Abstract
A geochemical study on thermal water has been carried out in Tianshui and its adjacent area, Gansu province, China. Chemical and isotopic contents were employed in the investigation on the origin and evolution of thermal water and the evaluation of reservoir temperature in the geothermal systems. Thermal waters in Wushan and Tianshui are characterized by outlet temperatures from 15 to 38°C and low TDS (226–255 mg/L), defined as bicarbonate water. Its origin may be attributed to the interaction between meteoric rain, biotite plagioclase gneiss and carbonate reservoir rocks. In contrast, thermal waters in Tongwei and Qingshui have higher outlet temperatures of 25–54.2°C and a moderate TDS of 915–1,793 mg/L, regarded as sulfate waters. These sulfate waters may arise from the interaction between meteoric water, granite and amphogneiss. Isotopic data presented here suggest that thermal waters in the study area have a meteoric origin without being significantly effected by water–rock isotope exchange. Chemical geothermometry indicates the existence of a deep geothermal reservoir of low-to-medium enthalpy (70–111°C) in the Tianshui study area.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
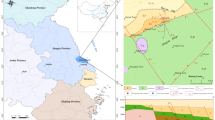
The Tianshui study area (34°21′N–35°39′N, 104°29′E–106°17′E) lies in Gansu province in the northwestern part of China. It is one of the major geothermal fields with an altitude of about 1,200 m above sea level (m a.s.l.). The topography of the Tianshui study area is highly rugged with a deep valley in the central part and steep slopes at the southern part (west Qinling Mountain), western part (Huajialing Mountain) and eastern part (Liupan Mountain). The Wei River is the main river in the study area while the rivers Shandan, Sandu, Hulu and Niutou form the major tributaries to the Wei River. The Tianshui study area includes Tianshui city, Dingxi city and 11 counties with a population density of 223 persons per km2. Most cities and counties depend predominantly on agriculture, while Tianshui city has some foundations in manufacturing of secondary industry and maintains good momentum of development in trade and business, finance and information of tertiary industries. Therefore, Tianshui city has strong secondary and tertiary industries (Fig. 1). Thermal waters of the study area have been used therapeutically since the Northern Wei Dynasty and are among the most popular thermal springs in Gansu province, improving the social and economic well being of the region. The natural discharge of the springs has recently decreased. Discharge volume of some wells has decreased sharply as a result of the increasing production volume over a period of years. Sustainable utilization of thermal waters in the study area is, therefore, of great concern.
The origin and evolutional process of low- to medium-enthalpy geothermal systems have long been studied. Most of these studies have focused on three aspects: (1) the interaction between thermal waters and wall rock has a fundamental control on the chemical characteristic of thermal waters (Gemici et al. 2004; Cruz and França 2006); (2) the origin of geothermal waters can be traced by using stable isotope composition (Ahmad et al. 2001; Papp and Niţoi 2006); and (3) the reservoir can be estimated through the use of chemical geothermometry (Ahmad et al. 2002). These advances in geothermal research provide tools for studying geothermal waters. Recently, attention has been focused on the distribution pattern and formation of geothermal waters (Chen and Li 2007; Zhang 2009) and subsurface geological formations and structures that form the geothermal reservoirs in this area (Zhang and Li 2006). In contrast, the hydrochemistry of thermal waters in Tianshui and adjacent area is less well known. Therefore, the objective of this paper is to analyze systematically hydrogeochemical properties, estimate reservoir temperature and propose a conceptual model for the origin of thermal waters in this area.
Geological setting
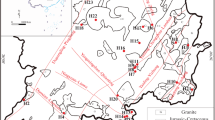
The Tianshui study area is an active zone tectonism and offers favorable setting uplift-fracture-convection type geothermal resources. The area is located within a complex geodynamic setting characterized by active tectonism and proximity to the triple junction between the north China block, the south China block and the Tibet plateau block, where neotectonism is remarkably active. Major structures include the east–west-trending faults of the Western Qilian–Qinling orogen (Fig. 2a, b). Bedrock geological formation mainly consists of Indosinian–Yanshanian granite (granodiorite, granosyenite and orthophyre), Devonian metamorphic rock (biotite quartz gneiss, biotite plagioclase gneiss) and Paleocene sandstone containing silty mudstone.
a Geological sketch map of Tianshui and adjacent area, China. Also denoted are major fault structural and sample locations from this study. Inset shows an approximate position of studied region in the Chinese tectonic system. b Generalized geologic cross section along Wushan–Tongwei and Tianshui–Qingshui
The distribution of thermal waters is primarily controlled by fractures associated with regional geological structures. Thermal waters in the study area occur mainly along the south–north-trending buried deep fault and the north–north–east trending regional fracture zone and particularly at the intersections of these major structures. For example, the Wushan and Tongwei springs (sample numbers 8, 9, 10, 11, 14 and 15) occur along the western uplift belt of Tongwei–Wushan–Zhouqu, while the Tianshui and Qingshui springs (sample numbers 5, 6, 12 and 13) occur along the eastern uplift belt of Qingshui–Chengxian (Fig. 1). All springs in the study area are defined as geothermal resources of hydrothermal convection, and their outlet temperatures range from 14.5 to 54.2°C. In China, springs with temperatures of 25°C or greater are classified as “hot springs”.
Method
A systematic geochemical survey of waters was carried out in the Tianshui study area. A total of 15 water samples consisting of hot springs/drill holes (drill holes are artificial wells in “hot spring” areas as a result of overproduction of hot spring), cold springs and domestic wells were analyzed both chemically and isotopically. All these water samples were collected from four sites of Tianshui, Wushan, Tongwei and Qingshui. They are greater than 300 m in depth. Hot spring water samples from drill holes in four sites were collected at various depths using a pre-rinsed stainless steel down-hole sampler. Cold springs were usually located near the valley floor where they were conspicuous by the wet ground and dense vegetation. Cold springs were sampled at the source where possible. The domestic well waters were sampled at household taps in four sites; water was allowed to run for several minutes prior to sample collection. Geothermal reservoir temperature was estimated using chemical geothermometry, and circulation depth was calculated according to the local geothermal gradient.
Sampling and analysis
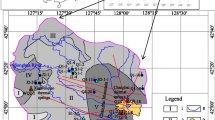
To investigate systematically the hydrochemistry of thermal waters in the Tianshui study area, a total of 15 samples were collected from different sites (Fig. 1), where 8 samples were taken from hot spring (sample numbers 5, 6, 9, 10, 11, 12, 13 and 15), 2 samples were collected from cold springs (sample numbers 8 and 14), and 5 samples were taken from domestic wells (sample numbers 1, 2, 3, 4 and 7). Temperature, pH, conductivity and total dissolved solids (TDS) were measured directly in the field using a Multi350i Instrument, with an accuracy of 0.1°C, 0.01 pH, 1 μS/cm and 0.01 mg/L, respectively. Conductivity of water is affected by temperature, so it is necessary to regulate temperature to 25°C when measuring conductivity. An acid–base titration was performed in the field to obtain the concentration of HCO3 − and CO3 2−. All samples were filtered (0.45 μm) and then stored in polyethylene bottles that were rinsed multiple times using deionized water. The 15 filtered samples were further divided into two sets. One set of samples was acidified by HNO3 solution to determine the concentrations of cation and SiO2. The non-acidified set was used for the analysis of anion concentration.
Laboratory measurements
The major chemical constituents of the 15 samples were analyzed through standard methods. The concentrations of Na+, K+, Ca2+, Mg2+ and SiO2 were determined with atomic absorption spectrophotometer (AAS), while the anion concentrations of Cl−, SO4 2− and NO3 − were measured with ion chromatograph, at Key Laboratory, Western China’s Environmental Systems (MOE), Lanzhou University. The isotopic determinations of δ18O and δ2H were carried out on an isotope mass spectrometer (IMS), at the Isotope Laboratory, Institute of Geography Science and Resource, Chinese Academy of Sciences. The δ18O compositions of water were determined using CO2 equilibrated with water with an accuracy of 0.2‰; the δ2H compositions of water were determined from the H2 generated by the Zn-reduction method with an accuracy of 2.0‰.
Water chemistry
Results of water analysis are tabulated in Table 1. Two principal groups of waters are defined in the Piper diagram (Fig. 3): (1) Ca–HCO3, Na·Ca–HCO3 and Na–HCO3 type of waters. All shallow groundwater in the study area, the Wushan and Tianshui springs (sample numbers 1, 2, 3, 4, 5, 6, 7, 8 and 9) fall within this group of water types. (2) Na·Ca–SO4, Na·Ca–SO4·Cl and Na–SO4·Cl type of waters and Na–HCO3·SO4·Cl type of waters are characterized by mixing. The Tongwei and Qingshui hot springs (sample numbers 10, 11, 12, 13 and 15) and the Tongwei cold spring (sample number 14) fall into these types of waters, respectively.
Piper plot showing the chemical variability of selected thermal and cold groundwaters in Tianshui and adjacent area, China. Number refers to Sample ID (see Table 1)
Bicarbonate water
All domestic well waters collected in the study area and springs from the Wushan and Tianshui fall into this group of bicarbonate water. Their temperature range is from 11 to 38°C, and pH falls in the interval of 7.53–9.06. HCO3 is the dominant component; therefore, these waters can be considered as HCO3 − type of water characterized by low TDS (224–378 mg/L). Hot springs and cold springs have higher TDS and conductivities than that of the domestic well waters, as would be expected due to their longer residence time in the subsurface.
Four domestic well water samples (1, 2, 3 and 7) are dominated by Ca and HCO3. Domestic well water (sample number 4) and Wushan cold spring (sample number 8) belong to Na·Ca–HCO3 type of water. Meteoric water can readily reach saturation with calcite composition during the initial stage of interaction with minerals including calcite. The dissolution rate of calcite is 2–6 orders of magnitude greater than that of Al-silicates, depending upon the pH (Stumm and Morgan 1996). The Tianshui and Wushan hot springs (sample numbers 5, 6 and 9) belong to Na–HCO3 water with a higher temperature ranging from 36.9 to 38°C. This type of water may be formed by the interaction between meteoric waters, biotite plagioclase gneiss and carbonate rocks in deeper reservoirs of the Tianshui and Wushan regions. SiO2 concentration (25.7–55.0 mg/L) of the Na–HCO3 and Na·Ca–HCO3 waters is much higher than that of Ca–HCO3 waters (11.4–16.5 mg/L), which suggests that deep circulation time and interaction degree between water and wall rock are greater for Na–HCO3 and Na·Ca–HCO3-type waters than for Ca–HCO3-type waters. This conclusion is further illustrated by the relatively high fluoride content in the Na–HCO3 and Na·Ca–HCO3 waters. In general, temperature within this group of thermal waters is slightly higher than the average annual air temperature of the local area. This phenomenon together with the chemical and physical characteristics of these waters indicate that they originate from shallow hydrological circulation and are typical of the first stages of the interaction between water and wall rock.
Sulfate water
SO4 is the primary anion (567.7–988.3 mg/L) in this group of sulfate water. Na·Ca–SO4 type of waters comprising samples 12 and 13 were taken from the Qingshui hot spring. These waters have an outlet temperature ranging from 25 to 54.2°C, and their TDS range is between 1,267 and 1,793 mg/L. Na·Ca–SO4·Cl and Na–SO4·Cl types of waters represented by samples 10, 11 and 15 were collected from the Tongwei hot spring. These waters are characterized by relatively high temperature ranging from 49.3 to 53°C and a moderate TDS range of 915–930 mg/L. Limited interaction between meteoric waters and gneissic rocks produces Ca–HCO3 and Na·Ca–HCO3 waters, while prolonged interaction between meteoric waters and the same rocks yields Na–SO4 waters (Pastorelli et al. 2001). Interaction between Na–HCO3 water and anhydrite or Ca–SO4 water and a local gneissic rock can also be used to explain the resultant Na–SO4 type of water (Parnachev et al. 1999). The chemical property of this group of sulfate waters may be attributed to the interaction between meteoric water, granite and amphogneiss in deep reservoir rocks of the Qingshui and Tongwei regions. The Qingshui and Tongwei hot springs smell of H2S, which may result from sulfate-reducing conditions. The Na–HCO3·SO4·Cl water with a lower temperature (14.5°C) and TDS (634 mg/L) represented by Tongwei cold spring (sample number 14) is probably attributed to mixing of cold water of low TDS and geothermal fluid of high temperatures and TDS.
There is no obvious trend of positive correlation between TDS and the concentration of main ions (Fig. 4), but the concentration of main ions increases with the increase of TDS except Mg. It is evident that sulfate waters have higher concentrations of Na, Ca, K, Cl, SO4, and SiO2 compared with the bicarbonate water. The reason why Mg content decreases with the increase of TDS is that on the one hand, main chemical components of core samples collected from wall rock of spring are mainly composed of SiO2 (70.7%) and Al2O3 (14.6%), and MgO only occupies a small percentage (0.26%); therefore, the source of Mg is scarce. On the other hand, Mg may be diminished remarkably during the chemical reaction with wall rock (Lambrakis and Kallergis 2005). Although there is a dramatic Na–Ca cation exchange occurring during thermal water circulation, the increase in Ca content with the increase of TDS is probably related to the dissolution of anorthite, since there is an amount of quartz, feldspar, mica and other aluminosilicate minerals in deep reservoir. Dotsika et al. (2006) has pointed out that the increase of Ca content is probably due to its being in equilibrium with a hydrothermal mineral (e.g. Na–k-feldspar, calcite, muscovite, etc.) at high temperature (>200°C) and CO2 pressure, instead of coming from the cold groundwater.
Stable isotope analysis
Isotopic compositions of hydrogen and oxygen of hot spring are regarded as effective proxies to trace water origin (Wang et al. 1995). The analytical results of the stable isotopes of hydrogen and oxygen from the Tianshui study area are tabulated in Table 2. The relationship between the δD and δ18O is shown in Fig. 5, which illustrates that most water samples lie along the global meteoric water line (GMWL) established by Craig (1961) and the meteoric water line of northwestern China established by Gao (1993). The correlation function for rainwater in northwestern China is δD = 7.38 δ18O + 7.16 (r = 0.978; n = 100). This function is established based on rain samples collected from five weather stations in northwestern China. The function seems to be close to the global meteoric water line but exhibits a smaller slope and delta D intercept, which indicates less precipitation and a more arid climate. It can be inferred from the distribution of hydrogen and oxygen isotopes that thermal waters are probably of meteoric origin in the study area. Domestic well water (sample number 7) collected from Wushan shows the sign of obviously positive delta 18O shift compared with the meteoric water line. This deviation seems to be related to a stronger dynamic evaporation process induced by relatively large surface area of the domestic well.
The stable isotope contents are quite variable. Cold spring and domestic well water have lower TDS and higher δ18O values compared with hot spring water, which suggests that cold spring and domestic well water experienced relatively short subsurface circulation. As thermal water experiences subsurface circulation and then is exposed on the surface, the isotope content of thermal water will suffer remarkable variation as evaporation is accompanied by the isotope fractionation. The change of isotope content is comparable in the subsurface circulation and in the surface evaporation processes (Shangguan et al. 1998). Therefore, the deeper the thermal water infiltrates, the more δD and δ18O contents decrease with increasing temperature. But there is no evidence for the positive shift of the δ18O. The scale of the δ18O shift is dependent on the reservoir temperature, the residence time and the degree of water–rock interaction (Motyka et al. 1993). However, the δD value is generally invariable due to little or even no hydrogen in reservoir wall rock. This low isotope exchange between water and its wall rock in this area may reflect a relatively low reservoir temperature, which is in agreement with the other medium–low temperature thermal systems (Marini et al. 2000; Gherardi et al. 2002; Koh et al. 2008). It can be concluded that thermal waters in the study area are of meteoric origin and not severely affected by water–rock isotope exchange.
Chemical geothermometry
Reservoir temperature which is an essential proxy to classify geothermal resources and evaluate potential of thermal water can be well constrained by geothermometry. Silica geothermometry and ionic solute geothermometry have been successfully used to estimate thermal reservoir temperature (Wang et al. 1993).
Water–mineral equilibria should be identified prior to the application of chemical geothermometers. The amount of silica dissolved in thermal waters is governed primarily by the solubility of chalcedony below the temperature of 110°C (Arnorsson 1975). The saturation index of chalcedony closer to zero than that of quartz for most water samples, which indicates equilibrium with chalcedony appears to be established. This conclusion on the equilibrium between water and chalcedony in this paper is in agreement with the study results of Arnorsson (1975). Therefore, chalcedony geothermometer free of vapor loss can be considered as a reliable method to evaluate reservoir temperature for the neutral thermal waters, in the low temperature condition with no boiling gasification. In contrast, the quartz geothermometer free of vapor loss offer a relatively high reservoir temperature (Table 3).
The Na–K–Mg ternary diagram proposed by Giggenbach (1988) was used to evaluate water–mineral equilibrium status and distinguish different types of water. Waters can be divided into three groups (fully equilibrated water, partially equilibrated water and immature water) in the ternary diagram. Thereby, the ternary diagram is widely employed to test whether water is suitable for the ionic solute geothermometer. In this study, most waters (sample numbers 5, 6, 9, 10, 11, 12 and 13) fall into the partially equilibrated field in the Na–K–Mg ternary diagram (Fig. 6), suggesting that they are representative of equilibration in the reservoir, whereas some waters (sample numbers 8, 14 and 15) tend to shift toward the Mg1/2 vertex and are considered as immature waters. These immature waters have relatively high Mg contents and similar isotopic values to the meteoric water, which suggests they may be attributed to being mixed with shallow groundwater and resultant limited water–rock reaction. So it is meaningless to apply the ionic solute geothermometer directly to these immature waters (Gemici and Filiz 2001).
Distribution of thermal waters in the Na–K–Mg triangular diagram (Giggenbach 1988) showing degree of water–rock equilibrium
Water–mineral equilibrium and geothermometry were performed to evaluate thermal reservoir temperature of thermal waters that fall into the partially equilibrated field, and calculated data of thermal reservoir temperature were tabulated in Table 4. It can be observed from this table that the K–Mg geothermometer yields higher reservoir temperatures than Na–K geothermometer for the Tianshui and Wushan hot springs (sample numbers 5, 6 and 9). The reason is that they are bicarbonate water with relatively low temperature, in which Na and K do not reach the cation exchange equilibrium. Therefore, it does not make sense to employ Na–K geothermometer to evaluate reservoir temperatures of these samples. Sepúlveda et al. (2004) have come to the same conclusion that the Na–K geothermometer is most applicable for the chloride-rich waters equilibrated at >180°C. The application of the Na–K–Ca geothermometer results in obviously high reservoir temperatures for the Tianshui and Wushan hot springs (sample numbers 5, 6 and 9). The reason is that Na–K–Ca geothermometer is not a mere temperature function, but is also controlled by PCO2, as it combines Na–K geothermometer and Na–Ca–CO2 indicator (Chiodini et al. 1991). Bicarbonate waters (sample numbers 5, 6 and 9) characterized by relatively high HCO3 content are rather sensitive to PCO2; therefore, their reservoir temperatures cannot be evaluated accurately by the Na–K–Ca geothermometer.
To sum up, the K–Mg and chalcedony geothermometers yield comparable reservoir temperatures, while other geothermometers result in significant deviations for the same water sample, indicating that certain geothermometer should be adopted according to special conditions. The Wushan and Tongwei cold springs (sample numbers 8 and 14) may suffer from being mixed with low-salinity waters, and then be re-equilibrated in the secondary reservoirs at about 30°C as suggested by chemical geothermometers. In general, the reservoir temperature range of thermal waters in Tianshui and its adjacent area is about 70–111°C using chemical geothermometers; this range is coincident with Fig. 6. Thermal waters in the study area can be regarded as an identical thermal system of medium–low temperature. Given that the geothermal gradient is about 35°C/km in eastern Gansu province (Lei et al. 1999), it can be inferred that the reservoir depth of these thermal waters fall into a depth between 1.9 and 3.1 km.
Conclusion
This research will provide a scientific basis on the evaluation and sustainable exploitation for thermal water in Tianshui and adjacent area. Stable isotope analysis suggests that thermal waters in Tianshui and its adjacent area are of meteoric origin. Compared with domestic well water, thermal waters in the study area have lower δD and δ18O values, indicating that they experienced a longer subsurface circulation. Meteoric water probably interacts with biotite plagioclase gneiss rock and carbonate in the reservoir when it infiltrates gradually along the fracture belts. Cold meteoric water turns into thermal water by absorbing the inner heat of the earth and then is exposed in river valleys around the Tianshui and Wushan regions via intricate fracture belts. These exposed springs are bicarbonate waters with an outlet temperature ranging from 15 to 38°C and low TDS (226–255 mg/L). In the Tongwei and Qingshui regions, meteoric water develops into sulfate waters in geothermal reservoir, probably due to the interaction with its wall rocks of granite and amphogneiss. These sulfate waters are characterized by higher outlet temperature ranging from 25 to 54.2°C and relatively high TDS (915–1,793 mg/L). Chemical and isotopic compositions of the cold springs (sample numbers 8 and 14) in Wushan and Tongwei indicate that they have been diluted by low-salinity cold water. The higher contents of Na, K, Ca, Cl, SO4 and SiO2 in thermal waters than those in cold waters are attributed to a prolonged interaction between water and its wall rock during longer subsurface circulation. Obviously, the chemical characteristics of thermal waters are mainly controlled by the chemistry of the wall rocks and the extent of water–rock reaction.
The equilibrium state of thermal waters should be identified prior to application of chemical geothermometers. In comparison with quartz, chalcedony approaches equilibrium. The Na–K geothermometer is suitable for chloride-rich waters characterized by longer residence time and higher temperature. Bicarbonate waters cannot be evaluated accurately using the Na–K–Ca geothermometer because they are sensitive to PCO2. The K–Mg geothermometer can be adopted in low-temperature geothermal systems. The Wushan and Tongwei springs (sample numbers 8 and 14) are mixed waters, originating from a second geothermal reservoir at a temperature of about 30°C. Chemical geothermometry records indicate that a main geothermal reservoir is present in the study area with a temperature range of 70–111°C, at depths ranging from 1.9 to 3.1 km, which is coincident with the local geothermal gradient of about 35°C/km. Climate change combined with overproduction by developers (Guo et al. 2005) is likely responsible for the decreasing discharge of these springs. In view of the mean annual precipitation of a mere 500 mm in the Tianshui region, sustainable utilization of the thermal water will be maintained only if the total withdrawal rate is reduced and an alternating pumping and non-pumping pattern is used, Otherwise, the thermal springs may experience gradual lowering of flows to the point where the springs dry up and production wells become the only source of thermal water.
References
Ahmad M, Akram W, Hussain SD, Sajjad MI, Zafar MS (2001) Origin and subsurface history of geothermal water of Murtazabad area, Pakistan—an isotopic evidence. Appl Radiat Isot 55:731–736
Ahmad M, Akram W, Ahmad N, Tasneem MA, Rafid M, Latif Z (2002) Assessment of reservoir temperatures of thermal springs of the northern areas of Pakistan by chemical and isotope geothermometry. Geothermics 31:613–631
Arnorsson S (1975) Application of the silica geothermometer in low temperature hydrothermal areas in Iceland. Am J Sci 275:763–783
Chen Y, Li Y (2007) Analyzing distribution and developmental prospect of thermal groundwater in eastern Gansu province. Ground Water 29(4):43–44 (in Chinese)
Chiodini G, Cioni R, Guidi M, Marini L (1991) Chemical geothermometry and geobarometry in hydrothermal aqueous solutions: a theoretical investigation based on a mineral-solution equilibrium model. Geochim Cosmoch Acta 55:2709–2727
Craig H (1961) Isotopic variations in meteoric waters. Science 133:1702–1703
Cruz JV, França Z (2006) Hydrogeochemistry of thermal and mineral water springs of the Azores archipelago (Portugal). J Volcanol Geotherm Res 151:382–398
Dotsika E, Leontiadis I, Poutoukis D, Cioni R, Raco B (2006) Fluid geochemistry of Chios geothermal area, Chios Island, Greece. J Volcanol Geotherm Res 154:237–250
Fournier RO (1977) A review of chemical and isotopic geothermometers for geothermal systems. In: Proceedings of the symposium on geothermal energy. Cento Scientific Programme, Ankara
Fournier RO, Truesdell AH (1973) An empirical Na–K–Ca geothermometer for natural waters. Geochim Cosmoch Acta 37:515–525
Gao Z (1993) Discussion on feature of isotope component from atmospheric water, groundwater and underground water in Northwest area, China. Acta Geology Gausu 2:94–101
Gemici Ü, Filiz Ş (2001) Hydrochemistry of the Çeşme geothermal area in western Turkey. J Volcanol Geotherm Res 110:171–187
Gemici Ü, Tarcan G, Çolak M, Helvaci C (2004) Hydrogeochemical and hydrogeological investigations of thermal waters in the Emet area (Kütahya, Turkey). Appl Geochem 19:105–117
Gherardi F, Panichi C, Yock A, Abaya JG (2002) Geochemistry of the surface and deep fluids of the Miravalles volcano geothermal system(Costa Rica). Geothermics 31:91–128
Giggenbach WF (1988) Geothermal solute equilibria derivation of Na–K–Mg–Ca geoindicators. Geochim Cosmoch Acta 52:2749–2765
Giggenbach WF, Confiantini R, Jangi BL, Truesdell AH (1983) Isotopic and chemical composition of Parbati valley geothermal discharges, north-west Himalaya, India. Geothermics 12:199–222
Guo Q, Wang Y, Ma T, Li L (2005) Variation of karst spring discharge in the recent five decades as an indicator of global climate change: a case study at Shanxi, northern China. Sci China Ser D-Earth Sci 48(11):2001–2010 (in Chinese)
Koh YK, Choi BY, Yun ST, Choi HS, Mayer B, Ryoo SW (2008) Origin and evolution of two contrasting thermal groundwaters (CO2-rich and alkaline) in the Jungwon area, South Korea: Hydrochemical and isotopic evidence. J Volcanol Geotherm Res 178:777–786
Lambrakis N, Kallergis G (2005) Contribution to the study of Greek thermal springs: hydrogeological and hydrochemical characteristics and origin of thermal waters. Hydrogeol J 13:506–521
Lei F, Dong Z, Liu B, Ma L, Zhao Y (1999) The geo-temperature field in Gansu–Ningxia–Qinghai area and its relation to earthquakes. J Gansu Sci 11:22–27 (in Chinese)
Marini L, Bonaria V, Guidi M, Hunziker JC, Ottonello G, Zuccolini MV (2000) Fluid geochemistry of the Acqui Terme-Visone geothermal area (Piemonte, Italy). Appl Geochem 15:917–935
Motyka RJ, Nye GJ, Turner DL, Liss SA (1993) The Geyser Bight geothermal area, Umnak Island, Alaska. Geothermics 22:301–327
Papp DC, Niţoi E (2006) Isotopic composition and origin of mineral and geothermal waters from Tuşnad Băi Spa, Harghita Mountains, Romania. Geochem Explor 89:314–317
Parnachev VP, Banks D, Berezovsky AY, Schönberg DG (1999) Hydrochemical evolution of Na–SO4–Cl groundwaters in a cold, semi-arid region of southern Siberia. Hydrogeol J 7:546–560
Pastorelli S, Marini L, Hunziker J (2001) Chemistry, isotope values (δD, δ18O, δ34Sso4) and temperatures of the water inflows in two Gotthard tunnels, Swiss Alps. Appl Geochem 16:633–649
Sepúlveda F, Dorsch K, Lahsen A, Bender S, Palacios C (2004) Chemical and isotopic composition of geothermal discharges from the Puyehue-Cordon Caulle area (40.5°S), Southern Chile. Geothermics 33:655–673
Shangguan Z, Dou J, Zang W, Wang J, Kong L, Gao S (1998) Modern geothermal fluid geochemistry in Tan-lu fault and Jiao-Liao fault blocks. Sci China (Ser D) 28:23–29 (in Chinese)
Stumm W, Morgan JJ (1996) Aquatic chemistry: chemical equilibria and rates in natural waters, 3rd edn. John Wiley and Sons, New York
Truesdell AH (1976) Summary of section III, geochemical techniques in exploration. In: Proceedings of 2nd UN symposium on the development and use of geothermal resources. San Francisco
Wang J, Xiong L, Pang Z (1993) Low medium temperature geothermal system of convection type. Science Press, Beijing (in Chinese)
Wang W, Zhang J, Hu G, Yang F, Xu Z (1995) Geochemistry of hot spring in Northwestern Hunan, China. Sci China (Ser B) 25:427–433 (in Chinese)
Zhang W (2009) Discussion on cause of formation of geothermal water in Wushan region. Gansu Metall 31:48–49 (in Chinese)
Zhang S, Li B (2006) The geological and geophysical characteristics of hot spring distribution in Tianshui and its NS adjacent areas. Northwest Seismol J 28:252–257 (in Chinese)
Acknowledgments
This research is financially supported by the National Natural Science Foundation of China (No. 50879033). The authors would like to thank two anonymous reviewers for their useful remarks. They are also grateful to James W. LaMoreaux, Editor-in-Chief, for his patience and constructive guidance during the completion of the manuscript.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Wen, Yh., Wang, Na. & Hu, Z. Hydrochemistry of geothermal water in Tianshui and adjacent area, Gansu province, China. Environ Earth Sci 67, 1281–1290 (2012). https://doi.org/10.1007/s12665-012-1571-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12665-012-1571-9