Abstract
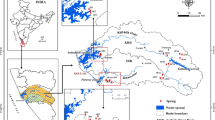
The Indian state of Odisha has a number of thermal springs. These thermal springs are located at eight places (Attri, Tarabalo, Deulajhari, Magarmuhan, Bankhol, Badaberena, Taptapani and Boden) and belong to Mahanadi Geothermal Province, which is an Archean/Pre-Cambrian Geothermal Province. The thermal water discharging from these springs shows moderately acidic to moderately alkaline character (pH: 5.05–8.93) and the temperature ranges from 28 (Boden) to 58 °C (Tarabalo). Total dissolved solids (TDS) also shows a wide variation between 16.9 (Bankhol) and 595 mg/L (Deulajhari). A wide variation in the chemical characteristics of the thermal waters has been observed as they are located in different geological settings. Based on water chemistry, all the thermal springs can broadly be grouped into three water types: Na–Cl, Ca–HCO3 and Na–HCO3. The thermal spring water from Attri, Tarabalo and Deulajhari belongs to Na–Cl water type which is due to the circulation through granitic rocks. Higher concentrations of Cl and F in these thermal waters further suggest limited mixing and longer residence time as compared to the other areas where Ca–HCO3 and Na–HCO3 water types were found. Anion variation diagram clearly indicates that the thermal waters from Attri, Tarabalo and Deulajhari are fast ascending and fall in the mature water field; thus, their chemical signatures can be used to determine the reservoir temperature. However, in other areas, water chemistry is shaped by near-surface groundwater mixing processes and thus the chemical geothermometers may not be applicable to determine the reservoir temperature. No appreciable temporal variations have been observed in the water chemistry of the thermal waters.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
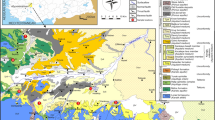
The demand for energy is increasing exponentially over the time. To meet this increasing demand in an eco-friendly way, alternative energy resources are being developed and geothermal energy forms an important part of it. Existences of vast reservoirs of geothermal energy at depths are manifested on the surface in form of thermal springs. In India, there exist around 400 geothermal springs (Chandrasekharam 2000). These geothermal springs are distributed across the country and are grouped into 10 geothermal provinces, namely Himalayan Geothermal Province; Naga Lushai Geothermal Province; Andaman–Nicobar Islands Province; West Coast Geothermal Province; Cambay Graben Geothermal Province; Aravalli Province; Son-Narmada-Tapti (SONATA) Geothermal Province; Godavari Geothermal Province; Mahanadi Geothermal Province and South Indian Cratonic Province (Fig. 1a) (Thussu 2002; Shanker et al. 1991; Chandrasekharam and Chandrasekhar 2010a). Around 50% of the thermal springs are located in the Himalayan Geothermal Province alone (Craig et al. 2013). The source of heat and the surface water temperature varies widely and thus their geothermal potential. The surface water temperature ranges from >35 to 100 °C. Maximum surface water temperature (75–100 °C) is observed in Himalayan and SONATA Geothermal Provinces (Fig. 1b) (Krishnaswamy 1975). Three geothermal provinces (i.e., Himalayan Geothermal Province; Naga Lushai Geothermal Province; Andaman–Nicobar Islands Province) fall in the orogenic regions and are associated with the provinces of Tertiary magmatism and metamorphism. The remaining seven geothermal provinces fall in non-orogenic or the peninsular regions. Most of these peninsular geothermal provinces are associated with graben structure (Thussu 2002). Literature archive shows that a lot of studies have been done on non-orogenic geothermal provinces of India with regard to their surface water chemistry, reservoir temperature and evaluation of their geothermal potential except for the Mahanadi Geothermal Province which is still one of the least studied as compared to the other geothermal provinces in India (Thussu 2002; Chandrasekharam and Chandrasekhar 2010a, b). The Mahanadi Geothermal province falls in the state of Odisha, and the thermal springs are manifested at eight locations, namely Attri, Tarabalo, Deulajhari, Magarmuhan, Bankhol, Badaberena, Taptapani and Boden. These springs belong to Archean/Pre-Cambrian Geothermal Province and the temperature of the surface water discharging from the thermal spring ranges from 28 to 58 °C. However, maximum temperature up to 85 °C has also been reported (Thussu 2002). In the case of Attri, Magarmuhan, Bankhol, Taptapani and Boden, the hot spring water discharges from a single spot, whereas in Tarabalo, Deulajhari and Badaberena, hot water discharges from multiple spots. The rate of discharge recorded at Attri, Tarabalo, Taptapani and Deulajhari thermal springs is 2.1, 2.7, 2–7 and 2.1 l/s, respectively (Thussu 2002), while for other springs it is not known. Although Mahanadi Geothermal Province did not get the required attention in the past but now is considered to be a potential site for developing both wet and enhanced geothermal system (EGS) geothermal projects in the future (Chandrasekharam and Chandrasekhar 2010b).
Map showing geothermal provinces of India and distribution of geothermal springs in Odisha. a Location of major geothermal provinces with heat flow values (Chandrasekharam and Chandrasekhar 2010a), b surface temperature of major geothermal springs (Krishnaswamy 1975), and c location of geothermal springs in the state of Odisha (Mahala et al. 2012)
Hydrogeochemistry of the spring water is a useful tool to decipher the reservoir temperature, mixing of the ascending water and the processes associated with it (Xilai et al. 2002; Bulbul 2015; Han et al. 2010; Chatterjee et al. 2017). The hydrogeochemistry of the thermal water mainly depends upon (1) the water type (i.e., the origin of water), (2) presence of structural lineaments, (3) mixing of ascending thermal water with the groundwater and (4) the rocks through which the thermal water interacts while it ascends. Studies have found that mixing of cold water with ascending thermal water modifies its chemical characters significantly (Han et al. 2010). The mixing may further be accelerated by groundwater exploitation near the faulted region (Bulbul 2015; Gokgoz and Akdagoglu 2016). However, in coastal regions, mixing of thermal water with seawater intruded through deeper faults may completely alter the chemical signatures of the thermal waters (Gemici and Feliz 2001). The origin of water/water type (connate, meteoric, and juvenile) has also an influence on the chemistry of the thermal waters. Thus, to draw any useful conclusion from the chemistry of thermal water, it is important to determine whether the water discharging from the thermal spring has preserved the chemical signatures of the geothermal reservoir or its chemical properties are modified by various processes (such as mixing with the groundwater, interaction with wall rock). It is also important to determine whether the chemistry of the thermal water changes seasonally. In the case of a prominent seasonal change in thermal water chemistry and extensive mixing, the inference drawn based on thermal water chemistry may not be very useful. A study has thus been undertaken with the following objectives: (1) to understand the water chemistry of the thermal springs located in different geological settings, (2) to determine the suitability of thermal spring waters to calculate reservoir temperature using chemical geothermometers, and (3) to determine whether the chemistry of thermal water varies due to seasonal changes in shallow groundwater chemistry.
Study area
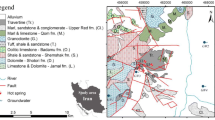
The study area lies in the state of Odisha with a total area of about 155,842 Sq.km and located along the east coast of India within latitudes 17°48′–22°34′N and longitude 81°24′–87°29′E. Geographically, the territory can be divided into four distinct regions: the Northern Plateau, the Central River Basin, the Eastern Ghats Hill Ranges and the Coastal Plains. The rocks of the state are mainly of Pre-Cambrian age with some Phanerozoic rocks, represented by the Gondwana Supergroup and minor patches of Tertiary and Quaternary formations (Chowdhury et al. 2011). The thermal springs manifested at eight locations are located in three different geological settings: Attri, Tarabalo, Deulajhari and Taptapani lie within Eastern Ghats Supergroup; Magarmuhan, Bankhol and Badaberena lie within Iron Ore Supergroup (IOSG); and Boden lies within Vindhyan Supergroup (Fig. 1c). The Mahanadi and Godavari Rift divides the Eastern Ghat Belt (EGB) into the northern, central and the southern segments (Sarkar and Nanda 1998) of which the northern segment and part of central segment lie within the state of Odisha. In Odisha sector, EGB is broadly divided into Khondalite Group, Charnockite Group and Migmatite Group, which from east to west lies in the sequence of Eastern Khondalite Zone, Central Migmatite Zone, Western Khondalite Zone, Western Charnockite Zone and Westernmost Transition Zone (Ramakrishnan 1987). The rock types encountered at Attri, Tarabalo, Deulajhari and Taptapani are Laterite, Khondalite, Charnockite and Augen gneiss (granitic), while at Magarmuhan and Bankhol mostly quartzite of IOSG is found (Mahala et al. 2012). The aerial photograph reveals the presence of NW–SE and NE–SW fractures/lineaments at Attri and Tarabalo (Thussu 2002). Deulajhari is located close to a NNE–SSW trending shear zone, and the thermal spring is located along banks of Mahanadi River which flows along a NW–SE fault in the area. One fracture trending NW–SE to WNW–ESE runs parallel to the Mahanadi lineament and passes very close to the Deulajhari thermal spring (Thussu 2002). A schematic geological profile illustrating the flow patterns of thermal springs located at the boundary of Gondwana Graben is shown in Fig. 2, and the generalized stratigraphic succession of the study area is given in Table 1. The state of Odisha has tropical climate characterized by high temperature, high humidity and medium-to-high rainfall. The annual rainfall varies from 1008 to 1988 mm with an average of 1481 mm across the state (Patra et al. 2012). The wind flow pattern developed in the upper atmosphere during the summer monsoon makes the state favorable for good rainfall (Mohapatra and Mohanty 2006). Rainfall data from the year 1871–2006 show that most of the rainfall occurs during the months of June, July, August and September (Patra et al. 2012).
Map showing a location of Eastern Ghat Belt (EGB) in India, b litho-tectonics units of EGB and adjacent cratons. Abbreviations used are MSZ Mahanadi Shear Zone, VSZ Vamshadhara Shear Zone, NSZ Nagavalli Shear Zone, SSZ Sileru Shear Zone, KSZ Koraput–Sonepur Shear Zone, and EGBSZ Eastern Ghats Boundary Shear Zone (Dasgupta et al. 2013), and c conceptual model with schematic geological profile and the flow pattern (Mahala et al. 2012; Sastri et al. 1981). EGSG Eastern Ghat Supergroup, IOSG Iron Ore Supergroup
Sampling and methodology
To investigate the water chemistry of the thermal springs, water samples were collected directly from the springs following the standard scientific protocol (APHA 1995). Water samples were collected from the thermal springs during the pre-monsoon, monsoon and post-monsoon seasons. In the areas where hot springs occur in clusters, a number of samples were collected randomly to represent the entire area. Additionally, shallow surface water samples were collected from the areas near to the thermal springs. Each sample collection site was georeferenced using a Garmin Global Positioning System (GPSMAP-76S) instrument. Water samples from all locations include the collection of: (1) filtered samples (0.45 µm cellulose nitrate filter) for analyses of major anions and (2) acidified (with 5 mL 14 M ultrapure HNO3/L) samples for major cation and trace element analyses. Samples were tightly sealed and stored at a low temperature until further analyses. Parameters like pH, temperature, electrical conductivity (EC) and total dissolved solids (TDS) were measured in the field. The pH value was measured with a portable pH meter (Orion 261S) using a combination electrode (pH C2401–7). EC and TDS were measured by a conductivity meter (Orion 250A+) with an operating range between 0 and 500 mS/cm. Alkalinity was measured by titration method using auto-titrator model Titrino Plus; fluoride and chloride were measured by ion analyzer with combination electrode Orion ionplus and sulfate concentrations were measured by UV–visible spectrophotometer. Analyses of cations were done with inductively coupled plasma–atomic emission spectroscopy (ICP-AES).
Results and discussion
The thermal spring water samples collected from the study area show moderately acidic to moderately alkaline characteristics. During the pre-monsoon season, pH values range from 5.1 to 8.8 (mean 7.5), while for monsoon and post-monsoon seasons, the value ranges from 5. 9 to 8.6 (mean 7.2) and 6.7 to 8.9 (mean 7.8), respectively. The pH does not show any season-specific trend. In some sample, higher pH value has been observed during post-monsoon, while in other samples higher pH values have been observed either during pre-monsoon or monsoon season. The temperatures of discharging thermal water do not show any significant temporal variation, and the temperature varies within a range of 1–4 °C for all the thermal springs. However, a significant spatial variation in the temperature of discharging thermal water has been observed, and the minimum temperature of 28 °C at Boden and maximum temperature of 58 °C at Tarabalo thermal spring have been observed. TDS values do not show much variation and remain same in all the seasons except for Tarabalo, where lower TDS values have been observed during monsoon season. However, a wide spatial variation in TDS values has been observed and it ranges from 16.9 (Bankhol) to 595 mg/L (Deulajhari). It is interesting to note that the major ion concentration also does not show much temporal variation, whereas a significant spatial variation has been observed in their concentrations. Sodium (Na) and chloride (Cl) are the dominant ions in the hot spring water discharging from Attri, Tarabalo and Deulajhari locations, whereas at other locations, bicarbonate (HCO3) is the most dominant species present in the thermal discharge. The concentration of Na and Cl ranges from 1.33 to 239.2 and 0.98–289 mg/L, respectively. Calcium (Ca), magnesium (Mg) and potassium (K) are present in much lesser concentrations, and their concentration varies between 0.15 and 65.45, <0.1–22.9 and 0.42–7.48 mg/L, respectively. The concentrations of HCO3 range between 9.4 and 306.6 mg/L. Higher HCO3 concentrations have been observed specifically in those locations where lower Cl concentration has been registered (Fig. 3). Sulfate (SO4) concentration ranges between 4.35 and 130 mg/L which is much lower than HCO3 and Cl concentrations. Among all the thermal springs, higher sulfate concentration has been observed at Attri, Tarabalo, and Deulajhari. In addition to major ions, lithium (Li) concentrations were also measured in the thermal water sample. The concentration of Li remains low in all the samples and ranges between <0.1 and 0.26 mg/L. The low Li/Na ratio indicates that the water is of shallow origin and had a limited interaction with the wall rock (White 1957). For better understanding of the temporal and spatial variation in the chemistry of the thermal waters, time series data of rainfall and surface temperature for different locations of study area have been obtained from Tropical Rainfall Measuring Mission (TRMM, 3B43) (Huffman et al. 2007) and European Centre for Medium-Range Weather Forecasts (ECMWF) (Dee et al. 2011), respectively. The plot of monthly average maximum temperature and monthly rainfall is shown in Fig. 4a, b. Despite significant seasonal changes in the surface temperature and rainfall, the hydrochemical characteristic of the thermal water remains the same. This can only happen when there is no appreciable seasonal change in supply and mixing of shallow water with the thermal water. The absence of a prominent seasonal variation in water chemistry of the thermal waters indicates that the thermal water sample can be collected in any season during the year and may be used to decipher the subsurface properties of the reservoir based on the chemical signature. However, it is important to note that the water from every single thermal spring cannot be used to determine the reservoir temperature or interpret other important subsurface parameters that depend upon the chemistry of the thermal water, until it fulfills some primary requirements. Anion variation diagram helps in determining the samples that can be used to determine the reservoir temperature using a cation geothermometer (Giggenbach 1988). The plotting of collected thermal water samples into anion variation diagram clearly shows that the water samples fall into two fields: Cl and HCO3 field (Fig. 5). The water samples belonging to Attri, Tarabalo and Deulajhari fall in and around the chloride/mature field and thus are considered as fast ascending waters with limited shallow surface mixing. Such type of thermal waters can be used to determine the reservoir temperature with a higher accuracy. The other thermal water falls in the HCO3 field which indicates the dominant influence of peripheral and shallow water in controlling the chemistry of the thermal waters. The SO4 field of the plot suggests mixing of volcanic gas with the thermal water (Singh et al. 2014). However, in the present study, none of the thermal spring water falls in SO4 field. This indicates that the subsurface geothermal reservoir is not emanating any gases. The HCO3:Cl ratio which indicates the degree of mixing of shallow water with ascending thermal water is given in Table 2. The physical and chemical parameters of shallow surface water samples collected from the surrounding areas of thermal springs are given in Table 3. The value of the HCO3:Cl ratio remains less than unity for thermal springs belonging to Attri, Tarabalo and Deulajhari. This further supports that there is little or no mixing of the ascending thermal waters with the shallow surface water. In the case of all other thermal springs, the HCO3:Cl ratio is more than 1, which indicates that the thermal water composition is modified by the secondary processes. In the case of Taptapani and Boden, the HCO3:Cl ratio is even above 20 which signifies severe mixing of shallow surface water in these thermal spring water. It is thus clear that the chemical geothermometers can be applied only to the thermal spring waters belonging to Attri, Tarabalo and Deulajhari locations.
The major ion composition of the thermal spring water samples (Table 4) was plotted in the Piper diagram using AquaChem 2012.1 software which is widely used in bringing out chemical relationships among the water samples and also to infer about their subsurface interaction with the surrounding rock (Piper 1944). The diagram consists of two triangles for plotting cations and anions. The values plotted in these triangles are projected so as to form a single point in a diamond-shaped field from which inferences are drawn. The plot clearly distinguishes the collected thermal water samples into three water types (Na–Cl type, Ca–HCO3 type and Na–HCO3 type). The thermal water samples from Attri, Tarabalo and Deulajhari fall in Field-II and are characterized as Na–Cl water type (Fig. 6). Such water type generally reflects the mixing of seawater with the ascending thermal waters (Gemici and Feliz 2001). The contribution from the sea not only causes an increase in Na and Cl concentrations but also results in higher TDS values. The value of TDS as high as 35,000 mg/L has been reported in the case of Na–Cl water where sea water mixing controlled the chemistry of thermal water (Gemici and Feliz 2001). However, in the present study maximum TDS value of 595 mg/L is registered. Such TDS value indicates that the Na and Cl concentrations are not contributed by seawater mixing but from some other sources. Chloride is considered as a good tracer in geothermal systems due to its conservative behavior (Michard 1990; Alcicek et al. 2016; Bulbul 2015); thus, the concentration of major ions from Attri, Tarabalo and Deulajhari was plotted against Cl (Fig. 7). A strong correlation of Cl with Na (R 2 = 0.77) and good correlation with K and Ca (R 2 = 0.57 and 0.51, respectively) clearly establish that the rock–water interaction is a dominant mechanism for controlling the concentrations of these ions in the system (Alcicek et al. 2016). The geophysical studies conducted in the area show the existence of a vertically faulted margin along with the presence of granite and syenite at the deeper level (Nayak et al. 1998). The water in a granitic terrain when circulates deeper through various structural lineaments (i.e., faults, shear zone) picks up Na and Cl ions (Chandrasekharam and Antu 1995; Savage et al. 1987; Hem 1985). Similar circulation through the fault intersecting the granitic rocks in the study area accounts for higher concentrations of Na and Cl in thermal waters. Further, low concentrations of calcium along with higher concentrations of fluoride have also been observed in the thermal waters. Due to lower calcium concentrations, the water does not reach the solubility product of fluorite (Ozsvath 2008; Chae et al. 2007; Farooqi et al. 2007) and thus results in higher fluoride concentrations (3.88–12.2 mg/L) in thermal waters collected from Attri, Tarabalo and Deulajhari. However, at other locations, the average fluoride concentrations are lower. The long residence time of the thermal water is one of the prime factors that contribute to higher fluoride concentration into the thermal waters (Hudak and Sanmanee 2003; Kim and Jeong 2005). In a granitic terrain, if the water resides for a prolonged period, the fluoride concentrations in the groundwater enrich continuously even after the groundwater reaches in equilibrium state with respect to fluorite (CaF2), mainly due to the removal of Ca by precipitation of calcite (CaCO3) (Kim and Jeong 2005; Chae et al. 2007). Higher fluoride concentrations are not uncommon in the geothermal waters and are reported from several geothermally active areas, such as the East African Rift Valley, New Zealand, France, Iceland, China, and parts of the western USA (Ozsvath 2008). Continuous discharge of higher fluoride-containing water from the geothermal spring of Attri, Tarabalo and Deulajhari may contaminate the local groundwater. The fluoride concentration higher than 1.5 mg/L in drinking water poses severe health risks to the population living the nearby areas (World Health Organisation (WHO) 2011; Centers for Disease Control and Prevention (CDCP) 1999).
In Bankhol and Magarmuhan, the discharging thermal water falls in Field-I and thus belongs to Ca–HCO3 water type (Fig. 6). The water from these thermal springs is poorly mineralized and has TDS values ranging between 16.9 and 20.9 mg/L. Shorter resident time in the subsurface coupled with heavy mixing with the shallow groundwater may cause the lowering of the TDS content (Bulbul 2015). However, studies have also established that vapors generated from the deep-seated high-temperature geothermal reservoir may get condensed at shallower depth and gives rise to thermal waters with lower TDS values (Glassley 2015). Such a condition is very unlikely in the study area as the temperature of the discharge water is quite low (36–45 °C). The outlet temperatures are very close to the average annual air temperature, suggesting that these waters do not circulate deeper and confine mainly to the shallow aquifers (Brombach et al. 2000).
The thermal waters discharging from Badaberena and Taptapani thermal spring belongs to Field-VI and is of Na–HCO3 type (Fig. 6). In general, except Attri, Tarabolo and Deulajhari, all other thermal spring water shows Na/Ca–HCO3 type characteristics which are a typical indicator of shallow groundwater. This suggests prominent mixing of shallow cold water which is believed to have been circulated within the alluvium or sedimentary formations with the ascending hot spring water (Singh et al. 2014). Higher concentrations of various ions have been observed in thermal waters from Attri, Tarabalo and Deulajhari, while, their concentrations remain very low for the mixed water type, especially those showing HCO3 characteristics (i.e., shallow groundwater). The near-surface water presumably has no residence time in the reservoir and only short or no contact with the wall rock, whereas the conditions for water in the deep reservoir are just opposite and thus they have considerably higher dissolved solid concentrations. The solute concentration of the thermal water tends to be directly related to the degree of mixing (Hem 1985). In the case of an extensive mixing with the near-surface water, the thermal water may nearly become as dilute as near-surface water. Mostly the near-surface water (i.e., shallow groundwater) has slightly higher ionic concentrations than the streams which typically contains ≈200 mg/L of dissolved salts (Hem 1985). Thus, it can be concluded that the thermal water of mixed water type (i.e., HCO3 water type) has undergone thorough mixing with shallow surface water. The difference in water chemistry of different thermal springs of the study area may also be due to the interaction with rocks of varied mineralogy, as the interaction of thermal water with the wall rock is another key factor that modifies the chemical characteristics of thermal water (Wen et al. 2012). It is also important to note that the thermal water from all the springs, irrespective of their water types, does not show a prominent seasonal variation and the concentration of most of the ions remains same throughout the year. Such behavior of thermal water has great implications, as the water from these thermal springs can be sampled any time during the year and generated data can be used for interpreting the subsurface parameters (i.e., reservoir temperature).
Conclusions
The thermal springs in the state of Odisha are clustered at eight places. The pH values of the thermal spring water have significant temporal and spatial variation showing moderately acidic (5.05) to moderately alkaline (8.93) character. The temperature of the thermal water ranges from 28 (Boden) to 58 °C (Tarabalo). Major ion concentrations and TDS in the thermal waters do not show much temporal variation, but significant spatial variation has been observed and the TDS concentration ranges from 16.9 (Bankhol) to 595 mg/L (Deulajhari). The absence of a significant seasonal variation in thermal water chemistry indicates that the water samples from these springs can be collected anytime during the year and can be used to determine various subsurface parameters (especially reservoir temperature) based on their chemical signatures. Anion variation diagram shows that the thermal waters from Attri, Tarabalo and Deulajhari fall in the chloride/mature water field; thus, chemical geothermometers can be applied to determine the reservoir temperature. The remaining thermal water samples fall in the immature water field, indicating that either the water has not been equilibrated with the geothermal reservoir or there is an extensive mixing of ascending thermal water with the shallow groundwater. Thus, the chemical geothermometers, if applied to these thermal waters, may not give the correct reservoir temperature. The plotting of thermal water samples into piper diagram further conforms that there is no appreciable seasonal change in the water chemistry of the thermal waters. The water samples from Attri, Tarabalo and Deulajhari fall in Na–Cl field of the piper diagram which indicates a deeper circulation through the granite rocks. However, the samples from other thermal springs fall in Na/Ca–HCO3 field indicating that the thermal water chemistry is shaped mainly by shallow water mixing processes. Further, higher fluoride concentration in thermal waters discharging from Attri, Tarabalo and Deulajhari springs poses a severe threat to local groundwater contamination.
References
Alcicek H, Bulbul A, Alcicek MC (2016) Hydrogeochemistry of the thermal waters from the Yenice Geothermal Field (Denizli Basin, Southwestern Anatolia, Turkey). J Volcanol Geotherm Res 309:118–138
American Public Health Association (APHA) (1995) Standard methods for the examination of water and wastewater, 19th edn. American Public Health Association, Washington, DC, pp 4–122
Brombach T, Marini L, Hunziker JC (2000) Geochemistry of the thermal springs and fumaroles of Basse-Terre Island, Guadeloupe, Lesser Antilles. Bull Volcanol 61:477–490
Bulbul A (2015) Mixing of geothermal and non-geothermal fluids in shallow aquifers in the Germencik-Nazilli area, Büyük Menderes Basin (SW Turkey). Geodin Acta 27(1):67–81
CDCP (Centers for Disease Control and Prevention) (1999) Achievements in public health, 1900–1999: fluoridation of drinking water to prevent dental caries. Morb Mortal Wkly Rep 48:933–940
Chae GT, Yun ST, Mayer B, Kim KH, Kim SY, Kwon JS, Kim K, Koh YK (2007) Fluorine geochemistry in bedrock groundwater of South Korea. Sci Total Environ 385(1–3):272–283
Chandrasekharam D (2000) Geothermal energy resources of India: Country Update. In: Proceedings world geothermal congress Kyushu-Tohoku, Japan
Chandrasekharam D, Antu MC (1995) Geochemistry of Tattapani thermal springs, Madhya Pradesh, India-field and experimental investigations. Geothermics 24(4):553–559
Chandrasekharam D, Chandrasekhar V (2010a) Geothermal energy resources, India: country update. World Geothermal Congress, Bali
Chandrasekharam D, Chandrasekhar V (2010b) Geochemistry of thermal springs of Orissa, India. GRC Trans 34:665–667
Chatterjee S, Sarkar A, Deodhar AS, Biswal BP, Jaryal A, Mohokar HV, Sinha UK, Das A (2017) Geochemical and isotope hydrological characterisation of geothermal resources at Godavari valley, India. Environ Earth Sci 76:97. doi:10.1007/s12665-017-6411-5
Chowdhury I, Ray B, Ali A (2011) Geology and mineral resources of Odisha. Geological Survey of India, Miscellaneous Publication No. 30 (3), Kolkata
Craig J, Absar A, Bhat G, Cadel G, Hafiz M, Hakhoo N, Kashkari R, Moore J, Ricchiuto TE, Thurow J, Thusu B (2013) Hot springs and the geothermal energy potential of Jammu and Kashmir State, N.W. Himalaya, India. Earth-Sci Rev 126:156–177
Dasgupta S, Bose S, Das K (2013) Tectonic evolution of the Eastern Ghats Belt, India. Precambrian Res 227:247–258
Dee DP, Uppala SM, Simmons AJ, Berrisford P, Poli P, Kobayashi S, Andrae U, Balmaseda MA, Balsamo G, Bauer P, Bechtold P, Beljaars ACM, van de Berg L, Bidlot J, Bormann N, Delsol C, Dragani R, Fuentes M, Geer AJ, Haimberger L, Healy SB, Hersbach H, Hólm EV, Isaksen L, Kållberg P, Köhler M, Matricardi M, McNally AP, Monge-Sanz BM, Morcrette JJ, Park BK, Peubey C, de Rosnay P, Tavolato C, Thépaut JN, Vitart F (2011) The ERA-Interim reanalysis: configuration and performance of the data assimilation system. Q J R Meteorol Soc 137:553–597
Farooqi A, Masuda H, Kusakabe M, Naseem M, Firdous N (2007) Distribution of highly arsenic and fluoride contaminated groundwater from east Punjab, Pakistan, and the controlling role of anthropogenic pollutants in the natural hydrological cycle. Geochem J 41:213–234
Gemici U, Feliz S (2001) Hydrochemistry of the Cesme geothermal area in western Turkey. J Volcanol Geotherm Res 110:171–187
Giggenbach WF (1988) Geothermal solute equilibria. Derivation of Na–K–Mg–Ca geoindicators. Geochica et Cosmochim Acta 52:2749–2765
Glassley WE (2015) Geothermal energy: renewable energy and the environment, 2nd edn. CRC Press, Taylor & Francis Group, Boca Raton, p 423. doi:10.1080/15567036.2015.1085286
Gokgoz A, Akdagoglu H (2016) Hydrogeology and hydrogeochemistry of a coastal low-temperature geothermal field: a case study from the Datca Peninsula (SW Turkey). Environ Earth Sci 75:1143. doi:10.1007/s12665-016-5957-y
Han DM, Liang X, Jin MG, Currell MJ, Song XF, Liu CM (2010) Evaluation of groundwater hydrochemical characteristics and mixing behaviour in the Daying and Qicun geothermal systems, Xinzhou Basin. J Volcanol Geotherm Res 189:92–104
Hem JD (1985) Study and interpretation of the chemical characteristics of natural water US. Geol Surv 2254:263
Hudak PF, Sanmanee S (2003) Spatial patterns of nitrate, chloride, sulfate, and fluoride concentrations in the woodbine aquifer of north-central Texas. Environ Monit Assess 82(3):311–320
Huffman GJ, Adler RF, Adler Bolvin DT, Gu G, Nelkin EJ, Bowman KP, Hong Y, Stocker EF, Wolff DB (2007) The TRMM multi-satellite precipitation analysis: quasi-global, multi-year, combined-sensor precipitation estimates at fine scale. J Hydrometeorol 8:38–55
Kim K, Jeong GY (2005) Factors influencing natural occurrence of fluoride-rich ground waters: a case study in the southeastern part of the Korean Peninsula. Chemosphere 58(10):1399–1408
Krishnaswamy VS (1975) Geothermal energy resources of India. In: Symposium on energy resources in India, Indian Science Congress, Abstracts
Mahala SC, Singh P, Das M, Acharya S (2012) Genesis of thermal springs of Odisha, India. Int J Earth Sci Eng 5(6):1572–1577
Michard G (1990) Behaviour of major elements and some trace elements (Li, Rb, Cs, Sr, Fe, Mn, W, F) in deep hot waters from granitic areas. Chem Geol 89:117–134
Mohapatra M, Mohanty UC (2006) Interannual variability of summer monsoon rainfall over Orissa in relation to tropospheric circulation features. Curr Sci 90:9–10
Nayak PN, Choudhury K, Sarkar B (1998) A review of geophysical studies of the Eastern Ghats Mobile Belt. Proc Workshop Eastern Ghats Mobile Belt Geol Surv India Spec Publ 44:87–94
Ozsvath DL (2008) Fluoride and environmental health: a review. Rev Environ Sci Biotechnol 8:59–79
Patra JP, Mishra A, Singh R, Raghuwanshi NS (2012) Detecting rainfall trends in twentieth century (1871–2006) over Orissa State, India. Clim Change 111:801–817
Piper M (1944) A graphic procedure in the geochemical interpretation of water-analyses. Am Geophys Union 25:914–923
Ramakrishnan M (1987) Stratigraphy, sedimentary environment and evolution of the late Proterozoic Indravati basin, Central India. Mem Geol Soc India 6:139–160
Sarkar A, Nanda JK (1998) Tectonic segments in the Eastern Ghats Precambrian mobile belt (Abs.) Dr. M.S. Krishnan Comm. National Seminar, Calcutta, pp 173–74
Sastri V, Bs Venkatachala, Narayanan V (1981) The evolution of the east coast Of India. Palaeogeogr Palaeoclimatol Palaeoecol 36:23–54
Savage D, Mark RC, Milodowski AE, George I (1987) Hydrothermal alteration of granite by meteoric fluid: an example for the Carnmenellies granite, United Kingdom. Contrib Mineral Petrol 96:391–405
Shanker R, Guha SK, Seth NN, Mathuraman K, Pitale UL, Jangi BL, Prakash G, Bandyopadhyay AK, Sinha RK (1991) Geothermal Atlas of India. Geol Surv India Spec Publ 19:144
Singh HK, Chandrasekharam D, Trupti G, Singh B (2014) Geochemical Investigations on Thermal and Cold Springs at Dumka District, Jharkhand, India. Int J Earth Sci Eng 07(1):190–194
Thussu JL (2002) Geothermal energy resources of India. Geol Surv India Spec Publ 69:210
Wen Y, Wang N, Hu Z (2012) Hydrochemistry of geothermal water in Tianshui and adjacent area, Gansu province, China. Environ Earth Sci 67(5):1281–1290
White DE (1957) Thermal waters of volcanic origin. Geol Soc Am Bull 68:1637–1658
WHO (2011) Guidelines for drinking-water quality, 4th edn. World Health Organization, Geneva
Xilai Z, Armannsson H, Yongle L, Hanxue Q (2002) Chemical equilibria of thermal waters for the application of geothermometers from the Guanzhong basin, China. J Volcanol Geotherm Res 113:119–127
Acknowledgements
The authors are thankful to the Ministry of Earth Sciences (MoES), Govt. of India for providing the financial support through Bay of Bengal Coastal Observatory (BoBCO). Horthing V. Zimik gratefully acknowledges the financial support recieved from the Council of Scientific & Industrial Research (CISR), India through the research fellowship (award number 09/1059(008)/2014-EMR-I). Indian Institute of Technology Bhubaneswar is specially thanked for providing necessary laboratory facility to carry out this research work. TRMM 3B43 monthly precipitation data used in this study are freely available at https://giovanni.sci.gsfc.nasa.gov/giovanni/. ECMWF surface temperature data are freely available at http://apps.ecmwf.int/datasets/data/interim-full-daily/levtype=sfc/. The authors thank the anonymous reviewer for the valuable suggestions and comments which helped improve the quality of the original manuscript.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Zimik, H.V., Farooq, S.H. & Prusty, P. Geochemical evaluation of thermal springs in Odisha, India. Environ Earth Sci 76, 593 (2017). https://doi.org/10.1007/s12665-017-6925-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12665-017-6925-x