Abstract
Nitrogen contamination is a global concern and has been a serious problem in agricultural areas. The present study was carried out in an intensively irrigated region of northwest China along the New Silk Road, Yinchuan Plain, where the residents depend on the groundwater as the primary source for drinking. To understand the nitrogen contamination in the aquifer system, the distribution of nitrate and ammonium and its controlling factors were studied based on hydrochemical, hydrogeological, and isotopic analyses. 11.37 and 40% of phreatic water samples are categorized as NO3–N and NH4–N pollution in accordance with the WHO standards. A total of 59.52% of confined water samples has high NH4–N values, exceeding the permissible limit for drinking purpose. The results indicate NO3–N predominates in the shallow water and NH4–N predominates in the deep water for the single phreatic aquifer. For the multilayer structure area, NO3–N predominates in the phreatic aquifer of the western and the southern parts of the plain; NH4–N predominates in the phreatic aquifer of the middle and the northern parts of the plain, and in the confined aquifers where groundwater pumping had been performed. The mixture of synthetic fertilizer and manure/sewage is primarily responsible for the phreatic water based on isotopic analysis. In the confined aquifers, higher NH4–N concentrations are mainly attributed to intensive pumping under higher pumping rates. The results of this study can be used as a scientific basis for the future research on nitrogen in the plain. They can also be used by scholars and decision makers who are interested in groundwater protection and sustainable development.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Groundwater is one of the most important sources of water for human due to its natural purity. The utilization of the finite groundwater resource, however, is being compromised with water of poor chemical quality, especially when it contains high level of nitrogen (N) (Heaton et al. 2012). Anthropogenic sources through agricultural activities, disposal of human and animal sewage, industrial wastes discharge, and landfill leachate, are major contributors to nitrogen contamination, resulting in adverse impacts on economy, ecosystem and human health (Böhlke et al. 2006; Umezawa et al. 2008; Yoshimoto et al. 2011; Wu and Sun 2015). For example, exposure to high level of nitrogen contamination through drinking water may lead to methemoglobinemia (blue baby syndrome) and cancer in humans (Low 1974; Ward et al. 2005; Li et al. 2014a; Marinov and Marinov 2014). Therefore, in most regions of the world, the significant increase of nitrogen in groundwater is a growing concern (Gustafson 1983; Li et al. 2006; Serhal et al. 2008; Subramani et al. 2010).
Nitrate (NO3 −) and ammonium (NH4 +) are the two most important forms of nitrogen. As NH4 + can be easily oxidized, nitrate is the most common contamination of aquifer systems (Umezawa et al. 2008; Welch et al. 2011). However, behavior of nitrogen in an aquifer depends heavily on multiple factors, such as nitrogen loading, biogeochemical processes, hydrogeological properties, and groundwater pumping. Land use type and fertilizer application rates largely determine the amount of nitrate in many agricultural areas (such as Diez et al. 2000; Thorburn et al. 2003; Ju et al. 2006; Kaown et al. 2009; Li et al. 2014a, 2016a). High concentrations of ammonium in shallow and deep groundwater are more often controlled by point sources and organic matter in sediment (Böhlke et al. 2006; Singh et al. 2006; Hinkle et al. 2007). Because biological cycling of nitrogen often occurs with changes in isotopic ratios, the combined use of δ 18O and δ 15N in nitrate has been widely reported for identification of actual sources and related processes (such as volatilization, nitrification and denitrification) (Mayer et al. 2002; Hosono et al. 2013; Pastén -Zapata et al. 2014; Zhang et al. 2014). In addition, many studies have pointed out the significant effects of local hydrogeological conditions on nitrogen behavior. For instance, the study conducted by Han et al. (2015) demonstrated that groundwater mean residence time determined redox conditions, significantly affecting the nitrate concentrations in the shallow Quaternary aquifers. Due to the presence of an quitard, the nitrate exhibited distinct charactetistics in the quifer of an agricultural area (Nishikiori et al. 2012). As pumping of groundwater can change the groundwater table and the characteristics of the flow system, it can also affect the fate of contamination (Li et al. 2014b, 2016a; Park et al. 2014). Dragon (2012) performed an investigation of nitrate in a regional Quaternary flow system, and found that the high concentrations of nitrate at great depth were caused by pumping. In recognition of these, before taking action to prevent groundwater contamination, it is quite important to understand the nitrogen distribution and the factors controlling its fate in aquifer system.
Yinchuan Plain is an important agricultural area in northwest China along the New Silk Road, where nearly three million population depend on groundwater for drinking. In the irrigated area, the excessive application of N fertilizer did help produce higher crop yields, but the nitrogen contents in groundwater were also elevated. Field investigations showed that the suitability of groundwater for drinking purpose was greatly affected by high nitrogen concentrations, which are above the permissible standards of World Health Organization (WHO 2011) (NO3–NN > 10 mg/L, NH4–NN > 0.2 mg/L). Furthermore, high rate of pumping over the recent decades has caused serious groundwater declines, and groundwater from the phreatic aquifer has been leaking into the confined aquifers, especially in the cone of depression areas (Qian et al. 2013; Chen et al. 2015). It is quite possible that groundwater with high nitrogen leaks into the confined aquifers, deteriorating the water quality. In this context, to build a new and sustainable Silk Road Economic Belt, it is necessary to recognize the threats of nitrogen in the aquifer system (Li et al. 2015a). Although several studies regarding soil and groundwater pollution have been carried out in and around the plain, including Li et al. (2014b, c, 2015b, 2016b), Qian and Li (2011), Qian et al. (2012, 2013, 2014), and Wu et al. (2014, 2015a), few nitrogen investigations have been reported. There is limited knowledge on nitrogen contamination at a regional scale. The distribution of nitrogen and the factors controlling its fate in the aquifer system remain unclear. All these make it more difficult to take effective action for contamination prevention.
The aim of this study is to gain a better understanding of nitrogen contamination in the aquifer system. In this study, the concentrations and distributions of NO3–N and NH4–N in the aquifer system were investigated. Based on the evaluation of the hydrogeological conditions, the redox conditions of the aquifer were studied to provide general insight into the nitrogen distribution along the flow path. The status of nitrogen pollution under different hydrogeological conditions and pumping conditions were evaluated. The findings from this paper can be used as a scientific basis for future research on nitrogen in the plain. They are valuable to scholars and decision makers who are interested in groundwater protection and sustainable development.
Description of the study area
Yinchuan Plain is located in the northern part of Ningxia Hui Autonomous Region, northwest China, covering an area of 6704.5 km2. Due to the fertile soils in the Yellow River alluvial plain, this region is used extensively for agriculture (Fig. 1). The land use (types of crops) and the agricultural practices (flood irrigation and N application rate) are relatively uniform in the agricultural area. The climate in the area is characterized by intense evaporation and little precipitation. The average annual precipitation and evaporation are 186.7 and 1834.44 mm, respectively.
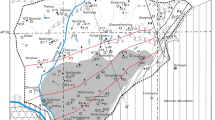
The Quaternary deposits are well developed in Yinchuan Plain with more than 2000 m in thickness (Qian et al. 2012). From west to east, the landforms are leaning pluvial plain, pluvial alluvial plain, and alluvial lacustrine plain, respectively (Fig. 2a). Overall, the groundwater flows in NNE direction. The pore water in loose rock mass, up to 250 m thick, is divided into two aquifer areas: (1) single phreatic aquifer zone and (2) multilayer structure area (Qian et al. 2013, 2014) (Fig. 2). The single phreatic aquifer zone is composed of mountain pluvial deposits and alluvial sands and gravel of the Yellow River mainly in the western and southern parts of the plain. The other regions covered by lacustrine and alluvial deposits all belong to the multilayer structure area (Fig. 3). It includes a shallow phreatic aquifer underlain by two confined aquifers (upper confined aquifer and lower confined aquifer). The thicknesses of the three aquifers are 30–45, 40–130, and 60–125 m, respectively. These aquifers are separated by aquitards, which are normally continuous and vary between 2 and 14 m in thickness. The depth from the ground surface to the water table is more than 10 m in the leaning pluvial plain and less than 4.6 m in the alluvial lacustrine plain.
A map showing landforms and sampling locations. A–A′ and B–B′ are two typical flow paths of phreatic water. The red lines (A–A′ and B–B′) also indicate the locations of the geological cross–sections presented in Fig. 3
Cross–sections of the geological contexts and aquifer systems of A–A′ (a) and B–B′ (b) (see Fig. 2a for location)
The water balance study between July 1, 2003, and June 30, 2004 is presented in Table 1 (Qian et al. 2012). The results show that the primary sources of recharge are from channels and irrigation infiltration. An extensive network of channels has been used to deliver water from the Yellow River for irrigation. The primary sources of discharge include evapotranspiration and artificial extraction (Table 1). Within the study area, there are thousands of pumping wells for extracting water for irrigation and drinking. In particular, the water in the confined aquifers is the primary source for municipal water supply systems that provide the drinking water for three million people living on the plain.
Groundwater chemistry is greatly affected by such factors as hydrogeological properties, chemistry of recharge water, soluble salt in soil, water–rock interactions, and evapotranspiration of phreatic water (Qian and Li 2011; Wang et al. 2013). The single phreatic aquifer zone is characterized by good permeability, high porosity, and a large hydraulic gradient. The water is mainly fresh water of HCO3·SO4–Ca·Mg and HCO3·SO4–Mg·Ca type with the total dissolved solids (TDS) generally less than 1 g/L. Variation in hydrochemical type of groundwater is observed in lacustrine and alluvial plain. In the south of Yongning, with larger hydraulic gradient and faster water cycle, the groundwater is mainly HCO3·SO4–Na·Mg and HCO3·SO4–Na·Ca type with low TDS (<1 g/L). In the north of Yongning, with smaller hydraulic gradient and the groundwater close to the ground surface, the groundwater is mainly HCO3·Cl–Mg·Na type with TDS between 1 and 2 g/L and SO4·Cl–Na·Mg type with TDS between 1 and 3 g/L. For some local areas, TDS can even be greater than 3 g/L partly due to intensive evapotranspiration and shallow water buried depth. Generally, groundwater quality becomes worse from SW to NE. Further, with the slower water circulation, the water in the confined aquifers is mainly HCO3·SO4–Mg·Na and HCO3·SO4–Na·Mg types in the south, HCO3·Cl–Na·Mg type in the middle and Cl·SO4–Na·Mg type in the north.
Sample collection and analyses
A total of 132 groundwater samples were taken from the private, irrigation, and public water supply wells in August 2013 on the study area (Fig. 2). They included 97 samples from the phreatic aquifer and 35 samples from the confined aquifers. Information on the use of the groundwater and the depth of the screen in the wells was acquired from the well managers and farmers. The sampling procedures, transportation, and conservation for all the samples followed the standard methods recommended by the Ministry of Water Resources of People’s Republic of China (PRC 1997). The water in the wells was pumped continuously for 10–15 min prior to collection of the samples. At the time of sampling, bottles were thoroughly rinsed 2–3 times with groundwater to be sampled. In addition, duplicates were introduced for QA/QC during the analysis. NO3–N and NH4–N were analyzed using the standard methods recommended by the Ministry Ministry of Health of PRC, Standardization Administration of PRC (2006) NO3–N and NH4–N were measured by spectrophotometry and ion chromatography at laboratory of Ningxia Environmental Monitoring Center, respectively. The detection limits of NO3–N and NH4–N were 0.02 and 0.025 mg/L, respectively. The analysis of 18O and 15N in nitrate for these samples was done by the State Key Laboratory of Environmental Geochemistry, Institute of Geochemistry Chinese academy of Science, Guizhou, China. Isotopic abundances of elements were determined using a Finnigan MAT 253 mass spectrometer and reported in the delta notation in per mill. The results of 18O and 15N were respectively expressed in δ‰ to Vienna Standard Mean Ocean Water (VSMOW) and N2 (air) as follows:
where R sample is the isotopic ratio 18O/16O or 15N/14N of the sample, and R standard is the isotopic ratio 18O/16O or 15N/14N of the standard. Analytical precisions for the δ 15N and δ 18O were estimated as ±0.2 and ±0.9‰, respectively.
Furthermore, the Institute of Survey and Monitoring of Land Resources of Ningxia (ISMLR) has carried out a large number of researches in Yinchuan Plain, and 165 samples (158 samples from the phreatic aquifer and 7 samples from the confined aquifers) were collected from private, irrigation, and monitoring wells on the plain during the same period. To provide an adequate number of data, the measured NO3–N and NH4–N values of these samples were used in this study (Fig. 2). The sampling methods and analysis methods for these samples also followed the national standards (Ministry of Water Resources of PRC 1997; Ministry of Health of PRC, Standardization Administration of PRC 2006). Thus, the data were reliable, and the concentrations of NO3–N and NH4–N from ISMLR can be used as the supplemental data to study the nitrogen contamination at a regional scale.
Results and discussion
Distribution of nitrate and ammonium
Phreatic aquifer
For the samples from the phreatic aquifer, the NO3–N concentrations range from below detection limit (BDL) to 158 mg/L with a mean level of 3.89 mg/L (Table 2). 11.37% of the samples (29 samples) exceed the acceptable limit of NO3–N for drinking purpose (>10 mg/L) (WHO 2011). The concentrations of 111 samples are BDL. The NH4–N concentrations vary from BDL to 31.08 mg/L with a mean level of 0.75 mg/L. It can be seen from Table 2 that 40% of them (102 samples) are not suitable for drinking without treatment due to excessive NH4–N (>0.2 mg/L) in accordance with the WHO standard (WHO 2011). The NH4–N concentrations BDL are found in 95 samples. Concerning the standard deviation, the most even values occurred in the phreatic aquifer for NO3–N and NH4–N with 12.62 and 2.93 values, respectively. Maximum values of NO3–N and NH4–N for all the samples (158 and 31.80 mg/L) were found in the phreatic wells, justifying the high variability.
As shown in Fig. 4 and Fig. 5, area-dependent variations of nitrogen species are observed in the phreatic aquifer. All the wells can be classified into three zones on the basis of the nitrogen species and its concentrations. (1) In the single phreatic aquifer, the vertical distribution of nitrate and ammonium concentrations is found among the samples (Fig. 5). Although no general trend between nitrogen concentration and depth of well in the phreatic aquifer, deep wells are susceptible to contamination in the zone. For the water supply wells in the southern part of the single phreatic aquifer, nitrate and ammonium contamination are still observed when the well depths are greater than 60 m. This area is designated as Zone I where the NO3–N and NH4–N concentrations are rather distinct with well depths. The levels of NO3–N are significant higher than that of NH4–N for the shallow phreatic water in Zone I. This subpart is designated as Zone Ia. On the contrary, the species of NH4–N is dominant and NO3–N concentrations are BDL or very low in deep wells (>100 m). It is designated as Zone Ib. (2) In the western and the southern parts of the plain of the multilayer structure area, NO3–N is the dominant species of nitrogen and the concentrations of NH4–N are very low (or even BDL). This area is designated as Zone II where NO3–N predominates in groundwater. (3) In the middle and the northern parts of the plain of the multilayer structure area, NH4–N is the dominant species of nitrogen and the levels of NO3–N are very low or undetectable. This area is designated as Zone III where NH4–N predominates in groundwater.
Variations of a NO3–N with well depth and b NH4–N and well depth. Gray dots denote the samples in Zone I (water sample n = 13), black dots denote the samples in Zone II (water sample n = 28), blue dots denote the samples in Zone III (water sample n = 56), and red dots denote the samples in confined aquifers (water sample n = 35)
A Mann–Whitney U test was performed to analyze the difference in NO3–N and NH4–N concentrations. The Mann–Whitney U test is a highly efficient nonparametric test to identify the difference between two independent samples using SPSS (version 22.0) (Miller and Miller 1988). The null hypothesis states that the two sample groups taken from a single population would have no consistent differences between the two sets of ranking. When the p value is less than 0.05, there is significant difference between them. The results indicate that the p values are respectively 0.024 for NO3–N and 0.016 for NH4–N between Zone Ia and Zone Ib, indicating the distinct nitrogen contamination (Table 3). In order to gain the NH4–N and NO3–N distribution of the shallow phreatic water at a regional scale, the difference of nitrogen contamination among Ia, Zone II and Zone III are obtained. As shown in Table 3, the difference between Ia and Zone II is not distinct (p = 0.316 for NO3–N and p = 0.12 for NH4–N), which is consistent with the nitrogen distribution in the two zones. By contrast, the p values are all less than 0.0001 for Zone Ia–Zone II and Zone II–Zone III, confirming the distinct different nitrogen distribution among these zones.
Confined aquifers
For the water samples from the confined aquifers, Table 2 shows that NH4–N has a wider range and higher frequencies of detection than those of NO3–N. Out of the 42 water samples from the confined aquifers, there are only four samples which have NO3–N detectable concentrations. In the upper confined aquifer, the concentration of NH4–N ranges from BDL to 1.4 mg/L, with a mean of 0.64 mg/L. In the lower confined aquifer, the concentration of NH4–N ranges from 0.11 to 1.86 mg/L, with a mean of 0.62 mg/L. 59.52% of them (25 samples) with excess NH4–N (>0.2 mg/L) are not suitable for drinking. Nitrogen contamination is only observed in the regions where pumping of groundwater has been carried out. NO3–N and NH4–N concentrations are undetected in the seven monitoring wells (i.e., W2, W6, W9, S1–S3 and S9) that were not affected by the pumping activities. Importantly, NH4–N predominates in the groundwater for these water supply wells.
Relations between hydrogeological properties and redox conditions
As the redox condition of water exerts a significant control on its chemistry (Ohio EPA 2014), it is necessary to understand the conditions in aquifer system. Nitrate, manganese, iron (Fe), and arsenic (As) in groundwater are often regarded as important indicators to understand redox conditions. On the basis of classification system of McMahon and Chapelle (2008), threshold values of redox indicators were 0.5 mg/L for NO3–N and 0.1 mg/L for Fe, respectively. According to the present study and previous researches (Li et al. 2011; Han et al. 2013), low NO3–N levels (< 0.5 mg/L) and high Fe concentrations (> 0.1 mg/L) have been observed in the phreatic water in the northern part of the study area and in the confined water, suggesting the anaerobic conditions. Meanwhile, high As concentrations (>10 μg/L) and oxidation–reduction potential (ORP) values that were also detected in the phreatic water in the northern part (Han et al. 2013) provide further demonstrations of the anaerobic environment, because the presence of dissolved As suggests that groundwater is relatively isolated from atmosphere and is not actively receiving oxygenated recharge (McMahon and Chapelle 2008; Ohio EPA 2014).
In the study area, the characteristics of hydrogeological factors are quite different along the flow path. The particle size of sediments, permeability, and groundwater flow velocity generally decrease from southwest to northeast in the phreatic aquifer (Qian et al. 2013). In such a situation, the groundwater residence time increases along the flow direction. The phreatic water at a depth of less than 60 m in the southern part of the plain has been estimated to be 6–25 years old, and for the phreatic water in the northern part of the plain has been estimated to be 45–57 years old (Wang et al. 2013). Groundwater circulates slowly and has lower renewal rates in the confined aquifers (Qian et al. 2013). In comparison to the distributions of aforementioned chemical elements, groundwater has long residence time and has a longer time to interact with electron donors in the sediments, resulting in anaerobic conditions. Consequently, age and chemistry data are consistent with anaerobic conditions in Zone III and in the confined aquifers. The close relations between hydrogeological properties and redox conditions have been also identified in other areas (such as Landon et al. 2011; Nishikiori et al. 2012; Han et al. 2015).
Factors controlling the fate of nitrogen in groundwater
Effects of hydrogeological properties on nitrogen contamination
This investigation suggests that NH4–N tends to occur in the groundwater in Zone Ib, Zone III and in confined aquifers. Generally, high NH4–N concentrations beneath irrigated fields can be attributed to two mechanisms: (1) sources of NH4–N and (2) the anaerobic and reducing conditions that activate the denitrification process and limit nitrification process in soil. In this agricultural area, urea (CO (NH2)2) and ammonium bicarbonate (NH4HCO3) are the major fertilizers applied in the fields. To maintain the yield and quality of crops, a large quantity of N fertilizer is commonly applied during cultivation. The average nitrogen fertilizer application rate per ha of cultivated land reached to 832.81 kg, which was far beyond the average rate in high fertilizer input region of China (339 kg/ha) (Chen et al. 2016). Given the high N fertilizer application and flood irrigation approach, the pollution status as reflected by NH4–N concentrations indicates that nitrification process is limited in the groundwater that is deep and near the end of groundwater flow path under the anaerobic conditions. The two mechanisms make the N nutrients prone to leaching into the groundwater in the form of NH4–N. Similar conclusion has been obtained in the typical agricultural catchment in central China, and NH4–N contamination caused by N–fertilizers can also be confirmed by the chemical data (Wang et al. 2015). Unlike nitrogen fertilizer, the point-source-contamination only has a local impact on groundwater (Singh et al. 2006; Umezawa et al. 2008). Given the high variability of NH4–N concentration in phreatic aquifer, the other potential sources such as domestic waste, sewage, and septic tanks seem to exist in the study area. Furthermore, NH4–N were undetected in monitoring wells (W9 and S9), strengthening the rejection of contamination by organic matter in the confined aquifers.
Nitrate contamination is completely different from ammonium. NH4–N tends to be rapidly converted to NO3–N in aerobic environment. Consequently, NO3–N predominates in Zone Ia and Zone II. As nitrate from different sources has characteristic δ 15N and δ 18O values, the isotopic compositions of NO3 − are used to identify nitrate origins of groundwater and the related behavior in this paper. The isotope compositions of groundwater NO3 − were detected in 36 of the 97 collected samples from phreatic aquifer. Although there is no baseline study on the estimation of natural background level of nitrate in Yinchuan Plain, NO3–N concentration above 3 mg/L is commonly known as anthropogenic sources (Babiker et al. 2004; Kaown et al. 2009). When excluding groundwater with NO3–N levels <3 mg/L, the δ 15N–NO3 and δ 18O–NO3 values of groundwater in Zone I, Zone II, and Zone III are plotted in Fig. 6.
Plot of δ 18O–NO3 values versus δ 15N–NO3 values for groundwater in the study area. The ranges of δ 18O and δ 15N in NO3 were from Kendall (1998) and Böttcher et al. (1990), Fukada et al. 2003 and Mayer et al. (2002). Gray, black, and blue dots denote the samples in Zone I, Zone II, and Zone II, respectively
Measured δ 15N values vary from +3.94 to +19.09‰ with a mean level of +9.72‰ in Zone I and Zone II. The δ 15N values range from +5.70 to +28.87‰ with a mean level of 16.35‰ in Zone III. Using the δ 15N–NO3 values for fertilizer (−4 to +4‰) and manure or sewage (+8 to +20‰) from literature data (Kendall 1998; Böttcher et al. 1990; Fukada et al. 2003), it appears that a mixture of synthetic fertilizer and manure/sewage are responsible for the observed δ 15N range between +4 and 20‰. The δ 18O data also exhibit direct derivation from different sources (Fig. 6). Considering the typical ranges of δ 18O value for fertilizers (+18 to +22‰) and for manure/sewage (+0 to +8‰), the measured δ 18O–NO3 values (+5.93 to +21.72‰) of groundwater in the phreatic aquifer further confirm the mixing between the two different sources. Further, the isotopic compositions of nitrogen and oxygen appear to increase at a ratio of about 2:1 (Fig. 6), indicating the occurrence of denitrification. The higher mean value of δ 15N in the remaining nitrate in Zone III tends to indicate the more intensive denitrification occurring in the zone.
To better elucidate the effects of hydrological properties on nitrogen contamination, the changes in NO3–N and NH4–N contents along the A–A′ and B–B′ flow paths in the phreatic aquifer are studied (Fig. 7). Coarse sediments are highly permeable and the hydraulic gradient is steep (1–5‰) in Zone I and Zone II (Fig. 3 and Fig. 8). Under the aerobic conditions, NH4–N oxidized rapidly, and NO3–N can move easily and predominates in A1 and B1 (Zone I and Zone II). Subsequently, as the groundwater enters Zone III, the contents of fine particles (such as clayey sand and sandy clay) below the sediments increase in the north direction and the hydraulic gradient simultaneously decreases (1–0.2‰) (Fig. 8). NH4–N that mainly derived from fertilizer is more easily to reach the water table where the depth to water is small. These conditions ensure a long resident time for the groundwater in A2 and B2–B4. Under the anaerobic conditions, NH4–N cannot to be oxidized and then become dominant in relation to the probable loss of nitrate through denitrification. Further, Fig. 8c shows that the slow water cycle in Zone III is also supported by the distribution of salinized land. All these findings indicate the major contribution of N–fertilizer on nitrogen contamination in groundwater, and the nitrogen species (NO3–N and NH4–N) are primarily controlled by the hydrological conditions.
Changes in NO3–N and NH4–N concentrations with groundwater flow a A–A′ and b B–B′. The vertical sections of the two lines (locations of the sections are shown in Fig. 2a are parallel to the flow directions
Maps of a hydraulic gradient of water in phreatic aquifer, b aquifer media, and c salinized land (data of (b) and (c) from Qian et al. (2012))
Influences of groundwater pumping on groundwater ammonium concentrations
The most severe consequence of intensive groundwater pumping is that the water table can be lowered. Due to the increasing vertical hydraulic gradient, pumped aquifer can be recharged by the adjacent aquifer through aquifer leakage. The situation is more likely to occur in drinking water sources and cone of depression areas (Qian et al. 2013; Wu et al. 2015a). As shown in Fig. 9, intensive extraction has caused serious decline in groundwater levels of the confined aquifers in Yinchuan plain, and a continuous decline of the water levels has been observed in recent years (Chen et al. 2015; Wu et al. 2015a). This implies that aquifer leakage from the phreatic aquifer to the upper confined aquifer or/and from the upper confined aquifer to the lower confined aquifer seems easier to occur in the pink and red areas (Fig. 9), inducing the alternation of the water chemicals.
In order to identify the effects of pumping rate on NH4–N, the hypothesis is that the high NH4–N is caused by leakage from the upper lying aquifer. The average concentrations of NH4–N from the water samples of the upper confined aquifer (Cupper) and the lower confined aquifer (Clower) are shown in Fig. 10. Under the anaerobic conditions, NH4–N is the dominant form in the groundwater. Comparing NH4–N concentrations, Cupper and Clower are close in the five water sources (Beijiao, Nanjiao, Helan water source, and NO.1 and NO.4 water plants) within the cone of depression, where the two confined aquifers were pumped simultaneously. However, Clower in Nanjiao water source is higher, which had a high pumping rates of 100,000 m3/day. Clower in the NO.1 and NO.4 water plants is low, which had a low pumping rate of less than 30,000 m3/day. Similarly, the higher concentrations of NH4–N in the lower confined water in the Helan and Beijiao water sources can be attributed to pumping as it had a high pumping rate of 100,000 m3/day. Therefore, even though these wells have similar depths and locate close to each other, the pumping has accelerated the downward migration of nitrogen contamination through aquitards under high pumping rate. The recent research conducted by Wu et al. (2015b) also proved the serious NH4–N contamination in the confined water for Beijiao water source. They found that the NH4–N concentrations varied from 0.116 to 0.362 mg/L in 14 water supply wells. The range of NH4–N is consistent with the observation in the present study. As the groundwater is the source of drinking water for residents, the potential contamination of the water in the confined aquifers is a “time bomb”. Even though the timing of the impending “explosion” cannot be predicted due to the insufficient evidence, it is however accelerated by the groundwater pumping.
Conclusions
The residents on Yinchuan plain depend heavily on the groundwater resource. This study has shown that there is excessive nitrogen in the groundwater, in both the phreatic aquifer and the confined aquifers. Hydrochemical, hydrogeological, and isotopic analyses have been carried out on pollution status of nitrogen in the aquifer system. The conclusions are:
-
(1)
The concentrations of NO3–N and NH4–N in 11.37 and 40% of phreatic water samples exceed the national allowable levels of nitrogen for drinking water based on the WHO standards. In confined aquifers, 59.52% of samples are not suitable for drinking due to excessive NH4–N; but NO3–N concentrations are considerably lower or undetectable.
-
(2)
The specific characteristics of nitrogen contamination in the aquifer system are as follows. For the single phreatic aquifer, NO3–N predominates in the shallow groundwater and NH4–N predominates in the deep groundwater. For the multilayer structure area, NO3–N predominates in the phreatic aquifer of the western and the southern parts of the plain; NH4–N predominates in the phreatic aquifer of the middle and the northern parts of the plain, and in the confined aquifers where groundwater pumping had been performed.
-
(3)
The distribution of nitrogen in groundwater is primarily controlled by the land use. For phreatic water, the isotopic values range from +3.94 to +28.87‰ for δ15N and from +5.93 to +21.72‰ for δ18O, respectively. The measured isotopic values reveal that the nitrogen contamination in the phreatic aquifer is mainly contributed by the mixture of synthetic fertilizer and manure/sewage. Groundwater pumping is an important reason for the nitrogen contamination in confined aquifers due to the observed higher NH4–N concentrations in the lower confined water under higher pumping rates.
-
(4)
The hydrogeological properties control nitrogen fates depending on the redox conditions at a regional scale. For the phreatic aquifer at the middle and northern parts of the plain and the confined aquifers, nitrification process is limited by the longer residence time, showing anaerobic conditions in the groundwater that is deep or near the end of flow path. The occurrence of denitrification is verified by isotopic analysis in the phreatic aquifer of the middle and the northern parts of the plain.
The results of this study have advanced the understanding of the nitrogen fate in the aquifer system. They can be used as a scientific basis for developing more guidelines on groundwater monitoring and pumping programs. Given the higher NH4–N concentrations in drinking water sources, water quality deterioration may be of the greatest health concern in future. To provide more information for quantifying N cycle in the surface water–soil–groundwater system, further research in the agricultural area is sorely needed.
References
Babiker IS, Mohamed AA, Terao H, Kato K, Ohta K (2004) Assessment of groundwater contamination by nitrate leaching from intensive vegetable cultivation using geographical information system. Environ Int 29:1009–1017
Böttcher J, Strebel O, Voerkelius S, Schmidt HL (1990) Using isotope fractionation of nitrate–nitrogen and nitrate–oxygen for evaluation of microbial denitrification in sandy aquifer. J Hydrol 114:413–424
Böhlke JK, Smith RL, Miller DN (2006) Ammonium transport and reaction in contaminated groundwater: application of isotope tracers and isotope fractionation studies. Water Resour Res 42:W05411
Chen J, Wu H, Qian H, Liu Q (2015) Analysis of evolvement for confined water cone of depression and its influence on groundwater resource sustainability in Yinchuan area. Adv Mater Res 1073–7076:1656–1659
Chen J, Wu, H, Qian H (2016) Groundwater nitrate contamination and associated health risk for the rural communities in an agricultural area of Ningxia, northwest China. Expo Health doi:10.1007/s12403–016–0208–8
Diez JA, Caballero R, Roman R, Tarquis A, Cartagena MC, Vallejo A (2000) Integrated fertilizer and irrigation management to reduce nitrate leaching in Central Spain. J Environ Qual 29:1539–1547
Dragon K (2012) Groundwater nitrate pollution in the recharge zone of a regional quaternary flow system (Wielkopolska region, Poland). Environ Earth Sci 68:2099–2109
Fukada T, Hiscock K, Dennis PF, Grischek T (2003) A dual isotope approach to identify denitrification in groundwater at a river–bank infiltration site. Water Res 37:3070–3078
Gustafson A (1983) Leaching of nitrate from arable land into groundwater in Sweden. Environ Geol 5:65–71
Han S, Zhang F, Zhang H, An Y, Wang Y, Xi W, Cheng W (2013) Spatial and temporal patterns of groundwater arsenic in shallow and deep groundwater of Yinchuan Plain, China. J Geochem Explor 135:71–78
Han D, Cao G, McCallum J, Song X (2015) Residence times of groundwater and nitrate transport in coastal aquifer systems: Daweijia area, northeastern China. Sci Total Environ 538:539–554
Heaton TH, Stuart ME, Sapiano M, Sultana MM (2012) An isotope study of the sources of nitrate in Malta’s groundwater. J Hydrol 414:244–254
Hosono T, Tokunaga T, Kagabu M, Nakata H, Orishikida T, Lin IT, Shimada J (2013) The use of δ 15N and δ 18O tracers with an understanding of groundwater flow dynamics for evaluating the origins and attenuation mechanisms of nitrate pollution. Water Res 47:2661–2675
Hinkle SR, Böhlke JK, Duffc JH, Morgana DS, Weick RJ (2007) Aquifer–scale controls on the distribution of nitrate and ammonium in ground water near La Pine, Oregon, USA. J Hydrol 333(2–4):486–503
Ju X, Kou C, Zhang F, Christie P (2006) Nitrogen balance and groundwater nitrate contamination: comparison among three intensive cropping systems on the North China Plain. Environ Pollut 143:117–125
Kendall C (1998) Tracing nitrogen sources and cycling in catchment. In: Kendall C, Mcdonnell JJ (eds) Isotope tracers in catchment hydrology. Elsevier, Amsterdam, pp 519–576
Kaown D, Koh D, Mayer B, Lee K (2009) Identification of nitrate and sulfate sources in groundwater using dual stable isotope approaches for an agricultural area with different land use (Chuncheon, mid–eastern Korea). Agric Ecosyst Environ 132:223–231
Landon M, Green C, Belitz K, Singleton M, Esser B (2011) Relations of hydrogeologic factors, groundwater reduction–oxidation conditions, and temporal and spatial distributions of nitrate, central–eastside San Joaquin valley, California, USA. Hydrogeol J 19:1203–1224
Li P, Wu Q, Wu J (2011) Groundwater suitability for drinking and agricultural usage in Yinchuan area, China. Int J Environ Sci 1(6):1241–1249
Li P, Qian H, Wu J (2014a) Origin and assessment of groundwater pollution and associated health risk: a case study in an industrial park, northwest China. Environ Geochem Health 36(4):693–712
Li P, Qian H, Wu J, Chen J, Zhang Y, Zhang H (2014b) Occurrence and hydrogeochemistry of fluoride in shallow alluvial aquifer of Weihe River, China. Environ Earth Sci 71(7):3133–3145
Li P, Qian H, Howard KWF, Wu J, Lyu X (2014c) Anthropogenic pollution and variability of manganese in alluvial sediments of the Yellow River, Ningxia, northwest China. Environ Monit Assess 186(3):1385–1398
Li P, Qian H, Howard KWF, Wu J (2015a) Building a new and sustainable “Silk road economic belt”. Environ Earth Sci 74:7267–7270
Li P, Qian H, Howard KWF, Wu J (2015b) Heavy metal contamination of Yellow River alluvial sediments, northwest China. Environ Earth Sci 73(7):3403–3415
Li P, Wu J, Qian H (2016a) Preliminary assessment of hydraulic connectivity between river water and shallow groundwater and estimation of their transfer rate during dry season in the Shidi River, China. Environ Earth Sci 75(2):99. doi:10.1007/s12665–015–4949–7
Li P, Wu J, Qian H, Zhang Y, Yang N, Jing L, Yu P (2016b) Hydrogeochemical characterization of groundwater in and around a wastewater irrigated forest in the southeastern edge of the Tengger Desert. Northwest China Expo Health. doi:10.1007/s12403–016–0193–y
Li X, Masuda H, Koba K, Zeng H (2006) Nitrogen isotope study on nitrate–contaminated groundwater in the Sichuan basin, China. Water Air Soil Poll 178:145–156
Low H (1974) Nitroso compounds: safety and public health. Arch Environ Health 29:256–260
Marinov I, Marinov AM (2014) A coupled mathematical model to predict the influence of nitrogen fertilization on crop, soil and groundwater quality. Water Resour Manag 28:5231–5246
Mayer B, Boyer EW, Goodale C et al (2002) Sources of nitrate in rivers draining sixteen watersheds in the northern US: isotopic constrains. Biogeochemistry 57–58:171–197
McMahon PB, Chapelle FH (2008) Redox processes and water quality of selected principal aquifer systems. Ground Water 46:259–271
Miller J, Miller JC (1988) Statistics for analytical chemistry, 2nd edn. Wiley, New York
Ministry of Health of PRC, Standardization Administration of PRC (2006) Standard examination methods for drinking water (GB/T5750–2006). Standards Press of China, Beijing (in Chinese)
Ministry of Water Resources of PRC (1997) Technical regulation of water quality sampling (SL 187–96). Standards Press of China, Beijing (in Chinese)
Nishikiori T, Takamatsu T, Kohzu A, Nakajima Y, Watanabe M (2012) Distribution of nitrate in groundwater affected by the presence of an aquitard at an agricultural area in Chiba, Japan. Environ Earth Sci 67:1531–1545
Park DK, Bae G, Kim S, Lee K (2014) Groundwater pumping effects on contaminant loading management in agricultural regions. J Environ Manag 139:97–108
Ohio Environmental Protection Agency (EPA) (2014) Reduction–oxidation (redox) control in Ohio’s ground water quality. J Environ Manag 139:97–108
Pastén -Zapata E, Ledesma-Ruiz R, Harter T, Ramírez AI, Mahlknecht J (2014) Assessment of sources and fate of nitrate in shallow groundwater of an agricultural area by using a multi–tracer approach. Sci Total Environ 470–471:855–864
Qian H, Li P (2011) Hydrochemical characteristics of groundwater in Yinchuan plain and their control factors. Asian J Chem 23(7):2927–2938
Qian H, Li P, Howard KW, Yang C, Zhang X (2012) Assessment of groundwater vulnerability in the Yinchuan plain, Northwest China using OREADIC. Environ Monit Assess 184:3613–3628
Qian H, Li P, Wu J, Zhou Y (2013) Isotopic characteristics of precipitation, surface and ground waters in the Yinchuan plain, Northwest China. Environ Earth Sci 70:57–70
Qian H, Wu J, Zhou Y, Li P (2014) Stable oxygen and hydrogen isotopes as indicators of lake water recharge and evaporation in the lakes of the Yinchuan Plain. Hydrol Process 28:3554–3562. doi:10.1002/hyp.9915
Serhal H, Bernard D, Khattabi JE, Sabine B–L, Shahrour I (2008) Impact of fertilizer application and urban wastes on the quality of groundwater in the Cambrai Chalk aquifer, Northern France. Environ Geol 57:1579–1592
Singh KP, Singh VK, Malik A, Basant N (2006) Distribution of nitrogen species in groundwater aquifers of an industrial area in alluvial Indo–Gangetic Plains—a case study. Environ Geochem Health 28:473–485
Subramani T, Rajmohan N, Elango L (2010) Groundwater geochemistry and identification of hydrogeochemical processes in a hard rock region, southern India. Environ Monit Assess 162:123–137
Thorburn PJ, Biggs JS, Weier KL, Keating BA (2003) Nitrate in groundwaters of intensive agricultural areas in coastal northeastern Australia. Agric Ecosyst Environ 94:49–58
Umezawa Y, Hosono T, Onodera S et al (2008) Sources of nitrate and ammonium contamination in groundwater under developing Asian megacities. Sci Total Environ 404:361–376
Wang L, Hu F, Yin L (2013) Hydrochemical and isotopic study of groundwater in the Yinchuan plain, China. Environ Earth Sci 69:2037–2057
Wang Y, Li Y, Li Y, Liu F, Liu X, Gong D, Ma Q, Li W, Wu J (2015) Intensive rice agriculture deteriorates the quality of shallow groundwater in a typical agricultural catchment in subtropical central China. Environ Sci Pollut Res 22:13278–13290
Ward MH, DeKok TM, Levallois P, Brender J, Gulis G, Nolan BT, VanDerslice J (2005) Workgroup report: drinking–water nitrate and health–recent findings and research needs. Environ Health Persp 113:1607–1614
Welch H, Green C, Coupe R (2011) The fate and transport of nitrate in shallow groundwater in northwestern Mississippi, USA. Hydrogeol J 19:1239–1252
WHO (2011) Guidelines for drinking–water quality (4th ed).
Wu J, Li P, Qian H, Fang Y (2014) Assessment of soil salinization based on a low–cost method and its influencing factors in a semi–arid agricultural area, northwest China. Environ Earth Sci 71(8):3465–3475
Wu J, Li P, Qian H (2015a) Hydrochemical characterization of drinking groundwater with special reference to fluoride in an arid area of China and the control of aquifer leakage on its concentrations. Environ Earth Sci 73:8575–8588
Wu H, Chen J, Qian H, Zhang X (2015b) Chemical characteristics and quality assessment of groundwater of exploited aquifers in Beijiao water source of Yinchuan, China: a case study for drinking, irrigation, and industrial purposes. J Chem 726340
Wu J, Sun Z (2015) Evaluation of shallow groundwater contamination and associated human health risk in an alluvial plain impacted by agricultural and industrial activities, mid–west China. Expo Health:1–19
Yoshimoto S, Tsuchihara T, Ishida S, Masumoto T, Imaizumi M (2011) Groundwater flow and transport and potential sources of groundwater nitrates in the Ryukyu limestone as a mixed flow aquifer in Okinawa island, Japan. Paddy Water Environ 9:367–384
Zhang Y, Li F, Zhang Q, Li J, Liu Q (2014) Tracing nitrate pollution sources and transformation in surface– and ground–waters using environmental isotopes. Sci Total Environ 490:213–222
Acknowledgements
The research was supported by the National Natural Science Foundation of China (41502234 and 41172212), the Special Fund for Basic Scientific Research of Central Colleges (310829151072), the Doctoral Postgraduate Technical Project of Chang’an University (2014G5290005), and the Foundation for the Excellent Doctoral Dissertation of Chang’an University (310829150002 and 310829165005). The editor and anonymous reviewers are also greatly acknowledged for their useful comments that have helped us to improve the quality of our paper.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Responsible editor: Boqiang Qin
Rights and permissions
About this article
Cite this article
Chen, J., Qian, H. & Wu, H. Nitrogen contamination in groundwater in an agricultural region along the New Silk Road, northwest China: distribution and factors controlling its fate. Environ Sci Pollut Res 24, 13154–13167 (2017). https://doi.org/10.1007/s11356-017-8881-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-017-8881-0