysis. Qual Quant 14:75–100. https://doi.org/10.1007/BF00154794
Fang HS (2009) Analysis of hydrogeology condition and the wellhead protection zoning division in Yinchuan area. Changan University, Xi’an (in Chinese)
Gao Y, Yu G, Luo C, Zhou P (2012) Groundwater nitrogen pollution and assessment of its health risks: a case study of a typical village in rural–urban continuum, China. PloS ONE 7:e33982. https://doi.org/10.1371/journal.pone.0033982
General Administration of Quality Supervision, Inspection and Quarantine of China, Standardization Administration of China (1993) Standards for groundwater quality (GB/T 14848-1993). Standards Press of China, Beijing (in Chinese)
Ghahremanzadeh H, Noori R, Baghvand A, Nasrabadi T (2017) Evaluating the main sources of groundwater pollution in the southern Tehran aquifer using principal component factor analysis. Environ Geochem Health. https://doi.org/10.1007/s10653-017-0058-8
Gibbs RJ (1970) Mechanisms controlling world water chemistry. Science 170:1088–1090. https://doi.org/10.1126/science.170.3962.1088
Han D, Cao G, Mccallum J, Song X (2015) Residence times of groundwater and nitrate transport in coastal aquifer systems: Daweijia area, northeastern China. Sci Total Environ 538:539–554. https://doi.org/10.1016/j.scitotenv.2015.08.036
He S, Wu J (2019) Hydrogeochemical characteristics, groundwater quality and health risks from hexavalent chromium and nitrate in groundwater of Huanhe Formation in Wuqi County, northwest China. Expo Health 11(2):125–137. https://doi.org/10.1007/s12403-018-0289-7
He X, Wu J, He S (2019) Hydrochemical characteristics and quality evaluation of groundwater in terms of health risks in Luohe aquifer in Wuqi County of the Chinese Loess Plateau, northwest China. Hum Ecol Risk Assess 25(1–2):32–51. https://doi.org/10.1080/10807039.2018.1531693
Heaton THE, Stuart ME, Sapiano M, Sultanac M (2012) An isotope study of the sources of nitrate in Malta’s groundwater. J Hydrol 414:244–254
Heatwole K, Mccray JE (2007) Modeling potential vadose-zone transport of nitrogen from onsite wastewater systems at the development scale. J Contam Hydrol 91:184–201. https://doi.org/10.1016/j.jconhyd.2006.08.012
Hossain MG, Lutfun-nessa M, Ahmed SS (2013) Factor and cluster analysis of water quality data of the groundwater wells of Kushtia, Bangladesh: implication for arsenic enrichment and mobilization. J Geol Soc India 81:377–384. https://doi.org/10.1007/s12594-013-0048-0
Hu K, Huang Y, Li H, Li B, Chen D, White RE (2005) Spatial variability of shallow groundwater level, electrical conductivity and nitrate concentration, and risk assessment of nitrate contamination in North China Plain. Environ Int 31:896–903. https://doi.org/10.1016/j.envint.2005.05.028
Kumar M, Ramanathan A, Rao MS (2006) Identification and evaluation of hydrogeochemical processes in the groundwater environment of Delhi, India. Environ Geol 50:1025–1039. https://doi.org/10.1007/s00254-006-0275-4
Lee J-S, Ko K-S, Kim T-K, Kim J-G, Cho S-H, Oh I-S (2006) Analysis of the effect of geology, soil properties, and land use on groundwater quality using multivariate statistical and GIS methods. Chin J Geochem 25:152. https://doi.org/10.1007/BF02840034
Li P, Qian H (2018a) Water resources research to support a sustainable China. Int J Water Resour Dev 34(3):327–336. https://doi.org/10.1080/07900627.2018.1452723
Li P, Qian H (2018b) Water resource development and protection in loess areas of the world: a summary to the thematic issue of water in loess. Environ Earth Sci 77(24):796. https://doi.org/10.1007/s12665-018-7984-3
Li P, Wu J (2019a) Drinking water quality and public health. Expo Health 11(2):73–79. https://doi.org/10.1007/s12403-019-00299-8
Li P, Wu J (2019b) Sustainable living with risks: meeting the challenges. Hum Ecol Risk Assess 25(1–2):1–10. https://doi.org/10.1080/10807039.2019.1584030
Li P, Wu J, Qian H, Lyu X, Liu H (2014a) Origin and assessment of groundwater pollution and associated health risk: a case study in an industrial park, northwest China. Environ Geochem Health 36(4):693–712. https://doi.org/10.1007/s10653-013-9590-3
Li P, Wu J, Qian H (2014b) Hydrogeochemistry and quality assessment of shallow groundwater in the southern part of the Yellow River alluvial plain (Zhongwei section), China. Earth Sci Res J 18(1):27–38. https://doi.org/10.15446/esrj.v18n1.34048
Li P, Li X, Meng X, Li M, Zhang Y (2016a) Appraising groundwater quality and health risks from contamination in a semiarid region of northwest China. Expo Health 8(3):361–379. https://doi.org/10.1007/s12403-016-0205-y
Li P, Wu J, Qian H, Zhang Y, Yang N, Jing L, Yu P (2016b) Hydrogeochemical characterization of groundwater in and around a wastewater irrigated forest in the southeastern edge of the Tengger Desert, Northwest China. Expo Health 8(3):331–348. https://doi.org/10.1007/s12403-016-0193-y
Li P, Wu J, Qian H (2016c) Hydrochemical appraisal of groundwater quality for drinking and irrigation purposes and the major influencing factors: a case study in and around Hua County, China. Arab J Geosci 9(1):15. https://doi.org/10.1007/s12517-015-2059-1
Li P, Tian R, Xue C, Wu J (2017a) Progress, opportunities and key fields for groundwater quality research under the impacts of human activities in China with a special focus on western China. Environ Sci Pollut Res 24(15):13224–13234. https://doi.org/10.1007/s11356-017-8753-7
Li P, Qian H, Zhou W (2017b) Finding harmony between the environment and humanity: an introduction to the thematic issue of the Silk Road. Environ Earth Sci 76(3):105. https://doi.org/10.1007/s12665-017-6428-9
Li P, Qian H, Wu J (2018a) Conjunctive use of groundwater and surface water to reduce soil salinization in the Yinchuan Plain, North–West China. Int J Water Resour Dev 34(3):337–353. https://doi.org/10.1080/07900627.2018.1443059
Li P, He S, Yang N, Xiang G (2018b) Groundwater quality assessment for domestic and agricultural purposes in Yan’an City, northwest China: implications to sustainable groundwater quality management on the Loess Plateau. Environ Earth Sci 77(23):775. https://doi.org/10.1007/s12665-2018-7968-3
Li P, Wu J, Tian R, He S, He X, Xue C, Zhang K (2018c) Geochemistry, hydraulic connectivity and quality appraisal of multilayered groundwater in the Hongdunzi Coal Mine, Northwest China. Mine Water Environ 37(2):222–237. https://doi.org/10.1007/s10230-017-0507-8
Li X, Wu H, Qian H, Gao Y (2018d) Groundwater chemistry regulated by hydrochemical processes and geological structures: a case study in Tongchuan, China. Water 10:338. https://doi.org/10.3390/w10030338
Li P, He X, Guo W (2019a) Spatial groundwater quality and potential health risks due to nitrate ingestion through drinking water: a case study in Yan’an City on the Loess Plateau of northwest China. Hum Ecol Risk Assess 25(1–2):11–31. https://doi.org/10.1080/10807039.2018.1553612
Li P, He X, Li Y, Xiang G (2019b) Occurrence and health implication of fluoride in groundwater of loess aquifer in the Chinese loess plateau: a case study of Tongchuan, Northwest China. Expo Health 11(2):95–107. https://doi.org/10.1007/s12403-018-0278-x
Li P, Tian R, Liu R (2019c) Solute geochemistry and multivariate analysis of water quality in the Guohua phosphorite mine, Guizhou Province, China. Expo Health 11(2):81–94. https://doi.org/10.1007/s12403-018-0277-y
Montety VD, Radakovicth O, Vallet-coulomb C, Blavoux B, Hermitte D, Valles V (2008) Origin of groundwater salinity and hydrogeochemical processes in a confined coastal aquifer: case of the Rhône delta (Southern France). Appl Geochem 23:2337–2349. https://doi.org/10.1016/j.apgeochem.2008.03.011
Peters WS (1987) Principal components and factor. Analysis. https://doi.org/10.1007/978-1-4612-4638-1_17
Qian H, Li P (2011) Hydrochemical characteristics of groundwater in Yinchuan plain and their control factors. Asian J Chem 23:2927–2938
Qian H, Li P, Howard KW, Yang C, Zhang X (2012) Assessment of groundwater vulnerability in the Yinchuan Plain, Northwest China using OREADIC. Environ Monit Assess 184:3613–3628. https://doi.org/10.1007/s10661-011-2211-7
Qian H, Li P, Wu J, Zhou Y (2013) Isotopic characteristics of precipitation, surface and ground waters in;the Yinchuan plain, Northwest China. Environ Earth Sci 70:57–70. https://doi.org/10.1007/s12665-012-2103-3
Qian H, Wu J, Zhou Y, Li P (2014) Stable oxygen and hydrogen isotopes as indicators of lake water recharge and evaporation in the lakes of the Yinchuan Plain. Hydrol Process 28:3554–3562. https://doi.org/10.1002/hyp.9915
Sebzalli YM, Wang XZ (2001) Knowledge discovery from process operational data using PCA and fuzzy clustering. Eng Appl Artif Intell 14:607–616. https://doi.org/10.1016/s0952-1976(01)00032-x
Sun Y, Hui Q, Wu X (2007) Hydrogeochemical characteristics of groundwater depression cones in Yinchuan City, Northwest China. Chin J Geochem 26:350–355. https://doi.org/10.1007/s11631-007-0350-x
Vega M, Pardo R, Barrado E, LuisDebán L (1998) Assessment of seasonal and polluting effects on the quality of river water by exploratory data analysis. Water Res 32:3581–3592. https://doi.org/10.1016/S0043-1354(98)00138-9
Willett J (1987) Similarity and clustering in chemical information systems. ISBN:0471914630
Wu J, Sun Z (2016) Evaluation of shallow groundwater contamination and associated human health risk in an alluvial plain impacted by agricultural and industrial activities, mid-west China. Expo Health 8(3):311–329. https://doi.org/10.1007/s12403-015-0170-x
Wu J, Li P, Qian H, Duan Z, Zhang X (2014) Using correlation and multivariate statistical analysis to identify hydrogeochemical processes affecting the major ion chemistry of waters: a case study in Laoheba phosphorite mine in Sichuan, China. Arab J Geosci 7:3973–3982. https://doi.org/10.1007/s12517-013-1057-4
Wu J, Li P, Qian H (2015) Hydrochemical characterization of drinking groundwater with special reference to fluoride in an arid area of China and the control of aquifer leakage on its concentrations. Environ Earth Sci 73(12):8575–8588. https://doi.org/10.1007/s12665-015-4018-2
Wu J, Wang L, Wang S, Tian R, Xue C, Feng W, Li Y (2017) Spatiotemporal variation of groundwater quality in an arid area experiencing long-term paper wastewater irrigation, northwest China. Environ Earth Sci 76(13):460. https://doi.org/10.1007/s12665-017-6787-2
Wu J, Li P, Wang D, Ren X, Wei M (2019) Statistical and multivariate statistical techniques to trace the sources and affecting factors of groundwater pollution in a rapidly growing city on the Chinese Loess Plateau. Hum Ecol Risk Assess. https://doi.org/10.1080/10807039.2019.1594156
Zabala M, Martinez S, Manzano M, Vives L (2016) Groundwater chemical baseline values to assess the Recovery Plan in the Matanza–Riachuelo River basin Argentina. Sci Total Environ 541:1516–1530. https://doi.org/10.1016/j.scitotenv.2015.10.006
Zhang X (2010) Analysis of hydrogeological influencing factors and risk assessment of groundwater source pollution in Yinchuan City. Changan University, Xi’an (in Chinese)
Zhang ZH (2014) Study on the Groundwater Pollution Simulation in Yinchuan Region. Chang’an University, Xi’an (in Chinese)
Zhang J (2015) Research on effect of Fe on the transfer and transformation of nitrogen in the groundwater. East China University of Technology, Shanghai (in Chinese)
Zhang X, Qian H, Chen J, Qiao L (2014) Assessment of groundwater chemistry and status in a heavily used semi-arid region with multivariate statistical analysis. Water 6:2212–2232. https://doi.org/10.3390/w6082212
Zhang Y, Wu J, Xu B (2018) Human health risk assessment of groundwater nitrogen pollution in Jinghui canal irrigation area of the loess region, northwest China. Environ Earth Sci 77:273. https://doi.org/10.1007/s12665-018-7456-9
Acknowledgements
The research is financially supported by the National Natural Science Foundation of China (41572236), Investigation and Evaluation of Groundwater Environment Status in Ningxia Hui Autonomous Region (220029160057).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
The authors declare no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is a part of the Topical Collection in Environmental Earth Sciences on “Water in Large Basins” guest edited by Peiyue Li and Jianhua Wu.
Rights and permissions
About this article
Cite this article
Liu, F., Qian, H., Shi, Z. et al. Long-term monitoring of hydrochemical characteristics and nitrogen pollution in the groundwater of Yinchuan area, Yinchuan basin of northwest China. Environ Earth Sci 78, 700 (2019). https://doi.org/10.1007/s12665-019-8695-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12665-019-8695-0